Abstract
Establishment of the embryonic mesoderm is dependent on integration of multiple signaling and transcriptional inputs. We report that the transcriptional regulator Foxd3 is essential for dorsal mesoderm formation in zebrafish, and that this function is dependent on the Nodal pathway. Foxd3 gain-of-function results in expanded dorsal mesodermal gene expression, including the Nodal-related gene cyclops, and body axis dorsalization. Foxd3 knockdown embryos displayed reduced expression of cyclops and mesodermal genes, axial defects similar to Nodal pathway loss-of-function, and Nodal pathway activation rescued these phenotypes. In MZoep mutants inactive for Nodal signaling, Foxd3 did not rescue mesoderm formation or axial development, indicating that the mesodermal function of Foxd3 is dependent on an active downstream Nodal pathway. A previously identified foxd3 mutant, sym1, was described as a predicted null mutation with neural crest defects, but no mesodermal or axial phenotypes. We find that Sym1 protein retains activity and can induce strong mesodermal expansion and axial dorsalization. A subset of sym1 homozygotes display axial defects and reduced cyclops and mesodermal gene expression, and penetrance of the mesodermal phenotypes is enhanced by Foxd3 knockdown. Therefore, sym1 is a hypomorphic allele, and reduced Foxd3 function results in a reduction of cyclops expression, and subsequent mesodermal and axial defects. These results demonstrate that Foxd3 is an essential upstream regulator of the Nodal pathway in zebrafish dorsal mesoderm development and establish a broadly conserved role for Foxd3 in vertebrate mesodermal development.
Keywords: Foxd3, Nodal, Forkhead, Mesoderm, Transcription, Zebrafish
Introduction
The vertebrate body plan forms in response to a network of signaling cascades that are integrated in time and space to induce and pattern the primary germ layers. These major signaling systems, including the Nodal, BMP, Wnt, FGF and other pathways, are subject to precise feedback and feedforward mechanisms that reinforce or inhibit signaling output (Kimelman, 2006). The modulation of signaling required for proper germ layer patterning is under the control of multiple extracellular signaling inhibitors, and a primary source of these inhibitors is the organizer, a major signaling center responsible for germ later patterning in the gastrula (De Robertis and Kuroda, 2004). The transcriptional networks initiated in response to these signaling pathways establish a spatial framework in the gastrula for further elaboration of the body plan. Defining the interplay between lineage-specific transcriptional networks and embryonic signaling inputs is essential for a mechanistic understanding of germ layer formation.
Nodal ligands, members of the TGFβ superfamily, are essential inducers of mesendoderm in the vertebrate embryo (Schier, 2003). In mouse, Nodal loss-of-function results in incomplete gastrulation, a failure of mesoderm formation, and developmental arrest (Conlon et al., 1994). Inhibition of Nodal signaling in Xenopus causes developmental arrest at gastrulation and a failure to form mesodermal and endodermal lineages (Osada and Wright, 1999). A zebrafish double mutant in two nodal genes (cyclops and squint) or a maternal zygotic mutant in the Nodal co-receptor one eyed pinhead (MZoep) fails to gastrulate, and lacks all head mesoderm, trunk mesoderm, and endoderm (Feldman et al., 2000; Whitman, 2001). Single mutants for cyclops or squint have a less severe phenotype, as do maternal or zygotic oep mutants (Dougan et al., 2003, Zhang et al., 1998). Spatial and temporal control of the Nodal pathway is dynamic and subject to multiple positive and negative inputs that reinforce Nodal activity in the mesodermal and endodermal domains and silence pathway activity in the adjacent ectodermal domain. While much is know about the inhibitory control of Nodal signaling, less is understood regarding the transcriptional mechanisms that restrict or silence the expression of nodal genes.
Foxd3, a member of the forkhead class of transcriptional regulators, has multiple roles in vertebrate embryogenesis, including maintenance of stem cell and progenitor cell populations, control of dorsal mesoderm formation in the gastrula, and regulation of neural crest development. Foxd3 is expressed in mouse and human embryonic stem cells, in mouse trophoblast stem cells, and in the epiblast cells of the preimplantation mouse embryo (Hanna et al., 2002; Sutton et al., 1996; Tompers et al., 2005). Neither embryonic stem cell lines nor trophoblast stem cell lines can be established from foxd3 null embryos, indicating an essential role for Foxd3 in controlling maintenance, survival, and differentiation of these stem cell populations (Hanna et al., 2002; Tompers et al., 2005). At the gastrula stage in Xenopus and zebrafish, foxd3 is expressed in the organizer (Odenthal and Nusslein-Volhard, 1998); Pohl and Knochel, 2001; Sasai et al., 2001) where it is coexpressed with multiple nodal-related genes. We have demonstrated in Xenopus that Foxd3 is necessary and sufficient for dorsal mesodermal development, and that Foxd3 functions as a repressor to maintain nodal expression and signaling activity in the Spemann organizer (Steiner et al., 2006; Yaklichkin et al., 2007). In the neural crest, studies in mouse, chick, zebrafish and Xenopus indicate that Foxd3 is required for the determination, migration, survival and/or differentiation of multiple neural crest lineages (Dottori et al., 2001; Kos et al., 2001; Sasai et al., 2001; Cheung et al., 2005; Whitlock et al., 2005; Lister et al., 2006; Montero-Balaguer et al., 2006; Stewart et al., 2006; Teng et al., 2008). Therefore, Foxd3 is an essential transcriptional regulator of diverse cell lineages at distinct stages of vertebrate development.
Early and late functions for Foxd3 have been described in both mouse and Xenopus (pregastrula or gastrula function early and neural crest function later). Surprisingly, despite conservation of foxd3 expression in the organizer domain, only a neural crest function has been described for zebrafish Foxd3. Knockdown and mutant studies in zebrafish have demonstrated a requirement for Foxd3 in the differentiation of neural crest derivatives, including craniofacial cartilage, peripheral neurons, glia, and iridophore pigment cells (Kelsh et al., 2000; Lister et al., 2006; Montero-Balaguer et al., 2006; Stewart et al., 2006). However, there have been no reports of defects in mesoderm formation, gastrulation or axial development for zebrafish Foxd3 knockdown or loss-of-function analyses. This apparent lack of gastrula function is especially striking in the foxd3 mutant sympathetic mutation 1 (sym1), a predicted null mutation (Stewart et al., 2006). These results suggest that, unlike mouse and Xenopus, Foxd3 function in the gastrula is not essential in the zebrafish, indicating an unexpected lack of developmental conservation. Another possible explanation for these results would be the presence of a second compensating foxd3 gene, but no second zebrafish foxd3 locus has yet been identified. These observations suggest either that Foxd3 is not essential in the zebrafish gastrula, or that the sym1 mutation is not a functional null for Foxd3.
Here we report gain-of-function, knockdown and mutant analyses that demonstrate an essential function for Foxd3 in zebrafish mesodermal development and axis formation, as well as the dependence of Foxd3 on an active, downstream Nodal signaling pathway. We show that the sym1 foxd3 mutation, previously predicted to be a functional null, is a hypomorphic allele with reduced function, resulting in partial penentrance of mesodermal defects. These studies define an early developmental requirement for Foxd3 in the zebrafish and confirm an essential conserved function of Foxd3 as a Nodal pathway regulator in the vertebrate gastrula.
Material and Methods
Zebrafish methods and microinjection
Zebrafish were raised under standard laboratory conditions as previously described (Mullins et al., 1994), and developmental stage was determined according to Kimmel et al. (1995). Microinjection of wild-type and sym1 (foxd3zdf10) embryos (a gift of Thomas Look; Stewart et al., 2006) was performed at the one-cell stage using standard methods (Westerfield, 1993).
FoxD3 expression plasmids and mutagenesis
A pCS2-myc-foxd3 plasmid (Lister et al., 2006) was used for expression of wild-type zebrafish Foxd3. For expression of Sym1, pCS2-myc-foxd3sym1 was generated by site-directed mutagenesis using pCS2-myc-foxd3 as template and the following mutagenic primers: Forward 5’-CGACCCCCAGTCGGAAGATATTTCGACAACGGTAGCTTTCTG-3’ and reverse 5’-CAGAAAGCTACCGTTGTCGAAATATCTTCCGACTGGGGGTCG-3’. For microinjection, in vitro transcribed mRNA was generated from linearized plasmid templates using the Ambion SP6 mMessage mMachine system (Austin, TX).
Morpholino oligonucleotides
Morpholino antisense oligonucleotides were obtained from Gene Tools (Philomath, OR). Lyophilized oligonucleotides were resuspended in water, then diluted into 1X Danieau buffer (Nasevicius and Ekker, 2000) and 1nl was injected into one-cell stage embryos. Two morpholino antisense oligonucleotides were designed to Danio rerio foxd3 (BC095603): foxd3MO1 (5'-TGCTGCTGGAGCAACCCAAGGTAAG-3') (a gift of David Raible; Lister et al., 2006) is complementary to nucleotides 160–184 of the 5’ UTR and foxd3MO2 (5’-TGGTGCCTCCAGA CAGGGTCATCAC-3’) is complementary to nucleotides 194–218 and overlaps the start codon. A mixture of the two oligonucleotides (total dosage 20ng per embryo) was used for knockdown experiments in wild-type embryos. Injection of either individual oligonucleotide at higher dosage (30–40ng) yielded similar results, but with some associated toxicity. As specificity controls, a mismatch oligonucleotide was injected at equal dosage (5’-TGGTcCCTaCAGAgAGGcTCATaA C-3’), and RNAs encoding Xenopus foxd3 (30pg) (Steiner et al., 2006) or zebrafish cyclops (20pg) (Feldman et al., 1998) were injected to rescue. For Foxd3 knockdown in sym1 embryos a mixture of FoxD2MO1 and FoxD3MO2 was injected at a total dosage of 2–4ng. Due to the slightly delayed development of morphants, embryos were stage matched for phenotypic and gene expression analyses.
Whole mount in situ hybridization
Whole-mount in situ hybridization was performed as previously described (Schulte-Merker et al., 1992), using the following digoxigenin-labeled antisense RNA probes: bmp7 (Schmid et al., 2000), chordin (Miller-Bertoglio et al., 1997), cyclops (Rebagliati et al., 1998), goosecoid (Stachel et al., 1993), no tail (Schulte-Merker et al., 1994), and sonic hedgehog (Krauss et al., 1993). All images were taken from an MZFLIII12.5 stereomicroscope (Leica) with a Retiga 1300 camera (Q-imaging) and processed using Adobe Photoshop.
