Abstract
Purpose
Measurement of distance stereoacuity may be useful in assessing strabismic patients, especially those with intermittent exotropia. We developed the Distance Randot Stereotest as an easily administered quantitative test for distance stereoacuity in children. Using a prototype,1 we reported testability, validity, and normative data. Here we report normative and validity data for the final, commercially available version of the test.
Method
We administered both the Prototype and the Final Version Distance Randot Stereotest to 156 normal volunteers (2–40 years of age) and 77 strabismic patients (4–62 years of age). Test–retest data were collected for the Final Version.
Results
Normative Final Version scores were similar to those obtained with the Prototype; 96% were ≤100 arcsec. Test–retests were identical in 82% and within one disparity level in 100%. Final Version scores were correlated with Prototype scores (rs = 0.64, p < 0.05). Among strabismic patients, 62.3% had abnormal stereoacuity; those with normal scores had incomitant or intermittent deviations. Nil stereoacuity was found in 27 patients, confirmed in 90.9%of retests; 17 had measurable stereoacuity, confirmed in 96.3%of retests. Patients with constant strabismus were more likely to have nil stereoacuity than patients who had intermittent strabismus (95% vs 12.2%).
Conclusions
Distance Randotscores from normal subjects have low variability within each age group and high test–retest reliability. There is little overlap between Distance Randot® scores from normal controls and strabismic patients. The Distance Randot Stereotest is a sensitive measurement of binocular sensory status that may be useful in monitoring progression of strabismus and/or recovery following strabismus surgery.
Introduction
The measurement of distance stereoacuity may be useful in assessing strabismic patients who have a difference in distance and near angle or frequency of deviation, including intermittent exotropia.2–5 We developed the Polaroid vectograph–based Distance Randot Stereotest as an easily administered quantitative test for measuring distance stereoacuity (3 meters) in children. The Distance Randot Stereotest provides a useful tool in measuring distance stereoacuity in patients with or without strabismus.1,4 Using a previous prototype Distance Randot Stereotest,1,4 we reported testability, validity, and normative data.1,2,4,6 The Distance Randot Stereotest appears very sensitive to disturbances of binocularity4,7 and has shown early promise for monitoring deterioration in intermittent exotropia and changes in distance stereoacuity after surgery for intermittent exotropia.2
Nevertheless, the prototype with which these previous data were gathered (henceforth “Prototype”) was composed of 4 separate test books and was administered with up to 4 trials (3 Randot® shapes and one blank) at each of 4 disparity levels (16 test plates). It was unwieldy to handle in the clinic and expensive to produce. To overcome these shortcomings, we designed a revised test (henceforth “Final Version”) containing 8 test plates (2 trials at each of 4 disparity levels) provided as a single book, which is easier to handle in the clinical setting and less expensive.
One aim of the present study was to report normative data and evaluate test–retest reliability and validity for children and adults using the commercially available Final Version of the Distance Randot Stereotest. A second aim was to determine whether the Distance Randot Stereotest provides a valid measure of binocular sensory status in strabismic patients.
Methods
Data were collected at multiple sites (Table 1). Informed consent was obtained from the adult subjects, or from one or both parents for children, prior to participation. This protocol was approved by the institutional review boards of the University of Texas Southwestern Medical Center, the Mayo Clinic, the University of Liverpool, and Memorial University.
Table 1.
Study population and data collection sites.
| Groups | N | Age range (mean ± SD years) | Location | HOTV or ETDRS visual acuity (logMAR) | Near Stereoacuity Randot® Preschool |
|---|---|---|---|---|---|
| Normal controls | 156 | 2–40 (14.7 ± 11) | RFSW UL MU |
−0.1 to 0.5 | 20″ to 400″ |
| Subset: 2–5 years | 54 | 2–5 (4.1 ± 0.8) | −0.1 to 0.5 | 30″ to 400″ | |
| Subset: 6–40 years | 102 | 6–40 (20.2 ± 9.7) | −0.3 to 0.1 | 20″ to 60″ | |
| Strabismic patients | 77 | 4–62 (21.5 ± 14.3) | RFSW Mayo UL |
−0.1 to 0.5 | 20″ to nil |
| Subset: constant | 20 | 5–50 (12.0 ± 10.7) | −0.1 to 0.5 | 400″ to nil | |
| Subset: intermittent | 57 | 4–62 (20.4 ± 13.5) | −0.1 to 0.5 | 20″ to nil |
RFSW, Retina Foundation of the Southwest (Dallas, TX); Mayo, Mayo Clinic (MN); UL, University of Liverpool (United Kingdom); MU, Memorial University (Canada).
