Abstract
We investigated the role of μ-opioid receptor (μ-OR) and dopamine receptor in the modulation of methamphetamine (METH)-induced expression of zif268 mRNA in the striatum of mice. Four groups of wild-type and μ-OR knockout mice were given a single daily intraperitoneal injection of saline (control; group 1) or METH (10 mg/kg; groups 2–4) for 7 consecutive days. On day 11 (after 4 abstinent days), groups 1 and 2 were challenged with saline, group 3 was challenged with METH (10 mg/kg), and group 4 was challenged with dopamine receptor antagonist haloperidol (0.06 mg/kg, subcutaneous injection) plus METH (10 mg/kg). Two hours after the last saline or METH injection, mouse brain tissues were taken for zif268 mRNA analysis using in situ hybridization histochemistry. In comparison to corresponding saline control group (group 1), striatal zif268 mRNA levels were unchanged in group 2 and increased in group 3 in both wild-type and μ-OR knockout mice and without genotype difference. METH challenge-enhanced expression of zif268 mRNA was completely abolished by pre-administration of haloperidol (group 4) in μ-OR knockout mice but not in wild-type mice. The results suggest a crosstalk of the two neurotransmitter systems in modulation of METH-induced IEG expression, because only in μ-OR knockout mice in which dopamine receptors were blocked were METH-induced zif268 expression abolished. METH-induced zif268 expression was not altered inμ-OR knockout mice without blockade of dopamine receptors or wild-type mice with blockade of dopamine receptors.
Keywords: methamphetamine, zif268 mRNA, in situ hybridization, μ-OR knockout mice, haloperidol
Introduction
Methamphetamine (METH) is a psychostimulant that activates several neurotransmitter systems (Miller and O’Callaghan, 1994; Shaw, 1999). As a member of the amphetamine family, METH has much more potent and longer lasting central effects than amphetamine. Chronic exposure to amphetamine or METH not only leads to addiction but also produces significant neurotoxicity in humans (Volkow et al., 2001) and experimental animals (Seiden and Sabol, 1996; Kaewsuk et al., 2009). METH-induced neurotoxicity is characterized by a persistent depletion of dopamine in the dopaminergic system and long-lasting behavioral abnormalities (Kita et al., 2000; Jeng et al., 2005). Recently, we found that repeated administration of METH decreases the striatal intensity of tyrosine hydroxylase (the rate-limiting enzyme of dopamine synthesis and the target for many neurotoxicants and drugs) in wild-type mice but not in the μ-OR knockout mice (unpublished data). Also, we observed that METH produces hyperlocomotor activity at a low dose (0.62 mg/kg) and stereotyped behaviors at high doses (2.5 and 10 mg/kg). Repeated administration of METH led to progressively enhanced and persistent locomotor and stereotyped behaviors, called behavioral sensitization. The μ-OR knockout mice were less sensitive than wild-type mice to METH-induced hyperlocomotor activity and stereotyped behaviors. Contrarily, the μ-OR knockout mice showed approximately 3-fold more sensitivity to the dopamine receptor antagonist haloperidol in counteracting METH-induced stereotyped behaviors (Shen et al., 2010). It remains unclear why there are genotype differences regarding METH-induced depletion of striatal tyrosine hydroxylase and behavioral responses.
Immediate early genes (IEGs) are actively, transiently and rapidly responsive to a wide variety of cellular stimuli (Davis et al., 2003). It has been reported that IEGs play a role in the transmission of information from cell surface receptors to the genetic material in many instances of neuronal plasticity, including development of seizure susceptibility, long-term potentiation and drug-induced behavioral changes (Robertson, 1992). Thus, studies on the inductions of IEGs may provide a useful tool in understanding the mechanisms and functions of transsynaptic activation of certain central neuronal pathways.
