Abstract
Objective
To test for a diurnal pattern in hot flashes in a multi-ethnic population living in a hot, humid environment. To examine rates of concordance between objective and subjective measures of hot flashes using ambulatory and laboratory measures.
Methods
Study participants aged 45–55 were recruited from the general population of Hilo, Hawaii. Women wore a Biolog hot flash monitor, kept a diary for 24-hours, and also participated in 3-hour laboratory measures (n=199). Diurnal patterns were assessed using polynomial regression. For each woman, objectively recorded hot flashes that matched subjective experience were treated as true positive readings. Subjective hot flashes were considered the standard for computing false positive and false negative readings. True positive, false positive, and false negative readings were compared across ethnic groups by chi-square analyses.
Results
Frequencies of sternal, nuchal and subjective hot flashes peaked at 15:00 ± 1 hour with no difference by ethnicity. Laboratory results supported the pattern seen in ambulatory monitoring. Sternal and nuchal monitoring showed the same frequency of true positive measures, but non-sternal electrodes picked up more false positive readings. Laboratory monitoring showed very low frequencies of false negatives. There were no ethnic differences in the frequency of true positive or false positive measures. Women of European descent were more likely to report hot flashes that were not objectively demonstrated (false negative measures).
Conclusions
The diurnal pattern and peak in hot flash occurrence in the hot humid environment of Hilo was similar to results from more temperate environments. Lack of variation in sternal vs. non-sternal measures, and in true positive measures across ethnicities suggests no appreciable effect of population variation in sweating patterns.
Keywords: Hot flashes, menopause, diurnal pattern, ambulatory monitoring, Japanese-American
Introduction
Hot flashes at midlife are the result of a heat dissipation response [1,2] apparently triggered by a core, hypothalamic mechanism within the context of declining estrogen levels [3]. Hot flashes are experienced as sudden, generally unpleasant, sensations of heat by up to 75% of women before or during menopause [4].
In a laboratory setting, sternal skin conductance provides the best measure of hot flash occurrence [5]. The sweating associated with hot flashes is measured by two electrodes set four inches apart on the surface of the skin near the sternum. Skin conductance levels increase with sweating, and hot flashes are indicated by sharp increases in skin conductance (at least 2 µmho) over a short period of time (30 seconds). Ambulatory hot flash monitors can also measure sweating by sternal skin conductance during the course of everyday activity [6–9].
Twenty-four hour diaries [10] and a 24-hour laboratory study [11] showed fewer hot flashes during the hours of sleep, suggesting a correlation with internal temperature. A diary study of hot flashes recorded in two-hour intervals demonstrated two peaks in the frequency of hot flashes [12]. Some women experienced a peak in the morning (04:00–09:59), others in the evening (16:00–21:59). Freedman et al. [13] applied ambulatory monitors to 10 symptomatic women to test the hypothesis that hot flashes would be most frequent when core body temperature peaked. Seven out of the 10 women demonstrated a diurnal rhythm in hot flash incidence. Pooled data showed a significant diurnal rhythm in hot flash incidence with a peak at about 18:30. The peak in hot flashes lagged behind the peak in the diurnal phase of core temperature by about 3 hours.
Two subsequent studies with ambulatory hot flash monitoring found an earlier peak in hot flash frequency. In 42 peri- or post-menopausal women with daily hot flashes, Thurston et al. [9] showed that, compared with evening flashes (18:00–22:00), hot flashes were more likely in late morning/early afternoon (10:00–13:59, OR 1.39) and late afternoon/early evening (14:00–15:59, OR 1.50). In 21 postmenopausal breast cancer survivors, a diurnal rhythm in hot flashes was found with a peak in hot flash frequency at 16:10 ±2 h, although results for individual participants indicated 8 hours of variability in the timing of the peak [14]. A follow up study suggested that variability may be due to a disrupted diurnal pattern of core body temperature in breast cancer survivors [15].
Ambulatory monitoring allows for the tracking of daily patterns in hot flash occurrence; however, ambulatory hot flash measures demonstrate lower levels of concordance between objective and subjective hot flashes compared with laboratory-based hot flash monitors [6,8,15–18]. In a study of 55 breast cancer survivors, Carpenter et al. [15] reported that diaries and event markers underestimated hot flash frequency by 50% or more. In Sylhet, Bangladesh, objective measures of hot flashes were matched by subjective experience only 20% of the time [8].
The study presented here examines diurnal rhythm and compares concordance rates between objective and subjective hot flashes using both laboratory and ambulatory hot flash measures. Participants, aged 45–55, drawn from a multi-ethnic population in Hilo, HI, were primarily of European, Japanese, and Mixed descent. Among women of Mixed descent, 45% listed Hawaiian in their ethnic background.
Hilo, Hawaii, has a tropical rain forest climate characterized by warm, moist conditions. Mean maximum temperatures have little variation, from a high of 79.3 degrees F in March to a high of 83.7 degrees F in September (www.worldclimate.com). Hilo receives an average of 128” of rain per year and has an annual relative humidity of 86% in the morning and 68% in the afternoon (Hawaii State Climate Office). Acclimatization to these conditions could affect sweating patterns; for instance, sweat onset occurs more rapidly [19] and appears first on limbs as opposed to on the trunk [20] in heat acclimatized people. Also, hydromeiosis, or sweat gland fatigue, can occur after profuse sweating in a humid environment [21]. These changes may influence the measurement of hot flashes by skin conductance. Also, ethnic variation in sweating patterns may influence the effectiveness of measuring hot flashes on the sternum alone [1].
To account for the possibility that sweating patterns may vary across populations, hot flashes were measured in this multi-ethnic sample at both sternal and nuchal sites. Ambulatory hot flash monitors were worn for approximately 24-hours. In addition, laboratory measurements were carried out for three hours in the afternoon. The choice of timing was based on previous studies of diurnal rhythm in hot flashes [13,14].
The first aim of this study was to determine the peak frequency of ambulatory hot flashes during the day, in part to confirm that the afternoon was the best time to measure hot flashes in a laboratory setting in a hot, humid environment. We examined the diurnal patterns of objective sternal, objective nuchal, and subjective hot flashes. The second aim was to compare the rates of concordance between sternal and subjective measures and between nuchal and subjective measures in ambulatory and laboratory settings. Finally, we examined potential ethnic variation in diurnal patterns and rate of concordance.
Methods
Participant recruitment
The Hilo Women’s Health Study examined hot flashes, blood pressure, and measures of stress in a multi-ethnic population on the Big Island of Hawaii. Criteria for study eligibility included age 45–55 years, no use of blood pressure medications or exogenous hormones (hormone therapy or birth control), and no history of hysterectomy, oophorectomy, or diabetes. Selection was not made on the basis of hot flash symptoms.