Genotyping
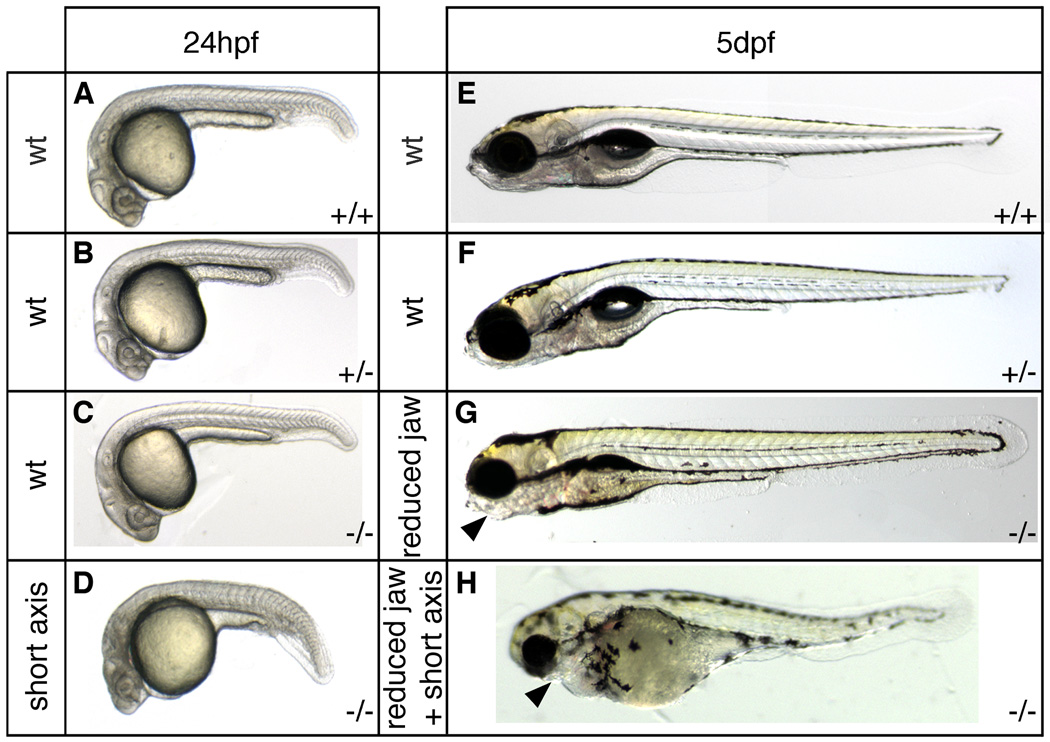
Heterozygous sym1 adults were crossed and individual progeny were harvested for genotyping at 5dpf. For each phenotypic class (wild-type, reduced jaw, short axis with reduced jaw) 7–14 individual embryos were analyzed. Genomic DNA was isolated as previously described (Westerfield, 1993) with the modification of incubating embryo lysates at 50°C overnight after the addition of extraction buffer. Primers flanking the position of the sym1 point deletion were used to PCR amplify this region of foxd3 from genomic DNA (forward 5’-GCGAATTCCTTCGTC AAGATCCCACG-3’; reverse 5’-CATATGGAATTCACCCGGCGAA TTCAG-3’) and products were subcloned into the pCR4-TOPO vector (Invitrogen). For each individual embryo 6–17 subclones were sequenced, and individual fish were assigned to genotypic categories based on the sequence of 6 or more subclones with matching top and bottom strands. For the phenotypically wild-type class, 14 individual embryos were analyzed and of these 7 were wild-type and 7 were sym1 heterozygotes. For the mutant classes, 7 “reduced jaw” embryos and 8 “short axis with reduced jaw” embryos were analyzed, and in every case were confirmed as sym1 homozygotes. The genotype of wild-type and sym1 heterozygous parents was also confirmed using this strategy.
Results
Foxd3 induces dorsal mesoderm and dorsalization of the body axis
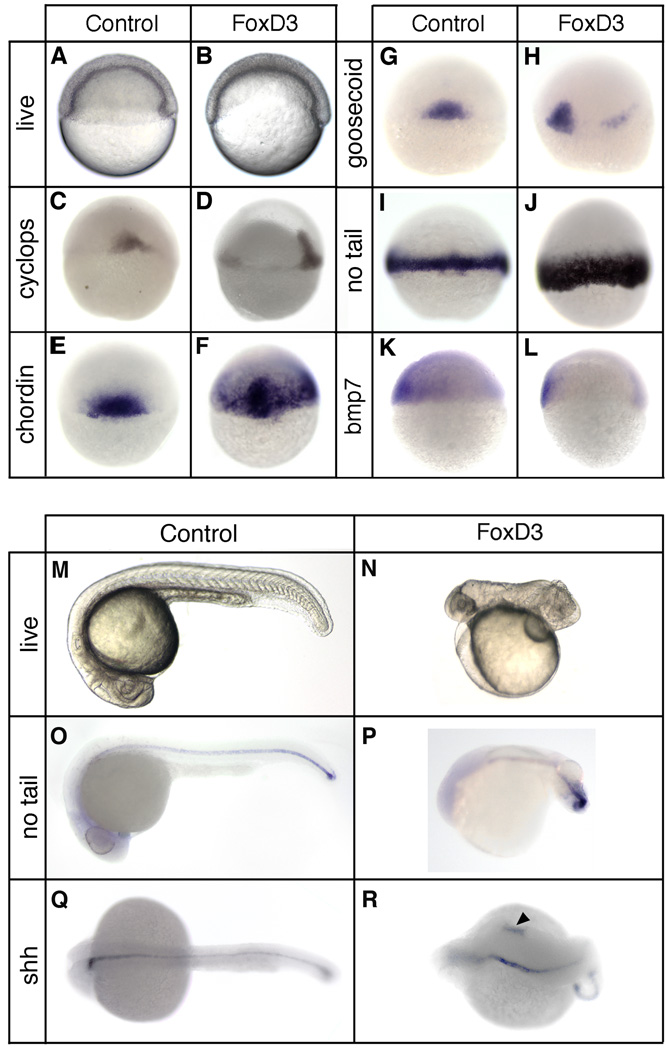
To determine the activity of Foxd3 in mesodermal development, gastrulation and axis formation, gain-of-function analysis was performed by mRNA injection, and embryos were examined at gastrula and 24hpf stages (Fig. 1). Wild-type embryos were injected at the one-cell stage with 25pg of foxd3 mRNA, and shield stage morphology was assessed in live embryos and mesodermal gene expression was evaluated by whole-mount in situ hybridization. In response to Foxd3, excessive convergence to the midline was observed as a thickening of the dorsolateral blastoderm and shield (94%, n=556), as compared to uninjected embryos (Fig. 1A,B). Expansion of dorsal or panmesodermal gene expression was observed in a majority of injected embryos, along with a reduction in ventral gene expression (Fig. 1C–L). For cyclops, chordin, and goosecoid (88%, n=637; 93%, n=473; 86%, n=641), genes normally restricted to the shield domain (Stachel et al., 1993; Miller-Bertoglio et al., 1997; Rebagliati et al., 1998), Foxd3 induced lateral expansion (Fig. 1D,F,H), as well as animal expansion for chordin (Fig. 1F) and ectopic lateral expression for goosecoid (Fig. 1H). The panmesodermal gene no tail is expressed throughout the margin at the shield stage (Schulte-Merker et al., 1992), and Foxd3 induced an expansion of no tail towards the animal pole (81%, n=634), resulting in a broader marginal domain of no tail expression (Fig. 1J). Expansion of squint expression was also observed in response to foxd3 overexpression (86%, n= 652) (data not shown), similar to the response of cyclops. Consistent with the expansion of dorsal gene expression, the ventrolateral domain of bmp7 expression (Schmid et al., 2000) was reduced and limited to the ventralmost margin in response to Foxd3 (95%, n=342) (Fig. 1L).
Fig 1.
Foxd3 induction of dorsal mesoderm and axial dorsalization. At the one-cell stage embryos were injected with 25pg of foxd3 mRNA and analyzed at the shield stage (6hpf) (A–L) or at 24 hpf (M–R). (A,B) Live embryos (lateral views, dorsal right) showing the shield and blastoderm structure in uninjected (A) and foxd3-injected (B) embryos. In situ hybridization of uninjected (C,E,G,I,K) and foxd3-injected (D,F,H,J,K) embryos showing expression of cyclops (C,D), chordin (E,F), goosecoid (G,H), no tail (I,J), and bmp7 (K,L). Views shown are dorsal (C,E,F,G,I,J), dorsal lateral (D,H), or lateral with dorsal right (K,L). Uninjected (M,O,Q) and foxd3-injected (N,P,R) embryos at 24hpf. Shown are live embryos (M,N), and embryos analyzed by in situ hybridization for no tail (O,P) or sonic hedgehog (Shh) (Q,R) expression. Views are lateral with anterior left (M–P) or dorsal with anterior left (Q,R). Ectopic expression of sonic hedgehog was observed in foxd3-expressing embryos (R, arrowhead).
Foxd3-injected embryos were strongly dorsalized at 24hpf (90%, n=230) (Fig. 1M,N), consistent with the expansion of dorsal mesodermal genes at the shield stage. To assess the formation of axial mesoderm at 24hpf, no tail and sonic hedgehog expression was examined (Fig. 1O–R). no tail expression was not perturbed throughout much of the body axis, but was disorganized in the tailbud (Fig. 1P), consistent with the morphogenetic disruption of posterior structures in dorsalized embryos (Holley, 2006). At 24hpf sonic hedgehog is expressed in the notochord, floor plate, and part of the diencephalon (Krauss et al., 1993), and Foxd3 induced expanded (83%, n=64) or ectopic (17%, n=13) expression (Fig. 1R), consistent with axial dorsalization and, in a minority of embryos, axial duplication. The morphological and gene expression changes observed at the shield and 24hpf stages demonstrate that Foxd3 can strongly induce the expansion of the dorsal mesoderm, resulting in a predicted dorsalization of the body axis. Furthermore, the embryonic response to Foxd3 is similar to that observed for Nodal pathway gain-of-function in the zebrafish (Feldman et al., 1998).
The gain-of-function studies show that zebrafish Foxd3 can influence mesodermal development within the intact embryo, but do not demonstrate an ability of Foxd3 to induce dorsal mesoderm de novo from competent tissue. To assess this function, zebrafish Foxd3 was expressed in Xenopus animal explants, which normally differentiate as atypical epidermis, but are competent to form mesoderm in response to appropriate inducers (Symes and Smith, 1987). At the one-cell stage, foxd3 mRNA (100pg) was injected into the animal pole, explants were isolated at the late blastula stage, cultured to the tailbud stage, and mesodermal gene expression was examined by RT-PCR. Zebrafish Foxd3 strongly induced the expression of muscle actin and collagen II, markers of somitic muscle and notochord, respectively (see Fig. S1 in supplementary materials). Therefore, zebrafish Foxd3 has potent dorsal mesoderm-inducing activity, identical to that previously described for Xenopus Foxd3 (Steiner et al., 2006).
