Abstract
During early mouse embryogenesis, multiple patterning and differentiation events require the activity of Nodal, a ligand of the transforming growth factor-beta (TGFβ) family. Although Nodal signaling is known to require activity of EGF-CFC co-receptors in many contexts, it has been unclear whether all Nodal signaling in the early mouse embryo is EGF-CFC dependent. We have investigated the double null mutant phenotypes for the EGF-CFC genes Cripto and Cryptic, which encode co-receptors for Nodal, and have found that they have partially redundant functions in early mouse development. Expression of Cripto and Cryptic is non-overlapping prior to gastrulation, since Cripto is expressed solely in the epiblast whereas Cryptic is expressed in the primitive endoderm of the late blastocyst and the visceral endoderm after implantation. Despite these non-overlapping expression patterns, Cripto; Cryptic double mutants display severe defects in epiblast, extraembryonic ectoderm, and anterior visceral endoderm (AVE), resulting in phenotypes that are highly similar to those of Nodal null mutants. Our results indicate that both Cripto and Cryptic function non-cell-autonomously during normal development, and that most if not all Nodal activity in early mouse embryogenesis is EGF-CFC-dependent.
Keywords: mouse, Nodal signaling, epiblast, extraembryonic ectoderm, visceral endoderm, anterior-posterior axis, non-cell-autonomy
The Nodal signaling pathway plays a central role in patterning the mouse embryo from pre-gastrulation through early somite stages. In particular, Nodal signaling is required for specification of the anterior-posterior and left-right body axes, as well as formation of the primary germ layers (reviewed in (Schier, 2003; Schier and Shen, 2000; Shen, 2007; Whitman, 2001)). Furthermore, recent studies have demonstrated that Nodal signaling plays critical roles in multiple patterning and differentiation events at pre-gastrulation stages of development (Brennan et al., 2001; Guzman-Ayala et al., 2004; Mesnard et al., 2006).
Following embryo implantation, Nodal is expressed in the epiblast, and mediates reciprocal interactions between the epiblast as well as two adjoining extraembryonic tissues: the visceral endoderm and extraembryonic ectoderm (ExE). Null mutants for Nodal display severe defects in all three of these tissues. In the epiblast, Nodal mutants display decreased expression of pluripotency markers, suggesting that Nodal signaling is required to maintain the undifferentiated state of the epiblast (Brennan et al., 2001; Mesnard et al., 2006). In the visceral endoderm, Nodal signaling is required for the specification of the distal visceral endoderm (DVE) as well as its subsequent translocation to the prospective anterior side, thereby establishing the anterior-posterior axis (Brennan et al., 2001; Norris and Robertson, 1999). In the ExE, Nodal signaling is essential for maintenance of the ExE and prevents differentiation of trophoblast stem cells (Brennan et al., 2001; Guzman-Ayala et al., 2004).
Nodal signaling is mediated by an activin receptor complex composed of a dimer of the type I serine-threonine receptor ALK4 (ActRIB) and a dimeric type II activin receptor, either ActRII or ActRIIB. Following receptor activation, Smad2 and/or Smad3 are phosphorylated and accumulate together with Smad4 in the nucleus to mediate transcriptional responses (Yan et al., 2002; Yeo and Whitman, 2001) together with the FoxH1 (FAST) winged-helix transcription factor (Chen et al., 1996; Chen et al., 1997), as well as members of the Mixer subfamily of homeodomain proteins (Germain et al., 2000). The Nodal pathway differs from that for Activin in that Nodal signaling requires the activity of EGF-CFC co-receptors (Reissmann et al., 2001; Yan et al., 2002; Yeo and Whitman, 2001), whereas Activin does not require a co-receptor for its signaling activity (Gritsman et al., 1999; Kumar et al., 2001; Yan et al., 2002). EGF-CFC co-receptors are small cysteine-rich extracellular proteins that are attached to the cell membrane by a glycosyl-phosphatidylinositol (GPI) linkage (Shen, 2007; Shen and Schier, 2000; Wechselberger et al., 2005). Two EGF-CFC genes are present in the mammalian genome, Cripto and Cryptic, while a varying number are present in other vertebrate species, including the single zebrafish gene, one-eyed pinhead (oep).
A central question regarding the function of EGF-CFC proteins has been whether they are essential for all aspects of Nodal signaling, as originally indicated by studies in the zebrafish (Gritsman et al., 1999). Analyses of null mutants in the mouse, however, have been less definitive on this issue. Although Cripto and Cryptic single mutants both display phenotypes associated with defects in Nodal signaling, it has been unclear whether all Nodal functions correspond to specific requirements for these EGF-CFC genes. Null mutants for Cripto lack embryonic mesoderm and definitive endoderm, and are defective in anterior translocation of the distal visceral endoderm (DVE) (Ding et al., 1998; Xu et al., 1999). In contrast, Cryptic null mutants survive until birth and display severe left-right laterality defects, but do not exhibit phenotypes associated with pre-gastrulation patterning and differentiation (Gaio et al., 1999; Yan et al., 1999). Thus, to a first approximation, Cripto is required for early Nodal functions prior to and including gastrulation, whereas Cryptic is required for later Nodal requirements in left-right specification.
However, null mutants for Cripto and Nodal display significant differences in their phenotypes (Brennan et al., 2001; Ding et al., 1998; Mesnard et al., 2006), indicating that some Nodal signaling activity is retained in the absence of Cripto. In particular, Cripto is only expressed in the epiblast, not the visceral endoderm or the ExE, and chimeras with a wild-type visceral endoderm and Cripto mutant epiblast still display a Cripto null phenotype (Ding et al., 1998; Kimura et al., 2001). Given that Cripto functions as a Nodal co-receptor, these observations have led to suggestions that Nodal signaling in the visceral endoderm and ExE may not require EGF-CFC activity (Ben-Haim et al., 2006; Liguori et al., 2008). For example, it has been proposed that signaling by the Nodal precursor from the epiblast to the ExE for maintenance of BMP4 expression is Cripto-independent, and possibly EGF-CFC independent as well (Ben-Haim et al., 2006). Similarly, the ability of a Cerberus-like (Cerl) null mutant to partially rescue the Cripto null phenotype has been interpreted as reflecting EGF-CFC-independent Nodal signaling (Liguori et al., 2008). Alternatively, these observations raise the possibility that Cryptic may have earlier functions than previously recognized, and also mediates Nodal signaling during early mouse embryogenesis.
In the studies described below, we show that in the absence of Cripto, there is an essential role for Cryptic in maintenance of the pluripotent epiblast and the extraembryonic ectoderm as well as formation of the distal visceral endoderm. Our findings suggest that EGF-CFC genes are essential for most if not all known aspects of Nodal function in the early mouse embryo.
Materials and Methods
Mouse strains and genotyping
The Cripto null allele and Cryptic null allele used in this study have been previously described (Ding et al., 1998; Yan et al., 1999). The Nodal-lacZ mice (Collignon et al., 1996) were generously provided by Liz Robertson.