The study had two phases: a postal questionnaire [22] and the ambulatory and laboratory measurement of hot flashes [23]. The cross-sectional postal survey was carried out in Hilo in 2004 and 2005. Questionnaires were sent to a random sample of households selected from parcel numbers obtained from a tax map of the district of Hilo. Of the 6401 survey packets delivered, 1824 questionnaires were completed and returned [22]. All participants in the first, cross-sectional phase of the study who met the age eligibility criteria were invited to participate in the second phase of the study. Of the 572 participants in the postal survey who met the age eligibility criteria, 84 participated in the second phase of ambulatory and laboratory hot flash monitoring.
The remaining participants in the second phase of study were recruited through word of mouth, through an advertisement placed in the local paper, the Tribune Herald, on ten different days from October 2006 through July 2007, and through telephone calls to local Japanese-American women1 who had participated in a previous study of blood pressure [24]. A final sample of 206 women was recruited; 200 women wore an ambulatory hot flash monitor, 203 participated in laboratory measures of hot flashes, and 199 did both between October 2005 and January 2008.
All women completed the Hilo Women’s Health Survey for demographic and biobehavioral information. The survey included a list of symptoms experienced during the two weeks before interview [22]. In addition, women were asked to check off ethnic groups included in their background and list a percentage for each (e.g., 50% Filipino, 25% Chinese, 25% European-American). For ethnic comparisons we selected women who described themselves to be 100% European-American (n=76), 100% Japanese-American (n=44), and self-described “Mixed” (n=63). Hot flash frequencies between European-American and Japanese-American women from this study have been previously reported [23].
Measurement of ambulatory hot flashes
Participants were asked to wear the Biolog hot flash monitor (UFI, Morro Bay, CA) for 24 hours. They came to the biological anthropology lab at UH-Hilo at their convenience2 to be outfitted with the portable monitor, and were instructed to wear two-piece comfortable clothing, to avoid strenuous activity, and to forego showering or bathing while wearing the monitors. Ag/AgCl hot flash monitor electrodes (1081-HFD, UFI, Morro Bay, CA) filled with 0.05 potassium choride Velvachol/glycol gel (Custom Med Apothecary, Indianapolis, IN), were placed about 4 inches apart on the upper chest between the second and third ribs to measure sternal skin conductance and about 4 inches apart on the back of the neck to measure nuchal skin conductance.3 Each set of electrodes conducted a 0.5 volt constant circuit and were attached to a two-channel Biolog monitor where skin conductance levels were recorded.
While wearing the ambulatory monitor, women were asked to record subjective hot flashes by pushing buttons on the monitor and recording in a diary the approximate start and end times of the hot flash, where it occurred on the body, and its intensity on a scale of 1 to 3 (1=mild, 3=severe). A researcher called each participant approximately midway through the 24 hour period to ask if the Biolog monitor was still displaying numbers, and if the electrodes were still attached to the body.
Measurement of hot flashes in the laboratory
In-lab measurements were carried out for three hours with a multi-channel physiological recorder (DataLab 2000, Lafayette Instruments). Women sat in a reclining chair in a small (10’ × 20’), quiet room that was not temperature controlled. Mean room temperature was 24.9 degrees C (s.d. 1.77) at the start, and rose to a mean of 25.5 degrees C (s.d 1.7) by the end of the 3-hour study. Humidity was 64% (s.d. 8.1). On average, women started the hot flash study at 13:03 (s.d. 1:06), ended at 15:59 (s.d. 1:04).
To monitor hot flashes, galvanic skin conductance amplifiers measured changes in level of sweating using a constant voltage between two sets of Ag/AgCl electrodes (1081-HFD, UFI, Morro Bay, CA) filled with 0.05 potassium choride Velvachol/glycol gel (Custom Med Apothecary, Indianapolis, IN). One set of electrodes was placed about 4 inches apart on the upper chest. Women were also asked, “Where do you feel hot flashes?” Depending on where women felt hot flashes, the second set of electrodes was placed on the back of the lower neck (85% of the time), on the face (9%), the upper neck (2%), and upper arm (1%). One individual had the second set of electrodes placed on a foot, one on the abdomen, and one on the lower back. If women did not give a specific site, then a second set of electrodes was placed about 4 inches apart on the back of the lower neck. After 30 minutes of baseline observation, a circulating water heating pad was placed on the lower abdomen and the participant was covered with a blanket. Participants were asked to not eat, drink, or use their cell phone during the study period.
A researcher sat in the same room to watch the computer monitor and ask the participant how she was feeling every 15 minutes and during objective hot flashes unless the participant was sleeping. Fifty-six percent of women fell asleep at some point during the measurement period. Women were instructed to notify the researcher of any subjective hot flash experience, including the location and duration of each hot flash. If the participant said she felt “warm,” she was asked if she would call it a hot flash at home. For each objective hot flash the researcher coded the participant’s subjective response as “no,” “yes” = subjective hot flash, “warm,” or “asleep.”
Scoring hot flashes
For ambulatory and laboratory studies, the criterion for an objective hot flash was a change in skin conductance level (SCL) of at least 2 µmho within 30 seconds [5,6]. For ambulatory studies, all objective hot flashes were coded by two individuals (AR and PM), with a third (LS) consulting to resolve ambiguities. The coding of objective hot flashes was made difficult by seemingly erratic changes in SCL, perhaps due to the highly humid conditions in Hilo. At times, the signal to the Biolog monitor shut down (SCL=0) from 1 to 10 seconds during a sweating episode. In addition to erratic downward spikes, there were quick upward spikes of 6 µmho or more, lasting just 2 seconds before returning to a low SCL. In an experiment, we observed extreme spikes in SCL when the monitor was held next to an activated cell phone. As a result of this observation, participants were told to keep cell phones on the opposite side of their body from the hot flash monitor.
For the ambulatory data, Biolog software was used to scan and identify objective hot flashes. A waiting period of 20 minutes was instituted between objective hot flash measurements to maximize the analysis of discrete events. With few exceptions (described below), if the rise in SCL was due to a recovery from a sudden, erratic fall in SCL, then the rise was not identified as a hot flash. Each objective hot flash was examined within a 10-minute, 1-minute, or 30-second window, to verify the validity of each hot flash [9]. The last 5 data points were initially averaged by the Biolog software to smooth the data.
Some of the hot flashes identified by the Biolog software would have been manually removed due to a downward spike except that they were concordant with subjective experience. Similarly, some hot flashes were identified by manually turning off the averaging of data points for the purpose of matching subjective experience. Nineteen percent of files included 1 or 2 hot flashes identified to maximize concordance. Eleven percent included 3 or more hot flashes identified to maximize concordance. Although some objective hot flashes were ambiguous and required discussion among investigators, all hot flashes used in this study met the criteria of a 2 µmho rise in SCL within 30 seconds.
