Abstract
The protozoan parasite Trypanosoma brucei lives in the bloodstream of vertebrates or in a tsetse fly. Expression of a GPI-phospholipase C polypeptide (GPI-PLCp) in the parasite is restricted to the bloodstream form. Events controlling the amount of GPI-PLCp expressed during differentiation are not completely understood. Our metabolic “pulse-chase” analysis reveals that GPI-PLCp is stable in bloodstream form. However, during differentiation of bloodstream to insect stage (procyclic) T. brucei, translation GPI-PLC mRNA ceases within 8 h of initiating transformation. GPI-PLCp is not lost precipitously from newly-transformed procyclic trypanosomes. Nascent procyclics contain 400-fold more GPI-PLCp than established insect stage T. brucei. Reduction of GPI-PLCp in early-stage procyclics is linked to parasite replication. Sixteen cell divisions are required to reduce the amount of GPI-PLCp in newly-differentiated procyclics to levels present in the established procyclic. GPI-PLCp is retained in strains of T. brucei that fail to replicate after differentiation of the bloodstream to the procyclic form. Thus, at least two factors control levels of GPI-PLCp during differentiation of bloodstream T. brucei; (i) repression of GPI-PLC mRNA translation, and (ii) sustained replication of newly-transformed procyclic T. brucei. These studies illustrate the importance of repeated cell divisions in controlling the steady-state amount of GPI-PLCp during differentiation of the African trypanosome.
INTRODUCTION
Trypanosoma brucei causes human African trypanosomiasis, and is transmitted to its vertebrate host through the bite of a tsetse fly. In natural settings, differentiation of the bloodstream form to the procyclic (insect stage) T. brucei is important for transmission of the parasite.
In the bloodstream, T. brucei is covered with a variant surface glycoprotein (VSG) whereas in a tsetse fly the parasite expresses procyclin (PARP) as the major surface protein. Differentiation of bloodstream to insect stage (procyclic) T. brucei (reviewed in (Fenn and Matthews, 2007)) is characterized by increased procyclin expression and loss of VSG within 6 h of initiating transformation (Roditi, et al., 1989). Cell division is arrested during differentiation, which is completed within 72 h.
In trypanosomatids, protein levels are controlled predominantly by post-transcriptional events, as initiation of transcription is rarely regulated in these organisms in (Clayton, 2002).
Glycosylphosphatidylinositol (GPI)-phospholipase C (GPI-PLC) is expressed in bloodstream form T. brucei. GPI-PLC is a virulence factor in pleomorphic T. brucei (Tasker, et al., 2000, Webb, et al., 1997). The enzyme stimulates endocytosis of transferrin in bloodstream T. brucei (Subramanya, et al., 2009), and is activated by mild acid or hypotonic conditions to cleave GPI at the endoplasmic reticulum (Subramanya and Mensa-Wilmot, 2006). GPI-PLC does not have a significant role in release of variant surface glycoprotein (VSG) from differentiating bloodstream T. brucei (Bülow, et al., 1989, Gruszynski, et al., 2006, Hanrahan, et al., 2009, Webb, et al., 1997).
In established procyclic T. brucei, GPI-PLC enzyme activity is not detectable (Bülow, et al., 1989, Mensa-Wilmot, et al., 1990). However, full-length mRNA of the GPI-PLC gene is present in established procyclic T. brucei (Carrington, et al., 1989, Mensa-Wilmot, et al., 1990), although the half-life of newly synthesized pre-mRNA is relatively short (Webb, et al., 2005). The basis for disappearance of GPI-PLC polypeptide (GPI-PLCp) from procyclic T. brucei is not known. As part of an effort to understand possible connections between steady-state level of GPI-PLCp and differentiation of bloodstream trypanosomes, we studied stability of GPI-PLCp and the kinetics of translation of GPI-PLC mRNA during differentiation of bloodstream to procyclic T. brucei.
We found, unexpectedly, that differentiation (alone) of bloodstream to the procyclic form is not sufficient to explain the difference in magnitude of GPI-PLCp level between established and newly-transformed procyclic cells. Arrest of translation of GPI-PLC mRNA occurs early during differentiation, in concert with a 50% loss of GPI-PLCp in newly-differentiated procyclic cells. However, multiple replication cycles are needed to reduce the level of GPI-PLCp from that found in newly-differentiated procyclic cells to that reported in established procyclic lines. These observations highlight the importance of cell replication in developmentally regulated expression of a trypanosome GPI-phospholipase C. Our results have implications the developmental regulation of proteins that are highly stable in bloodstream T. brucei.
EXPERIMENTAL PROCEDURES
Cells
Monomorphic T. brucei ILTat 1.3 and T. brucei 427, and the pleomorphic AnTat 1.1 were used. Culture-adapted bloodstream T. brucei 427 was a gift from Dr. C. C. Wang (University of California, San Francisco). Established procyclic T. brucei 427 was kindly provided by Dr. Jay Bangs (University of Wisconsin, Madison). Bloodstream form trypanosomes were harvested from infected rats and purified by DE-52 chromatography (Cross, 1975).
Mice were inoculated intraperitoneally with T brucei AnTat 1.1 and simultaneously injected with cyclophosphamide (Sigma) (300 mg/ kg body weight) (Gould, et al., 1986). Parasite density was approximately 4 × 107− 1 × 108/ml of blood between day 5 and day 6 when they were harvested.
Materials
Fetal bovine serum (FBS) was obtained from Life Technologies (Gaithersburg, MD). Citric acid and cis-aconitate were purchased from Sigma (St. Louis, MO). All protease inhibitors were from Boehringer Mannheim (Indianapolis, IN). 5-Bromo-4-chloro-indoyl phosphate (BCIP), p-nitroblue tetrazolium chloride (NBT), and alkaline phosphatase conjugated goat anti-rabbit IgG were purchased from BioRad (Richmond, CA). Procyclin (anti-GPEET) antibody was a gift from Dr. Isabel Roditi (Universitat Bern, Switzerland). Anti-VSG117 and anti-AnTat.1 antibodies were provided by Dr. Jay Bangs (University of Wisconsin, Madison). Anti-VSG221 (Hoek, et al., 1999) was a gift from Dr. George Cross (Rockefeller University). [35S]Methionine was purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Differentiation of Bloodstream T. brucei In Vitro
Monomorphic trypanosomes (strains 427 and ILTat1.3) were harvested from rat blood at a density of 2 × 109/ml. Following the preparation of a “buffy coat”, parasites were added to transformation medium (SDM-79 containing 10% heat-inactivated FBS, and 5 mM each of citric acid and cis-aconitate (Bülow, et al., 1989, Cunningham, 1977)) at the density indicated (see "Figure Legends"). Cultures were incubated at 27°C, and parasites counted at 12 or 24-h intervals. An aliquot (2 × 107) of parasites was harvested at each time point, washed with phosphate buffered saline (PBS; 140 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4), pelleted (3,000 × g, 5 min), and frozen at −80°C until use.