Subjects and Patients
Normative data for the Distance Randot Stereotest were obtained from 156 normal volunteers at 3 sites (Table 1). The inclusion criteria for normal controls were: (1) normal age-adjusted visual acuity measured by HOTV or E-ETDRS8,9; (2) no more than one line (0.1 logMAR) interocular difference in visual acuity; (3) normal Randot Preschool stereoacuity at near10; (4) no manifest tropia at distance or near fixation on the simultaneous prism and cover test; (5) no developmental delay or ocular or systemic disease; (6) no family history of ocular disorders.
Distance Stereoacuity was obtained from 77 patients with strabismus. The inclusion criteria for strabismic patients were: (1) constant (n = 20; tropia always present at distance and near) or intermittent (n = 57; intermittent tropia or constant tropia present only at distance or only at near) strabismus diagnosed by a pediatric ophthalmologist or orthoptist; (2) normal age-adjusted visual acuity measured by HOTV or E-ETDRS in the better seeing eye8,9; (3) visual acuity no poorer than 0.5 logMAR (20/60) in either eye; and (4) no developmental delay or coexisting ocular or systemic disease. Note that patients had a wide range of stereoacuities at near (Table 1).
Distance Randot Stereotest Protocol
The Final Version of the Distance Randot Stereotest is a Polaroid vectographic book (21 × 17 cm), presenting 2 geometric shapes at each of 4 disparities: 400, 200, 100, and 60 arcsec, which is the same as the Prototype. Subjects viewed the books at 3 meters in a normally illuminated room while wearing polarizing glasses (Stereo Optical Polarized Viewer). If the subject wore corrective spectacles, polarizing glasses were worn over his/her corrective lenses.
Pretest
The subject was asked to identify black-and-white pictures of the 4 geometric shapes (circle, triangle, square, and star) to confirm that they were able to name or match the shapes used in the test. The test proceeded only if the subject was able to name or match the shapes.
Test (Final Version)
Testing always began with the 400 arcsec level. If the child passed the pretest but could not identify or match both shapes at the 400 arcsec level, the test was scored as nil. If both responses were correct, testing proceeded to 200 arcsec, and so on, until the subject made an error. The smallest disparity at which the subject identified or matched both shapes correctly was recorded as stereoacuity. Combined with the pretest, the Final Version test took 0.5–3 minutes to complete.
Test (Prototype)
Testing always began with the 400 arcsec book. If the subject passed the pretest but could not identify or match 2 of 3 shapes at the 400 arcsec level, the test was scored as nil. If at least 2 responses were correct, testing proceeded to 200 arcsec, and so on, until the child could not identify at least 2 shapes of that level. The smallest disparity at which the child identified both shapes correctly was recorded as stereoacuity. Combined with the pretest, the Prototype test took 0.5–5 minutes to complete.
Data Analysis
Normative data from the Final Version of the Distance Randot Stereotest
Mean normal stereoacuity and 95% tolerance limits (±2 SD) were constructed for 7 age groups: 2–3 years (n = 23), 4–5 years (n = 31), 6–10 years (n = 24), 11–15 years (n = 19), 16–20 years (n = 17), 21–30 years (n = 22), and 31–40 years (n = 20).
Reliability of the Final Version of the Distance Randot Stereotest
Test–retest reliability of the Final Version of the Distance Randot Stereotest was assessed in a subgroup of 111 normal subjects (most of 2- to 5-year-old children were not retested) and all 77 patients with constant or intermittent strabismus. Test and retest were completed on the same day.
Validity of the Final Version of the Distance Randot Stereotest
Validity of the Final Version was evaluated in three ways. First, normative data obtained for the Final Version were compared to published normative data for the Prototype. Second, the concordance between stereoacuity obtained using the Final Version and the Prototype tests was determined for a subgroup of normal subjects (n = 80; 17.8 ± 9.6 years of age; range, 5–36 years) and patients (n = 42; 15 ± 16.1 years of age; range, 3.7–62 years) who were examined with both tests. Third, the distribution of Distance Randot Stereotest data from patients with constant strabismus at distance was compared with the data from patients with intermittent strabismus. We expected to find nil distance stereoacuity in patients with constant strabismus and a broad distribution of stereoacuity outcomes in those with intermittent strabismus.