Genotypes of 5.5, 6.5 and 7.5 dpc whole-mount embryos were determined by PCR using genomic DNA from embryo lysates. Embryos following in situ hybridization were similarly genotyped except a 45-cycle PCR program was used. Genotypes of embryo sections were deduced based on the strict correlation between the mutant phenotypes and their genotypes. PCR primers used were: Cripto wild type (forward) 5′-ACC TGC CCC ATG ACT TCT CTT ACA-3′, (reverse) 5′-CCC TGC TGC CCT TAT GCT ATT TTA-3′; Cripto mutant (forward) 5′-CCA TCC CCT GCC CGT CTA CAC G-3′, (reverse) 5′-GTC ACG CAA CTC GCC GCA CAT-3′; Cryptic wild type (forward) 5′-TTC CTG ACT CCA GCA CTT TGG GA-3′, (reverse) 5′-GGC TGA AAA ACA AGT TAG CAG G-3′; Cryptic mutant (forward) 5′-GTG GGG GTG GGG TGG GAT TAG AT-3′, (reverse) 5′-CCT CTG TTT TTG GTG ACT GTC GC-3′; Nodal wild type (forward) 5′-CCA CTC ACC ATT GAC ATT TTC CAC CAG-3′, (reverse) 5′-TGG ATG TAG GCA TGG TTG GTA GGA TG-3′; Nodal mutant (forward) 5′-ATA CTG CAC CGG GCG GGA AGG AT-3′, (reverse) 5′-CCG CGC TGT ACT GGA GGC TGA AG-3′.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as previously described (Shen et al., 1997). Mouse probes were as follows: Afp (Tilghman et al., 1979); Bmp4 (Winnier et al., 1995); Cer1 (Cerberus-like) (Belo et al., 1997); Cdx2 (Strumpf et al., 2005); Cripto (Ding et al., 1998); Cryptic (Shen et al., 1997); Hex (Hhex) (Thomas et al., 1998); Lefty1 (Meno et al., 1997); Lhx1 (Shawlot and Behringer, 1995); Mash2 (Guillemot et al., 1994); Nanog (Chambers et al., 2003). The Oct4 probe corresponded to a 1.0 kb HindIII-AccI fragment amplified from ES cell cDNA and cloned into pBluescript SK. All embryos were genotyped following in situ hybridization and photography, except for the embryos used for sectioning.
Results
Non-overlapping expression of Cripto and Cryptic in the pre-gastrulation embryo
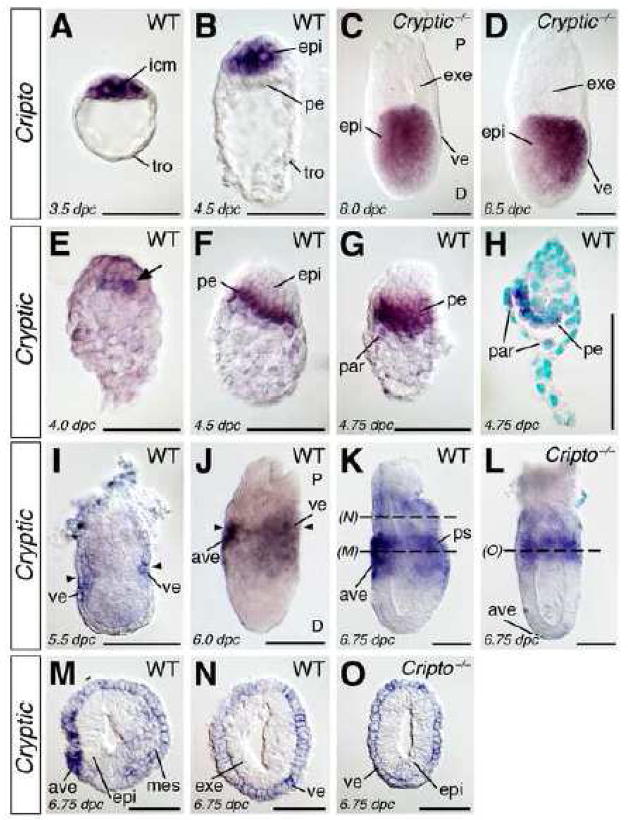
To investigate the potential redundant functions of Cripto and Cryptic, we first compared their expression at peri-implantation and pre-gastrulation stages of embryogenesis by whole-mount in situ hybridization (Fig. 1). In the blastocyst at 3.5 days post coitum (dpc), Cripto expression can be detected in the inner cell mass, but not the trophectoderm (Fig. 1A). Expression of Cripto is later found in the epiblast, but not the primitive endoderm or trophectoderm of the peri-implantation embryo at 4.5 dpc (Fig. 1B). At post-implantation stages, Cripto is expressed uniformly in the epiblast at 5.5 dpc, but not in the visceral endoderm or ExE, and is subsequently localized to the proximal epiblast at 6.0 dpc and posterior epiblast and nascent mesoderm at 6.75 dpc (data not shown), as we have previously described (Ding et al., 1998).
Figure 1.
Expression of Cripto and Cryptic in mouse embryos at 3.5 to 6.75 days post coitum (dpc). (A–D) Cripto expression in wild-type (A, B) and Cryptic null mutant (C, D) embryos. (A) Expression of Cripto in the wild-type blastocyst at 3.5 dpc is restricted to the inner cell mass, and is not found in the trophectoderm. (B) Cripto expression is restricted to the epiblast at 4.5 dpc, and is not found in the primitive endoderm. (C, D) Expression of Cripto in the proximal epiblast of a Cryptic null mutant at 6.0 dpc (C) and in the posterior epiblast at 6.5 dpc (D) is indistinguishable from the wild-type pattern (Ding et al., 1998). In panel D, proximal (P) and distal (D) are indicated. (E–O) Expression of Cryptic in wild-type (E–K, M, N) and Cripto null mutant (L, O) embryos. (E) Cryptic expression in cells of the nascent primitive endoderm (arrow) at 4.0 dpc. (F, G) Expression of Cryptic in the primitive endoderm and newly formed parietal endoderm at 4.5 dpc (F) and 4.75 dpc (G). (H) Longitudinal section of the embryo in (G), showing absence of expression in the epiblast; nuclei are counterstained with methyl green. (I) Cryptic expression at 5.5 dpc in the proximal visceral endoderm at the level of the boundary between extraembryonic ectoderm and epiblast (arrowheads). (J) Expression of Cryptic at 6.0 dpc in the proximal visceral endoderm, including the anterior visceral endoderm; the junction between extraembryonic ectoderm and epiblast is indicated (arrowheads); proximal (P) and distal (D) are indicated. (K) Cryptic expression in the embryonic and extraembryonic visceral endoderm at 6.75 dpc, including the anterior visceral endoderm. (L) Expression of Cryptic in the visceral endoderm is unaltered in a Cripto−/− embryo at 6.5 dpc. (M, N) Cross-sections of the embryo at the indicated levels in (K), showing Cryptic expression in the anterior visceral endoderm, and low-level expression in the visceral endoderm and nascent mesoderm. (O) Cross-section of the embryo in (L). Scale bars correspond to 100 microns. Abbreviations: ave, anterior visceral endoderm; epi, epiblast; exe, extraembryonic ectoderm; icm, inner cell mass; mes, mesoderm; par, parietal endoderm; pe, primitive endoderm; ps, primitive streak; tro, trophectoderm; ve, visceral endoderm.
In the case of Cryptic, we found that expression is first observed in nascent primitive endoderm cells at 4.0 dpc (Fig. 1E). Cryptic expression continues in the primitive endoderm and newly formed parietal endoderm of the peri-implantation and early post-implantation embryo at 4.5 and 4.75 dpc (Fig. 1F–H). Expression of Cryptic is subsequently found at low levels in the visceral endoderm around the embryonic-extraembryonic boundary at 5.5 dpc (Fig. 1I), and is subsequently up-regulated in the proximal embryonic visceral endoderm and distal extraembryonic visceral endoderm (Fig. 1J). Interestingly, Cryptic expression is not uniform within the embryonic visceral endoderm, but instead is expressed preferentially in the anterior visceral endoderm (AVE) (Fig. 1J, K). At early-mid streak stages of gastrulation, Cryptic is expressed in nascent mesoderm that will contribute to the axial and lateral mesoderm (Fig. 1K, M), consistent with our previous findings (Shen et al., 1997). Notably, Cryptic expression is never observed in the epiblast before gastrulation or in the ExE (Fig. 1I–K, M, N).
These results indicate that the expression patterns of Cripto and Cryptic do not overlap prior to mesoderm formation. Notably, Cripto expression in Cryptic null mutants (n=2) is indistinguishable from that in wild-type embryos prior to and during gastrulation (Fig. 1C, D) (Ding et al., 1998). Similarly, Cryptic continues to be expressed in Cripto null mutants (n=2) at these stages, although Cryptic expression is radially symmetric in the visceral endoderm, consistent with the lack of a properly positioned AVE in Cripto mutants (Fig. 1L, O). Therefore, there is no evidence for compensatory expression of either Cripto or Cryptic in the absence of function for the other gene.
