Summary
Ca2+ entering cells through store-operated channels (SOCs) affects most cell functions, and excess SOC is associated with pathologies. The molecular makeup of SOCs and their mechanisms of gating were clarified with the discovery of the Orais and STIM1. Another form of SOCs are the TRPCs. STIM1 gates both Orai and TRPC channels but does so by different mechanisms. Although the STIM1 SOAR domain mediates the binding of STIM1 to both channel types, SOAR is sufficient to open the Orais but the STIM1 polylysine domain mediates opening of the TRPC channels. This short review discusses recent findings on how STIM1 gates and regulates the Orais and TRPCs, and how the STIM1/Orai1/TRPCs complexes may function in vivo to mediate SOC activity.
Keywords: Orai, TRPC, channels, STIM1, gating
Introduction
The Ca2+ signal evoked by G protein-coupled receptor or tyrosine kinase receptor activation of phospholipase C is initiated by Ca2+ release from the ER and is rapidly followed by activation of plasma membrane Ca2+ influx channels [1–3]. The same Ca2+ channels can be activated by passive depletion of ER Ca2+, for example by Ca2+ ionophores or by inhibition of the ER Ca2+ pump (SERCA), and are therefore called store-operated Ca2+ channels (SOCs) [3]. The SOCs have many cellular functions and play a central role in various cell pathologies [3–7]. By virtue of supplying the cells with Ca2+ to sustain the physiological response of Ca2+ oscillations, the SOCs impact every cellular function mediated by Ca2+. In addition, Ca2+ influx by the SOCs refills the stores with Ca2+ when stimulation is terminated. SOCs-mediated Ca2+ influx also regulates apoptosis [3] and aberrant Ca2+ influx by the SOCs is common in many disease states. This is likely due to a sustained elevation of basal Ca2+ concentration that causes cell stress and activates autophagy and cell death [8,9].
The multiple roles of the SOCs in cell physiology and pathology focused attention on their molecular composition and their mechanisms of activation following receptor stimulation. Approaching these questions on the molecular level became possible in recent years with the discovery of STIM1 [10,11] and Orai channels [12–14], and the demonstration that STIM1 is important for gating of both Orai [14,15] and TRPC channels [16–18]. Several of these topics have been extensively reviewed in recent years. Here, we will discuss only recent findings on the gating of the Orai and TRPC channels by STIM1.
STIM1
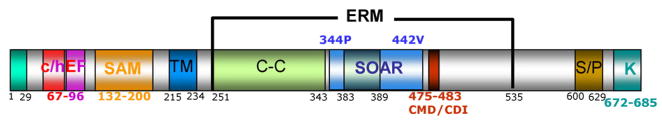
A key question in the SOC field was how information of the ER Ca2+ content is transmitted to the plasma membrane to regulate SOC channel opening. This was largely solved with the finding that STIM1 is a multidomain, ER resident, Ca2+ binding protein that opens SOCs [10,11]. Informatics and mutational analyses continue to discover domains and motifs in the STIM1 molecule that have specific roles in opening and regulation of the SOCs. Fig. 1 depicts the STIM1 domain/motifs identified to date.
Fig. 1.
The functional STIM1 domains identified so far include (from the N-terminus) the canonical and hidden EF hands Ca2+ binding domain, STIM1(67–96), which when bound with Ca2+ prevents oligomerization and clustering of STIM1. The SAM domain, STIM1(132–200), which participates in STIM1 oligomerization and clustering. The transmembrane domain, STIM1(215–234), which anchors STIM1 in the ER. The ERM domain, STIM1(251–535), which has two coiled-coil domains, STIM1(251–343) and STIM1(383–389). The second coiled-coil domain is within the SOAR domain, STIM1(344–442). SOAR mediates interaction of STIM1 with the Orais’ C-termini and opens the channels. CMD/CDI, STIM1(475–483), which together with the Orai1 conserved glutamates and calmodulin binding domain mediates fast Ca2+-dependent inactivation. Several serines in the S/P domain, STIM1(600–629), and other serine/threonine residues between the CMD/DCI and the S/P domain, are constitutively phosphorylated or their phosphorylation is altered during the cell cycle. The K-domain, STIM1(672–685), has multiple functions. It stabilizes STIM1 at the plasma membrane, it affects the voltage dependence of Orai1 when interacting with the Orai1 proline-rich region, and the K-domain gates the TRPC channels by electrostatic interaction with conserved aspartates/glutamates in the TRPCs C termini.