Changes in expression of IEGs induced by drugs of abuse have been proposed to be associated with specific behavioral changes (Harlan and Garcia, 1998; Shilling et al., 2006). Several lines of evidence suggest that METH and morphine induce the expression of IEGs in the brains of rodents. For example, acute METH enhances expression of c-fos and zif268 mRNA in the frontal cortex and striatum of mice (Hirata et al., 1998) as well as arc mRNA in the striatum and cortex of rats (Yamagata et al., 2000). Intra-striatum injection of c-fos antisense blocks METH-induced ambulatory locomotor activity in mice (Umekage et al., 1997). Morphine increases c-fos mRNA expression in the striatum of rats and this induction is completely abolished by a non-selective opioid receptor antagonist naloxone (Chang et al., 1988). Moreover, Horner and Keefe (2006) found that μ-OR antagonist clocinnamox blocks METH-induced expression of zif268 mRNA in the striatum of rats. Morphine is a μ-OR agonist whereas METH is an indirect dopamine receptor agonist. These data indicate that both μ-OR and dopamine receptor are implicated in modulation of the expression of IEGs.
This study is designed to determine the role of μ-OR and dopamine receptor in METH-induced expression of zif268 by using μ-OR knockout mice and dopamine receptor antagonist haloperidol. Zif268 is a mammalian transcription factor that is also called Egr1 (early growth response protein 1). It is known that zif268 is required for long-lasting behavioral effects of drugs of abuse (Valjent et al., 2006; EL Rawas et al., 2009). In the study, we tested the hypothesis that both of the two neurotransmitter receptors are involved in modulation of METH-induced expression of zif268 mRNA. The μ-OR knockout mice are less sensitive to METH in enhancing expression of zif268 but more sensitive to dopamine receptor antagonist in counteracting METH-induced zif268 expression.
Materials and methods
Chemicals
Deoxyadenosine 5′-(α-thio) triphosphate, [35S]-ATP (specific activity: 1250 Ci/mmol) were purchased from PerkinElmer Life Science (Boston, MA, USA). METH and other chemicals were purchased from Sigma (St. Louis, MO, USA).
Animals
The μ-OR knockout mice used in this study were developed by Loh et al. (1998) and maintained on a 1:1 hybrid genetic background (C57/BL6 and 129/Ola), as described. Mice were maintained in an animal room on a 12-h light/dark cycle and at constant temperature (22 ± 2°C). All procedures for animal care and breeding were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Mississippi Medical Center Animal Care and Use Committee.
Experimental protocol and sample preparations
Male wild-type and μ-OR knockout mice, ranging from 8 to 12 weeks old (body weight 20–25 g) were used in this study. Mice from each genotype were randomly divided into 4 groups with 8 mice each group. METH was freshly dissolved in saline before use and injected at a volume of 10 ml/kg of the body weight. For initiation of behavioral sensitization, mice were daily intraperitoneally (i.p.) injected with saline (control; group 1) or METH (10 mg/kg; group 2–4) for 7 consecutive days. On day 11, each group of mice was subcutaneously (s.c.) injected with saline (groups 1–3) or haloperidol (0.06 mg/kg; group 4) and then, 30 min later, injected with saline (groups 1 and 2) or METH (10 mg/kg, i.p.; groups 3 and 4). In the previous behavioral study, we found that acute administration of METH to mice produced significantly hyperlocomotor activity at low doses and stereotyped behavior at high doses (2.5 mg/kg and above). After repeated administration of METH, wild-type mice showed progressively enhanced locomotor activity (at 0.62 mg/kg) and stereotyped behaviors (at 2.5 and 10 mg/kg), as observed on experimental days 4 and 7, which suggests the development of behavioral sensitization (reverse tolerance) to the psychostimulant in these animals. METH at doses of 2.5 mg/kg and below did not induce behavioral sensitization but 10 mg/kg of METH produced a slight behavioral sensitization, as observed on day 7, in the μ-OR knockout mice (Shen et al., 2010). Behavioral sensitization is well known to be a long-lasting behavioral change. Animals remain sensitized for weeks or months after prolonged periods of abstinence (Chiu et al., 2005; Robinson and Berridge, 2008). Therefore, 10 mg/kg of METH and a 7-day METH initiation plus 4 abstinent day treatment protocol were used in this study.