For differentiation of AnTat 1.1 T. brucei, heparinized (infected) mouse blood was added to a 50% slurry of DE-52 beads prepared in bicine buffered saline (BBS) (10 ml bed vol/ml blood), following a modification by J. Bangs of a procedure developed by Overath and colleagues (Ziegelbauer, et al., 1990 Sep 11). Briefly, tubes containing the DE-52/blood slurry were inverted continuously for 5 min, centrifuged (1,500 × g, 1 min, 25°C), and the supernatant incubated at 27°C. (Cells adsorbed to the DE-52 beads were recovered by washing in fresh 1X BBS, and centrifuging at 1,500 × g for 20 seconds at room temperature. This second supernatant was pooled with the supernatant from the first centrifugation, and then centrifuged at 1,500 × g for 5 min to pellet the cells.) Parasites (108) were washed once with 1X BBS, resuspended in HMI-9 medium containing 15% FBS (Hirumi and Hirumi, 1994), and incubated at 37°C in 5% CO2 for 1 h before being added to 40 ml of transformation medium (SM containing 15% heat-inactivated FBS, 5 mM citric acid, and 5 mM cis-aconitate) (density of 2.5 × 106 cells/ml). Cells were incubated at 27°C. Beginning at inoculation and at time intervals indicated 107 cells were harvested. After washing with PBS, cells were frozen at −80°C until GPI-PLC enzyme assays were performed. In addition, at specified time intervals, 2 × 106 parasites were processed for immunofluorescence microscopy (see below).
Determination Of GPI-phospholipase C Activity
Parasites (2 × 107) were resuspended in 200 µl of hypotonic lysis buffer (10 mM sodium phosphate, 1 mM EDTA, pH 8) containing leupeptin (2.1 µM), N-tosyl-L-lysine chloro-methyl ketone (TLCK) (0.1 mM) and aprotinin (0.4 U) (Hereld, et al., 1986, Mensa-Wilmot, et al., 1994). Cells were kept on ice for 20 min. The lysate was centrifuged (14,000 × g, 4°C, for 10 min) and the supernatant discarded. The pellet was solubilized in 200 µl of 50 mM Tris-HCl, 5 mM EDTA, 1% NP40 (AB). Five µl of each sample was assayed for GPI-digestion activity using [3H]myristate-labeled membrane form VSG (mfVSG) as substrate (Mensa-Wilmot, et al., 1995). Duplicate reaction mixtures were incubated at 37°C for 15 min, released [3H]dimyristoylglycerol ([3H]DMG) was extracted with water-saturated butanol, quantitated by liquid scintillation counting. Several dilutions (in AB) of the solubilized membrane fractions were assayed in order to obtain values within the linear range (0.1–1 units) of GPI-PLC activity. Protein content of fractions was determined with bicinchoninic acid (BCA) reagent (Pierce).
SDS-PAGE And Western Blotting
For immunoblotting, proteins were transferred onto Immobilon P (Millipore) using a Trans-Blot Semi-Dry cell (BioRad) (Armah and Mensa-Wilmot, 1999). Rabbit antisera against VSGs (VSG117, VSGAntat1.1 or VSG221), procyclin (anti-GPEET), and sea urchin tubulin (anti-TUB) were used at a dilution of 1:3000 for western blotting (Mensa-Wilmot, et al., 1990).
Immunofluorescence
T. brucei AnTat1.1 bloodstream (2 × 106) were pelleted at 1,500 × g for 5 min and washed twice with 1X BBS. The cells were air dried on poly-L-lysine coated cover slips for 30 min at room temperature, and fixed in acetone at −20°C for 5 min. After rinsing twice with PBS for 5 min each time, fixed cells were blocked with 1% BSA (in PBS) for 25 min. Cover slips were incubated with primary antibody in blocking buffer for 2 h. Primary antibodies were against VSGAnTat1.1 (polyclonal anti-VSG AnTat 1.1 (1: 2000 dilution) (a gift from J. Bangs), and procyclin (mouse anti-GPEET antibody, CedarLane Laboratories) (1:200 dilution)). Cover slips were rinsed in PBS for 5 min (three times) and treated with blocking buffer for 25 min. Cells were then incubated with the following secondary antibodies in blocking solution for 2 h in the dark; goat anti-rabbit IgG-Alexa-Fluor 488 (Molecular Probes) (1:5000) to detect anti-VSG, and goat anti-mouse IgG – Alexa Fluor 594 (Molecular Probes) (1:5000) to localize anti-procyclin antibody. Cover slips were washed thrice with PBS (5 min each time), blotted dry, mounted on slides, and visualized with a Leica microscope (DMIRBE). Images were captured using an interline chip cooled CCD camera (Orca 9545 : Hamamatsu), and processed with Openlab 2.2 software (Improvision).
Flow Cytometry
T. brucei 427 (5 × 106 cells) was washed in two ml of PBS (pH 7.4). Cells were fixed with 2% paraformaldehyde for 5 min on ice, and washed with 0.5 M glycine for 5 min at room temperature. The cell pellet was washed once with PBS and then blocked with 1% BSA (in PBS) for 30 min at room temperature. Primary antibody against VSG (rabbit anti-VSG 221; 1:1000 dilution) and procyclin (mouse anti-GPEET antibody 1:1000 dilution) was added for 1 h. Cells were washed once in PBS, and incubated with secondary antibody (either goat anti-rabbit IgG-Alexa Flour 488 or goat anti-mouse IgG-Alexa Flour 488 594) at 1:1000 dilution for 1 h before washing once with PBS. Cells were resuspended in 1 ml PBS, and analyzed by flow cytometry on CyAn (DakoCytomation, USA). The fluorochromes were excited with 488 and 594 lasers. Data generated by the flow cytometer was analyzed with FlowJo software (Tree Star Inc.).
Metabolic Labeling, and Immunoprecipitation of [35S]Met-labeled GPI-PLCp
Parasites (2 × 108) were labeled with 500 µCi of [35S]methionine in 10 ml of methionine-free RPMI 1640 (Doering, et al., 1990) for 40 min at 37°C (untreated bloodstream form) or 27°C (differentiating T. brucei). Parasites were harvested, washed with PBS and lysed hypotonically (Armah and Mensa-Wilmot, 1999). Immunoadsorption of GPI-PLCp was performed as described (Armah and Mensa-Wilmot, 1999). [35S]Met-labeled GPI-PLCp was electrophoresed on SDS-PAG (12% minigel) and visualized by phosphorimaging (Personal Molecular Imager FX (BioRad)).
Half-Life Of GPI-PLCp In Bloodstream Form T. brucei
To determine the half-life of GPI-PLCp, freshly-harvested bloodstream cells (4.2 × 107) were labeled with [35S]Met for 40 min, and “chased” in HM1-9 medium at 37°C. An aliquot (3.5 × 106 cells) was withdrawn at 1 h intervals (up to 11 h). Cells were washed with PBS and frozen at −80°C until use. GPI-PLCp was immunoprecipitated (Armah and Mensa-Wilmot, 1999), and analyzed by SDS-PAGE/phosphorimaging.
