Abstract
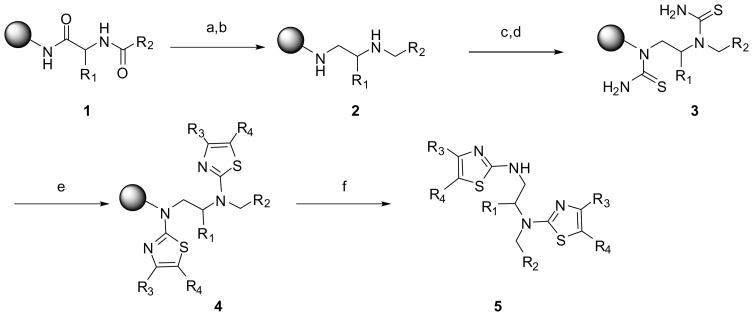
An efficient approach toward the parallel solid-phase synthesis of highly diversified chiral polyaminothiazoles employing Hantzsc’s thiazole synthesis is presented. The treatment of resin-bound chiral polyamines with Fmoc-isothiocyanates generated polythioureas which were further reacted with a variety of α-halogenoketones to afford following cleavage from the solid support the desired chiral polyaminothiazoles in good yield and purity.
The thiazole ring system is an important structural element found in numerous biologically active compounds.1 These have found applications in the development and preparation of drugs for the treatment of allergies,2 inflammation,3 schizophrenia,4 hypertension,5 as well as bacterial infections.6 Compounds containing the aminothiazole moiety are also known to be a ligand of estrogen receptors,7 adenosine receptor antagonists,8 while other analogues exhibit antitumoral properties.9 Moreover, thiazole derivatives are reported to be potential inhibitors of cyclin-dependent kinases (CDKs)10 and glycogen synthase kinase-3 (GSK-3).11 2-aminothiazoles were successfully employed as heterocyclic bioisosteres of the phenol moiety on dopamine agonists and the widely used antiparkinsonian agent pramipexole. These resulted in improved pharmacological properties including longer duration of action and improved bioavailability.12 Conjugated polyaminothiazole films were reported to display electrochemical properties with high thermal stability.13 Herein, we describe an efficient approach for the parallel synthesis of diversified oligoaminothiazoles. Staring from resin-bound peptides, a range of differing oligothiazoles were synthesized.
Thiourea is known to be a convenient starting material to prepare 2-amino-1,3-thiazoles.14, 15 Our approach using Hantzsc’s synthesis for the solid-phase synthesis of a variety of diaminothiazoles is outlined in Scheme 1. The parallel synthesis was performed starting from p-methylbenzhydrylamine (MBHA) resin bound acylated amino acid 1. Following reduction of the amide bonds in the presence of borane-THF,16 the corresponding resin-bound diamines 2 were treated with Fmoc-isothiocyanate to generate the corresponding di-thioureas 3. Following Fmoc deprotection, the resin-bound dithioureas were treated with a variety of α-halogenoketones to afford following cleavage of the solid-support, the desired di-aminothiazoles 5. The compounds were obtained in good yield (80 to 90%) and high purity (Table 1). The only byproduct observed was the mono-thiazole due to an incomplete reaction of the amine attached to the solid-support with Fmoc-isothiocyanate. We selected the following amino acids, alanine, proline, valine and phenylalanine, and three different halogenoketones, chloroacetone, 3-chloro-2-butanone and 2-chlorocyclohexanone. Similar results were obtained with all the amino acids utilized and we did not observe any detrimental effect of the amino acid side chains on the reaction. Some incomplete reaction was observed with the 2-Chlorocyclohexanone.
Scheme 1.
(a) BH3-THF, 65°C, 4 days; (b) piperidine, 65°C, overnight; (c) 6 equiv. Fmoc-NCS in DMF (0.3 M), RT, overnight; (d) 20% piperidine/DMF; (e) 20 equiv. α– halogenoketones in DMF (0.3 M), 70°C, overnight; (f) HF/anisole, 0°C, 90 min.
Table 1.