Severe post-implantation lethal phenotype of Cripto; Cryptic double mutants
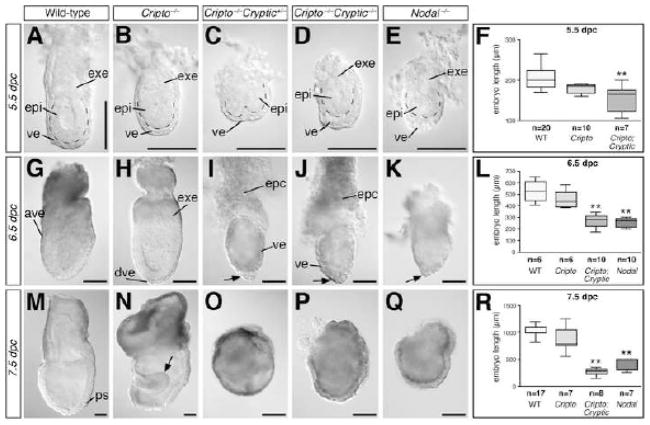
To investigate EGF-CFC functions in pre-gastrulation development, we analyzed the phenotypes of compound mutants for Cripto and Cryptic in intercrosses of Cripto+/−; Cryptic+/− double heterozygotes. At 5.5 dpc, embryos corresponding to all nine possible genotypes can be recovered in Mendelian ratios (Table 1). Morphological examination of the Cripto−/−; Cryptic−/− double mutant embryos at 5.5 dpc showed that they have a reduced ExE as well as thickened visceral endoderm relative to wild-type as well as Cripto−/− single mutants (Fig. 2A, B, D); interestingly, Cripto−/− mutants also showed a slightly smaller ExE relative to wild-type (Fig. 2A, B). At 6.5 dpc, Cripto−/−; Cryptic−/− double mutants lack any evidence of an anterior visceral endoderm, and are significantly smaller than their wild type or Cripto−/− single mutant littermates (Fig. 2G, H, J). By 7.5 dpc, Cripto−/−; Cryptic−/− embryos are highly abnormal, lacking mesoderm formation or evidence of anterior-patterning, and are in the process of resorbing (Fig. 2M, N, P). Unexpectedly, however, we also found that Cripto−/−; Cryptic+/− embryos were phenotypically similar to Cripto−/−; Cryptic−/− embryos (Table 2; Fig. 2C, I, O), indicating the haploinsufficiency of Cryptic in the absence of Cripto function; in contrast, Cripto+/−; Cryptic−/− embryos were phenotypically normal. Furthermore, the phenotypes of both Cripto−/−; Cryptic−/− and Cripto−/−; Cryptic+/− embryos were remarkably similar to that of Nodal−/− mutants at each stage examined (Fig. 2E, K, Q). Notably, the Cripto−/−; Cryptic−/− and Cripto−/−; Cryptic+/− double mutants at 6.5 dpc display an accumulation of visceral endoderm tissue at the distal tip that is characteristic of Nodal mutants (Fig. 2I–K).
Table 1.
Genotypes recovered from Cripto+/−; Cryptic+/− intercrosses at 5.5 dpc
| Genotype | Recovered | Expected |
|---|---|---|
| Cripto+/+; Cryptic+/+ | 3 | 4.75 |
| Cripto+/+; Cryptic+/− | 9 | 9.5 |
| Cripto+/+; Cryptic−/− | 5 | 4.75 |
| Cripto+/−; Cryptic+/+ | 9 | 9.5 |
| Cripto+/−; Cryptic+/− | 20 | 19 |
| Cripto+/−; Cryptic−/− | 12 | 9.5 |
| Cripto−/−; Cryptic+/+ | 5 | 4.75 |
| Cripto−/−; Cryptic+/− | 6 | 9.5 |
| Cripto−/−; Cryptic−/− | 7 | 4.75 |
| Totals | 76 |
Figure 2.
Morphology and epiblast length of Cripto; Cryptic double mutants relative to wild-type, Cripto, and Nodal null mutants. (A–E) Embryos of the indicated genotypes at 5.5 dpc; dashed lines indicate boundary between epiblast and visceral endoderm. (G–K) Embryos of the indicated genotypes at 6.5 dpc; arrows in I–K indicate regions of thickened visceral endoderm. (M–Q) Embryos of the indicated genotypes at 7.5 dpc; arrow in N indicates expanded neuroectoderm that is typical of Cripto−/− mutants (Ding et al., 1998). Scale bars correspond to 100 microns. (F, L, R) Box and whiskers plot of embryo length, measured from the base of the ectoplacental cone to the distal tip, in embryos of the indicated genotypes at 5.5, 6.5 and 7.5 dpc. Embryos of Cripto−/−; Cryptic−/− and Cripto−/−; Cryptic+/− genotypes are grouped together as Cripto; Cryptic. Abbreviations: ave, anterior visceral endoderm; epc, ectoplacental cone; epi, epiblast; exe, extraembryonic ectoderm; ps, primitive streak; ve, visceral endoderm.
Table 2.
Genotypes of abnormal embryos from Cripto+/−; Cryptic+/− intercrosses
| 5.5 dpca | 6.5 dpc | 7.5 dpc | ||||
|---|---|---|---|---|---|---|
| Genotype | Recovered | Expected | Recovered | Expected | Recovered | Expected |
| Cripto−/−; Cryptic+/+ | 5 | 4.75 | 3 | 2.7 | 3 | 2.6 |
| Cripto−/−; Cryptic+/− | 6b | 9.5 | 6b | 5.4 | 4b | 5.2 |
| Cripto−/−; Cryptic−/− | 7 | 4.75 | 3 | 2.7 | 7 | 2.6 |
| Other | 58 | 57 | 31 | 32.2 | 28 | 31.5 |
| Totals | 76 | 43 | 42 | |||
Data taken from Table 1.
Phenotype similar to that of Cripto−/−; Cryptic−/− mutants.
Given the severe epiblast defects observed in the Cripto−/−; Cryptic−/− double mutants, we measured the total length of embryos arising from Cripto+/−; Cryptic+/− intercrosses, as well as for Nodal−/− mutants. In these analyses, we measured the maximal length of the embryo along the proximal-distal axis from the distal tip to the base of the ectoplacental cone, followed by genotyping of the embryos examined (Fig. 2F, L, R). (Because Cripto−/−; Cryptic−/− or Cripto−/−; Cryptic+/− embryos have similar phenotypes, these genotypes were grouped together as “Cripto; Cryptic” embryos). Notably, we found that the embryo lengths of Cripto; Cryptic double mutants were significantly shorter than that of wild-type or Cripto−/− single mutants from 5.5 to 7.5 dpc (P < 0.01 for both comparisons at all three stages examined), and were similar to those of Nodal−/− single mutants at both 6.5 and 7.5 dpc. These findings indicate that the overall phenotype of Cripto; Cryptic double mutants is more severe than that of Cripto single mutants and is comparable to that of Nodal mutants.