The “enzyme activity half-life” of GPI-PLCp was determined as follows. Parasites (108) were resuspended in RPMI 1640 supplemented with 10% FBS and D-glucose (7 mg/ml). To block new protein synthesis, cells were treated with cycloheximide (1.2 mM) for 15 min at 37°C (Buxbaum, et al., 1996). A time-course was initiated during which 107 cells were harvested at 1 h intervals, washed once with cold PBS, lysed hypotonically, and GPI-PLCp activity assayed as described (Mensa-Wilmot, et al., 1995).
RESULTS
Newly-Differentiated Procyclic T. Brucei Contain GPI-PLCp
GPI-PLC enzyme activity is detectable in bloodstream form (BSF) T. brucei but not in the established insect stage (procyclic form, (PCF)) (Bülow and Overath, 1985, Mensa-Wilmot, et al., 1990). We hypothesized that GPI-PLCp is actively lost from bloodstream trypanosomes during their differentiation to the procyclic. To test this theory, we monitored enzyme activity after initiating transformation of bloodstream form to procyclic T. brucei. Two monomorphic strains (ILTat 1.3 and Lister 427) and one pleomorphic strain (AnTat 1.1) of the parasite were used for these studies. Although all three strains differentiate into the procyclic form, only AnTat 1.1 resumes cell division under conditions of our study. By using select parasite lines in appropriate media, we were able to arrest differentiation so that some events happening in the “early-stage” could be separated from others that require transition to a “late-stage” of transformation.
In monomorphic ILTat 1.3 T. brucei, total GPI-PLCp enzyme activity (per cell) remained constant for 72 hours after initiation of transformation (Fig. 1A). These results were unexpected because the enzyme activity is not found in established procyclics (i.e., procyclics that have been maintained in culture for long periods) (Bülow and Overath, 1985, Carrington, et al., 1989, Mensa-Wilmot, et al., 1990). And, the ILTat 1.3 cells were expected to have transformed into the procyclic stage within 48 of initiating differentiation (Bass and Wang, 1991, Mutomba and Wang, 1995, Roditi, et al., 1989, Ziegelbauer, et al., 1990 Sep 11). These data raised a concern that transformation of the cells to procyclic form had failed. To investigate this possibility, three characteristics of differentiating T. brucei were examined, namely (i) arrest of cell division, (ii) expression of procyclin, a marker of insect-stage cells (Clayton and Mowatt, 1989, Roditi, et al., 1989), and (iii) loss of variant surface glycoprotein (VSG), a bloodstream-specific polypeptide (Roditi, et al., 1989).
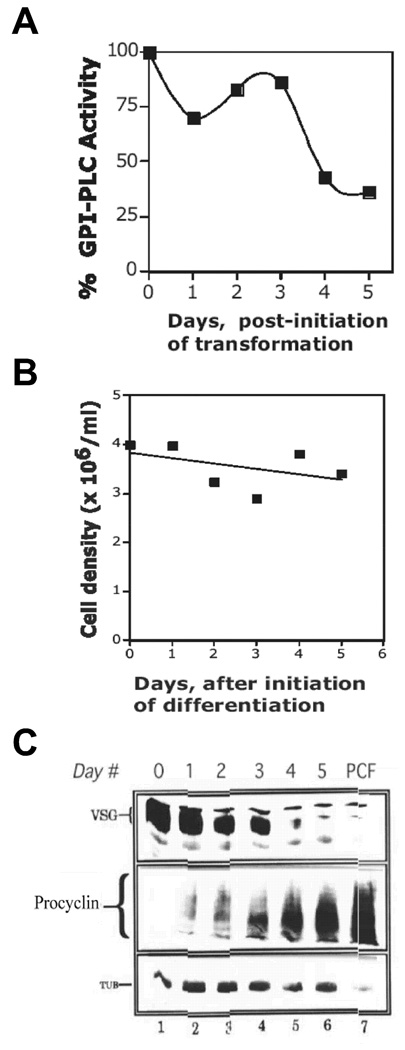
Figure 1. Newly Differentiated ILTat 1.3 Procyclic T. brucei Contain GPI-PLCp Activity.
T. brucei ILTat 1.3 was suspended in a transformation medium (SDM-79) at of 4 × 106/ml and incubated at 27°C. At 24 h intervals, 2 × 107 parasites were harvested. (A), Total GPI-PLCp activity (Units/107 cells) was determined. Activity in cells at day 0 of differentiation was assigned a value of 100%. (B), Cell density during differentiation of ILTat 1. (C), Western blotting with anti-VSG117 (106/lane), anti-procyclin (6 × 106/lane), or anti-tubulin antibody (2 × 106/lane). PCF, established procyclic; Day #, refers to days post initiation of differentiation; TUB, tubulin.
T. brucei cell division is arrested during transformation (Bass and Wang, 1991, Roditi, et al., 1989). Consistent with this property of differentiating parasites, cell density of ILTat 1.3 T. brucei was unchanged for five days under transformation conditions (Fig. 1B). VSG117 in differentiating ILTat 1.3 decreased 50% on day 2 (Fig. 1C, lanes 1–3), as determined by quantitative western blots using serial dilutions of the sample from day 0 as standard (not presented). By day 4, VSG117 was barely detectable (Fig. 1C, lane 5), similar to the situation in established procyclic T. brucei strain 427 (Fig. 1C, compare lanes 5 and 7). (The larger molecular weight polypeptide that adsorbs anti-VSG117 antibody in lanes 4 and 5 of Fig. 1C is not VSG117, because that band is detectable in procyclics that do not express VSG117).
Procyclin (PARP) polypeptide is expressed in insect form trypanosomes (Roditi, et al., 1989). Procyclin was initially undetectable in bloodstream ILTat1.3 (Fig. 1C, middle panel lane 1), but was present one day after initiation of differentiation (Fig. 1C, middle panel lane 2). In control experiments, similar amounts of tubulin were detected during differentiation (Fig. 1C, bottom panel lanes 1–6), indicating that equivalent numbers of cells were used in western blotting.
In conclusion, on day 3 of differentiation ILTat 1.3 T. brucei are early-stage procyclic. However, the cells do not replicate, and they contain as much as 40% of the GPI-PLCp in the bloodstream form. This quantity of residual GPI-PLCp in the nascent-procyclic T. brucei is 400-fold more than expected, since established procyclic form has a 1000-fold less, at least, enzyme activity than bloodstream form (Bülow and Overath, 1985, Carrington, et al., 1989, Mensa-Wilmot, et al., 1990).
An alternative hypothesis to explain the persistence of GPI-PLC polypeptide in procyclic cells is that mere differentiation is not sufficient to eliminate the protein from procyclic T. brucei. It is possible that the parasites failed to implement events that occur “late” in transformation and enable the cells to resume replication. To test these concepts, two other strains of T. brucei, namely monomorphic Lister 427 and the pleomorphic AnTat 1.1 strain were used in similar experiments. Whereas Lister 427 is not capable of replication after completion of transformation to the procyclic form in SDM-79 medium (Mutomba and Wang, 1995), AnTat 1.1 replicates prolifically in SM medium after differentiation in vitro (Rolin, et al., 1998). If parasite replication is needed for loss of GPI-PLCp from procyclic T. brucei, nascent-procyclic CA-427 are expected to retain GPI-PLCp.