The STIM1 N terminus includes the classical and hidden Ca2+ binding EF hands [19], and a SAM domain that reside in the ER lumen. Ca2+ binding to the EF hand(s) maintains STIM1 in a non-clustered form [20] at a distance from the plasma membrane that prevents interaction with the SOCs [21]. Ca2+ release from the ER results in clustering of STIM1 [10,11] that is facilitated by the SAM domain and requires dissociation of Ca2+ from the EF hand [20]. Although the clustering is not essential for activation of the SOCs [22,23], it generates ER/plasma membrane Ca2+ influx microdomains [24]. Sequences upstream of the EF hand [STIM1(1–65)] appear to participate in clustering and affect the rate of Orai1 activation [25].
The cytoplasmic portion of STIM1 that follows a single transmembrane domain has several structural and functional domains [26]. It begins with an ERM domain [STIM1(251–535)] that starts with a large coiled-coil domain [STIM1(251–343)]. A glutamate-rich domain [STIM1(270–336)] is present in the first coiled-coil domain, the function of which is not yet known. A second short coiled-coil domain [STIM1(383–389)] is encompassed within a highly conserved domain named SOAR [STIM1(334–442)] [23] (somewhat longer domains were named CAD [22], CCb9 [27]) that binds to and is sufficient to fully activate the Orai channels (see below). SOAR is also the domain through which STIM1 physically interacts with the TRPC channels (Lee, Yuan at al, unpublished results), but is not sufficient to open TRPC channels [18,28]. SOAR acts in combination with STIM1(450–485) to regulate the strength of SOAR interaction with Orai1 [23,29]. Thus, STIM1(450–470) enhances physical interaction of STIM1 with Orai1, while STIM1(470–485) weakens the interaction. A negatively charged stretch of seven amino acids just downstream of SOAR that was named CMD/CDI is required for fast, Ca2+-dependent inactivation of the Orai channels [30–32].
The next defined cytoplasmic STIM1 domain is a serine/proline-rich domain (the S/P domain). No function has yet been ascribed to the STIM1 stretch between the CMD/CDI and the S/P domains. Several of the serines in the S/P domain appear to be constitutively phosphorylated while the phosphorylation of several other serines changes during the cell cycle [33,34]. In one study no role could be determined for phosphorylation of the serines [33], whereas another study reported that phosphorylation of these sites inhibits STIM1 clustering and SOC activity [34]. The role of these phosphorylation sites remains to be clarifies since phosphomimetic mutations failed to reproduce the effect of STIM1 phosphorylation on the cell cycle [33,34]. The kinases phosphorylating STIM1 in vivo are not known with certainty.
A lysine-rich domain (the K-domain) is present at the most C terminus of STIM1. The K-domain helps in anchoring the STIM1 to the plasma membrane [35], perhaps by interacting with PIP2 and PIP3 at the plasma membrane [36]. In addition to the anchoring function, the K-domain modulates the voltage-dependence of Orai1 [23]. More importantly, the K-domain directly gates the TRPC channels ([18]- see below). Structure-function analysis of STIM1 continues to be extensively pursued and, undoubtedly, additional domains and motifs will be found in the near future.
Gating of Orai channels by STIM1
The Orais mediate the Ca2+-release activated Ca2+ (CRAC) current. The CRAC current was identified in RBL cells in 1992 [37], but the molecular nature of the channel mediating this current was not identified until 2006 by positional cloning and by genome screening [12–14]. Shortly thereafter, it was shown that the Orais are activated by STIM1 [14,15]. Three genes, termed orai 1, 2, 3, were subsequently identified in vertebrates and express closely related proteins [4,38]. The Orais are four transmembrane span proteins with relatively short N and C termini. Evidence that the Orais form the CRAC pore includes observations that expression of Orai 1 with STIM1 generates CRAC current [39], and mutations of charged residues in the transmembrane domains modifies the channel ionic selectivity [40–42].