Requirement of Cripto and Cryptic for epiblast and extraembryonic ectoderm maintenance
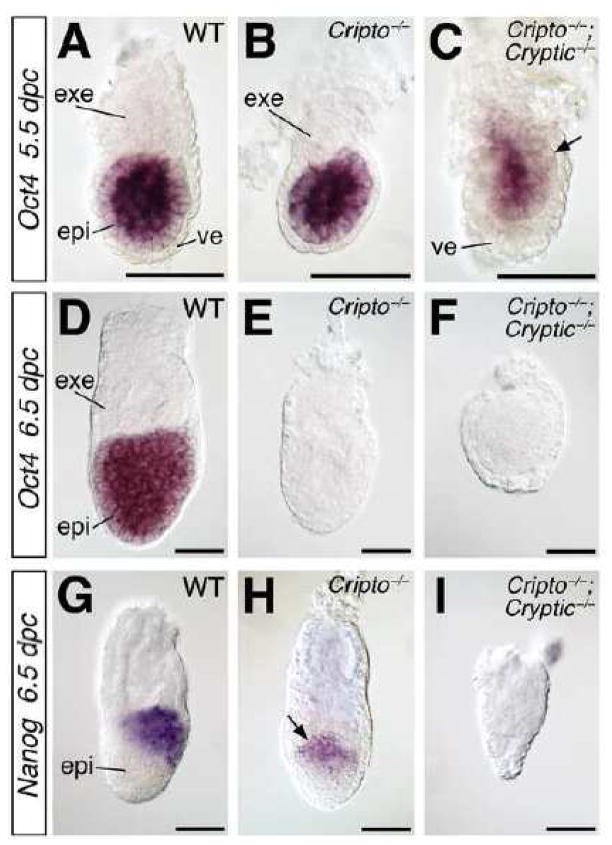
Based on the defects identified in our morphological analyses, we examined expression of epiblast-specific markers by in situ hybridization. The pluripotency marker Oct4 is expressed in wild-type epiblast at 5.5 and 6.5 dpc (Rosner et al., 1990), but is down-regulated in Cripto−/−; Cryptic−/− double mutants at 5.5 dpc (n=2) and is absent by 6.5 dpc (n=6) (Fig. 3A, C, D, F). Interestingly, Oct4 expression in Cripto−/− single mutants is relatively normal at 5.5 dpc (n=3), but is also down-regulated or absent at 6.5 dpc (n=4; 3 embryos lacked Oct4 expression, 1 embryo showed decreased expression) (Fig. 3B, E; data not shown). Furthermore, we found that Nanog expression in the posterior epiblast at 6.5 dpc (Morkel et al., 2003) is down-regulated in Cripto−/− mutants (n=3), and is absent in Cripto−/−; Cryptic−/− double mutants (n=3) (Fig. 3G–I). These findings are consistent with a severe epiblast defect in Cripto−/−; Cryptic−/− double mutants.
Figure 3.
Expression of epiblast markers in wild-type, Cripto, and Cripto; Cryptic mutants. (AC) Expression of Oct4 at 5.5 dpc; note the down-regulation in the Cripto−/−; Cryptic−/− double mutant (arrow). (D–F) Oct4 is expressed at 6.5 dpc in the wild-type epiblast (D), but not in the Cripto−/− or Cripto−/−; Cryptic−/− mutants (E, F). (G–I) Expression of Nanog at 6.5 dpc is down-regulated in the Cripto−/− mutant, but is absent in the Cripto−/−; Cryptic−/− double mutant. Nanog expression is localized to the posterior epiblast in the wild-type embryo (G), but is proximally localized in the Cripto−/− mutant (arrow in H), which lacks anterior-posterior polarity. Scale bars correspond to 100 microns. Abbreviations: epi, epiblast; exe, extraembryonic ectoderm; ve, visceral endoderm.
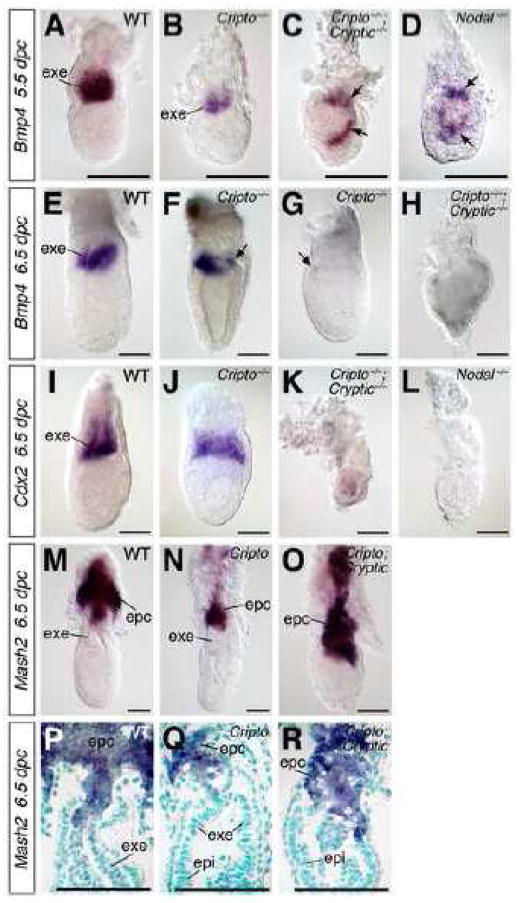
To assess the maintenance and differentiation of the ExE, we first examined the expression of Bmp4, which marks the ExE proximal to the epiblast in wild type embryos (Fig. 4A) (Coucouvanis and Martin, 1999). In Cripto−/− single mutants, the domain of Bmp4 expression in the ExE is reduced in size at 5.5 dpc, but is positioned correctly in relationship to the epiblast (n=6) (Fig. 4B). However, in Cripto−/−; Cryptic−/− double mutants, Bmp4-positive cells are found lateral or distal to the epiblast, indicating a disrupted proximal-distal axis (n=3) (Fig. 4C). Notably, a similar expression pattern is observed in Nodal−/− mutants (n=4) (Fig. 4D) as well as Smad2−/− mutants (Waldrip et al., 1998). At 6.5 dpc, Bmp4 expression is reduced or absent in the Cripto−/− mutants (n=3; 2 lacked Bmp4 expression, and 1 embryo displayed reduced but correctly positioned expression), and is completely absent in Cripto−/−; Cryptic−/− double mutants (n=3) (Fig. 4E–H). As is the case for Bmp4, expression of Cdx2 in the distal ExE at 6.5 dpc (Beck et al., 1995) is reduced or absent in Cripto−/− single mutants (n=4; 2 embryos lacked Cdx2 expression, and 2 showed reduced but correctly patterned expression), and is completely lost in Cripto−/−; Cryptic−/− double mutants (n=5), similar to Nodal−/− mutants (n=2) (Fig. 4I–L; data not shown).
Figure 4.
Expression of extraembryonic ectoderm (ExE) and ectoplacental cone markers in wild-type, Cripto, and Cripto; Cryptic mutants. (A–D) Bmp4 is expressed at 5.5 dpc in the ExE of wild-type (A), but is found only in the most distal part of the ExE in Cripto−/− mutants (B). Expression of Bmp4 is severely mislocalized in the presumptive epiblast (arrows) of Cripto−/−; Cryptic−/− double mutants (C) as well as Nodal−/− mutants (D). (E–H) At 6.5 dpc, Bmp4 is expressed in the distal ExE in wild-type (E), but is either reduced or nearly absent in Cripto−/− single mutants (arrows in F, G), and is lost entirely in Cripto−/−; Cryptic−/− double mutants (H). (I–L) Expression of Cdx2 at 6.5 dpc in the ExE in wild-type (I) is nearly normal in Cripto−/− mutants (J), but is absent in both Cripto−/−; Cryptic−/− double mutants (K) and Nodal−/− mutants (L). (M–O) Expression of Mash2 in the ectoplacental cone of wild-type (M), putative Cripto mutant (N) and putative Cripto; Cryptic double mutant (O) embryos, showing an expanded domain of Mash2 expression in the double mutant. (P–R) Longitudinal sections of the embryos shown in M–O, respectively; nuclei are counterstained with methyl green. In M–R, embryo genotypes were deduced from phenotypes, and the Cripto; Cryptic double mutant may correspond to either Cripto−/−; Cryptic−/− and Cripto−/−; Cryptic+/− genotypes. Scale bars correspond to 100 microns. Abbreviations: epc, ectoplacental cone; epi, epiblast; exe, extraembryonic ectoderm.
Next, we examined the expression of Mash2, which marks differentiating trophoblast cells of the ectoplacental cone in wild type embryos (Fig. 4M, P) (Guillemot et al., 1994). We found that the Mash2 expression domain is greatly expanded into the ExE region in putative Cripto; Cryptic double mutants at 6.5 dpc (n=2) (Fig. 4O, R), in contrast with the relatively normal expression pattern in putative Cripto single mutants (n=3) (Fig. 4N, Q). (Note that embryo genotypes were deduced from their phenotypes in this experiment since the embryos were sectioned and not genotyped; thus, Cripto; Cryptic double mutants may correspond to either Cripto−/−; Cryptic−/− and Cripto−/−; Cryptic+/− genotypes.) Overall, these data on marker expression in the ExE are consistent with an inability to maintain the population of trophoblast stem cells, which instead differentiate towards an ectoplacental cone fate; a similar phenotype was previously reported for Nodal mutants (Guzman-Ayala et al., 2004).