In T. brucei 427, a fifty percent reduction in total activity of GPI-PLCp was detected after 3 days in differentiation medium (Fig. 2A). No change in cell density was detectable during the 3 days of transformation (not presented) as previously reported (Mutomba and Wang, 1995). By western blot analysis, the cells lost VSG221 completely by day 3 (Fig. 2B, compare lane 1 to lane 4). Procyclin (PARP) was detectable on day 2 of transformation (Fig. 2B, lane 3), in agreement with a previous report (Roditi, et al., 1989). Comparable quantities of tubulin, a positive control, were detected at all stages of differentiation (Fig. 2B).
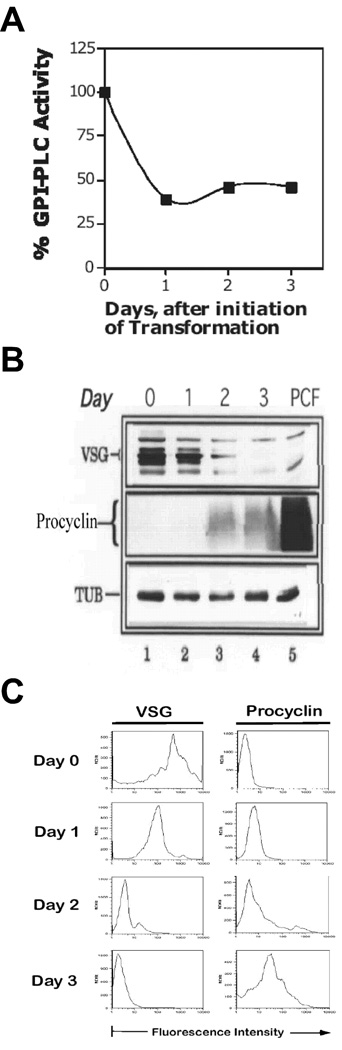
Figure 2. Early-stage Procyclic T. brucei 427 Retain GPI-PLCp.
Culture-adapted T. brucei 427 (CA-427) was resuspended in SDM-79 transformation medium (3.3 × 106/ml) and incubated at 27°C. Parasites (2 × 107) were harvested for analysis as described in the legend to Fig. 1. (A), Total GPI-PLCp activity (per 107 cells), expressed as a proportion of the activity of day zero cells. (B), Changes in protein expression. Cells (2 × 107) were withdrawn at the indicated time points, lysed hypotonically, and membrane proteins resolved by SDS-PAGE (12%, minigel). VSG, procyclin (PARP) and tubulin were analyzed as described in legend to Fig. 1C PCF, established procyclic; Day #, days post initiation of differentiation; TUB, tubulin, PARP, procyclin. (C), Analysis of procyclin and VSG Expression by Flow Cytometry. Cell surface expression of VSG and procyclin in differentiating T. brucei 427 was determined by flow cytometry (detailed in Materials and Methods).
Cell surface VSG221 and procyclin were analyzed with flow cytometry in differentiating T. brucei 427 (Fig. 2C). Loss of VSG221 was discernable beginning on day 1 of differentiation (Fig. 2C). Similarly, GPEET procyclin expression began on day 1 (Fig. 2C). These data confirm that on day 3, T. brucei 427 had differentiated to the procyclic form, in agreement with a previous study (Mutomba and Wang, 1995). More importantly, the data support conclusions reached earlier with ILTat 1.3; newly-differentiated procyclic T. brucei contain 400–500-fold more GPI-PLCp than established procyclic cells.
Loss Of GPI-PLCp Is Associated with Sustained Replication Of Nascent Procyclics
To test a hypothesis that cell division (post-differentiation) is important for continued loss of GPI-PLC activity from early-stage procyclic T. brucei, the AnTat 1.1 strain was studied, because that pleomorphic strain replicates vigorously after differentiation (Tasker, et al., 2000). Presence of GPI-PLCp in differentiating AnTat 1.1 was determined every 12 for the first 96 h, and then every 24 h thereafter (Fig. 3). In these studies, only the first ten days of differentiation were studied, because established procyclics lack GPI-PLCp (Bülow and Overath, 1985, Mensa-Wilmot, et al., 1990).
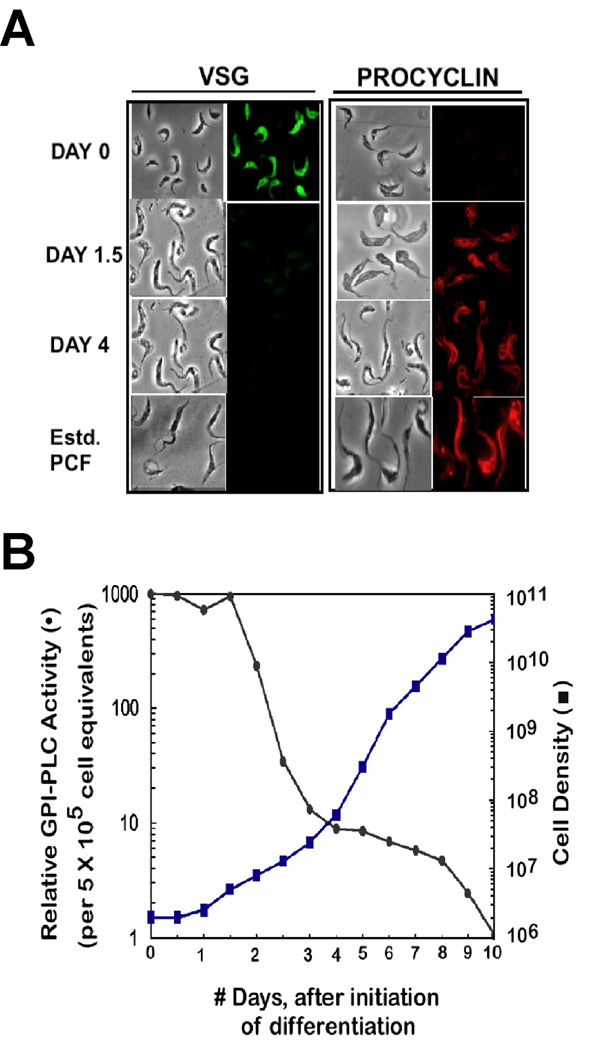
Figure 3. Differentiation, Cell Division, and Loss of GPI-PLCp in AnTat 1.1 T. brucei.
AnTat 1.1 T. brucei was resuspended in SM transformation medium (with 5 mM citrate and 5 mM cis-aconitate) at a density of 2 × 106/ml, and incubated at 27°C. At 12 h intervals, cells were counted, 2 × 107 parasites were harvested to be processed for GPI-PLCp activity or immunofluorescence assays (Materials and Methods). While in culture cells were counted and diluted to 105/ml when the density approached 107/ml. Cell density at any time point is calculated after correcting for the dilution factor. Relative GPI-PLC activity is plotted where amount of enzyme activity at Day 0 is assigned a value of 1000. (A), Detection of VSG and procyclin by immunofluorescence. (B), Replication of cells during and after transformation.