Although several STIM1-Orai1 interacting domains/motifs have been identified, it is still not known how STIM1 actually opens the Orais. Ca2+ store depletion results in co-clustering of STIM1 and Orai1. Moreover, Ca2+ store-independent clustering of STIM1 was reported to activate the native CRAC and Orai1 [43]. However, several STIM1 mutations allow STIM1 clustering and STIM1-Orai1 co-clustering but prevent activation of Orai1 by STIM1 [22,23], indicating that STIM1 clustering and STIM1-Orai1 co-clustering are not sufficient or necessary for activation of Orai1 by STIM1. Importantly, some of these STIM1 mutants retain the ability to activate TRPC1 [23], suggesting that the mutations did not simply disrupt the STIM1 structure.
The first indication that opening of Orai1 by STIM1 requires interaction of the STIM1 coiled-coil domains with the C terminus of Orai1 was obtained by mutations that are predicted to disrupt coiled-coil folds of the Orais [44,45]. A key subsequent finding was that SOAR within the STIM1 ERM domain [23] and the slightly longer STIM1 domain CAD and CCb9 [22,27] are sufficient to fully activate the Orais, indicating that SOAR is the STIM1 domain that opens the Orai channels and no other interaction is required for channel opening. Opening of the Orais is mediated by interaction of SOAR/CAD with the C terminus of Orai1 [22,23,30], while the Orai1 N terminus is not required for activation of Orai1 by STIM1. Thus, while deletion of most of the Orai1 N terminus (Orai1(Δ1-73)) reduces the rate and the extent of Orai1(Δ1-73) activation by STIM1 [23,46], Orai1(Δ1-73) is fully activated by SOAR/CAD [22,23]. The critical role of the Orai1 C terminus in opening of the channel is best illustrated in Fig. 2 (taken from [30]), in which activation of Orai chimeras by STIM1 and SOAR was examined. Translocation of the C termini of the Orais translocates the extent of channel opening. Thus, the Orai1 C terminus was much better than the native Orai2 and Orai3 C termini in opening these channels, while the Orai2 and Orai3 C termini poorly open Orai1.
Fig. 2. Orai1 C terminus potently opens Orai2 and Orai3.
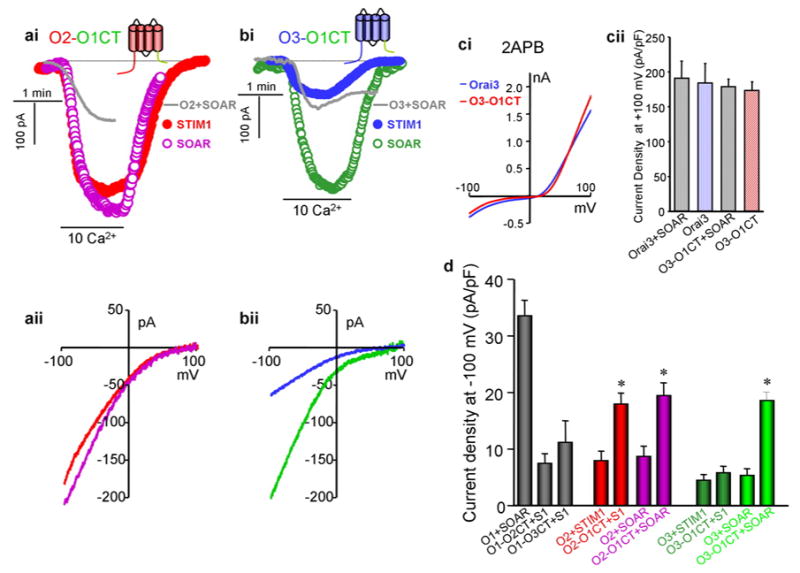
The CRAC current was measured in HEK cells transfected with the indicated chimera. (ai) shows the current mediated by Orai2-Orai1CT and STIM1 (●) or SOAR (○). The gray trace shows an example of a typical current mediated by Orai2+STIM1. (aii) shows examples I/V for Orai2-Orai1CT with STIM1 (red) and SOAR (purple). (bi) shows the current mediated by Orai3-Orai1CT and STIM1 (●) or SOAR (○). The gray trace shows an example of a typical current mediated by Orai3+STIM1. (bii) shows examples I/V for Orai3-Orai1CT with STIM1 (blue) and SOAR (green). (ci) shows the I/V of the current recorded in cells transfected with STIM1 and Orai3 (blue) or Orai3-Orai1CT (red) activated by 50 μM 2-ABP and (cii) shows the meam±s.e.m of 4 experiments at each condition. (d) is the mean±s.e.m of 4–6 experiments of the current density recorded under the indicated conditions. * denotes p<0.01 relative to the respective control. (Reproduced from [30]).