Two hours following the last injection, mice were sacrificed by decapitation. The brains were removed from the skull and immediately frozen in liquid nitrogen. Coronal sections of 14 μm thickness were cut in a microtome cryostat (Cyro 2000, Tissue-Tek) at −20°C. The sections were thaw-mounted on gelatin-coated slides and stored at −80°C until used.
Preparation of probes for in situ hybridization
The oligonucleotide probe (Invitrogen Corporation, CA, USA) was complementary to mRNAs encoding mouse zif268. The sequence for zif268 was 5′-GCG GCG AAT CGC GGC GGC TCC CCA AGT TCT GCG CGC TGG GAT CTC-3′ (Ekonomou et al., 2004). Oligonucleotide (10 pmol) was labeled at 3′ end with 5 μl of 35S-dATP using 35 U of terminal deoxynucleotidyltransferase (PerkinElmer Life Science 3′ end oligonucleotide labeling system) for 60 min at 37°C. The labeled probes were purified utilizing Centri-Spin columns (Princeton separation, NJ, USA) centrifuged at 3,000 rpm for 2 min. Two μl of this solution were counted by a liquid scintillation counter to assess the efficiency of labeling.
Hybridization
The slide-mounted sections were air-dried and fixed in 4% paraformaldehyde in 0.1 M phosphate-saline buffer for 15 min at 4°C, then rinsed with 0.1 M phosphate-saline buffer for 3 min at room temperature. Brain sections on the slides were immersed in 0.1 M triethanolamine-HCl and acetic acid for 10 min at 4°C. The sections were washed with 0.1 M phosphate-saline buffer for 3 min at room temperature, then dehydrated in a series of ascending concentration of ethanol (70 and 100% for 5 min each). The dehydrated sections were incubated with the hybridization mixture, which contained 50% formamide, 4 × SSC (1 × SSC = 0.15 M NaCl and 0.015 M sodium citrate), 10% Dextran sulfate, 5 × Denhardt’s, 0.25 mg/ml tRNA, 0.5 mg/ml ssDNA, 100 mM dithiothreitol, and 1 × 106 cpm/slide of 35S-labeled oligonucleotide. Slides were covered with hybridization coverslips and incubated overnight at 38°C in a humid chamber. After hybridization, the coverslips were floated off in 1 × SSC at room temperature. Then slides were washed in 1 × SSC for 15 min at 55°C twice and 0.5 × SSC for 15 min at 55°C twice, plus final wash in 0.5 × SSC for 10 min at room temperature twice. Slides were rinsed in distilled water, dehydrated in 70 and 100% ethanol for 5 min at room temperature each and immediately air-dried. Autoradiograms were obtained by juxtaposition of the tissue sections with Kodak BioMax MR Film (Eastman Kodak Co., Rochester, NY, USA) for 2 weeks at room temperature. The films were developed in Kodak D19 (Eastman Kodak Co., Rochester, NY, USA) and fixed.
Statistical analysis
The autoradiograms were analyzed using a scanning densitometer (Personal Densitometer, Molecular Dynamics, Sunnyvale, CA, USA), operating on the image acquisition and analysis program Image Quant 3.3 (Molecular Dynamics). Data were expressed as mean ± standard errors of the mean (S.E.M.). Each treated group was separately compared to that of the respective saline treated control. At the same treatment, μ-OR knockout mice were compared with that of the wild type controls. Statistical analysis was done by one-way ANOVA followed by a post-hoc Student-Newman-Keuls multiple comparison test. A difference was considered significant at P < 0.05.