All stereoacuity data were converted to log(arcsec). For convenience of statistics, we arbitrarily assigned nil a value of 10,000 arcsec (4 log(arcsec)). Since the range of possible stereoacuity scores included nil, nonparametric statistics were used for most of the statistical comparisons. The single exception is statistical analysis of the normative data set, which contained no nil values; in this case a two-way ANOVA was used for comparison of the Final Version and Prototype across age groups. Concordance between the Final Version and Prototype was evaluated and Spearman Rank Order correlation was used to test the validity of the Final Version.
Results
Normative Data
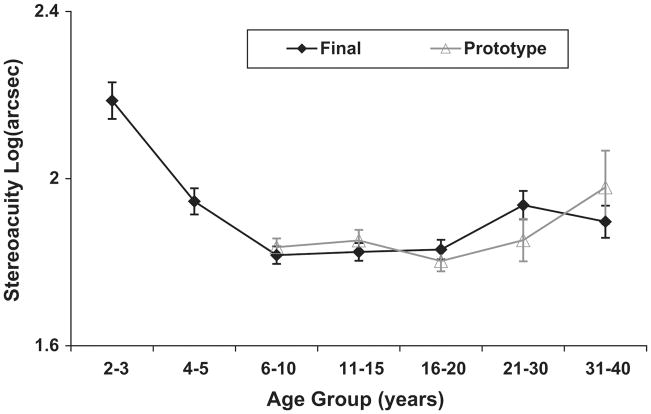
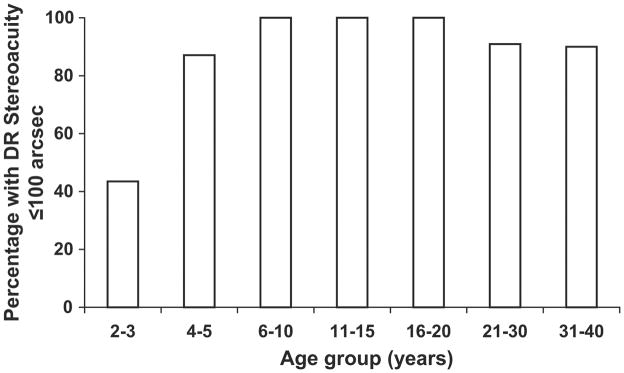
Distance stereoacuity obtained with the Final Version is shown as a function of age in Figure 1. Distance stereoacuity improves from a mean of 200 arcsec (2.3 log arcsec) at 2–3 years of age to a mean of 60 arcsec (1.78 log arcsec) by 6 years of age. Children <6 years old had a wider range of distance stereoacuity and were less likely to have distance stereoacuity of 60 arcsec (1.78 log arcsec) than older children and adults. Ninety-six percent of the normative cohort ≥6 years old had distance stereoacuity ≤100 arcsec (2 log arcsec) (Figure 2). On the other hand, 200 arcsec (2.3 log arcsec) is within the normal range of distance stereoacuities for 4–5 year old children and 400 arcsec (2.6 log arcsec) is within the normal range of distance stereoacuities for 2–3 year old children. Table 2 lists the mean stereoacuity and the 95% lower tolerance limit of normal stereoacuity for each age group.
FIG 1.
Normative stereoacuity obtained with the Final Version test and Prototype of Distance Randot Stereotest across age. The black diamond represents the mean of the Final Version; the gray triangle, the mean of the Prototype. Error bars are standard error. Note that Prototype data were collected only for children ≥6 years of age.
FIG 2.
Proportion of normal subjects with stereoacuity equal or less than 100 arcsec obtained with the Final Version of Distance Randot Stereotest.
Table 2.
Distance Randot® Stereoacuity norms.
| Age group (yr) | N | Mean (arcsec)a | Lower limit (arcsec)b |
|---|---|---|---|
| 2–3 | 23 | 200 | 400 |
| 4–5 | 31 | 100 | 200 |
| 6–10 | 24 | 60 | 100 |
| 11–15 | 19 | 60 | 100 |
| 16–20 | 17 | 60 | 100 |
| 21–30 | 22 | 100 | 200 |
| 31–40 | 20 | 100 | 200 |
Rounded to the next larger disparity level available in the Distance Randot® Test
Rounded score that includes 95% of the normative cohort
Reliability
Test and retest results for the Final Version were identical in 82.0% and within one disparity level in 100% of normal children and adults (Table 3A). Stereoacuity thresholds were well correlated between the Prototype and Final Version (Spearman Rank Order correlation: rs = 0.72, p < 0.001).