EGF-CFC function in visceral endoderm
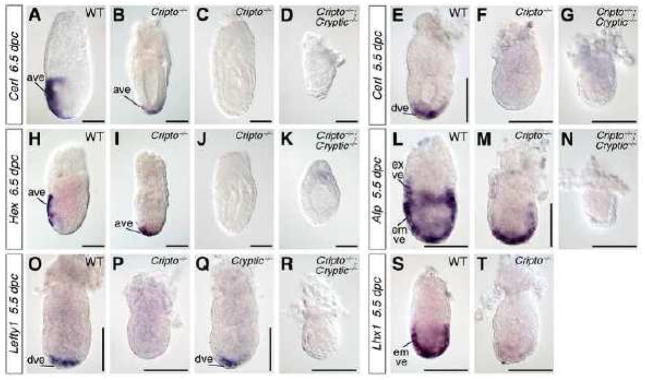
Finally, we examined the formation of the anterior visceral endoderm (AVE) in Cripto; Cryptic double mutants. First, we examined the expression of Cerberus-like (Cerl), which marks the AVE in wild-type embryos at 6.5 dpc, and found that expression was completely absent in Cripto−/−; Cryptic−/− double mutants (n=2) (Fig. 5A, D). Interestingly, we found that Cripto−/− single mutants display heterogeneous Cerl expression, with some embryos expressing Cerl in the distal visceral endoderm (n=2), and others with no detectable Cerl expression (n=3) (Fig. 5B, C). Similarly, Hex expression also marks the AVE in wild-type embryos at 6.5 dpc (Fig. 5H) (Thomas et al., 1998), but is lost in Cripto−/−; Cryptic−/− double mutants (n=4), and displays expression in the distal visceral endoderm (n=2) or is completely absent (n=4) in Cripto−/− single mutants (Fig. 5I–K).
Figure 5.
Expression of visceral endoderm markers in wild-type, Cripto, Cryptic, and Cripto; Cryptic mutants. (A–D) At 6.5 dpc, Cerl is expressed in wild-type embryos in the anterior visceral endoderm (A), but is either mislocalized distally or absent in Cripto−/− mutants (B, C), and is completely lost in Cripto−/−; Cryptic−/− double mutants (D). (E–G) At 5.5 dpc, Cerl is expressed in the distal visceral endoderm in wild-type embryos (E), but is absent in both Cripto−/− mutants and Cripto−/−; Cryptic−/− double mutants (F, G). (H–K) Expression of Hex at 6.5 dpc is found in the wild-type anterior visceral endoderm (H), but is either mislocalized distally or is lost in Cripto−/− mutants (I, J), and is absent in Cripto−/−; Cryptic−/− double mutants (K). (L–N) Afp (alpha-fetoprotein) is broadly expressed in the embryonic visceral endoderm (overlying the epiblast) and distal extraembryonic visceral endoderm (overlying the ExE) of wild-type embryos at 5.5 dpc (L), but is limited to the distal embryonic visceral endoderm in Cripto−/− mutants (M) and is absent in Cripto−/−; Cryptic−/− double mutants (N). (O–R) Lefty1 is expressed in the distal visceral endoderm at 5.5 dpc in wild-type as well Cryptic−/− mutants (O, Q), but is absent in Cripto−/− mutants and Cripto−/−; Cryptic−/− double mutants (P, R). (S, T) Lhx1 is expressed in the wild-type embryonic visceral endoderm at 5.5 dpc (S), but is absent in Cripto−/− mutants (T). Scale bars correspond to 100 microns. Abbreviations: ave, anterior visceral endoderm; dve, distal visceral endoderm; emve, embryonic visceral endoderm; exve, extraembryonic visceral endoderm.
Based on these findings, we further examined the visceral endoderm phenotype of Cripto; Cryptic double mutants as well as Cripto−/− and Cryptic−/− single mutants at 5.5 dpc. We found that expression of Cerl was not detectable in the distal visceral endoderm (DVE) of Cripto−/− mutants (n=5), and was absent in Cripto−/−; Cryptic−/− double mutants (n=2) (Fig. 5E–G). However, expression of alpha-fetoprotein (Afp) remains present in the embryonic visceral endoderm of Cripto−/− mutants at 5.5 dpc, although the expression in the extraembryonic visceral endoderm is lost (n=2) (Fig. 5L, M); in Cripto−/−; Cryptic−/− double mutants, Afp expression is completely absent (n=2) (Fig. 5N). Furthermore, expression of the DVE marker Lefty1 is also absent in Cripto−/− mutants (n=4) as well as Cripto−/−; Cryptic−/− double mutants (n=2) (Fig. 5O, P, R), consistent with the previously reported absence of Lefty1 expression in Cripto−/− mutants at 6.5 dpc (Kimura et al., 2001). In contrast, Lefty1 expression in the DVE appears normal in Cryptic−/− single mutants (Fig. 5Q), as expected from their wild-type phenotype at early embryonic stages. Finally, Lhx1 is broadly expressed in the embryonic visceral endoderm of wild-type embryos at 5.5 dpc (Perea-Gomez et al., 1999), yet its expression is absent in Cripto−/− mutants (n=3) (Fig. 5S, T).
These findings indicate that specification of the DVE and more broadly the embryonic visceral endoderm requires EGF-CFC function prior to 5.5 dpc, consistent with a previously described role of Nodal in this process (Mesnard et al., 2006). Interestingly, there is a significant role for Cripto in this requirement for EGF-CFC function, even though Cripto is not expressed in the visceral endoderm. Furthermore, since Cer1, Lefty1, and Lhx1 are believed to be directly or indirectly regulated by Nodal signaling, our results suggest that EGF-CFC genes are essential in specifying the DVE through the Nodal signaling pathway.
Discussion
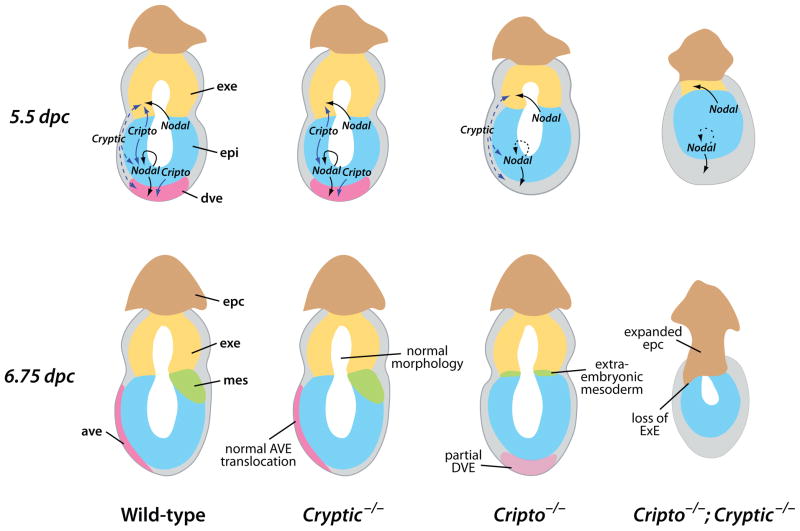
Taken together, our analyses have demonstrated that partially redundant activities of the EGF-CFC genes Cripto and Cryptic play essential roles in patterning the early mouse embryo. Notably, the Cripto; Cryptic double mutant phenotype is highly similar to that for Nodal null mutants, indicating that EGF-CFC function is required for most or all Nodal signaling during early embryogenesis. As is the case for Nodal, the combined functions of EGF-CFC genes are required for maintenance of the epiblast and ExE, as well as for formation of the DVE. However, the expression patterns of Cripto and Cryptic do not overlap in any of these tissues during pre-gastrulation development, indicating that their redundant activities are a consequence of non-cell-autonomous function.