Results
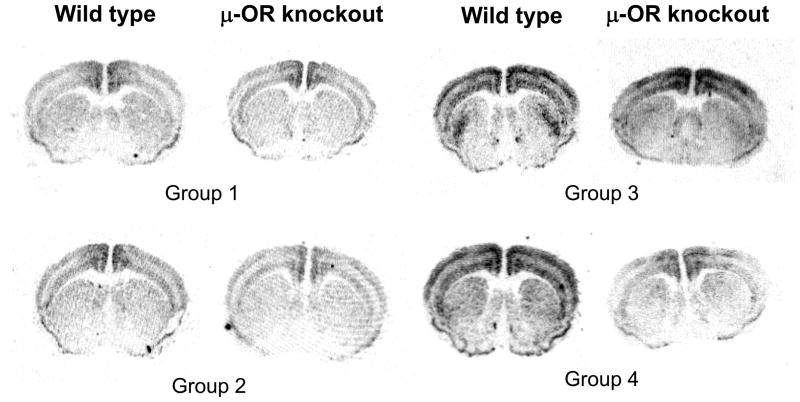
In situ autoradiograms and quantitative data of expression of zif268 mRNA in the striatum from the wild type and μ-OR knockout mice are shown in Fig. 1 and table 1.
Figure 1.
In situ film autoradiograms showing the expression of zif268 mRNA in the brain slides from the wild type and μ-OR knockout mice. Mice were daily intraperitoneally (i.p.) injected with saline (control; group 1) or METH (10 mg/kg; group 2–4) for 7 consecutive days. On day 11, each group of mice was subcutaneously (s.c.) injected with saline (groups 1–3) or haloperidol (0.06 mg/kg; group 4) and then, 30 min later, injected with saline (groups 1 and 2) or METH (10 mg/kg, i.p.; groups 3 and 4). Two hours following the last injection, mice were sacrificed by decapitation. The brain tissues were reserved for preparation of brain sections and in situ hybridization analysis.
There was no significant difference in expression of zif268 mRNA in all experimental groups except group 4 between wild type and μ-OR knockout mice. This result indicates that only lacking μ-OR in mice had no effect on METH-induced expression of zif268 mRNA.
No significant change in expression of zif268 mRNA was observed in 7 daily injections of METH followed by a 4-day drug free period in both wild type and μ-OR knockout mice (group 2, Table 1) when they were compared with that of the saline treated group (group 1, Table 1). However, there was a significant increase in zif268 mRNA expression in both genotypes of mice that were challenged with METH following 4 drug abstinent days (group 3, Table 1). These results reveal that METH-induced expression of zif268 mRNA is likely involved in early stages of target gene regulation in response to its exposure.
Haloperidol pretreatment 30 min before the challenge with METH on experimental day 11 completely blocked METH challenge-induced expression of zif268 mRNA in μ-OR knockout mice, but not in wild type controls (group 4, Table 1).
Discussion
In the present study, no genotype difference in expression of zif268 mRNA in the striatum was observed under basal (without METH treatment; group 1), METH-sensitized (group 2), and METH-sensitized with METH challenge conditions (group 3). The result indicated that lack of μ-OR in mice does not alter the magnitude of METH-induced expression of zif268 mRNA in the brains, although a previous study showed that single injection of the μ-OR antagonist, clocinnamox, blocked METH-induced expression of zif268 mRNA in the striatum of rats (Horner and Keefe, 2006). The difference results from the two studies may be due to the difference METH treatment protocols used or that there are certain compensatory changes in central neurotransmitter systems in μ-OR knockout mice since we noticed a higher dopamine receptor level in the striatum of naïve μ-OR knockout mice (Tien et al., 2003).
Recently, we found that METH produced hyperlocomotor activity at a low dose (0.62 mg/kg) and stereotyped behaviors at high doses (2.5 and 10 mg/kg). METH-induced behavioral responses were significantly attenuated in the μ-OR knockout mice in comparison to wild-type mice (Shen et al., 2010). Also, we noticed that repeated administration of METH (2.5 and 10 mg/kg) decreases the striatal intensity of tyrosine hydroxylase in wild-type mice but not in the μ-OR knockout mice (unpublished data). It has been proposed that the enhanced expression of IEGs is mediated with psychostimulant-produced behavioral abnormalities (Harlan and Garcia, 1998; Shilling et al., 2006). For instance, microinjection of c-fos antisense in the striatum blocks METH-induced ambulatory locomotor activity in mice (Umekage et al., 1997). Obviously, the present results do not support the idea that the difference of METH-produced neurotoxicity and behavioral abnormalities between wild-type and μ-OR knockout mice are related to the changes of IEGs in these animals.