Table 3.
| Table 3A. Test-retest repeatability in normal subjects (n = 111) | |||||
|---|---|---|---|---|---|
| Final version retest | Final version Distance Stereoacuity | ||||
| nil | 400 | 200 | 100 | 60 | |
| nil | 0 | ||||
| 400 | 0 | ||||
| 200 | 6 | 3 | |||
| 100 | 21 | 4 | |||
| 60 | 13 | 64 | |||
| Table 3B. Test-retest repeatability in patients (n = 77) | |||||
|---|---|---|---|---|---|
| Final version retest | Final version Distance Stereoacuity | ||||
| nil | 400 | 200 | 100 | 60 | |
| nil | 26 | ||||
| 400 | 1 | 5 | 1 | ||
| 200 | 2 | 6 | 2 | ||
| 100 | 2 | 5 | 10 | 2 | |
| 60 | 2 | 13 | |||
Note: Identical results on test and retest are highlighted in gray.
In a separate cohort of strabismic patients, 62.3% of patients had abnormal Final Version distance stereoacuity (>100 arsec). Nil distance stereoacuity was found in 27 (35%) patients and confirmed in 96.3% of retests. Measurable distance stereoacuity was found in 50 (65%) patients and confirmed in 100% of retests. Of the 29 strabismic patients with normal distance stereoacuity (60 or 100 arcsec [1.78 to 2 log(arcsec)]), 93% were confirmed on retest; all had intermittent strabismus. Overall, in patients with strabismus, 77.9% of test–retest results were identical; 97.4% of test–retest results were within one disparity level (Table 3B, and the scores on the two tests were correlated with prototype scores (Spearman Rank Order correlation: rs = 0.64, p < 0.001).
Validity
For comparison to the normative data obtained with the Final Version, Figure 1 includes data obtained with the Prototype for the same subjects (children ≥6 years old and adults). Overall, distance stereoacuity obtained with the Final Version was similar to distance stereoacuity obtained with the Prototype for children ≥6 years old and adults. There is no main effect of test version (Final Version vs Prototype: F1,169 = 0.01, p = 0.93). There was a significant main effect of age (F4,169 = 3.81, p = 0.005) but the interaction between age and test version was not significant (F4,169 = 1.42, p = 0.23).
Table 4A and 4B show the concordance of Final Version and Prototype in the normal group and patients. Tests for the Final Version and the Prototype were identical in 77.5% and within one disparity level in 97.5% of normal children and adults (Table 4A). Scores obtained using the two version of the test were correlated (Spearman Rank Order correlation: rs = 0.64, p < 0.001). Using the Final Version, nil level of distance stereoacuity was found in 26 patients and confirmed in 92.3% with the Prototype. Overall, in 76.1% of patients, stereoacuity thresholds obtained with the Final Version were identical to thresholds obtained with the Prototype (Table 4B), with 100% of results within one disparity level.
Table 4.
| Table 4A. Concordance of Final version and Prototype in normal subjects (n = 80) | |||||
|---|---|---|---|---|---|
| Prototype test | Final version Distance Stereoacuity | ||||
| nil | 400 | 200 | 100 | 60 | |
| nil | 0 | ||||
| 400 | 0 | 2 | |||
| 200 | 1 | 2 | |||
| 100 | 1 | 12 | 5 | ||
| 60 | 8 | 49 | |||
| Table 4B. Concordance of Final version and Prototype in patients (n = 42) | |||||
|---|---|---|---|---|---|
| Prototype test | Final version Distance Stereoacuity | ||||
| nil | 400 | 200 | 100 | 60 | |
| nil | 24 | ||||
| 400 | 2 | 1 | 1 | ||
| 200 | 2 | 3 | 2 | ||
| 100 | 1 | 0 | 1 | ||
| 60 | 1 | 4 | |||
Note: Identical results on Final version and Prototype are highlighted in gray.