Additional STIM1 domains that regulate Orai1 function are the K-domain and CMD/CDI domain. Deletion and/or mutation of these domains does not prevent full activation of the Orais by STIM1, but does change the regulation of the channels [23,30–32]. The CRAC current mediated by the Orais is strongly inward rectifying. The inward rectification appears to require interaction between the STIM1 K-domain and specific pairs of proline residues in the Orai1 N terminus [23]. Mutation of the pair of prolines 3, 5 or prolines 39, 40 of Orai1 markedly reduces the Orai1 current at negative voltages. This effect is eliminated by deletion of the K-domain or mutation of any lysine within the STIM1 K-domain [23].
The native CRAC current is inhibited by intracellular Ca2+. Two forms of inhibition by Ca2+ have been described; fast and slow Ca2+-dependent inactivation [47,48]. The mechanism of slow Ca2+-mediated inactivation is not known. Fast Ca2+-dependent inactivation (FCDI) is mediated by Ca2+ entering the channel that generates a Ca2+ microdomain next to the channel pore [3,47–49]. Ca2+ in this domain is regulated by the energetic state of the mitochondria [48,50] and by the glycolytic product and mitochondrial substrate pyruvate [49]. Interestingly, FCDI is specific to each Orai isoform and is affected by the Orai:STIM1 ratio. At a 1:1 plasmid expression ratio of Orai and STIM1, FCDI is most prominent with Orai3, less with Orai2 and is minimal, if any, with Orai1 [23,30,39]. The isoform-specific FCDI is determined by conserved C terminal glutamates in Orai2 and Orai3 that are only partially conserved in Orai1 [30]. Additional potential determinants of FCDI may be present at Orai1(284–301) since truncation of Orai1 at residue 283 increases FCDI [30]. The minimal FCDI of Orai1 becomes very prominent at 1:4 Orai1:STIM1 ratio [51]. Whether the Orai:STIM1 ratio affects FCDI of Orai2 and Orai3 is not known at present.
Recent work showed that STIM1 cooperates with Orai1 to mediate FCDI. A STIM1 sequence just C terminal of SOAR (STIM1(475DDVDDMDEE483)) has a cluster of 7 negative charges (highlighted) and was found to be critical for FCDI. Neutralization of groups or all the charges markedly reduces or eliminates FCDI of Orai1 [30–32] Orai2 and Orai3 [30]. Neutralization of only STIM1(482EE483) increased the rate of FCDI by an unknown mechanism [32].
A search for the Orai domains that account for the different modes of FCDI revealed that the C termini determine the mode of FCDI. Translocation of the C termini translocated the mode of FCDI [30]. Further analysis identified glutamates that are conserved in the three C termini of Orai2 and Orai3 but not in Orai1. Neutralization of these glutamates abolished FCDI of Orai2 and Orai3 [30]. An additional Orai1 domain critical for FCDI is a calmodulin binding site at the N terminus of Orai1 [32]. Mutations that disrupt calmodulin binding to this domain eliminate FCDI. This domain is completely conserved in Orai2 and Orai3, suggesting that Orai2 and Orai3 are also regulated by calmodulin.
Taken together, a model is emerging to suggest that Oria C-terminal glutamates are required to interact with and the N termini to form a site that binds calmodulin. Calmodulin may associate with the Orais at the resting state. The STIM1 SOAR binds to the Orai C termini to open the channel and present the adjacent STIM1(475-483) to the site formed by the glutamates and calmodulin binding site to stabilize and/or allow binding of Ca2+ entering the channels to this pocket. If calmodulin is not bound to the Orais in the resting state, it is likely that it is sequestered at a site close to the channel pore to allow the FCDI, since FCDI is mediated by Ca2+ in the close vicinity of the channel. In this case STIM1(475-483) may facilitate binding of the Ca2+-bound calmodulin to the Orai1 N terminus after binding of Ca2+ entering the channel to calmodulin. Further work is needed to clarify this point.