Differentiation of AnTat 1.1 in vitro was swift; 1.5 days after initiating transformation most of the VSG was lost and procyclin was detected (Fig. 3A). Replication of AnTat 1.1 cells begun at day 1, with an increase in cell density noted on day 1.5 (Fig. 3B).
GPI-PLCp remained in AnTat 1.1 through differentiation, when VSG was lost and procyclin was expressed on the cell surface (Fig. 3A and Fig. 3B). Reduction of GPI-PLCp amounts in early-stage procyclic T. brucei occurred after 1.5 days. Significant loss (65%) of GPI-PLCp was detected between day 1.5 and day 2 in concert with a doubling of the parasite population (Fig. 3B).
Clearly, one cell division is not sufficient to eliminate all GPI-PLCp from procyclic T. brucei, so the events are not linked to the cell cycle control per se. Gradual depletion of GPI-PLCp accompanied further cell replication. At day 4, following seven cell divisions (i.e. 100-fold increase in cell density) a 125-fold decrease in GPI-PLCp was found (Fig. 3B). From day 4 to day 8, the parasites divided seven more times but only a two-fold drop in GPI-PLCp occurred, for a total 250-fold loss of enzyme activity (i.e., from day 1 to day 8) (Fig. 3B). From day 8 to day 10, another 4-fold loss of GPI-PLCp took place, with two more cell divisions, for a total decrease of 1000-fold in the amount of enzyme present in bloodstream cells at the beginning of transformation.
Two conclusions may be drawn from these data. First, not all GPI-PLCp is lost from T. brucei after differentiation to the early-stage procyclic form. Second, the 1000-fold decrease in amount of GPI-PLCp in established procyclic T. brucei (Bülow and Overath, 1985, Mensa-Wilmot, et al., 1990) requires over 10 cell divisions of newly-differentiated procyclic AnTat 1.1 trypanosome. Thus, multiple cell divisions are crucial for down-regulation of GPI-PLC protein during differentiation of T. brucei. Trypanosome strains that failed to replicate after differentiation (e.g. ILTat 1.3 and Lister 427) retained a high proportion, as compared to established procyclics, of GPI-PLCp (Fig. 1 and Fig. 2).
Translation Of GPI-PLC mRNA Is Halted During Differentiation Of T. brucei
Steady-state level of GPI-PLC polypeptide in T. brucei is a balance between stability of preexisting protein and production of new polypeptide in bloodstream or differentiating cells. A shift of the balance between protein production and stability during transformation could affect the steady-state amount of the enzyme in newly-differentiated procyclics. Therefore, we determined the half-life of GPI-PLC protein in bloodstream cells and investigated whether it changed at the onset of differentiation of bloodstream to procyclic T. brucei.
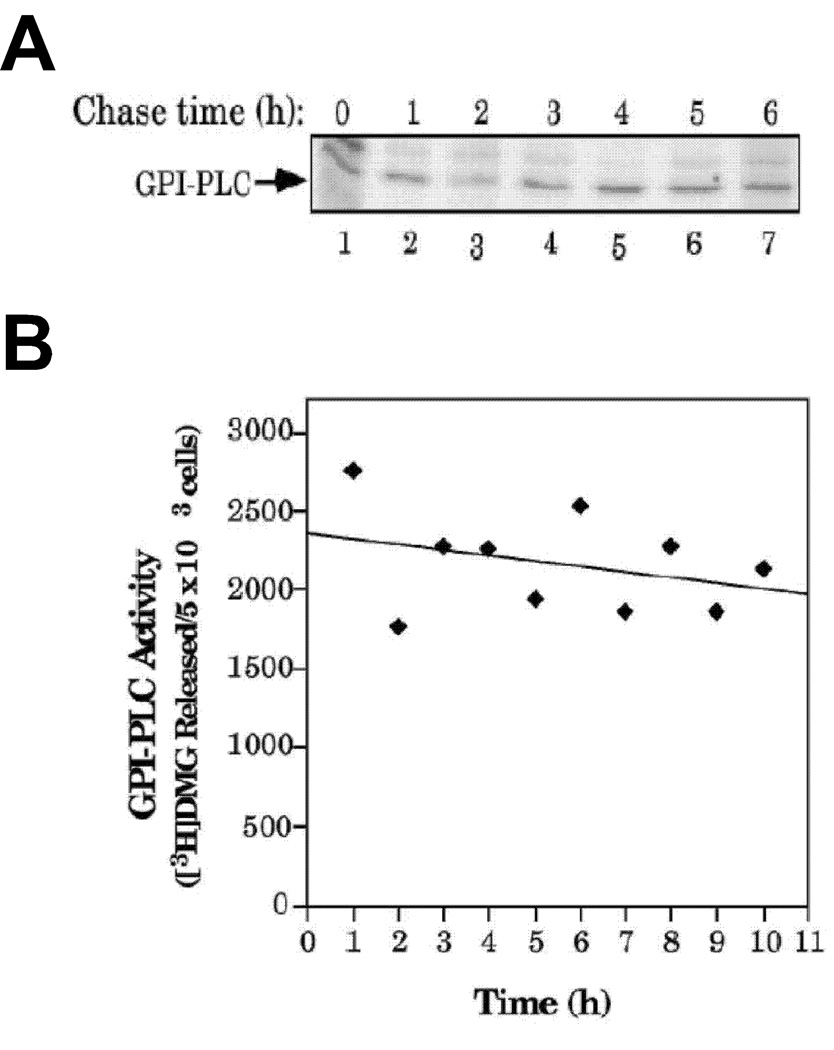
The half-life of [35S]Met-labeled (i.e. newly-synthesized) GPI-PLCp was greater than 6 h in bloodstream T. brucei (Fig. 4A), in a ‘pulse-chase’ study. A second method was used to determine the half-life of pre-existing GPI-PLCp in bloodstream T. brucei. Here, protein synthesis was inhibited with cycloheximide and the amount of GPI-PLCp remaining was quantitated using enzyme assays (Fig. 4B). Similar to results from the previous assay (Fig. 4A) the half-life of GPI-PLCp in bloodstream T. brucei was greater than 6 h (Fig. 4B). Together, these observations indicate that GPI-PLCp is not degraded significantly in bloodstream T. brucei because the cells divide every 8 h or so.
Figure 4. Half-life of GPI-PLCp in Blood stream T. brucei.
(A), T. brucei 427 was pulsed with [35S]Met at 37°C and chased in HM1-9 medium for 6 h. Parasites were harvested at 1 h intervals during the “chase”. GPI-PLCp (from 3.5 × 106 parasites) was immunoprecipitated and analyzed by 12% SDS-PAGE followed by phosphorimaging. (The upper band in Fig. 6A was due to non-specific adsorption to the antibody since it is not detectable when stringent wash conditions are employed after immunoprecipitation of GPI-PLCp (see Fig. 4A)). (B), T. brucei 427 bloodstream form was treated with 1.2 mM cycloheximide, and then incubated at 37°C for varying amounts of time. Parasites were lysed hypotonically and assayed for GPI-PLC activity.