Foxd3 is essential for mesodermal and axial development
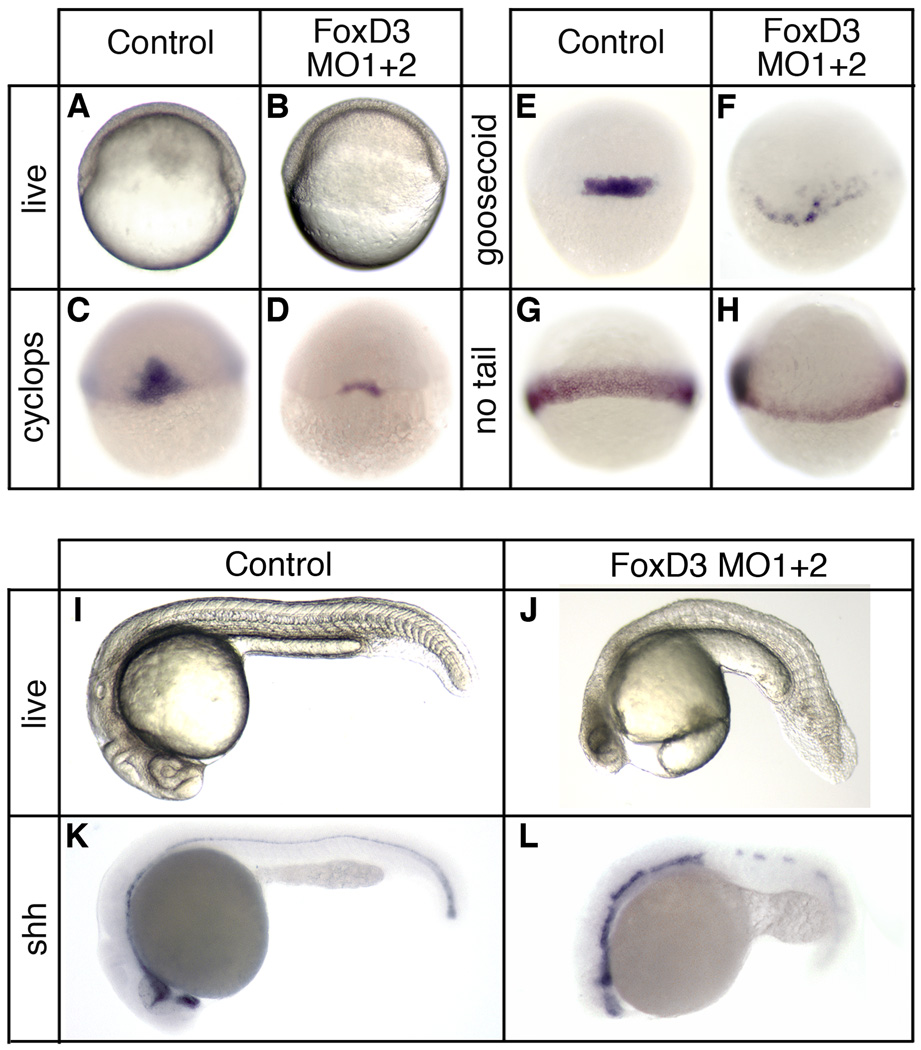
To determine the requirement for Foxd3 in zebrafish mesodermal development, knockdown studies were performed using morpholino antisense oligonucleotides. A mixture of two oligonucleotides, one targeting the foxd3 5’UTR and one overlapping the initiator codon (see Materials and Methods), were injected at the one-cell stage (total dosage 20ng), and embryos were analyzed at the shield and 24hpf stages (Fig. 2). At the shield stage, Foxd3 knockdown embryos had reduced or absent shield structures (93%, n=593) (Fig. 2A,B), and a significant reduction of dorsal mesodermal gene expression (Fig. 2C–H). cyclops and goosecoid expression was detectable, but substantially reduced in a majority of knockdown embryos (86%, n=438 and 73%, n=554, respectively) (Fig. 2D,F), and no tail expression was reduced to a thin marginal expression domain (70%, n=523) (Fig. 2H). In addition, chordin and squint expression in the shield was strongly reduced (79%, n=431 and 81%, n=633, respectively) (data not shown).
Fig. 2.
Foxd3 is essential for mesodermal development and axis formation. At the one-cell stage embryos were injected with a mixture of two foxd3-specific antisense morpholino oligonucleotides (MO1+2, total dosage 20ng) and analyzed at the shield stage (6hpf) (A–H) or at 24 hpf (I–L). (A,B) Live embryos (lateral views, dorsal right) showing the shield and blastoderm structure in uninjected (A) and Foxd3 knockdown (B) embryos. In situ hybridization of uninjected (C,E,G) and FoxD3 knockdown (D,F,H) embryos showing expression of cyclops (C,D), goosecoid (E,F), and no tail (G,H) (dorsal views). Uninjected (I,K) and Foxd3 knockdown (J,L) embryos at 24hpf. Shown are live embryos (I,J), and embryos analyzed by in situ hybridization for sonic hedgehog (Shh) (K,L) expression (lateral views with anterior left). A foxd3 oligonucleotide with multiple mismatches did not produce mesodermal or axial phenotypes at a dosage (20–40ng) equal to or greater than the perfect match oligonucleotides (data not shown). See Supplementary Materials for rescue experiments (Fig. S2).
At 24hpf, Foxd3 knockdown embryos display reduced head structures, notochord defects, loss of trunk somites, and retention of tail somites (78%, n=330) (Fig. 2I,J). Analysis of sonic hedgehog expression indicated disruption of notochord and floor plate development (87%, n=138) (Fig. 2K,L). Moreover, the mesodermal and axial defects observed in Foxd3 knockdown embryos are similar to the single cyclops or squint mutants, as well as the zygotic oep mutant (Feldman et al., 1998; Gritsman et al., 1999), consistent with the reduction of cyclops and squint expression in knockdown embryos.
Specificity controls for the knockdown studies included injection of a morpholino mismatch oligonucleotide, as well as knockdown rescue experiments. At a dosage (20–40ng) equal to or greater than the perfect match oligonucleotides, a foxd3 oligonucleotide with multiple mismatches did not produce any mesodermal or axial phenotypes (data not shown). In addition, injection of knockdown embryos with Xenopus foxd3 (30pg) rescued normal development in most embryos (81%, n=126) (see Fig. S2 in supplementary materials). Taken together, the observations indicate that a specific knockdown of endogenous foxd3 results in severe mesodermal and axial defects, strongly supporting an essential and conserved role for Foxd3 in mesodermal development. Furthermore, the similarity of phenotypes for Foxd3 knockdown and Nodal pathway partial loss-of-function is consistent with the predicted role of Foxd3 in promoting Nodal expression and signaling (Steiner et al., 2006). The regulatory relation of Foxd3 and the Nodal pathway is further supported by the ability of injected cyclops mRNA (20pg) to fully rescue normal development in Foxd3 knockdown embryos (73%, n=202) (see Fig. S2 in supplementary materials).
It is important to note that previous Foxd3 knockdown attempts in the zebrafish resulted only in neural crest defects, and not in the mesodermal phenotypes we report (Kelsh et al., 2000; Lister et al., 2006; Montero-Balaguer et al., 2006; Stewart et al., 2006). This difference may simply reflect the efficacy of Foxd3 knockdown. In our studies, a mixture of two foxd3-specific oligonucleotides was used, while the previously studies made use of only a single oligonucleotide. If our approach results in a more complete knockdown of Foxd3 protein, it would suggest that neural crest development is more sensitive to Foxd3 dosage than is mesodermal development, which could account for the differences in results obtained. Consistent with this idea, injection of lower doses of either single or combined morpholinos can recapitulate the neural crest defects previously reported (Kelsh et al., 2000; Lister et al., 2006; Montero-Balaguer et al., 2006; Stewart et al., 2006), in the absence of axial phenotypes (data not shown). However, a difference in knockdown efficiency cannot account for the absence of mesodermal phenotypes in the sym1 mutant, which has been reported to be a foxd3 null mutation (Stewart et al., 2006).