For in-lab studies, every hot flash was coded by three investigators (AG, LM, and PM) using the DataLab 2000 software. A fourth investigator (LS) consulted to resolve ambiguities. There were very few ambiguities associated with the laboratory methods.
Statistics: Comparison of hot flash frequencies
Eighty-three percent of the in-lab studies were started by 12:00, and 93% had finished by the end of the 17:00 hour; therefore, the numbers of sternal, nuchal, and subjective in-lab hot flashes were compared with the numbers of sternal, nuchal, and subjective ambulatory hot flashes measured during the hours of 12:00 to 17:00. Mann-Whitney tests were used to compare the number of hot flashes.
The number of ambulatory hot flashes was also compared using two-way Mann-Whitney tests across ethnic groups (European, Japanese, and Mixed descent) and by hot flash report (yes/no) on the standardized questionnaires.
Diurnal rhythms: coding ambulatory data
The numbers of objective hot flashes experienced on the sternum and on the back of the neck, as well as the number of subjective hot flashes, were computed for each participant for each hour of the 24 hours monitored. If the Biolog was not worn for at least 20 minutes of an hour, that hour was counted as missing data. For sternal measures, the highest frequency of missing data was at 08:00, the lowest at 18:00. If the Biolog was worn, the number of hot flashes during that hour (0 to 3) was recorded for each woman.
Subjective experience was measured by either the monitor event marker or diary entry. Women made entries about posture and mood into a diary every 20 minutes as part of a study of blood pressure; therefore, if a woman did not make any entries and no event marker was pressed, we recorded the hour as missing data unless she indicated that she was asleep. For subjective hot flash measures, the highest frequency of missing data was at 08:00, the lowest at 19:00. If a woman was asleep, a “0” was recorded for that hour because she was not awakened by a hot flash.
Statistics: Diurnal rhythms
Ambulatory hot flash data were analyzed using polynomial regression to show the presence or absence of a quadratic in pooled responses for objective sternal, objective nuchal, and subjective hot flashes. Peaks in hot flash frequencies were compared across ethnic groups (European-American, Japanese-American, and mixed-ethnicity) after scaling the frequencies of hot flashes as percents of the daily totals within each group. The maximal incidence rate was estimated by taking a derivative of the quadratic curve.
The timing of hot flashes – laboratory data
The numbers of objective sternal and nuchal hot flashes, as well as the number of subjective hot flashes, were computed for each participant for each hour of the three hours monitored. Data were pooled and the number of hot flashes recorded was divided by the total number of women observed at each hour from 12:00 to 17:00. This was done to examine whether the peak frequency found by ambulatory methods was supported by the laboratory data.
Statistics: Concordance
For both ambulatory and laboratory data, we recorded the occurrence (yes/no) of a subjective hot flash within 20 minutes before or after each objective sternal hot flash and objective nuchal hot flash. In addition we recorded the occurrence of an objective hot flash (either sternal or nuchal) within 20 minutes before or after each subjective hot flash. We examined rates of true positives, false positives, and false negatives by treating each woman as an independent sampling unit and calculating the following: (1) objectively recorded hot flashes that matched subjective experience (the monitor gave a true positive reading),4 (2) objectively recorded hot flashes that were not subjectively experienced (the monitor gave a false positive reading), and (3) subjectively reported hot flashes that were not measured by a monitor (the monitor gave a false negative reading). Subjective hot flashes were treated as the standard. Scores between 0.0 and 1.0 indicated the percentage of true positives, false positives, or false negatives. True positive scores were transformed into an individual having had no true positive readings vs. at least one true positive reading. The same was done for false positive and false negative readings. The frequencies of having true positive, false positive, or false negative readings were compared across sternal vs. non-sternal (generally nuchal) sites, and across ethnic groups (Japanese, European, Mixed), by chi-square analyses.
Results
Sample Characteristics
Sample characteristics are shown in Table 1. Most participants self-identified as 100% European-American (38%), 100% Japanese (22%), or Mixed (32%) and were on average married (72%), financially OK or comfortable (74%), with some college education or a college degree (63%). About half grew up in Hawaii (52%), and 63% reported that they played sports or exercised. Mean BMI was slightly overweight (25.9 kg/m2).
Table 1.
Sample characteristics, Hilo, Hawaii (n=206).
| Variables | Mean (s.d.) or % |
|---|---|
| Mean age (s.d.) | 50.6 (3.0) |
| Self-described ethnicity | |
| European-American | 38% |
| Japanese | 22% |
| Mixed | 32% |
| Other (African-American, Chinese, Filipino, Hispanic, Korean, Pacific Islander) |
8% |
| Menopause status | |
| Pre-menopausal | 29% |
| Early menopausal transition | 9% |
| Late menopausal transition | 31% |
| Post-menopausal | 31% |
| Marital status | |
| Single | 10% |
| Married | 72% |
| Divorced/Separated | 17% |
| Widowed | 1% |
| Education | |
| High school or less | 13% |
| Any college and/or degree | 63% |
| Any post-graduate and/ or degree | 25% |
| Income | |
| <$30,000 | 28% |
| $30–79,999 | 48% |
| >=$80,000 | 25% |
| Financial comfort | |
| Struggling | 23% |
| OK | 42% |
| Comfortable | 32% |
| Well off | 3% |
| Mean parity (s.d.) | 2.2 (1.5) |
| Childhood | |
| In Hawaii | 52% |
| In US, not Hawaii | 39% |
| Not in US | 9% |
| Mean age at move to HI (s.d.) | 30.9 (13.3) n=111 |
| BMI (wt/ht2) (s.d.) | 25.9 (5.3) |
| Use of HT Never | 89% |
| In the past | 10% |
| Smokes | 11% |
| Plays sports or exercises | 63% |
Comparison of hot flash frequencies
Twenty-four hour ambulatory hot flash monitors detected a mean number of 3.7 sternal hot flashes (range 0 to 25, s.d. 5.0) and 5.7 nuchal hot flashes (range 0 to 30, s.d. 6.6) per woman. Twenty-three percent of participants demonstrated no objective hot flashes. Subjective hot flashes, recorded by diary or event marker, averaged 2.9 hot flashes per individual (range 0 to 21, s.d. 4.1); 44% reported no hot flashes.