We investigated the possibility that differentiating bloodstream cells synthesized new GPI-PLC protein. For this purpose, transformation was initiated, cells were retrieved at various times, pulsed with [35S]Met, and newly synthesized [35S]Met–labeled GPI-PLCp was quantitated (Fig. 5).
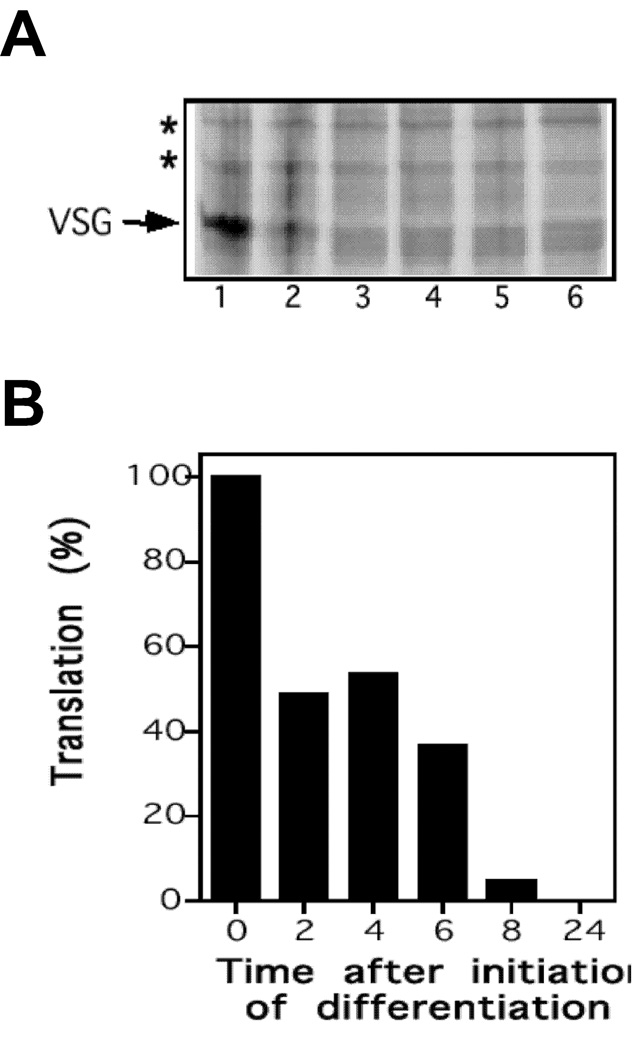
Figure 5. Synthesis of GPI-PLCp after initiation of Differentiation.
T. brucei 427 bloodstream form was placed in a SDM-79 differentiation medium at 27°C. At specified intervals, 1.4 × 108 cells were labeled metabolically with [35S]methionine at 27°C. All samples were analyzed by SDS-PAGE and phosphorimaging. (A), affinity-adsorbed GPI-PLCp. (B), membrane protein profile (2 × 107 per lane). (C), quantitation of data presented in panel A.
Translation of GPI-PLCp was arrested in differentiating cells. Two hours after initiation of transformation, synthesis of new GPI-PLCp decreased 50% (Fig. 5A and Fig. 5B), as compared to the degree of translation in bloodstream form (i.e. time = 0 min). By 8 h only 6% new GPI-PLCp synthesis occurred (Fig. 5A and 5B), and after 24 h translation of GPI-PLCp was not detectable (Fig. 5A). We confirmed with quantitative dot blot analysis that 70–80%, as compared to the amount found in bloodstream T. brucei, of GPI-PLC mRNA was detectable during the differentiation period (not presented). As control, synthesis of VSG117 during differentiation was monitored. A 70% decrease in VSG translation was observed 2 h post-initiation of differentiation, and new VSG synthesis was not detected beyond 4 h of incubation in differentiation medium (data not presented).
DISCUSSION
Multiple Events Contribute to Loss of GPI-PLCp in Established Procyclic T. brucei
In trypanosomatids, gene expression during differentiation is controlled by post-transcriptionally (reviewed in (Clayton, 2002, Haile and Papadopoulou, 2007)). These general principles regulation of GPI-PLCp during transformation of bloodstream to procyclic trypanosomes. Although GPI-PLCp is detected in bloodstream but not in established procyclic T. brucei (Carrington, et al., 1989, Mensa-Wilmot, et al., 1990), significant GPI-PLC mRNA is present in established procyclic cells (Carrington, et al., 1989, Mensa-Wilmot, et al., 1990) although initial transcripts are unstable (Webb, et al., 2005). Mechanisms used to limit accumulation of GPI-PLCp in established procyclics are not well understood.
Two processes, at least, contribute to developmental control of the steady-state level of GPI-PLC polypeptide in procyclic stage T. brucei. During differentiation, translation of GPI-PLC mRNA translation is inhibited (Fig. 5) as the cells lose VSG polypeptide (by proteolysis from the plasma membrane (Bülow, et al., 1989, Grandgenett, et al., 2007, Gruszynski, et al., 2006)) and produce procyclin protein (Fig. 1, Fig. 2 and Fig. 3) (Roditi, et al., 1989). Although fifty-to-sixty percent of GPI-PLCp is lost during transformation (Fig. 1, Fig. 2 and Fig. 3), a further 500-fold decrease of GPI-PLCp in early-stage procyclic cells is needed to account for the thousand-fold difference in enzyme level between bloodstream and established procyclic T. brucei (Bülow and Overath, 1985, Mensa-Wilmot, et al., 1990).
Loss of GPI-PLC protein from newly-differentiated (i.e. early-stage) procyclic T. brucei is not precipitous; it requires multiple cell divisions, the second major contributor to down-regulation of the enzyme in insect stage T. brucei. In the AnTat1.1 strain, each round of the first four cell divisions decreases GPI-PLCp by approximately 50% (Fig. 3). In the absence of new synthesis of GPI-PLCp (Fig. 4), a significant fraction of GPI-PLCp is lost apparently by the “dilution of cell constituents” that accompanies cell duplication events. In theory, nine cell divisions (a dilution factor of 29) are needed for the 1000-fold reduction of GPI-PLCp in established procyclics (as compared to blood stream T. brucei) (Bülow and Overath, 1985, Carrington, et al., 1989, Mensa-Wilmot, et al., 1990). Experimentally, it takes 17 cell divisions in AnTat1.1 to achieve this effect, partly because after the eighth division loss of GPI-PLCp is not directly (inversely) proportional to the number of replication events. We speculate that GPI-PLCp is lost from the procyclic cells through the action of an inactivator (e.g. protease) that has a short half-life and whose expression is linked to replication of the parasite.
Our observations with T. brucei transformation may have relevance for other models of differentiation in which transformation is accompanied by or followed with division of the differentiated cells in parasites and vertebrates (e.g., differentiation of T cell subclasses), especially during animal development. (Our cell differentiation model cannot apply to those transformation events, which produce cells that do not replicate (e.g., red blood cell maturation). In the former models of differentiation, it is highly likely that cell transformation is not finalized until residual marker proteins of pre-differentiated cells are eliminated either by proteolysis or serial dilution of cytoplasmic content.