Foxd3 function is dependent on an active Nodal signaling pathway
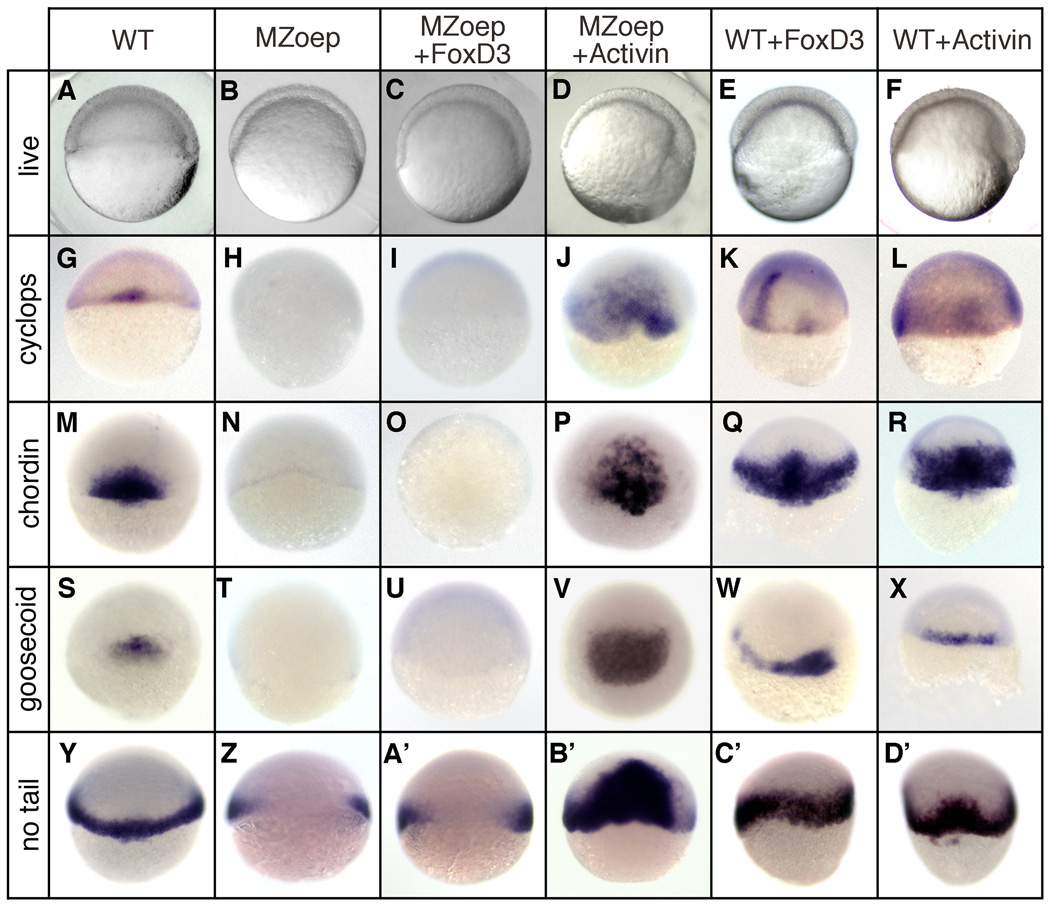
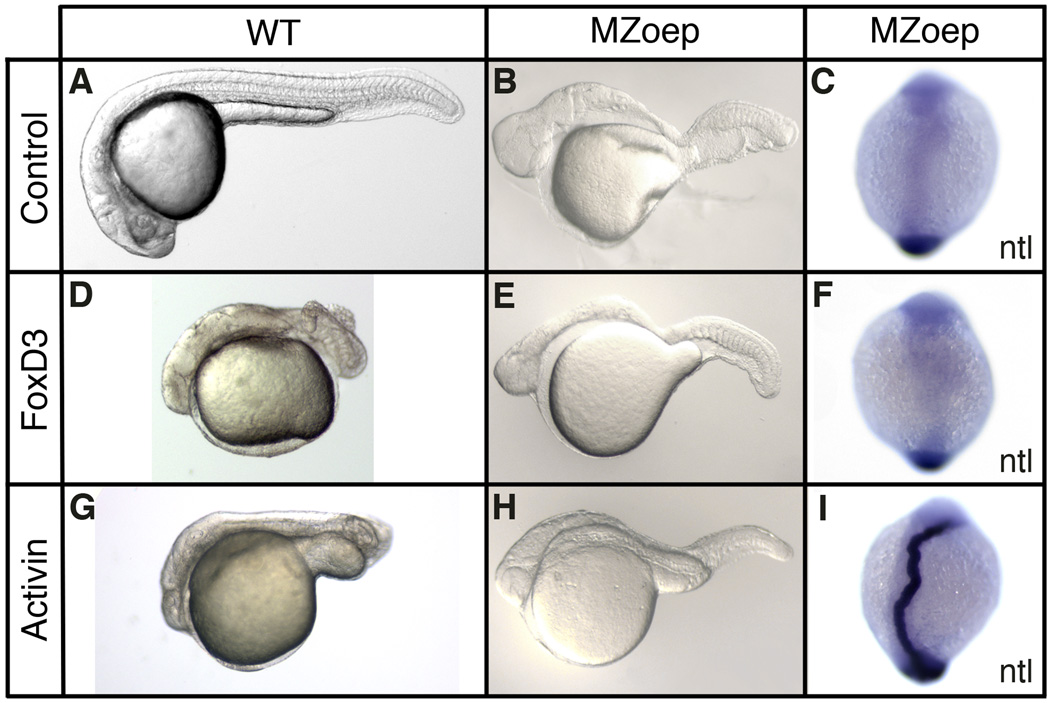
The Foxd3 gain-of-function and knockdown results support a role for Foxd3 in maintaining Nodal expression and activity in the organizer, where foxd3 and nodal genes are coexpressed during gastrulation (Odenthal and Nusslein-Volhard, 1998). To further examine the regulatory relation of Foxd3 and the Nodal pathway, we made use of MZoep mutant zebrafish that lack an essential Nodal coreceptor (Gritsman et al., 1999). In MZoep embryos, Nodal cannot bind its functional receptor complex and the elimination of Nodal signaling output results in a loss of dorsal mesodermal gene expression (Fig. 3H,N,T,Z). If Foxd3 acts solely as an upstream positive regulator of the Nodal pathway in the gastrula, it is predicted that the mesodermal activity of Foxd3 would be fully suppressed in MZoep embryos. At the one-cell stage, MZoep embryos were injected with foxd3 (25pg) and mesodermal gene expression was examined at the shield stage (Fig. 3). As predicted, Foxd3 did not rescue or induce the expression of cyclops (100%, n=132), goosecoid (100%, n=120), chordin (100%, n=114), or no tail (100%, n=102) in any of the injected embryos (Fig. 3I,O,U,A’). As a control for rescue, MZoep embryos were injected with activin (25pg), a TGFβ ligand that activates the Nodal pathway independent of the oep coreceptor requirement (Gritsman et al., 1999), and strong rescue of mesodermal gene expression was observed (Fig. 3J,P,V,B’). As positive controls for Foxd3 and Activin function, wild-type embryos injected with either foxd3 or activin showed strong induction of mesodermal gene expression (Fig. 3K,L,Q,R,W,X,C’,D’). Injected MZoep embryos were also examined at later stages for axial and midline rescue (Fig. 4) and Foxd3 failed to rescue head, trunk or notochord development (Fig. 4E,F), while Activin partially rescued head and trunk, and fully rescued notochord (Fig. 4H,I) (Gritsman et al., 1999).
Fig. 3.
Foxd3 induction of mesoderm is dependent on an active Nodal signaling pathway. Wild-type (WT) or MZoep mutant embryos were injected at the one-cell stage with zebrafish foxd3 (25pg) or Xenopus ActivinβB (25pg) mRNA and analyzed at the shield stage (6hpf). (A–F) Live embryos (lateral views, dorsal right) showing the shield and blastoderm structure in uninjected (A,B), foxd3-injected (C,E), and Activin-injected (D,F) embryos. In situ hybridization showing expression of cyclops (G–L), chordin (M–R), goosecoid (S–X), and no tail (Y–D’) (dorsal views).
Fig. 4.
Foxd3 regulation of axis formation is dependent on the Nodal signaling pathway. Wild-type (WT) or MZoep mutant embryos were injected at the one-cell stage with zebrafish foxd3 (25pg) or Xenopus ActivinβB (25pg) mRNA and analyzed at the 24hpf stage (A,B,D,E,G,H) or early somitogenesis stage (11hpf) (C,F,I). Uninjected (A,B), foxd3-injected (D,E), and Activin-injected (G,H) wild-type (A,D,G) and MZoep (B,E,H) embryos at 24hpf are shown (lateral views of live embryos with anterior left). In situ hybridization showing notochord expression of no tail (ntl) (C,F,I) in uninjected (C), FoxD3-injected (F), and Activin-injected (I) MZoep embryos at early somitogenesis stage (11hpf) (dorsal views with anterior up).
Further support for these conclusions was obtained by coexpressing foxd3 and antivin, an atypical TGFβ-related protein that inhibits Nodal signaling by sequestration of the Oep coreceptor (Thisse and Thisse, 1999). At the shield stage, Antivin fully suppressed mesodermal gene expression and coinjection of Foxd3 did not rescue expression of cyclops (100%, n=160), goosecoid (100%, n=132), or no tail (100%, n=116) (see Fig. S3 in supplementary materials). These results demonstrate that the mesodermal function of Foxd3 in the gastrula is completely dependent on a functional Nodal signaling pathway, consistent with a model in which Foxd3 acts upstream of Nodal in the organizer domain to promote mesodermal development.
Reexamination of the foxd3 sym1 mutant reveals mesodermal and axial defects
The sympathetic mutation 1 (sym1) is a foxd3 mutant (foxd3zdf10) identified in a genetic screen for mutations that disrupt the development of sympathetic neurons (Stewart et al., 2006). Homozygous sym1 embryos have defects in multiple neural crest lineages, including peripheral neurons, glia and cartilage, resulting in major craniofacial abnormalities. The molecular lesion present in sym1 is a point deletion (G537) that results in a short frameshift and premature stop, truncating the Foxd3 protein within the C-terminal region of the winged helix DNA-binding domain. This truncation is predicted to disrupt wing 2 of the winged helix, a region required for minor groove contacts, site recognition specificity and transcriptional function (Berry et al., 2005). Based on the predicted truncation and inactivation of the DNA-binding domain, Stewart and colleagues conclude that sym1 is functional null of foxd3. Subsequent to the identification of sym1, a conserved C-terminal Groucho corepressor interaction motif was identified in Xenopus Foxd3 that is essential for transcriptional and developmental function (Yaklichkin et al., 2007). The truncated sym1 product, with impaired DNA-binding function and absence of an essential transcriptional effector domain, is strongly predicted to be a functional null allele of foxd3.
Given the evidence from our zebrafish and Xenopus knockdown studies that Foxd3 is an essential regulator of mesodermal development, the reported absence of mesodermal phenotypes in sym1 embryos is difficult to accommodate. In an attempt to resolve this conundrum, we reexamined the phenotypic consequences of the sym1 mutation. Mating pairs of heterozygous sym1 adults were obtained and cross progeny (n=351) were examined at 24hpf and 5dpf, and assigned to phenotypic classes (Fig. 5). At 24hpf, a subset of cross progeny (10%, n=119) displayed axial phenotypes with reduced head structures, shortened axes, and expanded ventral tail somites (Fig. 5D), consistent with the Foxd3 knockdown phenotype (Fig. 2). Craniofacial defects are not morphologically apparent at this early stage. At 5dpf (n=494), while most embryos fit the previously identified phenotypic classes − 77% wild-type (Fig. 5E,F) and 14% with craniofacial defects (Fig. 5G) – a previously unreported phenotypic class was observed (9%) with both craniofacial defects and axial defects, including curved or shortened axes (Fig. 5H). This novel phenotypic class was not previously reported for the sym1 mutant (Stewart et al., 2006), but the nature of the axial abnormalities at 24hpf and 5dpf is consistent with Foxd3 function in mesoderm formation. To correlate each of these phenotypic classes with Foxd3 genotype, multiple individual embryos from each class were subjected to genotyping analysis. At 24hpf, the axial defect class consisted exclusively of sym1 homozygotes, and at 5dpf, the craniofacial defect class and the craniofacial-axial defect class consisted only of sym1 homozygotes (see Materials and Methods). At 24hpf, the phenotypically wild-type class consisted of wild-type, heterozygous and homozygous embryos, while at 5dpf, the phenotypically wild-type class consisted of wild-type and heterozygous embryos. Importantly, these results show that a genetic loss-of-function in Foxd3 does lead to phenotypic defects consistent with an essential function for Foxd3 in mesoderm and axis formation.
Fig. 5.
Axis formation defects in sym1 embryos. Heterozygous sym1 adults were crossed and axial development of progeny was analyzed at ~24hpf and ~5dpf. Representative samples of the two phenotypic classes observed at 24hpf (n=119): wild-type (90%) (A,B,C), and axial defects (10%) (D), and representative samples of the three phenotypic classes observed at 5dpf (n=494): wild-type (77%) (E,F), craniofacial defects (14%) (G), and craniofacial defects together with axial defects (9%) (H). Genotyping analysis indicated that at 24hpf (A–D) the wild-type phenotypic class consisted of genotypically wild-type (+/+), sym1 heterozygotes (+/−), and sym1 homozygotes (−/−), while axial defect class consisted only of sym1 homozygotes (−/−). At 5dpf (E–H) the wild-type phenotypic class consisted of both genotypically wild-type (+/+) and sym1 heterozygotes (+/−), while the craniofacial defect and the craniofacial with axial defect classes consisted only of sym1 homozygotes (−/−). Arrowhead indicates reduced jaw structures in the craniofacial defect class (G) and in the craniofacial defects together with axial defect class (H). Phenotypic class is indicated to the left of each panel and genotype is shown on the bottom right of each panel.