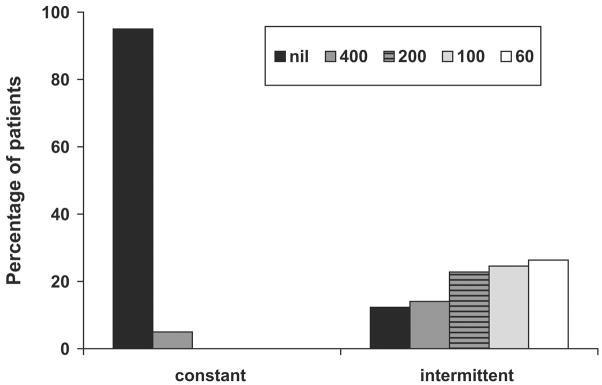
As expected, patients with constant strabismus were more likely to have nil stereoacuity than patients with intermittent strabismus (Figure 3). The percentage of nil stereoacuity was 95% in 20 patients with constant strabismus; the one patient with constant strabismus with stereoacuity of 400 arcsec had a 4Δ esotropic microtropia. Among the 57 patients with intermittent strabismus the percentage of nil stereoacuity was 12.2%. The difference is significant (z = 7.93, p < 0.001).
FIG 3.
Proportion of patients with strabismus with measurable Distance Randot Stereotest stereoacuity.
Discussion
Thcis paper provides a normative data set gathered at three sites for the Distance Randot® Stereotest. The tight clustering and high test–retest reliability of normative data in children age 6 years and older suggest that the Distance Randot Stereotest may be particularly suited to test such a population. While children as young as 2–3 years of age often are testable, the range of normal distance stereoacuity scores is broad and it is only at 6 cyears of age that 96% of the normative data reach the adult level. Thus at age 2–3 years, the Distance Randot Stereotest may only be useful for determining presence or abscence of distance stereoacuity. Note (in Table 2) that data from 21- to 30-year-old and 31- to 40-year-old normal adults have larger variance than data from children age 6–20 years. The reason is unknown. The normative data from the current study showed excellent concordance with normative data obtained using the Prototype test, supporting the validity of the simplified test protocol used in the Final Version.
The Prototype Distance Randot Stereotest already has been shown to be a useful tool to measure distance stereoacuity in patients with or without strabismus.1,4,7,11–14 Holmes and colleagues7 reported that distance stereoacuity thresholds are degraded in some patients with intermittent exotropia, as measured using the Prototype. In the present study, distance stereoacuities obtained with the Final Version of the Distance Randot® Stereotest were shown to have excellent agreement with the results collected with the Prototype Distance Randot Stereotest. In agreement with the literature,1,2,5,7,11–16 we found that Distance Randot stereoacuity was poorer in patients with intermittent exotropia compared with normal controls and that, with the exception of a single patient with constant microtropia, patients with constant strabismus had nil Distance Randot® stereoacuity. Since true stereopsis is possible when the horizontal deviation is up to 4Δ,6 the finding of measurable distance stereoacuity in a patient with microtropia is reasonable. Distance stereoacuity varies considerably over the time course of one day in some patients with intermittent exotropia, including change from nil on one assessment to measurable later the same day.11 This might explain the slightly higher test–retest variability found among patients as compared with the normal group in the present study.
The Final Version of the Distance Randot Stereotest was successful in some children as young as 2 years old. Since fewer trials per disparity level are required with the Final Version of the test compared to the Prototype, it is faster to complete, and therefore may be more appropriate for young children (2–6 years of age), who have short attention spans. Compared with real depth tests (Frisby and Frisby-Davis 2), random dot tests (such as the Distance Randot Stereotest) are highly sensitive to refractive error, blur, heterophoria and strabismus.12–14,17 Moreover, stereoacuity thresholds are more easily degraded by reduced monocular visual acuity with the use of random dot tests than real depth tests.4,14
Measurement of distance stereoacuity may be particularly important in types of strabismus where the deviation or control at distance differs from the deviation or control at near. The most common condition that fits this description is intermittent exotropia, which has an incidence of 32.1 per 100,000 in children less than 19 years of age.18 Measurement of distance stereoacuity has been used previously to assess the severity of intermittent exotropia and to determine whether deterioration has occurred.5,7 In addition, changes in distance stereoacuity have been suggested for use as an indicator of need for surgical treatment in patients with intermittent exotropia as well as an outcome measure of surgical success.2,5,15,16 The Distance Randot Stereotest is a simple and efficient approach to making reliable and valid measurements of distance stereoacuity in such a clinical setting.
Acknowledgments
supported by National Institutes of Health Grant EY-005236.