Gating of TRPC channels by STIM1
TRPC channels also function as SOCs. The role of TRP channels in Ca2+ influx was discovered in Drosophila with the discovery of the role of TRPL in the response to light [52,53]. This lead to cloning of the mammalian TRPC channels, and demonstration that the TRPCs mediate receptor-stimulated Ca2+ influx [54]. Since these seminal findings, numerous studies examined the role of TRPC channels in both receptor-stimulated and store-dependent Ca2+ influx [55]. Recent studies have revealed that STIM1 regulates TRPC channel opening, and that TRPCs and Orais are co-functional.
Before the discovery of STIM1 and Orai1, most studies used knockdown of TRPCs by antisense or siRNA or expression of dominant negative channels to study the function of TRPCs as SOCs. It is not possible to cite here all these studies, however this was shown for most TRPCs and the specific references can be found in several reviews (for example [3,55,56]). Although many of these studies supported a role for the TRPCs in SOCs, this appeared to be at least in part cell or condition specific.
With the discovery of STIM1, we asked whether TRPC channels are gated by STIM1. In the first studies, it was demonstrated that STIM1 binds to multiple TRPC channels and gates TRPC1 [16]. Interaction of STIM1 with TRPC1 was confirmed in several subsequent studies [57–60]. Interaction of STIM1 with the TRPC channels is mediated by the STIM1 ERM domain [16]. Importantly, the cytosolic C-terminus of STIM1 was sufficient to activate the TRPC channels, indicating that no ER resident sequence was required for gating of the TRPC (and Orai) channels [16]. More recently, we found that SOAR within ERM is the domain that binds to the TRPC channels (Lee and Yuan et al, not shown).
Regulation of TRPC1 by STIM1 was extended to other TRPC channels to show that TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 (but not TRPC7) are gated by STIM1 [17]. However, STIM1 binds to and appears to directly regulate TRPC1, TRPC4 and TRPC5. On the other hand, regulation of TRPC3 and TRPC6 by STIM1 appears indirect and involves STIM1-dependent heteromultimerization of TRPC1-TRPC3 and TRPC4-TRPC6, in which TRPC1 and TRPC4 presents STIM1 to TRPC3 and TRPC6, respectively [17]. However, knockdown of TRPC1 and TRPC4 did not inhibit TRPC3 and TRPC6 activities, but rather, resulted in STIM1-independent activity of TRPC3 and TRPC6 [17]. This important finding indicates that TRPC3 and TRPC6 have two operating modes, STIM1-dependent and STIM1-independent modes. It follows that interaction of STIM1 with these TRPC channels tunes their function as SOCs or as non-SOCs. Nevertheless, both modes can be activated by receptor stimulation, and the extent of interaction with STIM1 determines whether they function as SOCs or as purely receptor-stimulated channels (ROCs). This model predicts that TRPC channel gating as SOC vs ROC will be determined by the level of endogenous STIM1 in a given cell type, and may rationalize earlier studies that indicated TRPCs do not contribute to SOC.
Structure function analysis showed that the STIM1 K-domain is not required for binding of STIM1 to TRPC channels but it is essential for activation of the TRPCs by STIM1 [16]. In search of a mechanism by which STIM1 gates the TRPC channels, we noticed that the K-domain is predicted to fold as an α helix with the lysines positive charges on one side of the α helix. We searched for complementary sequences in TRPC channels and identified two conserved negative charges at the end of the α helical TRP box1 [18]. Neutralization or charge swapping of the positive charges in the STIM1 K-domain or the negative charges in TRPC1 C terminus resulted in inhibition of channel activity [18]. The key findings in these studies was that opposite charges in STIM1 and TRPC1 independent of charge type always resulted in active channel. This is summarized in table 1. Hence, STIM1 lysines and arginines can be matched with TRPC1 aspartates or glutamates or vice versa to generate active channels. On the other hand, charge neutralization or matching positive or negative charges in STIM1 and TRPC1 always resulted in dead channels (table 1). These findings indicate that STIM1 gates TRPC1 by electrostatic interaction of the positively charged STIM1 lysines with the negatively charged TRPC1 aspartates. It is likely that the conserved aspartates/glutamates in the other TRPCs serve similar role. Preliminary results suggest that this is the case.