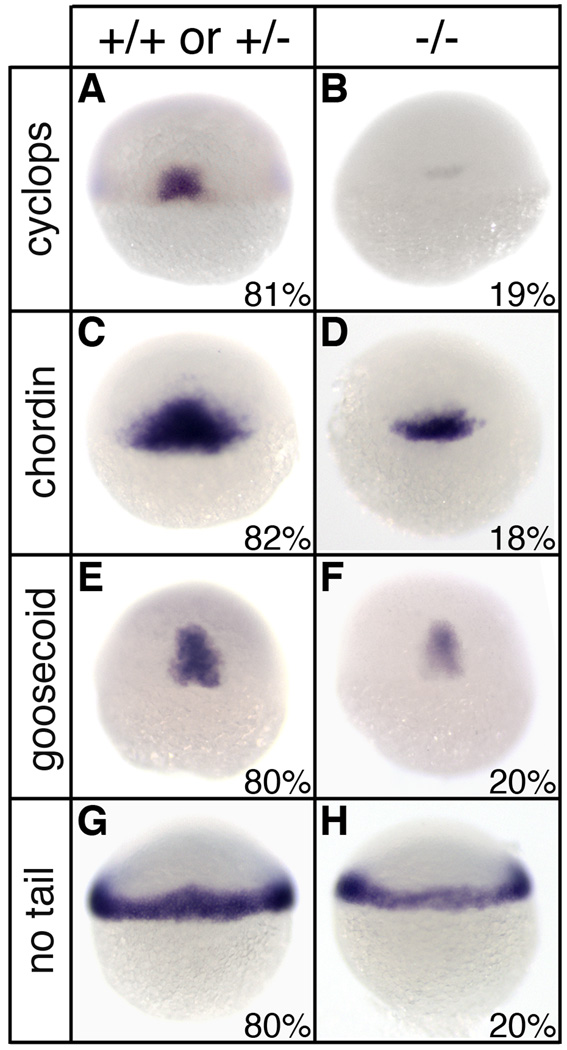
To determine the underlying developmental origins of the axial defects present in sym1 homozygotes, mesodermal gene expression was examined in cross progeny at the shield stage (Fig. 6). While ~80% of cross progeny had normal mesodermal gene expression, ~20% of the embryos displayed a substantial reduction of cyclops (n=321), chordin (n=299), goosecoid (n=317), and no tail (n=253) expression (Fig. 6B,D,F,H). Following in situ hybridization, embryos from each class were genotyped, and while embryos with normal gene expression were either wild-type or sym1 heterozygotes, embryos with reduced mesodermal gene expression were homozygous for sym1 (data not shown). Importantly, these results confirm the requirement for Foxd3 in dorsal mesodermal development at the gastrula stage. It is interesting to note that while most of the predicted 25% homozygous embryos show mesodermal gene expression deficits at the shield stage, the phenotypic severity appears to diminish during development such that by 24hpf only 9% of embryos display axial defects. This difference in penetrance of gene expression and axial phenotypes may reflect a developmental threshold with reduced levels of mesodermal gene expression being sufficient to support normal axial development in some embryos. Alternatively, compensation or regulation, during gastrulation or thereafter, may moderate the consequence of Foxd3 loss-of-function in some, but not all, homozygous embryos.
Fig. 6.
Reduced mesodermal gene expression in sym1 mutants. Heterozygous sym1 adults were crossed and progeny were examined for mesodermal gene expression by in situ hybridization at shield stage (6hpf). For cyclops (A,B), chordin (C,D), goosecoid (E,F), and no tail (G,H), two phenotypic classes were observed: wild-type (A,C,E,G) and reduced gene expression (B,D,F,H). For each mesodermal gene analyzed, distribution of progeny into the two classes was ~80% for the wild-type class and ~20% for the reduced class (exact distribution for each gene is indicated in lower right of each panel). Representative embryos were selected for genotyping analysis following in situ hybridization and this indicated that the wild-type phenotypic class consisted of both genotypically wild-type (+/+) and sym1 heterozygotes (+/−), while the reduced gene expression class consisted only of sym1 homozygotes (−/−). Dorsal views are shown.
sym1 is a hypomorphic allele of Foxd3 with partial penetrance of mesodermal defects
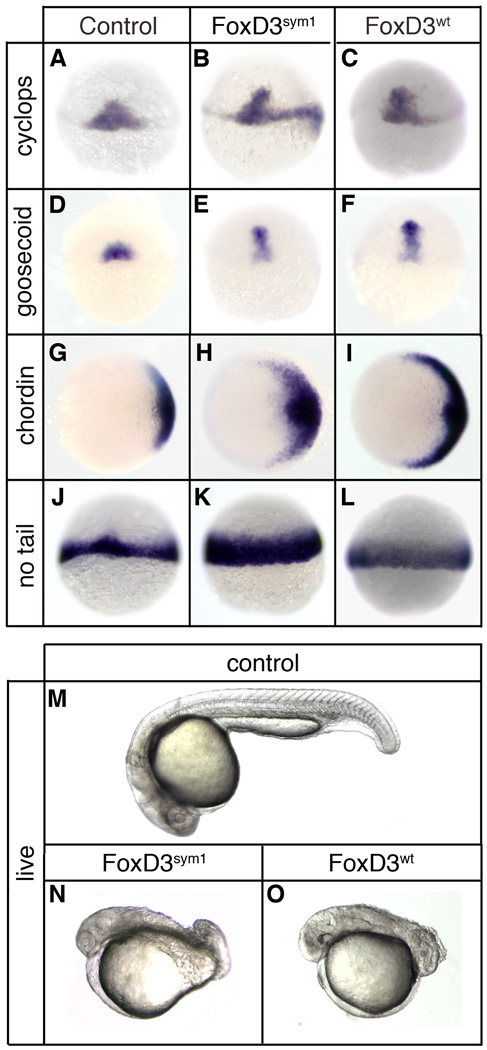
Despite the strong prediction that sym1 is a functional null allele of foxd3, the partial penetrance of mesodermal defects at both the gastrula and 24hpf stages raises the possibility that the sym1 product may retain some level of activity. To assess the developmental activity of the sym1 product, the point deletion was introduced into the wild-type foxd3 cDNA, and sym1 mRNA (150pg) was injected into wild-type embryos at the one-cell stage. Injected embryos were examined for mesodermal gene expression at the shield stage and for axis formation at 24hpf (Fig. 7). Surprisingly, sym1 injection resulted in expanded expression of cyclops (75%, n=310), goosecoid (73%, n=316), chordin (76%, n=328), and no tail (66%, n=334) in most embryos (Fig. 7B,E,H,K). Similarly, sym1 induced strong dorsalization of the body axis at 24hpf (76%, n=330) (Fig. 7N). When sym1 mRNA was injected at doses 6-fold higher than wild-type foxd3, the embryonic response was indistinguishable (Fig. 7C,F,I,L,O). This retention of activity indicates that sym1 is a hypomorphic allele, not a null, despite the strong prediction otherwise.
Fig. 7.
sym1 retains mesoderm induction and axis dorsalizing activity. At the one-cell stage embryos were injected with 150pg of sym1 mRNA (Foxd3sym1) (B,E,H,K,N) or 25pg of wild-type foxd3mRNA (Foxd3wt) (C,F,I,L,O) and analyzed at the shield stage (6hpf) (A–L) or at 24 hpf (M–O). In situ hybridization at shield stage of uninjected (A,D,G,J), sym1-injected (B,E,H,K), and foxd3-injected (C,F,I,L) embryos showing expression of cyclops (A–C), goosecoid (D–F), chordin (G–H), and no tail (J–L). Views shown are dorsal (A–F, J–L) or animal with dorsal right (G–I). Uninjected (M), sym1-injected (N), and foxd3-injected (O) embryos at 24hpf. Shown are live embryos (lateral views with anterior left).
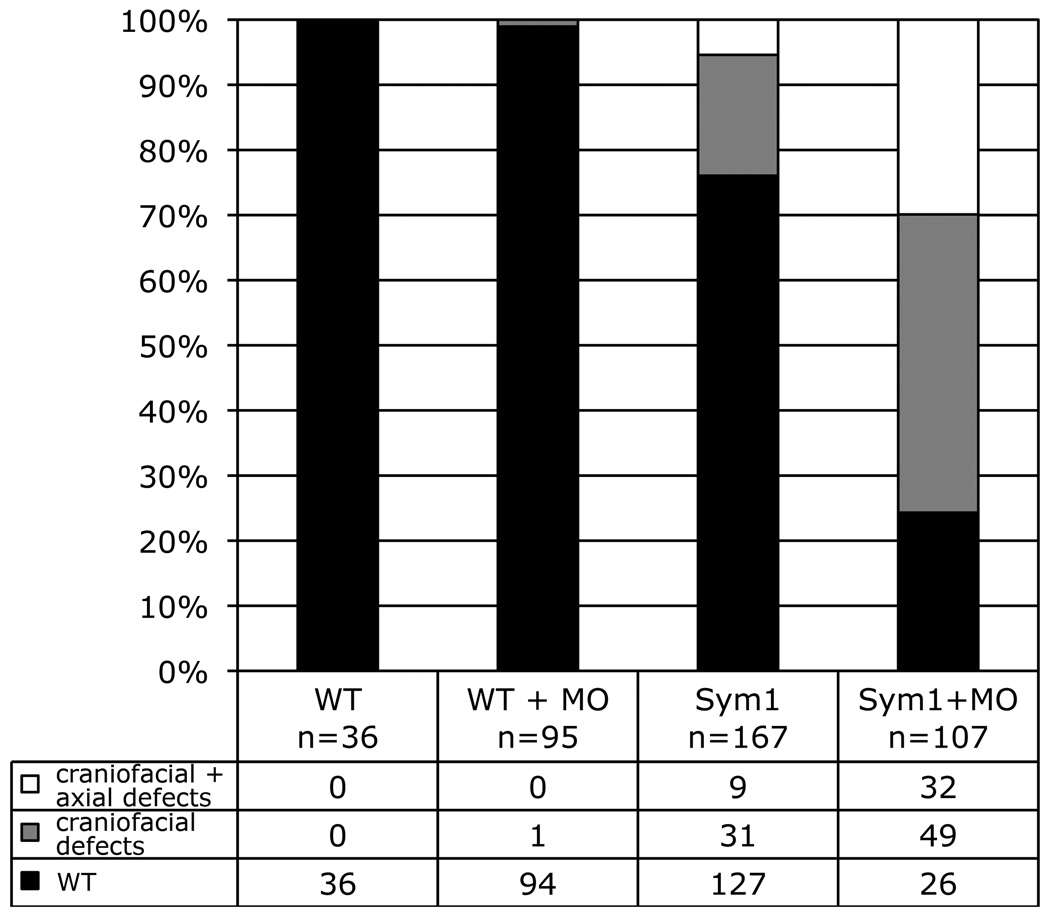
To confirm the retention of a low level of Foxd3 function in sym1 mutants, we attempted to further knockdown Foxd3 function in sym1 cross progeny. If sym1 is indeed a hypomorphic allele, it is predicted that knockdown of the sym1 product would result in increased penetrance of the axial phenotype. The mixture of two foxd3-specific oligonucleotides was injected at low dosage (2.5ng) into the one-cell stage progeny of sym1 het crosses. At this low dosage no phenotypic response was observed in wild-type embryos (Fig. 8). In contrast, injection of this low dosage of oligonucleotides into sym1 progeny resulted in a dramatic increase in both the craniofacial only and craniofacial-axial phenotypic classes. In these experiments, the phenotypic distribution of uninjected sym1 progeny was 76% wild-type, 18% craniofacial only, and 5% craniofacial-axial (n=167) (Fig. 8). Foxd3 knockdown in cross progeny resulted in 24% wild-type, 45% craniofacial only, and 29% craniofacial-axial (n=107) (Fig. 8). Genotyping confirmed that the expanded craniofacial only and craniofacial-axial phenotypic classes consisted entirely of sym1 heterozygotes and homozygotes (data not shown). Taken together, these results indicate that functional Foxd3 protein is retained in sym1 homozygotes, and that knockdown of the remaining Foxd3 function results in increased penetrance of axial phenotypes, confirming that Foxd3 function is essential for zebrafish mesodermal development.