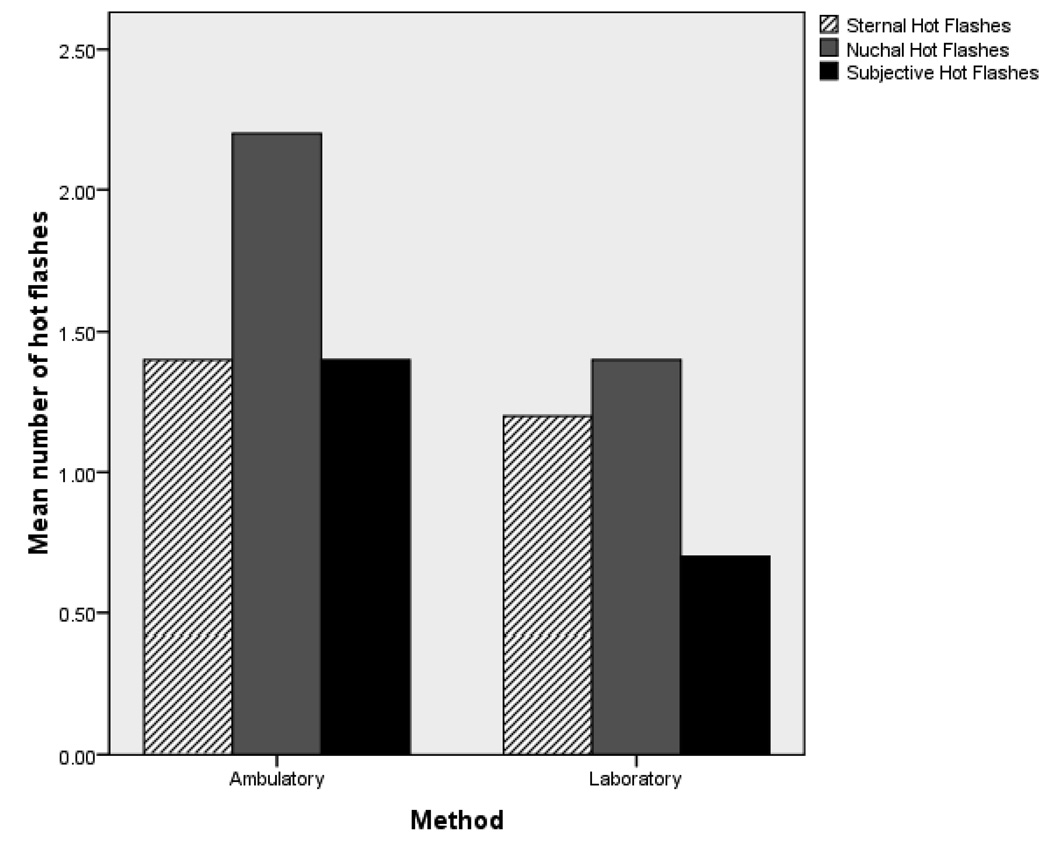
With ambulatory data limited to the hours of 12:00 to 17:00, there were no significant differences by Mann-Whitney tests between the number of objective sternal or objective non-sternal hot flashes recorded per participant by ambulatory (p=0.857) or laboratory (p=0.053) methods. In contrast, more subjective hot flashes were reported during ambulatory compared to laboratory studies (p=0.002). For illustrative purposes, Figure 1 shows the mean number of hot flashes experienced by each woman in laboratory and ambulatory measures.
Figure 1.
Comparison of sternal, nuchal, and subjective hot flash frequencies between ambulatory and laboratory methods of hot flash monitoring during the hours of 12:00 to 17:00.
Over half of the women fell asleep during the laboratory monitoring; however, there were no significant differences by Mann-Whitney tests in the number of sternal, nuchal, or subjective hot flashes between those who fell asleep (n=113) and those who did not (n=84). Among both sleepers and non-sleepers, 64% reported no hot flashes at all during the period of laboratory monitoring.
As expected, women who reported that they had experienced a hot flash during the two weeks prior to interview reported more subjective hot flashes (4.9, s.d. 4.5) during the 24-hour ambulatory study compared to women who had not been symptomatic (1.4, s.d. 3.0). The difference was significant using a Mann-Whitney test (p=0.000). The total number of objective hot flashes also differed. Women who reported no hot flash during the past two weeks (7.3, s.d. 10.2) had fewer objective hot flashes compared to women who reported a hot flash (10.1, s.d, 11.3; Mann-Whitney test, p=0.028).