Table 1. Effect of mutation of STIM1(684KK685) and of TRPC1(639DD640) on TRPC1 channel activity.
HEK cells were transfected with the TRPC1 mutants alone (first column) or with the indicated STIM1 mutants. The results are the mean±s.e.m. (n≥4 for each condition) of the current in pA recorded at −100 mV and extracted from the −100 to +100 RAMPs. Highlighted in red are full rescues and in blue partial rescues. ND=not determined. (reproduced from [18]).
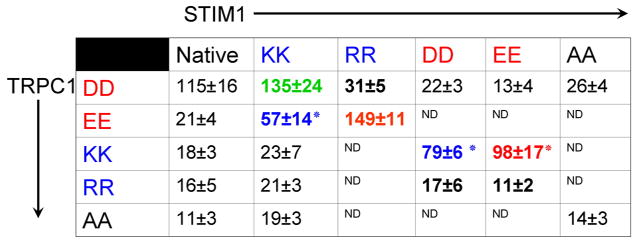 |
P<0.05 relative to wild-type TRPC1+STIM1
ND = Not determined
A critical question is whether the native, and not only ectopically expressed TRPCs, can function as SOCs in vivo. Recently, it became possible to address these questions with the development of mice lacking specific TRPCs. Indeed, knockout of TRPC4 resulted in reduced SOC activity in endothelial cells [61] and impaired airway intercellular permeability [62]. Knockout of TRPC1 markedly reduced receptor-stimulated and SOC-mediated Ca2+ influx in salivary gland cells (and in pancreatic acini, Kim MS et al, unpublished observation) and receptor-stimulated salivary secretion [63]. Knockout of TRPC3 inhibited receptor-stimulated and SOC-mediated Ca2+ influx in pancreatic and salivary gland acinar and duct cells, and strongly blunted acute pancreatitis due to excessive SOC-mediated Ca2+ influx [9]. An elegant recent study showed that expression of TRPC3 and TRPC6 in skeletal muscle resulted in marked increase in SOC activity to cause muscle dystrophy. Most notably, knockdown of TRPC6 in mice lacking dystrophin and deletion of the δ-sarcoglycan markedly reduced the augmented SOC activity and muscle dystrophy in the two mice strains [64].
Although one report claims that knockdown of STIM1 does not affect the activity of several TRPCs [65], there is now evidence from several laboratories that STIM1 regulates TRPC channels and mediates their SOC function. These include the following: TRPCs a) interact directly or indirectly with STIM1 and the SOAR domain in the cytoplasmic C terminus of STIM1; b) activity is reduced in cells treated with siSTIM1; c) are inhibited by the dominant negative STIM1(ΔERM) and STIM1(ΔK); d) are made spontaneously active by STIM1(D76A) and STIM1-C terminus; e) are inhibited by scavenging the native STIM1 with the dominant negative Orai1(R91W) [66]; f) are inhibited by mutations of the conserved DD/E in TRPCs C terminus and by mutations in the STIM1 polybasic domain; g) the TRPCs(KK) mutants are not active but are rescued by the STIM1(EE) mutant; h) at high expression levels TRPC1(KK)+STIM1(EE) can be activated by passive store depletion in cells treated with siOrai1 [67]; i) SOC activity is reduced in cells treated with antisense or siRNA targeting many of the TRPCs [for example [68,69]]; j) SOC activity is reduced in cells obtained from TRPC1−/−, TRPC3−/− and TRPC4−/− mice, and deletion of these genes has the expected physiological phenotype of reduced Ca2+-mediated function. Together, these findings indicate that TRPC channels function as STIM1-gated SOCs.