However, the present study reveals the genotype difference of haloperidol counteracting METH-enhanced expression of zif268 mRNA. Pre-administration of haloperidol completely eliminated METH challenge-induced expression of zif268 mRNA in the μ-OR knockout mice but not in wild type controls (group 4, Table 1). Evidence indicates that haloperidol (1 mg/kg) enhanced expression of zif268 mRNA in the striatum of rats that peaked 30–45 min after injection and returned to baseline within 2 hours (Nguyen et al., 1992). METH (4 mg/kg)-induced expression of zif268 mRNA in the striatum of rats shows similar results, peaking at 45 min and returning to baseline within 3 hours after injection (Wang and McGinty, 1995). In the present study, we collected the brain tissue for determining zif268 mRNA at 2.5 hours after the injection of haloperidol and 2 hours after METH administration. Thus, results from this study may represent an antagonistic effect of haloperidol on METH challenge-induced expression of zif268 mRNA in METH-sensitized mice. The μ-OR knockout mice are more sensitive to haloperidol than wild type controls in counteracting METH challenge-induced expression of zif268 mRNA. The change is consistent with our behavioral study results, in which haloperidol produced a more potent effect in counteracting METH-induced stereotyped behaviors in μ-OR knockout mice than that in wild-type controls (Shen et al., 2010).
In a previous study, we treated mice with different doses of METH for 7 consecutive days and sacrificed them on day 11 (4 days after last injection) for neurochemical measurements. METH at doses of 0.62 and 2.5 mg/kg produced significant decrease in D1 and D2 dopamine receptor ligand binding in the striatum and nucleus accumbens in the μ-OR knockout mice but not in wild-type mice. METH at a dose of 10 mg/kg also decreased D1 receptor ligand binding in these brain regions in the μ-OR knockout mice but not in wild-type mice, and increased D2 receptor ligand binding in both genotypes of mice (Tien et al., 2007). The results suggest that the METH-induced decrease in D1 dopamine receptor in the μ-OR knockout mice may be a characteristic change. Haloperidol is a D1/D2 receptor antagonist with relatively higher affinity for D2 sites. Because repeated METH exposure decreases D1 (probably D2 as well) receptor levels in the brains of μ-OR knockout mice, a higher dopamine receptor occupancy by haloperidol in the brains of μ-OR knockout mice would be expected, which may explain a more potent efficacy of the antagonist counteracting METH-induced IEG expression and stereotyped behaviors in these mice. This possibility as well as how dopamine and μ-opioid receptor-mediated signal transduction cascades modulate METH-induced IEG expression, and thus leads to behavioral changes, need to be verified by further study.
Conclusion
Expression of zif268 mRNA was not altered in μ-OR knockout mice without blockade of dopamine receptors or wild-type mice with blockade of dopamine receptors. However, METH-induced zif268 expression was abolished in μ-OR knockout mice in which dopamine receptors were blocked. The results suggest a crosstalk of μ-opioid system and dopamine system in modulation of expression of zif268 induced by METH.