There were no significant differences in objective or subjective hot flash frequencies during ambulatory studies in two-way comparisons among women of European, Japanese, and Mixed descent.
Diurnal pattern
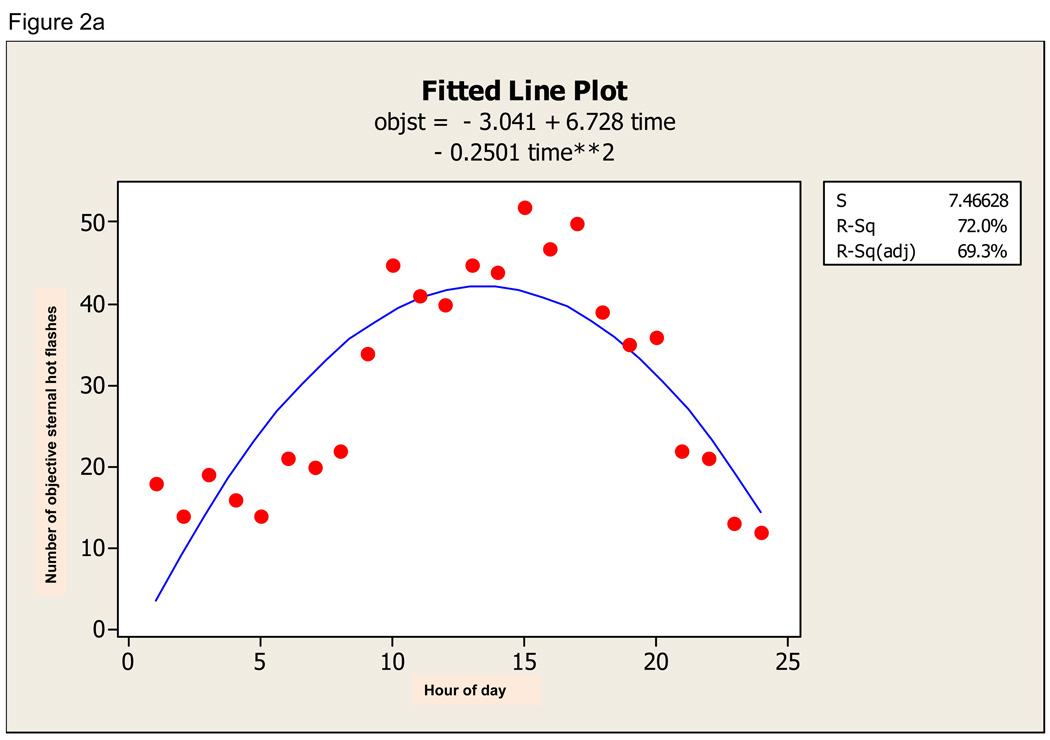
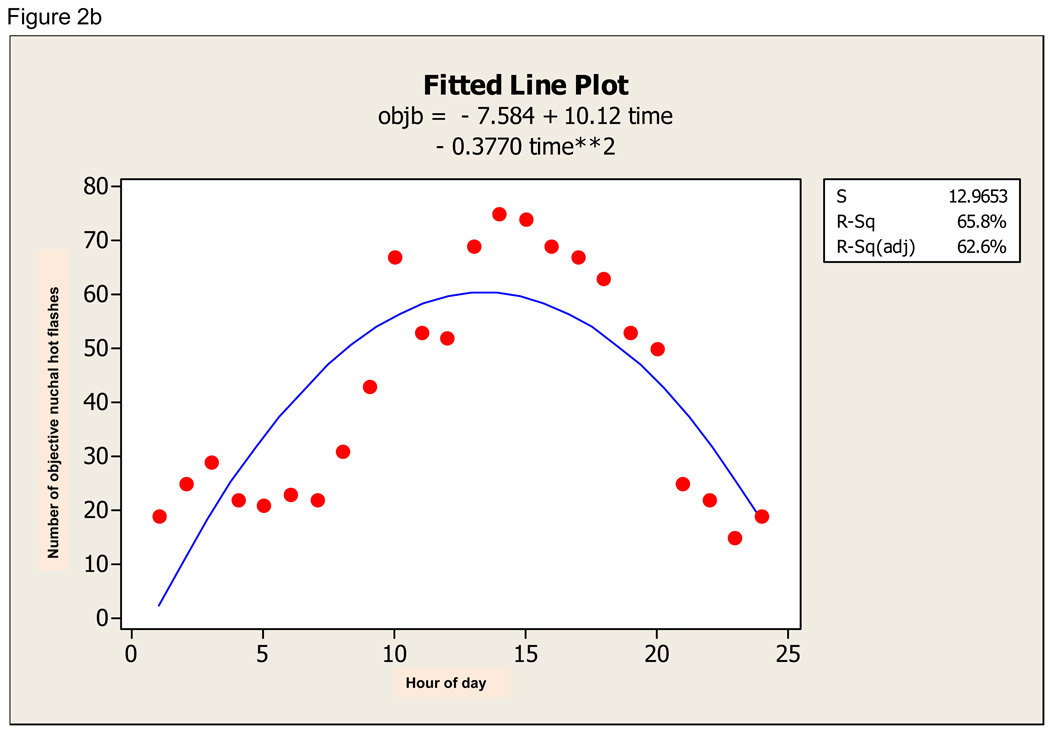
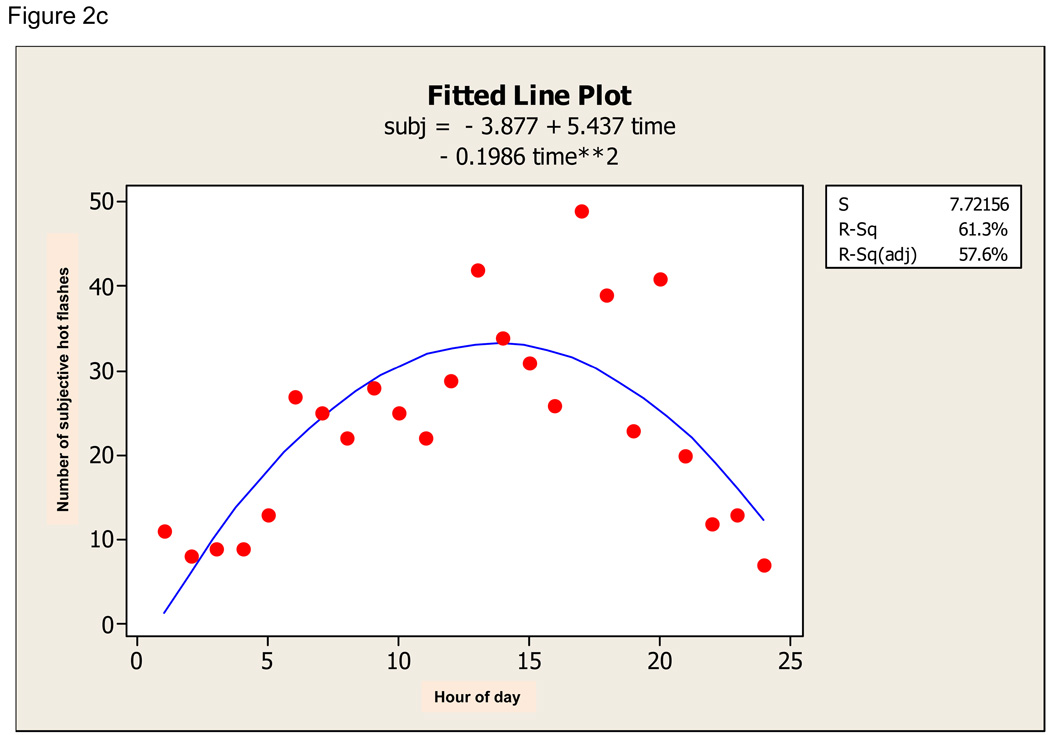
There was a definite diurnal pattern to the ambulatory data. For sternal hot flashes, the quadratic regression was significant (F 49.21, p=0.000) and time of day explained 69.3% of the variation in hot flash incidence (Figure 2a). For nuchal hot flashes, the quadratic regression was also significant (F 37.08, p=0.000) and time of day explained 62.6% of variation in hot flash incidence (Figure 2b). For subjective hot flashes, the quadratic regression was also significant (F 29.00, p=0.000) and time of day explained 57.6% of variation in hot flash incidence (Figure 2c). Furthermore, there was significant diurnal variation in concordance between sternal and subjective, nuchal and subjective, and sternal and nuchal hot flashes. All fitted curves demonstrate a peak at about 15:00 +/− 1 hour.
Figure 2.
a. Quadratic fit of objective sternal hot flash data (objst) across 24-hour period. b. Quadratic fit of objective nuchal hot flash data (objb) across 24-hour period. c. Quadratic fit to subjective hot flash data (subj) across 24-hour period.