Orai1, TRPC channels and STIM1
Although Orai1 and TRPCs can clearly function independently of each other, and have distinct channel properties when activated by STIM1 [18], growing evidence suggests that the native channels exist with Orai and TRPC in the same complex and that these proteins may depend on the activity of each other. The first suggestion of biochemical and functional association between the Orai and TRPC channels was the evidence that transgene expression of low level of Orai1 in cells stably or transiently expressing TRPC1, TRPC3 and TRPC6 resulted in enhanced SOC activity [66,70]. Orai1 appears to interact with both the C- and N-termini of TRPCs [70]. Further analysis showed that STIM1 mediates/assembles the Orai1/TRPC1 complex [71,72]. Moreover, knockdown of TRPC1, STIM1 and Orai1 similarly reduced SOC activity, while over-expression of TRPC1 increased SOC activity in salivary gland cells [73].
Further support for the interdependence of TRPC and Orai channel function was provided by the finding that functional SOC formed by TRPC1-STIM1 requires channel-competent Orai1 [67,73]. Hence, knockout of Orai1 or expression of dominant negative Orai1 reduced TRPC1 activity [67,73]. The reciprocal dependence can also be demonstrated when Orai1 and TRPC1 are expressed at very low, physiological levels [67]. Expression of low levels of either Orai1 or TRPC1 in the presence of native STIM1 results in minimal SOC activity and CRAC current. However, when the same low levels of Orai1 and TRPC1 are co-expressed, normal SOC activity is restored. Remarkably, restoration of SOC required both functional Orai1 and functional TRPC1 [67]. Expression level also affects the function of other TRPC channels. This is the case for TRPC3 [17,74] and TRPC6 [17]. At high expression levels, their spontaneous activity increases and they function as STIM1-independent channels [17]. As indicated above, STIM1 heteromultimerizes the TRPCs by assembling complexes of TRPCs that bind STIM1 (TRPC1 and TRPC4) with TRPCs that do not binds STIM1 (TRPC3 and TRPC6, respectively) Knockdown of TRPC1 and TRPC4 converts TRPC3 or TRPC6 activity from STIM1-dependent to STIM1-independent [17]. Hence, the accumulating evidence indicates that several TRPC channels can function both as STIM1-dependent and as STIM1-independent, depending on the complexes that they form and possibly their cellular localization. In this respect, two studies examined localization of the TRPC1, Orai1 and STIM1 in cellular microdomains and reported assembly of the complexes in lipid raft domains [57,72]. Moreover, it appears that intact lipid rafts are required for the function of TRPC1 as SOC, whereas TRPC1 complexes outside the lipid rafts may function as non-SOC channels [57,72]. In the rafts TRPC1 seems to interact with caveolin in the resting state. Activation of TRPC1 requires dissociation from caveolin to allow its interaction with and activation by STIM1 [75].
The overall findings with STIM1, Orai1 and TRPCs suggest that the stoichiometry of the proteins and the composition of the complexes determine their function as SOCs or as non-SOCs. At physiological expression levels, STIM1, Orai1 and TRPCs appear to exist in the same molecular complex to function as SOCs. The requirement for functional Orai1 and TRPC1 for SOC activity would suggest that Ca2+ entering each of the channels is required to stabilize the complex and allow its regulation by STIM1. The channels can also function independent of each other. Orai1 mediates CRAC current when interacting with STIM1 in the absence of TRPCs. TRPCs function as SOCs when interacting with STIM1 directly or with the aid of other TRPCs, but as non-SOC when not associated with STIM1. In this manner STIM1 functions to tune the SOC activity of the TRPCs. To examine this and other scenarios requires further studies on the relationships between TRPCs, the Orais and STIM1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–9. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–56. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113–20. doi: 10.1016/j.tips.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 7.Ng SW, Nelson C, Parekh AB. Coupling of Ca(2+) microdomains to spatially and temporally distinct cellular responses by the tyrosine kinase Syk. J Biol Chem. 2009;284:24767–72. doi: 10.1074/jbc.M109.011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer-Hansen M, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–17. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 13.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SL, et al. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peinelt C, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 17.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–45. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–48. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ Depletion-induced Oligomerization of Stromal Interaction Molecule 1 (STIM1) via the EF-SAM Region: AN INITIATION MECHANISM FOR CAPACITIVE Ca2+ ENTRY. J Biol Chem. 2006;281:35855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 21.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–90. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 22.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Mancarella S, Wang Y, Yue C, Ritchie M, Gill DL, Soboloff J. The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J Biol Chem. 2009;284:19164–8. doi: 10.1074/jbc.C109.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindl R, Muik M, Fahrner M, Derler I, Fritsch R, Bergsmann J, Romanin C. Recent progress on STIM1 domains controlling Orai activation. Cell Calcium. 2009;46:227–32. doi: 10.1016/j.ceca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang G, et al. ER stress disrupts Ca2+-signaling complexes and Ca2+ regulation in secretory and muscle cells from PERK-knockout mice. J Cell Sci. 2006;119:153–61. doi: 10.1242/jcs.02731. [DOI] [PubMed] [Google Scholar]