Acknowledgments
The authors thank Ms. Cindy Wang for her technique help on preparation of brain slides. The project was supported by the Center for Psychiatric Neuroscience at the University of Mississippi Medical Center which is supported by NIH Grant P20 RR0l7701 (T. Ma, subproject PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chang SL, Squinto SP, Harlan RE. Morphine activation of c-fos expression in rat brain. Biochem Biophys Res Commun. 1988;157:698–704. doi: 10.1016/s0006-291x(88)80306-1. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Ma T, Ho IK. Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull. 2005;67:100–109. doi: 10.1016/j.brainresbull.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Poulou PD, Matsokis N, Angelatou F. Stimulation of adenosine A2A receptors zif/268 and NMDA epsilon2 subunit mRNA expression in cortex and striatum of the “weaver” mutant mouse, a genetic model of nigrostriatal dopamine deficiency. Neurosci. 2004;123:1025–1036. doi: 10.1016/j.neuroscience.2003.10.043. [DOI] [PubMed] [Google Scholar]
- EL Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacol. 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Hirata H, Asanuma M, Cadet JL. Superoxide radicals are mediators of the effects of methamphetamine on Zif268 (Egr-1, NGFI-A) in the brain: evidence from using CuZn superoxide dismutase transgenic mice. Brain Res Mol Brain Res. 1998;58:209–216. doi: 10.1016/s0169-328x(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Horner KA, Keefe KA. Regulation of psychostimulant-induced preprodynorphin, c-fos and zif/268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur J Pharmacol. 2006;532:61–73. doi: 10.1016/j.ejphar.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Jeng W, Wong AW, Ting-A-Kee R, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med. 2005;39:317–326. doi: 10.1016/j.freeradbiomed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Kaewsuk S, Sae-ung K, Phansuwan-Pujito P, Govitrapong P. Melatonin attenuates methamphetamine-induced reduction of tyrosine hydroxylase, synaptophysin and growth-associated protein-43 levels in the neonatal rat brain. Neurochem Int. 2009;55:397–405. doi: 10.1016/j.neuint.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Kita T, Matsunan Y, Saraya T, Shimada K, O’Hara K, Kubo K, Wagner GC, Nakashima T. Methamphetamine-induced striatal dopamine release, behavior changes and neurotoxicity in BALB/c mice. Int J Develop Neurosci. 2000;18:521–530. doi: 10.1016/s0736-5748(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. μ-Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci USA. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HA. Immediate-early genes, neuronal plasticity, and memory. Biochem Cell Biol. 1992;70:729–737. doi: 10.1139/o92-112. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr. 1996;163:251–276. [PubMed] [Google Scholar]
- Shaw KP. Human methamphetamine-related fatalities in Taiwan during 1991–1996. J Forensic Sci. 1999;44:27–31. [PubMed] [Google Scholar]
- Shen X, Purser C, Tien L-T, Chiu C-T, Paul IA, Baker R, Loh HH, Ho IK, Ma T. The μ-opioid receptor knockout mice are insensitive to methamphetamine-induced behavioral sensitization. J Neurosci Res. 2010 doi: 10.1002/jnr.22386. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling PD, Kuczenski R, Segal DS, Barrett TB, Kelsoe JR. Differential regulation of immediate-early gene expression in the prefrontal cortex of rats with a high vs low behavioral response to methamphetamine. Neuropsychopharmacology. 2006;31:2359–67. doi: 10.1038/sj.npp.1301162. [DOI] [PubMed] [Google Scholar]
- Tien LT, Park Y, Fan LW, Ma T, Loh HH, Ho IK. Increased dopamine D2 receptor binding and enhanced apomorphine-induced locomotor activity in mu-opioid receptor knockout mice. Brain Res Bull. 2003;61:109–115. doi: 10.1016/s0361-9230(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Tien LT, Ho IK, Loh HH, Ma T. Role of mu-opioid receptor in modulation of preproenkephalin mRNA expression and opioid and dopamine receptor binding in methamphetamine-sensitized mice. J Neurosci Res. 2007;85:673–680. doi: 10.1002/jnr.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekage T, Namima M, Fukushima K, Sugita S, Watanabe Y. c-fos antisense blocks methamphetamine-induced ambulatory activity reversibly. NeuroReport. 1997;8:407–410. doi: 10.1097/00001756-199701200-00005. [DOI] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbillé AG, Brami-Cherrier K, Caboche J, Topilko P, Girault JA, Herve D. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat forebrain. J Neurochem. 1995;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Suzuki K, Sugiura H, Kawashima N, Okuyama S. Activation of an effector immediate-early gene arc by methamphetamine. Ann N Y Acad Sci. 2000;914:22–32. doi: 10.1111/j.1749-6632.2000.tb05180.x. [DOI] [PubMed] [Google Scholar]