Fig. 8.
Foxd3 knockdown in sym1 embryos enhances penetrance of mesodermal defects. Cross progeny of wild-type (WT) or sym1 heterozygous (Sym1) adults were injected at the one-cell stage with a mixture of foxd3MO1 and foxd3MO2 (MO) (total dosage 2.5ng) and craniofacial and axial phenotypes were assessed at 5dpf. Quantification of the three phenotypic classes is shown as a percentage of total: Wild-type (wt, black), craniofacial defect (reduced jaw, gray), craniofacial defects together with axial defects (short, white).
Discussion
The control of mesodermal development is an area of intense study, and these efforts have provided broad mechanistic insight to the signaling and transcriptional processes that establish and pattern the major lineages of the vertebrate embryo (Kimelman, 2006; Chang and Kessler, 2007). Here we show that the transcriptional protein, Foxd3, is an essential regulator of mesodermal and axial development in the zebrafish. Foxd3 function is required for maintenance of cyclops expression in the shield, formation of dorsal mesodermal lineages, and development of normal axial structure. The mesodermal activity of Foxd3 is fully dependent on a functional Nodal signaling pathway, indicating that Foxd3 acts as an upstream positive regulator of Nodal pathway activity. Furthermore, we demonstrate that sym1, a foxd3 mutant previously reported to be a null allele with no mesodermal or axial defects (Stewart et al., 2006), does indeed exhibit reduction of cyclops expression, loss of dorsal mesodermal lineages, and disruption of axis formation, albeit at reduced penetrance. Despite predictions that sym1 is a functional null, we find that knockdown of residual Foxd3 activity in sym1 embryos strongly enhances the mesodermal and axial phenotypes, indicating that sym1 is a hypomorphic allele of foxd3. These studies define the developmental requirement for Foxd3 in the zebrafish gastrula and demonstrate that Foxd3 is an upstream regulator of the Nodal pathway that is essential for dorsal mesoderm development.
Foxd3 regulation of the Nodal pathway
With the onset of gastrulaton in the zebrafish, foxd3 expression is initiated in the shield domain (Odenthal and Nusslein-Volhard, 1998) where it is coexpressed with the Nodal ligands, cyclops and squint. Our developmental analyses demonstrate that Foxd3 function is necessary and sufficient for Nodal expression in the gastrula. Foxd3 gain-of-function resulted in ectopic and/or expanded cyclops expression, while in knockdown and sym1 embryos, expression of cyclops was substantially reduced (Figs. 1,2,6). A similar Foxd3-dependence was observed for squint expression in the shield (data not shown). Given that the onset of Nodal expression in the dorsal domain precedes the initiation of FoxD3 expression in the shield, the results suggest that Foxd3 is not required for the initiation of Nodal expression, but rather for the maintenance of Nodal expression during gastrulation.
The developmental importance of the Foxd3-Nodal interaction is apparent from the mesodermal and axial defects resulting from Foxd3 loss-of-function, and the similarity of these phenotypes to Nodal pathway loss-of-function. Foxd3 loss-of-function, either by knockdown or sym1 mutation, results in a reduction of dorsal mesodermal gene expression and axial defects that include reduction of head structures, reduction of trunk mesoderm, disruption of midline structures, and expansion of tail somites (Figs. 2,5,6). This phenotypic spectrum corresponds to those reported for Nodal pathway loss-of-function in either the single cyclops or squint mutants or the zygotic oep mutant (Feldman et al., 1998; Zhang et al., 1998), consistent with the reduction of cyclops and squint expression in Foxd3 morphants and sym1 mutants. This phenotypic similarity suggests that the developmental consequences of Foxd3 loss-of-function are largely due to a reduction of downstream Nodal pathway activity. Consistent with this idea, injection of cyclops mRNA fully rescued axial development in both Foxd3 knockdown and sym1 mutant embryos (Fig. S2 and data not shown).
Strong evidence of the dependence of Foxd3 function on Nodal pathway activity comes from the analysis of Foxd3 activity in the MZoep mutant, which is inactive for Nodal signaling. In the MZoep background, Foxd3 had no detectable activity, even when expressed at levels sufficient to strongly dorsalize wild-type embryos (Figs. 3,4). Foxd3 could rescue neither dorsal mesodermal gene expression nor axis formation in MZoep embryos, indicating that in the zebrafish gastrula, the mesodermal activity of Foxd3 was completely dependent on a functional downstream Nodal pathway. These results provide functional and genetic support for the essential role of Foxd3 as an upstream positive regulator of Nodal pathway activity in the zebrafish gastrula. Furthermore, these studies strongly confirm and extend our model of Foxd3-Nodal interaction in the Xenopus gastrula (Steiner et al., 2006), suggesting that this regulatory pathway may play an essential conserved role in vertebrate mesodermal development. It will be of great interest to explore the interaction of Foxd3 and the Nodal pathway in the mouse, where the gastrula-specific function of Foxd3 has yet to be examined.
sym1 is a hypomorphic allele of foxd3
In striking contrast to our findings, prior knockdown and mutant studies of Foxd3 in the zebrafish revealed no mesodermal or axial phenotypes. Using translation-blocking oligonucleotides, several groups described neural crest lineage defects resulting from Foxd3 knockdown, each reporting largely similar phenotypes, but no mesodermal or axial defects were observed (Whitlock et al., 2005; Lister et al., 2006; Montero-Balaguer et al., 2006; Stewart et al., 2006; Ignatius et al., 2008; Curran et al., 2009). In these studies, it is likely that the Foxd3 knockdown conditions were optimized for neural crest-related phenotypes, and that these conditions were not sufficient for generating mesodermal defects at significant frequency. It should be noted, however, that one group does remark on an apparent dorsalizing activity of overexpressed Foxd3 (Lister et al., 2006), consistent with our results. We observed mesodermal defects at high frequency by injection of a mixture of two foxd3-specific oligonucleotides, suggesting that a more complete knockdown of Foxd3 may be necessary to disrupt mesodermal development. When injected at lower doses, the single or combined oligonucleotides did produce the previously described neural crest defects in the absence of mesodermal or axial phenotypes. Therefore, the absence of mesodermal phenotypes in previous knockdown studies may merely reflect differences in experimental design that result in more or less complete knockdown of Foxd3.
The discrepancy in obtaining mesodermal phenotypes resulting from Foxd3 knockdown prompted a reevaluation of the identified zebrafish foxd3 mutants. Two foxd3 mutants, sym1 and mos, were identified in screens for regulators of neural crest development (Montero-Balaguer et al., 2006; Stewart et al., 2006). In both cases, these mutations result in defects in craniofacial structures, the peripheral nervous system, and pigment cells, but not mesodermal or axial phenotypes. The molecular lesion in sym1 is predicted to truncate the DNA-binding domain and eliminate an essential C-terminal transcriptional effector domain, so it was concluded that sym1 was a functional null allele of foxd3 (Stewart et al., 2006). Given that our knockdown studies in Xenopus (Steiner et al., 2006) and zebrafish (this study) demonstrate a requirement for Foxd3 in mesodermal development, the absence of mesodermal defects in sym1 suggested either the presence of redundant and/or compensating activities in the sym1 mutant or that sym1 was not a functional null allele.
The reanalysis of sym1 provides definitive evidence of mesodermal and axial defects in this mutant, with ~39% of sym1 homozygotes displaying both craniofacial and axial phenotypes (Fig. 5), and ~80% of homozygotes with reduced cyclops and dorsal mesodermal gene expression at the shield stage (Fig. 6). The penetrance of the mesodermal defects is enhanced by specific knockdown of Foxd3 in mutant embryos (Fig. 8), indicating that sym1 is a hypomorphic allele of foxd3, and not a null. In gain-of-function studies sym1 retains dorsalizing activity (Fig. 7), consistent with a hypomorphic character for the sym1 mutation. Taken together, these observations demonstrate that reduction of Foxd3 activity in the zebrafish results in mesodermal and axial defects, establishing an essential role for Foxd3 in zebrafish mesodermal development.
The mos mutation, in contrast to sym1, does not reside in the foxd3 coding region, but is a mutation in a distal regulatory element required for foxd3 expression in neural crest lineages (Montero-Balaguer et al., 2006). In mos embryos, foxd3 expression is lost in neural crest progenitor cells, resulting in neural crest phenotypes identical to those observed in sym1 embryos and in Foxd3 knockdown embryos. At the gastrula stage, however, foxd3 expression in the shield is normal and no defects in mesodermal or axial development are observed in mos embryos. Therefore, gastrula expression and function of Foxd3 is maintained in mos embryos, resulting in normal development of the mesoderm and body axis.
In contrast to the reduced penetrance of mesodermal phenotypes in sym1 embryos, neural crest phenotypes are fully penetrant in sym1 and equal in severity to that observed in mos mutant embryos, which are null for foxd3 expression in the neural crest. This suggests that sym1 is a functional null for neural crest development, despite the sufficiency of this hypomorphic allele to support mesodermal developmental in most embryos. This difference in penetrance likely reflects differing dosage sensitivities in the two lineages, with greater Foxd3 activity required for neural crest development. More recent studies in the zebrafish have examined the genetic interaction of Foxd3 with other neural crest regulators, and conclusions drawn from these studies are based on the assumption that sym1 is a true null allele of foxd3 (Ignatius et al., 2008; Arduini et al., 2009; Cooper et al., 2009). Our demonstration that sym1 is a hypomorph that retains biological activity should prompt a careful reevaluation of these genetic interaction studies. Identification of a true null allele for zebrafish foxd3 is necessary to provide a definitive analysis of Foxd3 function in both mesodermal and neural crest lineages.