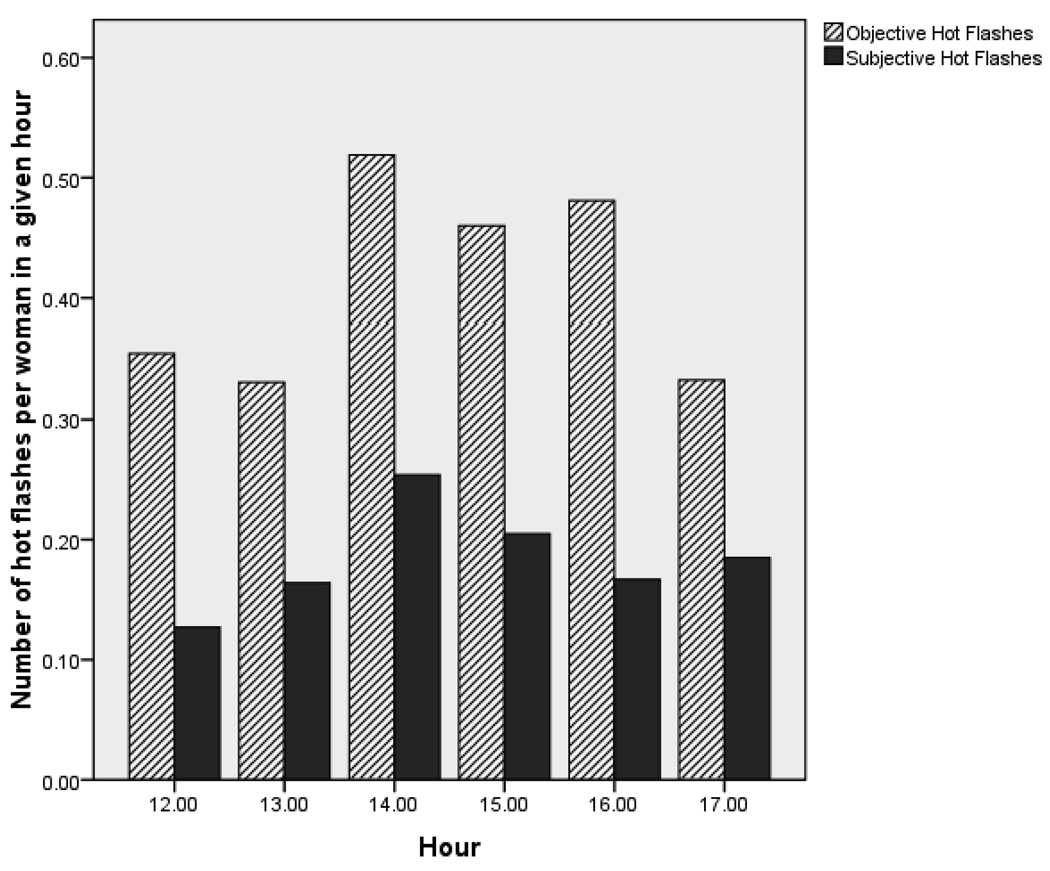
The pattern of hot flashes experienced during the laboratory session was consistent with the 24-hour ambulatory results. In laboratory studies, women wore the hot flash monitor for only three hours during the afternoon hours of 12:00 to 17:00. The highest frequencies in objective hot flashes were during the hours of 14:00, 15:00, and 16:00. Highest frequencies in subjective hot flash report were during the hours of 14:00 and 15:00 (Figure 3).
Figure 3.
Frequency of objective and subjective hot flashes per woman during each hour of laboratory study.
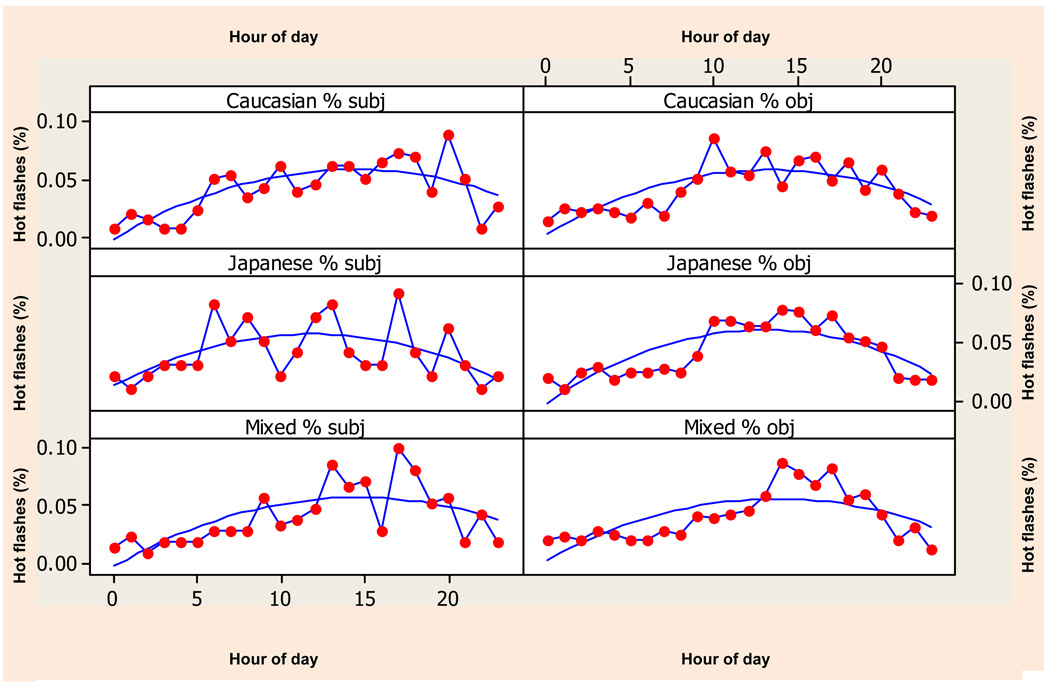
There were no ethnic differences in diurnal pattern of objective or subjective hot flashes (Figure 4). Japanese subjective hot flash data demonstrated the weakest fit to the quadratic curve (F 10.06, p=0.005). Among Japanese participants, only 27% of the variation in subjective report was explained by time, compared to 43% of the variation explained among women of both European and Mixed descent.
Figure 4.
Subjective (on left) and objective (on right) hot flashes recorded during 24-hour ambulatory monitoring by ethnic group with fitted quadratic. Hot flashes are scaled as percent of daily totals to show how the quadratic structure of each variable for each group is very similar.
Concordance
Table 2 shows the percentage of women who showed true positive, false positive, and false negative measures.
Table 2.
Percentages of true positive, false positive, and false negative results for ambulatory and laboratory monitoring.
| Ambulatory monitoring | Laboratory monitoring | |||||||
|---|---|---|---|---|---|---|---|---|
| N | True positives subj = obj /total obj |
False positives subj ≠ obj /total obj |
False negatives subj ≠ obj /total subj |
N | True positives subj = obj /total obj |
False positives subj ≠ obj /total obj |
False negatives subj ≠ obja /total subj |
|
| Sternal | 199 | 39% | 57% | 49% | 197 | 25% | 36% | 6% |
| Nuchal | 185 | 40% | 70% | 46% | 169 | 25% | 42% | |
| Comparisonsb | NS | P=0.008 | NS | NS | NS | |||
| Sternal measures |
12% 0% 5% P=0.031 |
|||||||
| Ethnicity | ||||||||
| European | 77 | 38% | 52% | 53% | 74 | 28% | 29% | |
| Japanese | 44 | 36% | 61% | 41% | 44 | 20% | 42% | |
| Mixed | 63 | 44% | 57% | 51% | 60 | 32% | 33% | |
| Comparisonsb | NS | NS | NS | NS | NS | |||
| Nuchal measures |
||||||||
| Ethnicity | ||||||||
| European | 73 | 40% | 70% | 52% | 67 | 19% | 31% | |
| Japanese | 38 | 40% | 68% | 42% | 35 | 23% | 49% | |
| Mixed | 60 | 40% | 72% | 45% | 53 | 36% | 42% | |
| Comparisonsb | NS | NS | NS | NS | NS | |||
Because of the low number of false negatives in laboratory measures, subjective hot flashes were examined in relation to either sternal or nuchal objective hot flashes
All comparisons were done with chi-square analysis.