Non-autonomous EGF-CFC function
The existence of two EGF-CFC genes in mammalian genomes and only one gene (oep) in zebrafish suggests that Cripto and Cryptic originated from a common ancestor and subsequently acquired functional divergency. However, our studies have shown that these two EGF-CFC genes retain common redundant functions, and that in the absence of Cripto, Cryptic is haploinsufficient. In addition, we have observed a defect in maintenance of pluripotent marker expression in Cripto null mutants alone, consistent with the previously described premature neural differentiation of epiblast (Ding et al., 1998; Kimura et al., 2001; Liguori et al., 2003); a similar phenotype has been described for Nodal mutants (Camus et al., 2006; Lu and Robertson, 2004; Mesnard et al., 2006).
Previous studies have shown that Cripto mutants form a partially functional DVE that fails to translocate, based on the expression of Hex and Cerl in the distally mispositioned AVE at 6.75 dpc (Ding et al., 1998). In the present work, we observe that this mispositioning of Hex and Cerl expression in the distal visceral endoderm is heterogeneous in Cripto mutants, with some embryos lacking expression altogether; similar heterogeneity was found for marker expression in the epiblast and ExE. This phenotypic heterogeneity may be due to a different strain background from our previous study, since the Cripto mutant mice have now been backcrossed extensively against C57BL/6 in our laboratory. In addition, our data suggest that expression of Cer1 may be developmentally delayed in Cripto mutants, since it was not detected at 5.5 dpc. Thus, the failure of DVE movement in Cripto mutants may be due at least in part to the absence of Lefty1 and Cer1 expression at 5.5 dpc, which are believed to drive DVE movement (Yamamoto et al., 2004). However, the ability of Cripto mutants to form a partial DVE is likely to result from the activity of Cryptic expressed in the proximal visceral endoderm.
The lack of overlapping expression for Cripto and Cryptic is particularly striking given the multiple phenotypes of Cripto; Cryptic double mutants that are not observed in the individual single mutants. Thus, the ability of Cryptic expressed in the visceral endoderm to promote formation of a DVE (albeit defective) in Cripto mutants presumably reflects a cell-autonomous activity of Cryptic. In contrast, the ability of Cripto expressed in the epiblast to promote normal AVE formation and movement in Cryptic mutants would be non-cell-autonomous. Moreover, the ability of Cryptic to promote epiblast maintenance in the absence of Cripto is presumably non-cell-autonomous, since Cryptic is only expressed in the visceral endoderm. Finally, neither Cripto nor Cryptic are expressed in the ExE, yet these two genes are required for maintenance of undifferentiated ExE. The simplest explanation for these observations is that both Cripto and Cryptic act non-cell-autonomously during early embryogenesis, as previously shown for Cripto in axial mesendoderm formation during gastrulation (Chu et al., 2005), and more generally in embryonic development (Xu et al., 1999). Thus, we propose that Cripto and/or Cryptic act non-cell-autonomously in distinct contexts in epiblast maintenance, ExE maintenance, and AVE formation and movement (Fig. 6).
Figure 6.
Model for non-autonomous redundant functions of Cripto and Cryptic. Schematic diagrams of the phenotype of wild-type, Cryptic null mutant, Cripto null mutant, and Cripto; Cryptic double mutant embryos at 5.5 and 6.75 dpc. Suggested pathways of Nodal and EGF-CFC activity are shown (arrows). Note that the proposed signaling interactions shown at 5.5 dpc could in principle occur at earlier stages, and that the arrows may correspond to direct or indirect signaling events. Abbreviations: ave, anterior visceral endoderm; dve, distal visceral endoderm; epc, ectoplacental cone; epi, epiblast; exe, extraembryonic ectoderm; mes, mesoderm.
In principle, these non-cell-autonomous functions of Cripto and Cryptic could reflect direct or indirect mechanisms. In the former situation, EGF-CFC proteins could be released from cells of one tissue and act in a paracrine manner on cells of a neighboring tissue. In the latter case, downstream targets of Cripto or Cryptic could mediate the relevant intercellular interactions. In the case of Cripto, our genetic analyses have previously suggested that the non-cell-autonomous function of Cripto in axial mesendoderm reflects a direct, trans-acting activity of Cripto protein, thus favoring the first model (Chu et al., 2005). However, it remains unclear whether the roles of EGF-CFC proteins in visceral endoderm and the ExE are direct or indirect, particularly since their temporal requirements during embryogenesis are also unknown. Perhaps consistent with a direct role, Lefty1 is expressed normally in the DVE of Cryptic null mutants at 5.5 dpc (Fig. 5Q), which is notable since the relevant Lefty1 promoter element contains essential FoxH1 binding sites, indicating that it is responsive to Nodal signaling (Takaoka et al., 2006). Also favoring a direct role for EGF-CFC proteins, weak phospho-Smad2 immunoreactivity can be detected in the ExE at 5.5 dpc, and is abolished by culture in the presence of the ALK4/ALK5/ALK7 inhibitor SB431542 (Yamamoto et al., 2009). On the other hand, this phospho-Smad2 immunoreactivity may be due to a different TGFβ signaling factor that does not require EGF-CFC activity, such as Activin. Indeed, a recent study has suggested that Activin, but not Nodal, is required for ExE as well as trophoblast stem cell maintenance, and that the ExE requirement for Nodal (and presumably EGF-CFC) function may reflect an autoregulatory loop for epiblast expression of FGF4, which in turn acts as a paracrine signal to the ExE (Natale et al., 2009).
Although EGF-CFC proteins were originally identified as strictly cis-acting co-receptors for Nodal ligands that act cell-autonomously in zebrafish (Gritsman et al., 1999; Schier et al., 1997; Strahle et al., 1997), there is considerable evidence for their trans-acting functions in cell culture and in vivo (Chu et al., 2005; Minchiotti et al., 2001; Parisi et al., 2003; Yan et al., 2002). For example, overexpression of a truncated form of zebrafish Oep that lacks the C-terminal GPI anchor in the extraembryonic yolk syncytial layer can rescue the embryonic defects of oep mutants, indicating non-autonomous function (Gritsman et al., 1999; Minchiotti et al., 2001). In principle, such paracrine responses to Cripto can be due to activation of the Nodal pathway, or can be Nodal-independent in other contexts (Bianco et al., 2002; Bianco et al., 2003). Cripto can be released from the cell membrane after cleavage of its GPI-linkage by GPI-phospholipase D (Minchiotti et al., 2000; Watanabe et al., 2007), while activity of human Cryptic is modulated by a C-terminal hydrophilic extension that can lead to formation of both GPI-linked and soluble forms of the protein (Watanabe et al., 2008). However, the resulting soluble EGF-CFC proteins may be less efficient at signaling since they compete for Nodal binding with secreted inhibitors (Blanchet et al., 2008; Constam, 2009). Thus, paracrine activity of Cripto and Cryptic may be tightly regulated by Cerl as well as Lefty; notably, Lefty proteins can also interact directly with EGF-CFC proteins (Chen and Shen, 2004; Cheng et al., 2004).
Partial functional redundancy of EGF-CFC genes in mediating Nodal signaling
Our findings demonstrate the strong similarity of the Cripto; Cryptic double mutant phenotype to the Nodal null phenotype. These Nodal mutant phenotypes include the defective maintenance of the extraembryonic ectoderm (Brennan et al., 2001; Guzman-Ayala et al., 2004), as well as the precocious neural differentiation of epiblast (Camus et al., 2006), which is related to a requirement for epiblast proliferation and size control in vivo (Mesnard et al., 2006). Furthermore, Nodal signaling is required for maintenance of undifferentiated human ES cells and mouse epiblast stem cells (Brons et al., 2007; James et al., 2005; Tesar et al., 2007), most likely through up-regulation of Nanog expression (Vallier et al., 2009; Xu et al., 2008). In each case, the Nodal mutant phenotype is highly similar to the Cripto; Cryptic double mutant phenotype, supporting the conclusion that EGF-CFC proteins mediate all aspects of Nodal signaling in vivo.