Transcriptional function of Foxd3 in development
Foxd3 has multiple roles in the developing vertebrate embryo, including the maintenance of embryonic and trophoblast stem cell lineages in the mouse (Hanna et al., 2002; Sutton et al., 1996; Tompers et al., 2005), regulation of neural crest determination, migration, and/or differentiation in the mouse, chick, Xenopus, and zebrafish (Dottori et al., 2001; Kos et al., 2001; Sasai et al., 2001; Cheung et al., 2005; Whitlock et al., 2005; Lister et al., 2006; Montero-Balaguer et al., 2006; Stewart et al., 2006; Teng et al., 2008; Arduini et al., 2009; Cooper et al., 2009), and promotion of nodal expression and activity during mesodermal development in Xenopus (Steiner et al., 2006) and zebrafish (this study). Given the many roles of Foxd3, defining the molecular mechanisms of Foxd3 action, including transcriptional activity and target gene identity, is essential for understanding the common and lineage-specific functions of Foxd3.
The transcriptional function of Foxd3 has been examined in a variety of cell culture and embryonic systems and these developmental and transcriptional studies provide strong evidence that Foxd3 functions as a transcriptional repressor. In Xenopus neural crest and mesodermal lineages, a fusion protein containing the Engrailed repressor domain and the Foxd3 winged helix DNA-binding domain mimicked the activity of native Foxd3, while a VP16 activator-Foxd3 fusion antagonized Foxd3 function (Pohl and Knochel, 2001; Sasai et al., 2001; Steiner et al., 2006). Similarly, an Engrailed-Foxd3 fusion protein can mimic the mesodermal and axial activities of native Foxd3 in the zebrafish, and VP16-Foxd3 disrupts zebrafish mesoderm and axis formation (L.L.C. and D.S.K., unpublished). In cell culture, Xenopus mesoderm and zebrafish neural crest, transcriptional reporter assays indicate that Foxd3 directly represses transcription of natural and heterologous target genes (Sutton et al., 1996; Freyaldenhoven et al., 1997; Yaklichkin et al., 2007; Ignatius et al., 2008; Curran et al., 2009). Consistent with a function as an obligate transcriptional repressor, all Foxd3 orthologs contain a conserved Groucho corepressor interaction motif, which in Xenopus Foxd3 is essential for Groucho recruitment, transcriptional repression activity, and mesoderm induction (Yaklichkin et al., 2007). Taken together, these observations provide compelling evidence that Foxd3 functions as a transcriptional repressor to regulate mesodermal development in the zebrafish gastrula.
Despite the strong evidence that Foxd3 functions as a repressor, transcriptional activation function for mouse FOXD3 has been reported in 293 cells and ES cells (Guo et al., 2002; Pan et al., 2006). Given that mouse FOXD3 can also function as a repressor in 293 cells (Sutton et al., 1996), the transcriptional activity of FOXD3 may be dependent on promoter context and/or transcriptional cofactors that facilitate activation of certain targets and repression of others. These results suggest that FOXD3 may have a dual transcriptional function, with context-dependent or lineage-specific activation of a subset of target genes. It should also be noted that the mammalian FOXD3 proteins contain several polyalanine and polyglycine sequences that are not present in other Foxd3 orthologs, and these sequences may confer additional transcriptional functions on the mammalian proteins.
An activation function for Foxd3 has also been reported in zebrafish somitogenesis (Lee et al., 2006). In a yeast one-hybrid screen, zebrafish Foxd3 bound a somite-specific regulatory element of myf5, and a transcriptional reporter containing that element was weakly activated by Foxd3 in cell culture studies. Foxd3 is coexpressed with myf5 in somites, and Foxd3 knockdown resulted in a loss of myf5 expression in somites, indicating that Foxd3 activates myf5 expression in newly formed somites. Therefore, the transcriptional function of Foxd3 may differ in distinct lineages of the zebrafish, perhaps due to the lineage-specific expression of coactivators, corepressors, or other interacting factors. However, in conflict with these results, we have found that when introduced into zebrafish embryos by microinjection, the myf5 reporters were unresponsive to overexpressed Foxd3 at the shield and somite stages, arguing against a direct role for Foxd3 in myf5 regulation (L.L.C. and D.S.K., unpublished).
The Nodal pathway is subject to multiple levels of negative regulation that strictly limit signaling activity, both spatially and temporally (Schier, 2003; Shen, 2007). Due to the non-autonomous and autoregulatory function of Nodal signaling, negative regulatory inputs are essential to limit the amplification and spread of Nodal activity, thus ensuring proper embryonic organization. The ability of Foxd3 to promote nodal expression as a transcriptional repressor suggests that Foxd3 functions as an indirect activator by repressing a negative regulator(s) of nodal expression and/or activity in the shield. Foxd3 may repress antagonists of Nodal ligand-receptor interaction or signal transduction, or repressors of Nodal transcription. If Foxd3 were to repress antagonists of Nodal signaling, it might be expected that the transcriptional response to Foxd3 would be mediated largely by the conserved autoregulatory enhancer within intron 1 of the nodal locus (Osada et al., 2000). In preliminary analyses of the Xenopus nodal-related-1 gene, we identified a distal Foxd3-responsive region upstream of the start site of transcription, while the autoregulatory intron 1 element was unresponsive to Foxd3 (Qun Lu and D.S.K., unpublished). We therefore favor a role for Foxd3 in repressing a transcriptional repressor of nodal expression in the shield.
How does sym1 retain biological activity?
The reanalysis of sym1 demonstrates that this hypomorphic allele of foxd3 results in mesodermal and axial defects with reduced penetrance, but one confounding question remains unanswered. How does the protein product of sym1 retain biological activity? The molecular lesion of the sym1 mutation is a point deletion within the C-terminus of the winged helix DNA-binding domain (Stewart et al., 2006). This results in a short frameshift followed by a premature stop, truncating much of the W2 region of the DNA-binding domain and eliminating a distal Groucho interaction motif (GEH). Disruption of either the W2 or GEH domains has been shown to result in a near complete loss-of-function (Berry et al, 2005; Yaklichkin et al., 2007), and disruption of both is strongly predicted to result in a functionally inactive Foxd3 protein. So how is it that the product of sym1 retains biological activity, as shown in our studies?
In considering this question we note that the coding capacity for full-length Foxd3 protein is present within the sym1 mRNA, which is not truncated, so it may be that corrective mechanisms bypass the premature stop to generate a Foxd3 protein containing the essential C-terminal domains. In support of such a corrective mechanism, when truncated at the position of the predicted premature stop, the sym1 cDNA has no dorsalizing activity (data not shown). Therefore, sequences distal to the C-terminus of the predicted sym1 product are required for the biological activity of sym1, raising the possibility that full-length functional Foxd3 protein may be produced from the sym1 cDNA. Suppression of the premature stop would not be sufficient to restore Foxd3 function as the downstream reading frame has been shifted due to the point deletion. On the other hand, translational frameshifting, prior to the premature stop, would correct the open reading frame and allow production of full-length Foxd3 protein. Ongoing efforts focus on defining the molecular mechanisms responsible for the unexpected retention of biological activity for sym1.
Foxd3 is an essential transcriptional regulator for the development of multiple lineages in the vertebrate embryo. A number of important questions remain to be to explored, including the identification of Foxd3 targets that mediate the distinct functions of Foxd3 in specific lineages. It will be especially interesting to determine whether lineage-specific targets and mechanisms mediate Foxd3 function in the mesoderm, neural crest and stem cell populations, or if an underlying common regulatory pathway is utilized in each of these lineages. Ongoing studies of Foxd3 in the organizer, neural crest, and stem cell populations will provide further insight into the developmental and molecular mechanisms of vertebrate embryogenesis.
Supplementary Material
Supplementary Figure 1. Zebrafish Foxd3 induces mesoderm in Xenopus animal explants. At the one-cell stage, Xenopus embryos were injected in the animal pole with 100pg of Xenopus foxd3 (xFoxD3, lane 2) or zebrafish foxd3 (zFoxD3, lane 3), explants were prepared at the late blastula stage (stage 9) and analyzed by RT-PCR at the tailbud stage (stage 25) for the expression of Muscle Actin (somites) and Collagen II (notochord). EF1α is a control for RNA recovery and loading, intact embryos (Embryo, lane 4) served as a positive control and an identical reaction without reverse transcriptase controlled for PCR contamination (Embryo-RT, lane 5).
Supplementary Figure 2. Rescue of Foxd3 knockdown by Xenopus foxd3 or cyclops. At the one-cell stage, wild-type embryos were injected with a mixture of foxd3MO1 and foxd3MO2 (MO1+2, total dosage 20ng) alone (D), or in combination with 30pg of Xenopus foxd3 (XFoxD3) (E) or 20pg of cyclops (Cyc) (F) mRNA, and axial development was assessed at 24hpf (A–F). Embryos were phenotypically classified as Knockdown (D), Wild-Type (E,F) or Dorsalized (not shown), and these classes are quantified in (G). Knockdown embryos have reduced head structures, reduction of trunk mesoderm, and expanded tail somites (D), while rescued embryos are normal (E) or near normal (F) in all aspects of axis formation. Injection of MO1+2 alone resulted in 88% (n=396) of embryos with the knockdown phenotype, while 82% (n=126) of Xfoxd3-injected and 73% (n=202) of cyclops-injected embryos were phenotypically wild-type. A minority of knockdown embryos rescued by injection of Xfoxd3 (16%) or cyclops (21%) mRNA displayed a dorsalized body axis. Injection of Xfoxd3 or cyclops mRNA alone strongly dorsalized control embryos (B,C). Shown are lateral views, anterior left, of live embryos.
Supplementary Figure 3. Antivin blocks the mesodermal activity of FoxD3. At the one-cell stage, wild-type embryos were injected with Antivin (10pg) (B,F,J,N), FoxD3 (25pg) (D,H,L,P), or a mixture of both mRNAs (C,G,K,O). At the shield stage (6hpf) live embryos were examined for shield and blastoderm structure (A–D), and fixed embryos were analyzed by in situ hybridization for cyclops (E–F), goosecoid (I–L), and no tail (M–P) expression. Shown are lateral views with dorsal right (A–D), dorsal views (E–L), and animal views with dorsal right (M–P).