Footnotes
The authors have no financial interest in the Distance Randot® Stereotest. Stereo Optical Company, Inc. (Chicago, IL) provided the prototype and final versions of the Distance Randot® Stereotest to us free of charge.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu VL, Birch EE, Holmes JM. Assessment of a new Distance Randot stereoacuity test. J AAPOS. 2006;10:419–23. doi: 10.1016/j.jaapos.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Adams WE, Leske DA, Hatt SR, et al. Improvement in distance stereoacuity following surgery for intermittent exotropia. J AAPOS. 2008;12:141–4. doi: 10.1016/j.jaapos.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes JM, Fawcett SL. Testing distance stereoacuity with the Frisby-Davis 2 (FD2) test. Am J Ophthalmol. 2005;139:193–5. doi: 10.1016/j.ajo.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Leske DA, Birch EE, Holmes JM. Real depth vs randot stereotests. Am J Ophthalmol. 2006;142:699–701. doi: 10.1016/j.ajo.2006.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stathacopoulos RA, Rosenbaum AL, Zanoni D, et al. Distance stereoacuity. Assessing control in intermittent exotropia. Ophthalmology. 1993;100:495–500. doi: 10.1016/s0161-6420(93)31616-7. [DOI] [PubMed] [Google Scholar]
- 6.Leske DA, Holmes JM. Maximum angle of horizontal strabismus consistent with true stereopsis. J AAPOS. 2004;8:28–34. doi: 10.1016/j.jaapos.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Holmes JM, Birch EE, Leske DA, Fu VL, Mohney BG. New tests of distance stereoacuity and their role in evaluating intermittent exotropia. Ophthalmology. 2007;114:1215–20. doi: 10.1016/j.ophtha.2006.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drover JR, Felius J, Cheng CS, Morale SE, Wyatt L, Birch EE. Normative pediatric visual acuity using single surrounded HOTV optotypes on the Electronic Visual Acuity Tester following the Amblyopia Treatment Study protocol. J AAPOS. 2008;12:145–9. doi: 10.1016/j.jaapos.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotter SA, Chu RH, Chandler DL, et al. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to <13 years old. Am J Ophthalmol. 2003;136:655–61. doi: 10.1016/s0002-9394(03)00388-x. [DOI] [PubMed] [Google Scholar]
- 10.Birch E, Williams C, Hunter J, Lapa MC. Random dot stereoacuity of preschool children. ALSPAC “Children in Focus” Study Team. J Pediatr Ophthalmol Strabismus. 1997;34:217–22. doi: 10.3928/0191-3913-19970701-08. quiz 247–8. [DOI] [PubMed] [Google Scholar]
- 11.Hatt SR, Mohney BG, Leske DA, Holmes JM. Variability of stereoacuity in intermittent exotropia. Am J Ophthalmol. 2008;145:556–61. doi: 10.1016/j.ajo.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laird PW, Hatt SR, Leske DA, Holmes JM. Stereoacuity and binocular visual acuity in prism-induced exodeviation. J AAPOS. 2007;11:362–6. doi: 10.1016/j.jaapos.2007.01.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laird PW, Hatt SR, Leske DA, Holmes JM. Distance stereoacuity in prism-induced convergence stress. J AAPOS. 2008;12:370–74. doi: 10.1016/j.jaapos.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odell NV, Hatt SR, Leske DA, Adams WE, Holmes JM. The effect of induced monocular blur on measures of stereoacuity. J AAPOS. 2009;13:136–41. doi: 10.1016/j.jaapos.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neal TD, Rosenbaum AL, Stathacopoulos RA. Distance stereo acuity improvement in intermittent exotropic patients following strabismus surgery. J Pediatr Ophthalmol Strabismus. 1995;32:353–7. doi: 10.3928/0191-3913-19951101-06. discussion 358. [DOI] [PubMed] [Google Scholar]
- 16.Yildirim C, Mutlu FM, Chen Y, Altinsoy HI. Assessment of central and peripheral fusion and near and distance stereoacuity in intermittent exotropic patients before and after strabismus surgery. Am J Ophthalmol. 1999;128:222–30. doi: 10.1016/s0002-9394(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 17.Rutstein RP, Swanson MW. Atypical fixation preference with anisometropia. Optom Vis Sci. 2007;84:848–51. doi: 10.1097/OPX.0b013e3181559d9a. [DOI] [PubMed] [Google Scholar]
- 18.Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: A population-based study. Ophthalmology. 2005;112:104–8. doi: 10.1016/j.ophtha.2004.07.033. [DOI] [PubMed] [Google Scholar]