Our results are relevant for previous studies that have addressed whether Nodal signaling always requires EGF-CFC function. For example, one recent study showed that purified Nodal precursor protein can act on ExE explants, which lack EGF-CFC expression, suggesting that Nodal precursor signals from the epiblast to the ExE in an EGF-CFC independent manner (Ben-Haim et al., 2006). However, an alternative explanation for this observation is that these ExE explants may have already been “primed” for Nodal responsiveness by paracrine Cripto and Cryptic by 5.5 dpc, and might not require EGF-CFC activity thereafter. Furthermore, our results can also account for the Cripto-independent Nodal signaling that was deduced from the ability of a Cerl null mutant to partially suppress the Cripto mutant phenotype (Liguori et al., 2008). In this case, we propose that the removal of a Nodal antagonist in Cripto; Cerl double mutants may result in greater range and/or activity of Nodal protein and possibly of trans-acting Cryptic protein as well. Thus, in the absence of Cerl, Nodal could mediate Cryptic-dependent rescue of the Cripto mutant phenotype.
Although Cripto and Cryptic display functional redundancy, our findings suggest that their activities are not wholly equivalent. The embryonic lethality of Cripto null mutants suggests that Cripto is more essential than Cryptic, and therefore that Cripto may be more important than Cryptic in mediating Nodal function, a conclusion that is also consistent with the observed haploinsufficiency of Cryptic in the absence of Cripto function. This difference between Cripto and Cryptic may simply be due to relative differences in expression levels, or the expression of Cripto in the epiblast, where the requirement for mediating Nodal activity is greatest. Alternatively, this difference may reflect a mechanistic distinction between Cripto and Cryptic with respect to their non-autonomous activities.
Our findings are also relevant for assessing whether EGF-CFC proteins may have activities that are independent of Nodal in early embryogenesis. In particular, recent work has shown that EGF-CFC proteins can mediate signaling by additional TGFβ ligands during early vertebrate embryogenesis (Chen et al., 2006; Cheng et al., 2003). One of these TGFβ ligands, GDF3 (Growth-Differentiation Factor-3), has a Nodal-like activity in Xenopus embryos as well as EGF-CFC-dependent signaling activity in cell culture, while Gdf3 null mutants display phenotypes resembling those of Nodal hypomorphs (Andersson et al., 2007; Andersson et al., 2008; Chen et al., 2006); however, we note that other studies in Xenopus embryos have concluded that GDF3 primarily acts as a BMP inhibitor in vivo (Levine and Brivanlou, 2006; Levine et al., 2009). Furthermore, the TGFβ ligand GDF1 also stimulates Nodal pathway activity in an EGF-CFC-dependent manner (Andersson et al., 2006; Cheng et al., 2003), and can heterodimerize with Nodal to potentiate Nodal activity, possibly by increasing long-range signaling (Tanaka et al., 2007).
Finally, it remains conceivable that EGF-CFC proteins may have activities that are entirely independent of the TGFβ/Activin/Nodal pathway. For example, previous work has suggested a Nodal-independent pathway that involves interaction of Cripto with glypican-1 and subsequent activation of c-Src in mammary epithelial cells (Bianco et al., 2003), while a recent study has shown that Cripto can facilitate signaling through Notch receptors in embryonal carcinoma cells (Watanabe et al., 2009). In addition, Cripto and the Xenopus EGF-CFC protein FRL-1 have been proposed to mediate signaling by Wnt11 through the canonical Wnt/β-catenin signaling pathway (Tao et al., 2005). Notably, the analysis of a Cripto hypomorphic allele containing a point mutation that eliminates activity in cell culture assays for Nodal function has suggested that many of the in vivo functions of Cripto are due to Nodal pathway-independent activities (D’Andrea et al., 2008). However, given the overall similarity of the Cripto; Cryptic double mutant phenotype with that of Nodal null mutants, if such Nodal pathway-independent activities of EGF-CFC proteins occur during early embryogenesis, they should be largely redundant with Nodal pathway activity. Of course, these data do not exclude the possibility that EGF-CFC proteins may have Nodal pathway-independent activities at later stages of development, or in cancer or other disease processes. Investigation of such Nodal-independent EGF-CFC functions will be of continuing interest in future studies.
Acknowledgments
We are grateful to Jixiang Ding for his initial observations on the Cripto; Cryptic double mutant phenotype. We thank Richard Behringer, Eddy De Robertis, Hiroshi Hamada, Brigid Hogan, Liz Robertson, Janet Rossant, and Austin Smith for reagents, and Antonella Galli, Marianna Kruithof-de Julio, and David-Emlyn Parfitt for comments on the manuscript. This work was supported by NIH grant HD42837 (M.M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson O, Bertolino P, Ibanez CF. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol. 2007;311:500–511. doi: 10.1016/j.ydbio.2007.08.060. [DOI] [PubMed] [Google Scholar]
- Andersson O, Korach-Andre M, Reissmann E, Ibanez CF, Bertolino P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci U S A. 2008;105:7252–7256. doi: 10.1073/pnas.0800272105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O, Reissmann E, Jornvall H, Ibanez CF. Synergistic interaction between Gdf1 and Nodal during anterior axis development. Dev Biol. 2006;293:370–381. doi: 10.1016/j.ydbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The Nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates Nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, Margolis RU, Sanicola M, Salomon DS. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- Blanchet MH, Le Good JA, Oorschot V, Baflast S, Minchiotti G, Klumperman J, Constam DB. Cripto localizes Nodal at the limiting membrane of early endosomes. Sci Signal. 2008;1:ra13. doi: 10.1126/scisignal.1165027. [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Camus A, Perea-Gomez A, Moreau A, Collignon J. Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol. 2006;295:743–755. doi: 10.1016/j.ydbio.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chen C, Shen MM. Two modes by which Lefty proteins inhibit Nodal signaling. Curr Biol. 2004;14:618–624. doi: 10.1016/j.cub.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development. 2006;133:319–329. doi: 10.1242/dev.02210. [DOI] [PubMed] [Google Scholar]
- Chen X, Rubock MJ, Whitman M. A transcriptional partner for MAD proteins in TGF-beta signalling. Nature. 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Bennett JT, Brivanlou AH, Schier AF. EGF-CFC proteins are essential coreceptors for the TGF-beta signals Vg1 and GDF1. Genes Dev. 2003;17:31–36. doi: 10.1101/gad.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Brivanlou AH, Schier AF. Lefty blocks a subset of TGFbeta signals by antagonizing EGF-CFC coreceptors. PLoS Biol. 2004;2:215–226. doi: 10.1371/journal.pbio.0020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Ding J, Jeays-Ward K, Price SM, Placzek M, Shen MM. Non-cell-autonomous role for Cripto in axial midline formation during vertebrate embryogenesis. Development. 2005;132:5539–5551. doi: 10.1242/dev.02157. [DOI] [PubMed] [Google Scholar]
- Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- Constam DB. Running the gauntlet: an overview of the modalities of travel employed by the putative morphogen Nodal. Curr Opin Genet Dev. 2009:302–307. doi: 10.1016/j.gde.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- D’Andrea D, Liguori GL, Le Good JA, Lonardo E, Andersson O, Constam DB, Persico MG, Minchiotti G. Cripto promotes A-P axis specification independently of its stimulatory effect on Nodal autoinduction. J Cell Biol. 2008;180:597–605. doi: 10.1083/jcb.200709090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Gaio U, Schweickert A, Fischer AN, Muller T, Ozcelik C, Lankes W, Strehle M, Britsch S, Blum M, Birchmeier C. A role of the cryptic gene in the correct establishment of the left-right axis. Curr Biol. 1999;9:1339–1342. doi: 10.1016/s0960-9822(00)80059-7. [DOI] [PubMed] [Google Scholar]