Acknowledgments
We are grateful to Shannon Fisher, Peter Klein, Mary Mullins, Michael Pack, and Christine Reid for critical reading of the manuscript. We thank David Raible and Mary Mullins for providing plasmids, Thomas Look and John Kanki for providing sym1 zebrafish, and Michael Granato, Mary Mullins and Michael Pack for helpful advice on genotyping. We thank Rebecca Burdine and her colleagues for providing access to oep zebrafish and for assistance in the MZoep rescue experiments. We thank Qun Lu for performing the Xenopus animal explant studies. We are grateful to David Cobb and the zebrafish facility staff for maintaining the lines used in these studies. This work was supported by grants from the NIH (T32-GM007229 and F31-GM069003) to L.L.C. and by grants from the NIH (R01-GM64768) and NSF (IOS-0718961) to D.S.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arduini BL, Bosse KM, Henion PD. Genetic ablation of neural crest cell diversification. Development. 2009;136:1987–1994. doi: 10.1242/dev.033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry FB, Tamimi Y, Carle MV, Lehmann OJ, Walter MA. The establishment of a predictive mutational model of the forkhead domain through the analyses of FOXC2 missense mutations identified in patients with hereditary lymphedema with distichiasis. Hum. Mol. Genet. 2005;14:2619–2627. doi: 10.1093/hmg/ddi295. [DOI] [PubMed] [Google Scholar]
- Chang LL, Kessler DS. Formation of the embryonic mesoderm. In: Moody SA, editor. Principles of Developmental Genetics. San Diego: Elsevier Inc; 2007. pp. 258–294. [Google Scholar]
- Chen C, Shen MM. Two modes by which Lefty proteins inhibit nodal signaling. Curr. Biol. 2004;14:618–624. doi: 10.1016/j.cub.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Cooper CD, Linbo TH, Raible DW. Kit and foxd3 genetically interact to regulate melanophore survival in zebrafish. Dev. Dyn. 2009;238:875–886. doi: 10.1002/dvdy.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran K, Raible DW, Lister JA. Foxd3 controls melanophore specification in the zebrafish neural crest by regulation of Mitf. Dev. Biol. 2009;332:408–417. doi: 10.1016/j.ydbio.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. The winged helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Dougan ST, Warga RM, Kane DA, Schier AF, Talbot WS. The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development. 2003;130:1837–1851. doi: 10.1242/dev.00400. [DOI] [PubMed] [Google Scholar]
- Feldman B, Dougan ST, Schier AF, Talbot WS. Nodal-related signals establish mesendodermal fate and trunk neural identity in zebrafish. Curr. Biol. 2000;10:531–534. doi: 10.1016/s0960-9822(00)00469-3. [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Freyaldenhoven BS, Freyaldenhoven MP, Iacovoni JS, Vogt PK. Avian winged helix proteins CWH-1, CWH-2 and CWH-3 repress transcription from Qin binding sites. Oncogene. 1997;15:483–488. doi: 10.1038/sj.onc.1201189. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Guo Y, Costa R, Ramsey H, Starnes T, Vance G, Robertson K, Kelley M, Reinbold R, Scholer H, Hromas R. The embryonic stem cell transcription factors Oct-4 and FoxD3 interact to regulate endodermal-specific promoter expression. Proc. Natl. Acad. Sci. USA. 2002;99:3663–3667. doi: 10.1073/pnas.062041099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley SA. Anterior-posterior differences in vertebrate segments: specification of trunk and tail somites in the zebrafish blastula. Genes Dev. 2006;20:1831–1837. doi: 10.1101/gad.1453706. [DOI] [PubMed] [Google Scholar]
- Ignatius MS, Moose HE, El-Hodiri HM, Henion PD. colgate/hdac1 repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev. Biol. 2008;313:568–583. doi: 10.1016/j.ydbio.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh RN, Dutton K, Medlin J, Eisen JS. Expression of zebrafish fkd6 in neural crest-derived glia. Mech. Dev. 2000;93:161–164. doi: 10.1016/s0925-4773(00)00250-1. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Mesoderm induction: from caps to chips. Nat. Rev. Genet. 2006;7:360–372. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kos R, Reedy MV, Johnson RL, Erickson CA. The winged helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–1479. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lee HC, Huang HY, Lin CY, Chen YH, Tsai HJ. Foxd3 mediates zebrafish myf5 expression during early somitogenesis. Dev. Biol. 2006;290:359–372. doi: 10.1016/j.ydbio.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Lister JA, Cooper C, Nguyen K, Modrell M, Grant K, Raible DW. Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Dev. Biol. 2006;290:92–104. doi: 10.1016/j.ydbio.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Liu Y, Labosky PA. Regulation of ES cell self renewal and pluripotency by Foxd3. Stem Cells. 2008;26:2475–2484. doi: 10.1634/stemcells.2008-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Bertoglio VE, Fisher S, Sanchez A, Mullins MC, Halpern ME. Differential regulation of chordin expression domains in mutant zebrafish. Dev. Biol. 1997;192:537–550. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- Montero-Balaguer M, Lang MR, Sachdev SW, Knappmeyer C, Stewart RA, De La Guardia A, Hatzopoulos AK, Knapik EW. The mother superior mutation ablates foxd3 activity in neural crest progenitor cells and depletes neural crest derivatives in zebrafish. Dev. Dyn. 2006;235:3199–3212. doi: 10.1002/dvdy.20959. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr. Biol. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. forkhead domain genes in zebrafish. Dev. Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Osada SI, Wright CV. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- Osada SI, Saijoh Y, Frisch A, Yeo CY, Adachi H, Watanabe M, Whitman M, Hamada H, Wright CV. Activin/nodal responsiveness and asymmetric expression of a Xenopus nodal-related gene converge on a FAST-regulated module in intron 1. Development. 2000;127:2503–2514. doi: 10.1242/dev.127.11.2503. [DOI] [PubMed] [Google Scholar]
- Pan G, Li J, Zhou Y, Zheng H, Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. FASEB J. 2006;20:1730–1732. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- Pohl BS, Knochel W. Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mech. Dev. 2001;103:93–106. doi: 10.1016/s0925-4773(01)00334-3. [DOI] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Haffter P, Dawid IB. cyclops encodes a nodal-related factor involved in midline signaling. Proc. Natl. Acad. Sci. USA. 1998;95:9932–9937. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu. Rev. Cell Dev. Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schmid B, Furthauer M, Conners SA, Trout J, Thisse B, Thisse C, Mullins MC. Equivalent genetic roles of bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho KW, De Robertis EM, Nusslein-Volhard C. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development. 1994;120:843–852. doi: 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Ho RK, Herrmann BG, Nusslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992;116:1021–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Stachel SE, Grunwald DJ, Myers PZ. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- Steiner AB, Engleka MJ, Lu Q, Piwarzyk EC, Yaklichkin S, Lefebvre JL, Walters JW, Pineda-Salgado L, Labosky PA, Kessler DS. FoxD3 regulation of Nodal in the Spemann organizer is essential for Xenopus dorsal mesoderm development. Development. 2006;133:4827–4838. doi: 10.1242/dev.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RA, Arduini BL, Berghmans S, George RE, Kanki JP, Henion PD, Look AT. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev. Biol. 2006;292:174–188. doi: 10.1016/j.ydbio.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J. Biol. Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- Symes K, Smith JC. Gastrulation movements provide an early marker of mesoderm induction in Xenopus laevis. Development. 1987;101:339–349. [Google Scholar]
- Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–1624. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Tompers DM, Foreman RK, Wang Q, Kumanova M, Labosky PA. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Dev. Biol. 2005;285:126–137. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene: University of Oregon Press; 1993. [Google Scholar]
- Whitlock KE, Smith KM, Kim H, Harden MV. A role for foxd3 and sox10 in the differentiation of gonadotropin-releasing hormone (GnRH) cells in the zebrafish Danio rerio. Development. 2005;132:5491–5502. doi: 10.1242/dev.02158. [DOI] [PubMed] [Google Scholar]
- Whitman M. Nodal signaling in early vertebrate embryos: themes and variations. Dev. Cell. 2001;1:605–617. doi: 10.1016/s1534-5807(01)00076-4. [DOI] [PubMed] [Google Scholar]
- Yaklichkin S, Steiner AB, Lu Q, Kessler DS. FoxD3 and Grg4 physically interact to repress transcription and induce mesoderm in Xenopus. J. Biol. Chem. 2007;282:2548–2557. doi: 10.1074/jbc.M607412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Zebrafish Foxd3 induces mesoderm in Xenopus animal explants. At the one-cell stage, Xenopus embryos were injected in the animal pole with 100pg of Xenopus foxd3 (xFoxD3, lane 2) or zebrafish foxd3 (zFoxD3, lane 3), explants were prepared at the late blastula stage (stage 9) and analyzed by RT-PCR at the tailbud stage (stage 25) for the expression of Muscle Actin (somites) and Collagen II (notochord). EF1α is a control for RNA recovery and loading, intact embryos (Embryo, lane 4) served as a positive control and an identical reaction without reverse transcriptase controlled for PCR contamination (Embryo-RT, lane 5).
Supplementary Figure 2. Rescue of Foxd3 knockdown by Xenopus foxd3 or cyclops. At the one-cell stage, wild-type embryos were injected with a mixture of foxd3MO1 and foxd3MO2 (MO1+2, total dosage 20ng) alone (D), or in combination with 30pg of Xenopus foxd3 (XFoxD3) (E) or 20pg of cyclops (Cyc) (F) mRNA, and axial development was assessed at 24hpf (A–F). Embryos were phenotypically classified as Knockdown (D), Wild-Type (E,F) or Dorsalized (not shown), and these classes are quantified in (G). Knockdown embryos have reduced head structures, reduction of trunk mesoderm, and expanded tail somites (D), while rescued embryos are normal (E) or near normal (F) in all aspects of axis formation. Injection of MO1+2 alone resulted in 88% (n=396) of embryos with the knockdown phenotype, while 82% (n=126) of Xfoxd3-injected and 73% (n=202) of cyclops-injected embryos were phenotypically wild-type. A minority of knockdown embryos rescued by injection of Xfoxd3 (16%) or cyclops (21%) mRNA displayed a dorsalized body axis. Injection of Xfoxd3 or cyclops mRNA alone strongly dorsalized control embryos (B,C). Shown are lateral views, anterior left, of live embryos.
Supplementary Figure 3. Antivin blocks the mesodermal activity of FoxD3. At the one-cell stage, wild-type embryos were injected with Antivin (10pg) (B,F,J,N), FoxD3 (25pg) (D,H,L,P), or a mixture of both mRNAs (C,G,K,O). At the shield stage (6hpf) live embryos were examined for shield and blastoderm structure (A–D), and fixed embryos were analyzed by in situ hybridization for cyclops (E–F), goosecoid (I–L), and no tail (M–P) expression. Shown are lateral views with dorsal right (A–D), dorsal views (E–L), and animal views with dorsal right (M–P).