Absence of GPI-PLCp Marks a “Late Stage” in Differentiation of Bloodstream Trypanosomes
In most studies of bloodstream T. brucei differentiation, molecular markers are monitored up to 72 h, since transformation to the procyclic form, depending on the strain, is “completed” in 72 h (Bass and Wang, 1991, Roditi, et al., 1989) (Fig. 1, Fig. 2, Fig. 3 and Fig. 4). GPI-PLCp is bloodstream-specific(Bülow and Overath, 1985, Mensa-Wilmot, et al., 1990), yet the protein persists in newly-differentiated procyclics (Fig. 1, Fig. 2, Fig. 3, and Fig. 4). To reconcile these facts we postulate an “early” and a “late stage”, at least, during differentiation of bloodstream to procyclic T. brucei.
“Early” events in differentiation of T. brucei bloodstream form to the procyclic stage (reviewed (Fenn and Matthews, 2007, Matthews, et al., 2004)) may include arrest of cell division, transient up-regulation of zinc finger proteins (TbZFP’s) (Hendriks, et al., 2001), proteolysis of VSG, synthesis of GPEET procyclin protein (Bülow, et al., 1989, Roditi, et al., 1989), appearance of glycolipid PP3 (Gruszynski, et al., 2006), and translational arrest of GPI-PLCp (Fig. 4). Not all bloodstream-specific markers are actively eliminated (e.g. proteolyzed) from newly-differentiated procyclic cells; those proteins may be lost in a “late stage” of transformation.
Cells may enter a “late stage” of differentiation if replication of the early-stage procyclic form T. brucei resumes. In support of this proposal, cells that are not capable of post-differentiation replication retain protein markers from the bloodstream stage (e.g. GPI-PLCp) although they are morphologically procyclic and express procyclin protein (Fig. 1 and Fig. 2). For proteins that are not totally degraded during differentiation (exemplified by GPI-PLCp), a minimum of 9 cell divisions may be necessary to reduce their steady-state level by a thousand-fold.
We propose that complete loss of GPI-PLCp from early-stage procyclic T. brucei signifies successful entry into a “late-stage” of bloodstream differentiation. Cells may complete the “early” stage of transformation (marked by loss of VSG and expression of procyclin) but fail to enter the “late” stage, in which case GPI-PLCp is retained in procyclic cells beyond the initial transformation phase when VSG is lost and procyclin is expressed on the cell surface, as observed during differentiation of strains ILTat 1.3 and Lister 427 in SDM-79 medium (Fig. 1 and Fig. 2).
Finally, we acknowledge the value of using the correct T. brucei strain and medium combination for investigating early events in differentiation of the parasite. In SDM-79 some strains of T. brucei (e.g. Lister 427 and ILTat1.3) cannot sustain cell division after transforming into the early stage procyclic (Mutomba and Wang, 1995) (Fig. 1 and Fig. 2). (We have made similar observations with SM medium (not presented)). The features of early-stage procyclics may be studied under such conditions because sufficient cell numbers will be available for molecular studies. In contrast, T. brucei AnTat 1.1 bypasses the replication-limiting conditions and replicates rapidly after differentiation in SM medium (Fig. 3). Similarly, T. brucei 427 bloodstream form may transform and replicate efficiently in DTM or modified HMI-9 medium (Li, et al., 2003, Muller, et al., 2002). The last set of cell and medium combinations are not recommended for studying early-stage procyclics.
Acknowledgments
The work was supported by National Institutes of Health grant # AI070141 to KM-W.
Abbreviations used are
- BSF
bloodstream form trypanosomes
- GPI
glycosylphosphatidylinositol
- GPI-PLC
GPI-specific phospholipase C
- GPI-PLCp
polypeptide encoded by GPI-PLC gene, PCF, procyclic (insect stage) T. brucei
- VSG
variant-specific surface glycoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cited References
- 1.Armah DA, Mensa-Wilmot K. S-Myristoylation of a GPI-Phospholipase C in Trypanosoma brucei. Journal of Biological Chemistry. 1999;274:5931–5939. doi: 10.1074/jbc.274.9.5931. [DOI] [PubMed] [Google Scholar]
- 2.Bass KE, Wang CC. The in vitro differentiation of pleomorphic Trypanosoma brucei from bloodstream into procyclic form requires neither intermediary nor short-stumpy stage. Molecular and Biochemical Parasitology. 1991;44:261–270. doi: 10.1016/0166-6851(91)90012-u. [DOI] [PubMed] [Google Scholar]
- 3.Bülow R, Griffiths G, Webster P, Stierhof Y-D, Opperdoes FR, Overath P. Intracellular localization of the glycosyl-phosphatidylinositol-specific phospholipase C of Trypanosoma brucei. Journal of Cell Science. 1989;93:233–240. doi: 10.1242/jcs.93.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Bülow R, Nonnengasser C, Overath P. Release of the variant surface glycoprotein during differentiation of bloodstream to procyclic forms of Trypanosoma brucei. Molecular and Biochemical Parasitology. 1989;32:85–92. doi: 10.1016/0166-6851(89)90132-1. [DOI] [PubMed] [Google Scholar]
- 5.Bülow R, Overath P. Synthesis of a hydrolase for the membrane-form variant surface glycoprotein is repressed during transformation of Trypanosoma brucei. Federation of European Biochemical Societies Letters. 1985;187:105–110. doi: 10.1016/0014-5793(85)81223-0. [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum LU, Milne KG, Werbovetz KA, Englund PT. Myristate exchange on the Trypanosoma brucei variant surface glycoprotein. Proceedings of the National Academy of Sciences (USA) 1996;93:1178–1183. doi: 10.1073/pnas.93.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrington M, Bülow R, Reinke H, Overath P. Sequence and expression of the glycosyl-phosphatidylinositol-specific phospholipase C of Trypanosoma brucei. Molecular and Biochemical Parasitology. 1989;33:289–296. doi: 10.1016/0166-6851(89)90091-1. [DOI] [PubMed] [Google Scholar]
- 8.Clayton CE. Life without transcriptional control? From fly to man and back again. European Molecuar Biology Organization Journal. 2002;21:1881–1888. doi: 10.1093/emboj/21.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clayton CE, Mowatt MR. The procyclic acidic repetitive proteins of Trypanosoma brucei. Journal of Biological Chemistry. 1989;264:15088–15093. [PubMed] [Google Scholar]
- 10.Cross GAM. Identification, purification, and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975;71:393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham I. New Culture Medium for Maintenance of Tsetse Tissues and Growth of Trypanosomatids. Journal of Protozoology. 1977;24(2):325–329. doi: 10.1111/j.1550-7408.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 12.Doering TL, Raper J, Buxbaum LU, Hart GW, Englund PT. Biosynthesis of glycosyl phosphatidylinositol protein anchors. Methods. 1990;1:288–296. [Google Scholar]