In ambulatory monitoring, there was no significant difference in the frequency of true positive measures between nuchal (%) and sternal (%) measurements. In ambulatory monitoring, nuchal measurements recorded significantly more false positives (when women demonstrated a hot flash that they did not report) compared to sternal measures (70% vs. 57%%, p=0.008). Frequency of false positive readings did not significantly differ between sternal and nuchal measures in the laboratory. Laboratory monitoring showed a very low frequency of false negatives (when women felt a hot flash that was not demonstrated by the machine).
There were no ethnic differences in the frequency of true positives or false positives using either sternal or nuchal measures, in either ambulatory or laboratory settings. While the number of false negatives was generally low in the laboratory setting, European-American women were more likely to report hot flashes that they did not demonstrate objectively (12%) compared to women of Japanese and Mixed descent (0% and 5%, p=0.031).
Discussion
Number of hot flashes
The mean number of objective sternal hot flashes recorded by 24-hour ambulatory monitors in this study (3.7, s.d. 5.0) was lower than the mean number of waking hot flashes reported by Thurston et al. [9] (8.8, s.d. 5.6). The low mean in this study is due in part to the inclusion of women (23% of the sample) who demonstrated no sternal hot flashes. The range of objective sternal hot flashes (0 to 25) was very similar to the range reported by Freedman et al. [13] of 1 to 22 hot flashes per day, Carpenter et al. [14] of 1 to 30 hot flashes per day, and Thurston et al. [9] of 1 to 25 waking hot flashes per day. When both nuchal and sternal hot flashes were counted, a subgroup (4%) of women demonstrated 40 to 55 hot flashes per day. This finding may be unique to the hot, humid environment of Hilo, Hawaii.
During the afternoon hours of 12:00 to 17:00, the total number of objective sternal hot flashes did not differ by ambulatory or air conditioned laboratory methods of measurement, suggesting that ambulatory measures were not negatively affected by the hot, humid environment of Hilo. More non-sternal hot flashes were recorded by ambulatory measures. This finding allayed our initial concern that emotional sweating (e.g., on the face) could falsely increase the number of objective hot flashes among the participants who chose non-sternal and non-nuchal sites for the placement of the second electrodes.
During the hours of 12:00 to 17:00, women were more likely to report subjective hot flashes during the ambulatory study compared to the laboratory study. It has been hypothesized that low rates of concordance between subjective and objective hot flashes in ambulatory studies could be due to inaccuracies in filling out paper diaries [12,15,16]. In this study diaries were kept for reasons of blood pressure monitoring as well as hot flash monitoring, and this may have helped women to remember to note hot flashes or press the event markers. Still, it is of interest that fewer women reported subjective hot flashes while sitting in the laboratory in the company of a researcher. Although over half of the women fell asleep during the laboratory monitoring, there was no difference in the number of subjective hot flashes between those who fell asleep and those who did not. Perhaps women were more likely to interpreted feelings of warmth to be hot flashes during their daily activities rather than while sitting in a laboratory, focusing on their sensations.
Not surprisingly, women who said on a standardized questionnaire that they had experienced a hot flash during the two weeks before interview reported significantly more subjective hot flashes (4.9 hot flashes) compared to women who had not had a history of hot flashes (1.4, p=0.000). The difference in number of objective hot flashes between women who, on a questionnaire, said they had not experienced hot flashes (7.3) and women who had (10.1) was also significant (but at a higher p value, p=0.028). This finding somewhat contradicts the observations of Carpenter et al. [15] that survey results about hot flash experience do not accurately reflect objective hot flash experience.
The lack of difference in numbers of hot flashes among ethnic groups is consistent with the findings of Thurston et al. [9]. Further comparison of hot flash experience between women of European and Japanese descent from this study in Hilo is detailed elsewhere [23].
Diurnal patterns
One of the primary questions prompting this study was whether the diurnal pattern of hot flashes varied in the warm, humid climate of Hilo compared to the temperate climates of Detroit [13], Nashville [14], or North Carolina [9]. We identified a definite diurnal pattern with the ambulatory data for sternal objective, nuchal objective, and subjective hot flashes. For all three, the quadratic regression was significant and time of day explained more than 50% of the variation in hot flash incidence. There was also significant diurnal variation in concordance between objective and subjective measures. Fitted curves demonstrated a peak at about 15:00 +/− 1 hour, earlier than the peak identified in Detroit [13] and closer to the findings of Carpenter et al. [14] and Thurston et al. [9]. In addition, the abbreviated time period of laboratory study demonstrated a temporal pattern consistent with the diurnal rhythm suggested by the ambulatory data. There were no significant differences in diurnal pattern among women of European, Japanese, and Mixed descent.
Concordance
The low frequency of true positive measures was due, in part, to the high frequency of women who reported no hot flashes during the study period. The use of nuchal electrodes did not result in a higher frequency of true positive measures compared to the use of sternal electrodes in either ambulatory or laboratory studies. There appears to be no added advantage to the monitoring of objective hot flashes with nuchal measures. Nuchal electrodes recorded more false positive readings compared to sternal electrodes, suggesting that nuchal electrodes picked up more sweating episodes that met the hot flash criteria, but women did not consider these sweating episodes to be hot flashes.
There was no variation in the frequency of true positive measures across ethnicity. In the laboratory, the frequencies of sternal and nuchal true positive measures were almost the same for women of European (38% vs. 40%), Japanese (36% vs. 40%), and Mixed descent (44% vs. 40%), suggesting no appreciable effect of variation in sweating patterns.
Laboratory monitoring showed a very low frequency of false negatives (when women felt a hot flash that was not demonstrated by the machine; 6%). In the laboratory setting, European-American women were more likely to report hot flashes that they did not objectively demonstrate compared to women of Japanese and Mixed descent (12% vs. 0% and 5%, p<0.05). This difference may reflect a difference in interpreting and/or reporting sensations of warmth. The difference in frequency of false negative measures between European- and Japanese-American women was not significant with ambulatory sternal (53% vs. 41%) or nuchal (52% vs. 42%) measures, although the direction of the relationship was the same.