- Germain S, Howell M, Esslemont GM, Hill CS. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes Dev. 2000;14:435–451. [PMC free article] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for Nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- Guzman-Ayala M, Ben-Haim N, Beck S, Constam DB. Nodal protein processing and fibroblast growth factor 4 synergize to maintain a trophoblast stem cell microenvironment. Proc Natl Acad Sci U S A. 2004;101:15656–15660. doi: 10.1073/pnas.0405429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- Kimura C, Shen MM, Takeda N, Aizawa S, Matsuo I. Complementary functions of Otx2 and Cripto in initial patterning of mouse epiblast. Dev Biol. 2001;235:12–32. doi: 10.1006/dbio.2001.0289. [DOI] [PubMed] [Google Scholar]
- Kumar A, Novoselov V, Celeste AJ, Wolfman NM, ten Dijke P, Kuehn MR. Nodal signaling uses activin and transforming growth factor-beta receptor-regulated Smads. J Biol Chem. 2001;276:656–661. doi: 10.1074/jbc.M004649200. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. GDF3, a BMP inhibitor, regulates cell fate in stem cells and early embryos. Development. 2006;133:209–216. doi: 10.1242/dev.02192. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Levine ZJ, Brivanlou AH. GDF3 is a BMP inhibitor that can activate Nodal signaling only at very high doses. Dev Biol. 2009;325:43–48. doi: 10.1016/j.ydbio.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori GL, Borges AC, D’Andrea D, Liguoro A, Goncalves L, Salgueiro AM, Persico MG, Belo JA. Cripto-independent Nodal signaling promotes positioning of the A-P axis in the early mouse embryo. Dev Biol. 2008;315:280–289. doi: 10.1016/j.ydbio.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Liguori GL, Echevarria D, Improta R, Signore M, Adamson E, Martinez S, Persico MG. Anterior neural plate regionalization in cripto null mutant mouse embryos in the absence of node and primitive streak. Dev Biol. 2003;264:537–549. doi: 10.1016/j.ydbio.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Lu CC, Robertson EJ. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev Biol. 2004;273:149–159. doi: 10.1016/j.ydbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Meno C, Ito Y, Saijoh Y, Matsuda Y, Tashiro K, Kuhara S, Hamada H. Two closely-related left-right asymmetrically expressed genes, lefty-1 and lefty-2: their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Genes Cells. 1997;2:513–524. doi: 10.1046/j.1365-2443.1997.1400338.x. [DOI] [PubMed] [Google Scholar]
- Mesnard D, Guzman-Ayala M, Constam DB. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development. 2006;133:2497–2505. doi: 10.1242/dev.02413. [DOI] [PubMed] [Google Scholar]
- Minchiotti G, Manco G, Parisi S, Lago CT, Rosa F, Persico MG. Structure-function analysis of the EGF-CFC family member Cripto identifies residues essential for nodal signalling. Development. 2001;128:4501–4510. doi: 10.1242/dev.128.22.4501. [DOI] [PubMed] [Google Scholar]
- Minchiotti G, Parisi S, Liguori G, Signore M, Lania G, Adamson ED, Lago CT, Persico MG. Membrane-anchorage of Cripto protein by glycosylphosphatidylinositol and its distribution during early mouse development. Mech Dev. 2000;90:133–142. doi: 10.1016/s0925-4773(99)00235-x. [DOI] [PubMed] [Google Scholar]
- Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, Taketo MM, Behringer RR, Shen MM, Birchmeier W. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- Natale DR, Hemberger M, Hughes M, Cross JC. Activin promotes differentiation of cultured mouse trophoblast stem cells towards a labyrinth cell fate. Dev Biol. 2009;335:120–131. doi: 10.1016/j.ydbio.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Norris DP, Robertson EJ. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev. 1999;13:1575–1588. doi: 10.1101/gad.13.12.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi S, D’Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol. 2003;163:303–314. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Gomez A, Shawlot W, Sasaki H, Behringer RR, Ang S. HNF3beta and Lim1 interact in the visceral endoderm to regulate primitive streak formation and anterior-posterior polarity in the mouse embryo. Development. 1999;126:4499–4511. doi: 10.1242/dev.126.20.4499. [DOI] [PubMed] [Google Scholar]
- Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PWJ, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SCF, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR. Requirement for Lim1 in head-organizer function. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Shen MM, Schier AF. The EGF-CFC gene family in vertebrate development. Trends Genet. 2000;16:303–309. doi: 10.1016/s0168-9525(00)02006-0. [DOI] [PubMed] [Google Scholar]
- Shen MM, Wang H, Leder P. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development. 1997;124:429–442. doi: 10.1242/dev.124.2.429. [DOI] [PubMed] [Google Scholar]
- Strahle U, Jesuthasan S, Blader P, Garcia-Villalba P, Hatta K, Ingham PW. one-eyed pinhead is required for development of the ventral midline of the zebrafish (Danio rerio) neural tube. Genes Funct. 1997;1:131–148. doi: 10.1046/j.1365-4624.1997.00010.x. [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Shiratori H, Meno C, Rossant J, Saijoh Y, Hamada H. The mouse embryo autonomously acquires anterior-posterior polarity at implantation. Dev Cell. 2006;10:451–459. doi: 10.1016/j.devcel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Sakuma R, Nakamura T, Hamada H, Saijoh Y. Long-range action of Nodal requires interaction with GDF1. Genes Dev. 2007;21:3272–3282. doi: 10.1101/gad.1623907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal Wnt11 activates the canonical Wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Brown A, Beddington RSP. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125:85–94. doi: 10.1242/dev.125.1.85. [DOI] [PubMed] [Google Scholar]
- Tilghman SM, Kioussis D, Gorin MB, Ruiz JP, Ingram RS. The presence of intervening sequences in the alpha-fetoprotein gene of the mouse. J Biol Chem. 1979;254:7393–7399. [PubMed] [Google Scholar]
- Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, Brons G, Pedersen RA. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Bianco C, Strizzi L, Hamada S, Mancino M, Bailly V, Mo W, Wen D, Miatkowski K, Gonzales M, Sanicola M, Seno M, Salomon DS. Growth factor induction of Cripto-1 shedding by glycosylphosphatidylinositol-phospholipase D and enhancement of endothelial cell migration. J Biol Chem. 2007;282:31643–31655. doi: 10.1074/jbc.M702713200. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nagaoka T, Lee JM, Bianco C, Gonzales M, Castro NP, Rangel MC, Sakamoto K, Sun Y, Callahan R, Salomon DS. Enhancement of Notch receptor maturation and signaling sensitivity by Cripto-1. J Cell Biol. 2009;187:343–353. doi: 10.1083/jcb.200905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Nagaoka T, Strizzi L, Mancino M, Gonzales M, Bianco C, Salomon DS. Characterization of the glycosylphosphatidylinositol-anchor signal sequence of human Cryptic with a hydrophilic extension. Biochim Biophys Acta. 2008;1778:2671–2681. doi: 10.1016/j.bbamem.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechselberger C, Bianco C, Strizzi L, Ebert AD, Kenney N, Sun Y, Salomon DS. Modulation of TGF-beta signaling by EGF-CFC proteins. Exp Cell Res. 2005;310:249–255. doi: 10.1016/j.yexcr.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Whitman M. Nodal signaling in early vertebrate embryos. Themes and variations. Dev Cell. 2001;1:605–617. doi: 10.1016/s1534-5807(01)00076-4. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–494. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R, Thomson JA. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Beppu H, Takaoka K, Meno C, Li E, Miyazono K, Hamada H. Antagonism between Smad1 and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. J Cell Biol. 2009;184:323–334. doi: 10.1083/jcb.200808044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, Ang SL, Hamada H, Meno C. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- Yan Y-T, Gritsman K, Ding J, Burdine RD, Corrales JD, Price SM, Talbot WS, Schier AF, Shen MM. Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev. 1999;13:2527–2537. doi: 10.1101/gad.13.19.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YT, Liu JJ, Luo YEC, Haltiwanger RS, Abate-Shen C, Shen MM. Dual roles of Cripto as a ligand and coreceptor in the Nodal signaling pathway. Mol Cell Biol. 2002;22:4439–4449. doi: 10.1128/MCB.22.13.4439-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]