- 29.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J Biol Chem. 2009;284:8421–6. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci U S A. 2009;106:14687–92. doi: 10.1073/pnas.0904664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derler I, Fahrner M, Muik M, Lackner B, Schindl R, Groschner K, Romanin C. A Ca2(+)release-activated Ca2(+) (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2(+)-dependent inactivation of ORAI1 channels. J Biol Chem. 2009;284:24933–8. doi: 10.1074/jbc.C109.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci U S A. 2009;106:15495–500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci U S A. 2009;106:17401–6. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth JT, Petranka JG, Boyles RR, Dehaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009 doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J. 2009 doi: 10.1042/BJ20090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–6. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 38.Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 Are Store-Operated Ca(2+) Channels with Distinct Functional Properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 41.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–42. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muik M, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–22. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 45.Frischauf I, Muik M, Derler I, Bergsmann J, Fahrner M, Schindl R, Groschner K, Romanin C. Molecular determinants of the coupling between STIM1 and Orai channels: differential activation of Orai1–3 channels by a STIM1 coiled-coil mutant. J Biol Chem. 2009;284:21696–706. doi: 10.1074/jbc.M109.018408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–56. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 47.Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995;105:209–26. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parekh AB. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547:333–48. doi: 10.1113/jphysiol.2002.034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakowski D, Parekh AB. Regulation of store-operated calcium channels by the intermediary metabolite pyruvic acid. Curr Biol. 2007;17:1076–81. doi: 10.1016/j.cub.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 50.Gilabert JA, Bakowski D, Parekh AB. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. Embo J. 2001;20:2672–9. doi: 10.1093/emboj/20.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scrimgeour N, Litjens T, Ma L, Barritt GJ, Rychkov GY. Properties of Orai1 mediated store-operated current depend on the expression levels of STIM1 and Orai1 proteins. J Physiol. 2009 doi: 10.1113/jphysiol.2009.170662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–3. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 53.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–51. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 54.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–71. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 55.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 56.Kiselyov K, Kim JY, Zeng W, Muallem S. Protein-protein interaction and functionTRPC channels. Pflugers Arch. 2005;451:116–24. doi: 10.1007/s00424-005-1442-2. [DOI] [PubMed] [Google Scholar]
- 57.Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: Moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008 doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–23. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–64. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 60.Jardin I, Lopez JJ, Salido GM, Rosado JA. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J Biol Chem. 2008;283:25296–304. doi: 10.1074/jbc.M802904200. [DOI] [PubMed] [Google Scholar]
- 61.Freichel M, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–7. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 62.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–6. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- 63.Liu X, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A. 2007;104:17542–7. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, Molkentin JD. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW., Jr TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009;587:2275–98. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim MS, Zeng W, Yuan J, Shin DM, Worley P, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem. 2009 doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–69. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Babnigg G, Villereal ML. Functional significance of human trp1 and trp3 in store-operated Ca(2+) entry in HEK-293 cells. Am J Physiol Cell Physiol. 2000;278:C526–36. doi: 10.1152/ajpcell.2000.278.3.C526. [DOI] [PubMed] [Google Scholar]
- 70.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–7. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ong HL, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–16. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283:17333–40. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–40. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vazquez G, Lievremont JP, St JBG, Putney JW., Jr Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc Natl Acad Sci U S A. 2001;98:11777–82. doi: 10.1073/pnas.201238198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, Ambudkar IS. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]