Conclusions
The first aim of this study was to determine the peak frequency of ambulatory hot flashes during the day. We examined the diurnal patterns of objective sternal, objective nuchal, and subjective hot flashes to show a peak at around 15:00 ±1 hour. There was no ethnic variation in diurnal pattern among women of European-, Japanese-, and Mixed descent.
The second aim was to examine the rates of concordance between objective and subjective measures of hot flashes in ambulatory and laboratory settings. We showed that the frequency of true positive measures was highly consistent between sternal/ subjective and nuchal/subjective measures. Nuchal monitoring resulted in more false positive measures (when women did not report a hot flash but a hot flash was recorded by the ambulatory monitor.) There were very few false negative measures (when women reported a hot flash that was not recorded by the monitor) by laboratory (6%) methods. There was no ethnic variation in frequency of true positive and false positive measures in ambulatory or laboratory settings. European-American women were more likely to report a hot flash that was not demonstrated than were women of Japanese or Mixed descent.
Acknowledgments
Supported by NIH grant No. S06-GM08073-32.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest/disclosures for any of the authors.
Seven Japanese-American women were recruited from this previous study.
Fifty-three percent of the sample started to wear the monitor between 07:00 and 09:59, 19% started between 10:00 and 12:59, 12% between 13:00 and 15:59, and 12% between 16:00 and 18:59. Six women started before 07:00 in the morning and only one woman started after 18:00 in the evening.
Fourteen women wore just sternal electrodes.
For example, one woman demonstrated sternal hot flashes at 9:15, 9:53, 10:20, 11:01, and 18:01 and subjective hot flashes at 10:01, 18:00, 18:54, and 20:45. The frequency of true positives was computed as 3/5 or 0.6 because 3 of her objective experiences were matched by a subjective experience (within 20 minutes). The sternal hot flashes at 9:53 and 10:20 were both concurrent with the subjective experience reported at 10:01. The frequency of false positives was computed as 2/5 or 0.4 (the hot flashes at 9:15 and 11:01 were demonstrated but not felt). The frequency of false negatives was computed as 2/4 or 0.5 (the hot flashes at 18:54 and 20:45 were subjectively felt but not objectively demonstrated.)
References
- 1.Sievert LL. Variation in sweating patterns: implications for studies of hot flashes through skin conductance. Menopause. 2007;14(4):742–751. doi: 10.1097/gme.0b013e3180577841. [DOI] [PubMed] [Google Scholar]
- 2.Sturdee DW. The menopausal hot flash – Anything new? Maturitas. 2008;60(1):42–49. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:117–125. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- 4.Gold E, Colvin A, Avis N, Bromberger J, Greendale G, Powell, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Am J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26(5):573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RR, Woodward S, Norton D. Laboratory and ambulatory monitoring of menopausal hot flushes: comparison of symptomatic and asymptomatic women. J Psychophysiol. 1992;6:162–166. [Google Scholar]
- 8.Sievert LL, Begum K, Sharmeen T, Chowdhury O, Muttukrishna S, Bentley G. Patterns of occurrence and concordance between subjective and objective hot flashes among Muslim and Hindu women in Sylhet, Bangladesh. Am J Human Biology. 2007;20:598–604. doi: 10.1002/ajhb.20785. [DOI] [PubMed] [Google Scholar]
- 9.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67(1):137–146. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 10.Kronenberg F, Downey JA. Thermoregulatory physiology of menopausal hot flashes: a review. Can J Physiol Pharmacol. 65:1312–1324. doi: 10.1139/y87-208. [DOI] [PubMed] [Google Scholar]
- 11.Kronenberg F. Menopausal hot flashes: randomness or rhythmicity. Chaos. 1991;1(3):271–278. doi: 10.1063/1.165840. [DOI] [PubMed] [Google Scholar]
- 12.Albright DL, Voda AM, Smolensky MH, His B, Decker M. Circadian rhythms in hot flashes in natural and surgically-induced menopause. Chronobiol Int. 1989;6:279–284. doi: 10.3109/07420528909056929. [DOI] [PubMed] [Google Scholar]
- 13.Freedman RR, Norton D, Woodward S, Cornélissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80(8):2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter JS, Gautam S, Freedman RR, Andrykowski M. Circadian rhythm of objectively recorded hot flashes in postmenopausal breast cancer survivors. Menopause. 2001;8(3):181–188. doi: 10.1097/00042192-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter JS, Monahan PO, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104:1322–1326. doi: 10.1097/01.AOG.0000143891.79482.ee. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter JS, Rand KL. Modeling the hot flash experience in breast cancer survivors. Menopause. 2008;15(3):469–475. doi: 10.1097/gme.0b013e3181591db7. [DOI] [PubMed] [Google Scholar]
- 17.Otte JL, Flockhart D, Hayes D, Storniolo AM, Stearns V, Schneider B, Henry NL, Azzouz F, Nguyen A, Lemler S, Hayden J, Jeter S, Wright L, Carpenter JS. Comparison of subjective and objective hot flash measures over time among breast cancer survivors initiating aromatase inhibitor therapy. Menopause. 2009;16(4):653–659. doi: 10.1097/gme.0b013e3181a5d0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurston RC, Matthews KA, Hernandez J, De La Torre F. Improving the performance of physiologic hot flash measures with support vector machines. Psychophysiology. 2009;46(2):285–292. doi: 10.1111/j.1469-8986.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna JM, Brown DE. Human heat tolerance: An anthropological perspective. Ann Rev Anthropol. 1983;12:259–284. [Google Scholar]
- 20.Hofler W. Changes in regional distribution of sweating during acclimatization to heat. J Appl Physiol. 1968;25:503–506. doi: 10.1152/jappl.1968.25.5.503. [DOI] [PubMed] [Google Scholar]
- 21.Kerslake D. The Stress of Hot Environments. London: Cambridge University Press; 1972. [PubMed] [Google Scholar]
- 22.Sievert LL, Morrison LA, Reza AM, Brown DE, Kalua E, Tefft HAT. Age-related differences in health complaints: The Hilo Women’s Health Study. Women and Health. 2007;45(3):31–51. doi: 10.1300/J013v45n03_03. [DOI] [PubMed] [Google Scholar]
- 23.Brown DE, Sievert LL, Morrison LA, Reza AM, Mills PS. Do Japanese American women really have fewer hot flashes than European Americans? The Hilo Women’s Health Study. Menopause. 2009;16 doi: 10.1097/gme.0b013e31819d88da. xxx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown DE, Sievert LL, Aki SL, Mills PS, Etrata MB, Paopao RN, James GD. Effects of age, ethnicity and menopause on ambulatory blood pressure: Japanese-American and Caucasian school teachers in Hawaii. Am J Hum Biol. 2001;13(4):486–493. doi: 10.1002/ajhb.1080. [DOI] [PubMed] [Google Scholar]