- 13.Fenn K, Matthews KR. The cell biology of Trypanosoma brucei differentiation. Current Opinion in Microbiology. 2007;10:539–546. doi: 10.1016/j.mib.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould CL, De Gee AL, Mansfield JM, Sonnenfeld G. Trypanosoma brucei rhodesiense infection in mice prevents virus-induced diabetes: possible role of interferon and immunological mechanisms. J Interferon Res. 1986;6:499–506. doi: 10.1089/jir.1986.6.499. [DOI] [PubMed] [Google Scholar]
- 15.Grandgenett PM, Otsu K, Wilson HR, Wilson ME, Donelson JE. A function for a specific zinc metalloprotease of African trypanosomes. Public Library of Science Pathogens. 2007;3:1432–1445. doi: 10.1371/journal.ppat.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruszynski AE, van Deursen FJ, Albareda MC, Best A, Chaudhary K, Cliffe LJ, del Rio L, Dunn JD, Ellis L, Evans KJ, Figueiredo JM, Malmquist NA, Omosun Y, Palenchar JB, Prickett S, Punkosdy GA, van Dooren G, Wang Q, Menon AK, Matthews KR, Bangs JD. Regulation of surface coat exchange by differentiating African trypanosomes. Mol Biochem Parasitol. 2006;147:211–223. doi: 10.1016/j.molbiopara.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Haile S, Papadopoulou B. Developmental regulation of gene expression in trypanosomatid parasitic protozoa. Current Opinion in Microbiology. 2007;10:569–577. doi: 10.1016/j.mib.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Hanrahan O, Webb H, O'Byrne R, Brabazon E, Treumann A, Sunter JD, Carrington M, Voorheis HP. The glycosylphosphatidylinositol-PLC in Trypanosoma brucei forms a linear array on the exterior of the flagellar membrane before and after activation. Public Library of Science Pathogens. 2009;5:e1000468. doi: 10.1371/journal.ppat.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriks EF, Robinson DR, Hinkins M, Matthews KR. A novel CCCH protein which modulates differentiation of Trypanosoma brucei to its procyclic form. European Molecuar Biology Organization Journal. 2001;20:6700–6711. doi: 10.1093/emboj/20.23.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hereld D, Krakow JL, Bangs JD, Hart GW, Englund PT. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. Journal of Biological Chemistry. 1986;261:13813–13819. [PubMed] [Google Scholar]
- 21.Hirumi H, Hirumi K. Axenic culture of African trypanosome bloodstream forms. Parasitology Today. 1994;10:80–84. doi: 10.1016/0169-4758(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 22.Hoek M, Xu H, Cross GA. Trypanosoma brucei: generation of specific antisera to recombinant variant surface glycoproteins. Experimental Parasitology. 1999;91:199–202. doi: 10.1006/expr.1998.4369. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Li Z, Wang CC. Differentiation of Trypanosoma brucei may be stage nonspecific and does not require progression of cell cycle. Molecular Microbiology. 2003;49:251–265. doi: 10.1046/j.1365-2958.2003.03575.x. [DOI] [PubMed] [Google Scholar]
- 24.Matthews KR, Ellis JR, Paterou A. Molecular regulation of the life cycle of African trypanosomes. Trends in Parasitology. 2004;20:40–47. doi: 10.1016/j.pt.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Mensa-Wilmot K, Hereld D, Englund PT. Genomic Organization, Chromosomal Localization, and Developmentally Regulated Expression of the Glycosyl-Phosphatidylinositol-Specific Phospholipase C of Trypanosoma brucei. Molecular and Cellular Biology. 1990;10:720–726. doi: 10.1128/mcb.10.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mensa-Wilmot K, LeBowitz JH, Chang KP, Al-Qahtani A, McGwire BS, Tucker S, Morris JC. A glycosylphosphatidylinositol (GPI)-negative phenotype produced in Leishmania major by GPI phospholipase C from Trypanosoma brucei: Topography of two GPI pathways. Journal of Cell Biology. 1994;124:935–947. doi: 10.1083/jcb.124.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mensa-Wilmot K, Morris JC, Al-Qahtani A, Englund PT. Purification and use of recombinant glycosylphosphatidylinositol phospholipase C. Methods in Enzymology. 1995;250:641–655. doi: 10.1016/0076-6879(95)50102-9. [DOI] [PubMed] [Google Scholar]
- 28.Muller IB, Domenicali-Pfister D, Roditi I, Vassella E. Stage-specific requirement of a mitogen-activated protein kinase by Trypanosoma brucei. Molecular Biology of the Cell. 2002;13:3787–3799. doi: 10.1091/mbc.E02-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mutomba MC, Wang CC. Differentiation of a culture-adapted mutant bloodstream form of Trypanosoma brucei into the procyclic form results in growth arrest of the cells. Molecular and Biochemical Parasitology. 1995;72:215–225. doi: 10.1016/0166-6851(95)00081-b. [DOI] [PubMed] [Google Scholar]
- 30.Roditi I, Schwarz H, Pearson TW, Beecroft RP, Liu MK, Richardson JP, Buhring HJ, Pleiss J, Bulow R, Williams RO. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. Journal of Cell Biology. 1989;108:737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolin S, Hanocq-Quertier J, Paturiaux-Hanocq F, Nolan DP, Pays E. Mild acid stress as a differentiation trigger in Trypanosoma brucei. Molecular and Biochemical Parasitology. 1998;93:251–262. doi: 10.1016/s0166-6851(98)00046-2. [DOI] [PubMed] [Google Scholar]
- 32.Subramanya S, Hardin CF, Steverding D, Mensa-Wilmot K. Glycosylphosphatidylinositol-specific phospholipase C regulates transferrin endocytosis in the African trypanosome. Biochemical Journal. 2009;417:685–694. doi: 10.1042/BJ20080167. [DOI] [PubMed] [Google Scholar]
- 33.Subramanya S, Mensa-Wilmot K. Regulated cleavage of intracellular glycosylphosphatidylinositol in a trypanosome. Peroxisome-to-endoplasmic reticulum translocation of a phospholipase C. Federation Of European Biochemical Societies Journal. 2006;273:2110–2126. doi: 10.1111/j.1742-4658.2006.05225.x. [DOI] [PubMed] [Google Scholar]
- 34.Tasker M, Wilson J, Sarkar M, Hendriks E, Matthews K. A novel selection regime for differentiation defects demonstrates an essential role for the stumpy form in the life cycle of the African trypanosome. Molecular Biology of the Cell. 2000;11:1905–1917. doi: 10.1091/mbc.11.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb H, Burns R, Ellis L, Kimblin N, Carrington M. Developmentally regulated instability of the GPI-PLC mRNA is dependent on a short-lived protein factor. Nucleic Acids Research. 2005;33:1503–1512. doi: 10.1093/nar/gki298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb H, Carnall N, Vanhamme L, Rolin S, Van Den Abbeele J, Welburn S, Pays E, Carrington M. The GPI-phospholipase C of Trypanosoma brucei is nonessential but influences parasitemia in mice. Journal of Cell Biology. 1997;139:103–114. doi: 10.1083/jcb.139.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegelbauer K, Quinten M, Schwarz H, Pearson TW, Overath P. Synchronous differentiation of Trypanosoma brucei from bloodstream to procyclic forms in vitro. European Journal of Biochemistry. 1990 Sep 11;192:373–378. doi: 10.1111/j.1432-1033.1990.tb19237.x. [DOI] [PubMed] [Google Scholar]