Individual products of dithiazolo derivatives
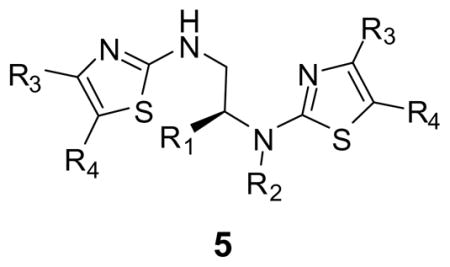
| Entry | R1 | R2 | R3 | R4 | MW obtaineda | Purity (%)b |
|---|---|---|---|---|---|---|
| 5a | —CH3 | —H | —CH3 | —H | 269.08 (MH+) | 88 |
| 5b | —CH3 | —H | —CH3 | —CH3 | 297.11 (MH+) | 84 |
| 5c | —CH3 | —H | —(CH2)4– | 349.19 (MH+) | 85 | |
| 5d | —CH2C6H5 | —H | —CH3 | —H | 345.13 (MH+) | 91 |
| 5e | —CH2C6H5 | —H | —CH3 | —CH3 | 373.15 (MH+) | 86 |
| 5f | —CH2C6H5 | —H | —(CH2)4– | 425.24 (MH+) | 82 | |
| 5g | —CH(CH3)2 | —H | —CH3 | —H | 297.10 (MH+) | 90 |
| 5h | —CH(CH3)2 | —H | —CH3 | —CH3 | 325.14 (MH+) | 88 |
| 5i | —CH(CH3)2 | —H | —(CH2)4– | 377.25 (MH+) | 85 | |
| 5j | —CH3 | —CH2CH3 | —CH3 | —H | 297.08 (MH+) | 92 |
| 5k | —CH3 | —CH2CH3 | —CH3 | —CH3 | 325.11 (MH+) | 87 |
| 5l | —CH3 | —CH2CH2C6H5 | —CH3 | —CH3 | 401.17 (MH+) | 89 |
| 5m | —CH3 | —CH2CH2C6H5 | —(CH2)4– | 453.17 (MH+) | 84 | |
| 5n | —CH2C6H5 | —CH2CH3 | —CH3 | —H | 373.17 (MH+) | 90 |
| 5o | —CH2C6H5 | —CH2CH3 | —CH3 | —CH3 | 401.13 (MH+) | 92 |
| 5p | —CH2C6H5 | —CH2CH2C6H5 | —CH3 | —CH3 | 477.20 (MH+) | 88 |
| 5q | —CH2C6H5 | —CH2CH2C6H5 | —(CH2)4– | 529.30 (MH+) | 84 | |
| 5r | —CH(CH3)2 | —CH2CH 3 | —CH3 | —H | 325.13 (MH+) | 93 |
| 5s | —CH(CH3)2 | —CH2CH3 | —CH3 | —CH3 | 353.15 (MH+) | 85 |
| 5t | —CH(CH3)2 | —CH2CH2C6H5 | —CH3 | —CH3 | 429.21 (MH+) | 89 |
| 5u | —CH(CH3)2 | —CH2CH2C6H5 | —(CH2)4– | 481.28 (MH+) | 87 | |
| 5v | —CH2)3c— | —CH3 | —CH3 | 322.13 (MH+) | 94 | |
Determined by ESI-MS.
The products were run on a Vydac column, gradients 5 to 95% formic acid in ACN in 7 min. The purity was estimated on analytical traces at λ = 214 nm and 254 nm.
Derived from proline.
The same approach was employed for the synthesis of different lengths of chiral polyaminothiazoles from their corresponding resin-bound chiral polyamines.17 Table 2 shows examples of tetrathiazoles obtained from resin-bound tripeptides. All compounds were analyzed by LC-MS and selected ones by 1H-NMR and 13C-NMR.
Table 2.
Individual products of tetrathiazole derivatives
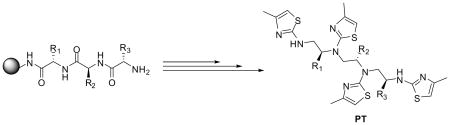
| Entry | R1 | R2 | R3 | MW obtaineda | Purity (%)b |
|---|---|---|---|---|---|
| PT-1 | —CH2C6H5 | —CH2C6H5 | —CH2CH(CH3)2 | 771 (MH+) | 83 |
| PT-2 | —CH2C6H5 | —CH2C6H4OH | —CH2CH(CH3)2 | 787 (MH+) | 85 |
| PT-3 | —CH2C6H5 | —CH2CH(CH3)2 | —(CH2C6H5 | 771 (MH+) | 88 |
| PT-4 | —CH2C6H5 | —CH2CH(CH3)2 | —CH2CH(CH3)2 | 737 (MH+) | 85 |
| PT-5 | —CH2C6H4OH | —CH2C6H5 | —CH2C6H5 | 821 (MH+) | 88 |
| PT-6 | —CH2C6H4OH | —CH2C6H5 | —CH2CH(CH3)2 | 787 (MH+) | 85 |
| PT-7 | —CH2C6H4OH | —CH2CH(CH3)2 | —(CH2CH(CH3)2 | 752 (MH+) | 82 |
Determined by ESI-MS.
The products were run on a Vydac column, gradients 5 to 95% formic acid in ACN in 7 min. The purity was estimated on analytical traces at λ = 214 nm and 254 nm.
Due to the well-understood chemistry, the availability of a wide diversity of chiral amino acids and the excellent synthetic purity and yields obtained during the solid- phase synthesis of peptides, the work presented offers a unique approach toward the synthesis of chiral polyaminothiazoles using resin-bound amino acids, peptides, and peptidomimetics as starting materials.
Supplementary Material
Acknowledgments
The authors would like to thank the State of Florida Funding, NIH (1R03DA025850 -01A1, Nefzi), NIH (5P41GM081261-03, Houghten) and NIH (3P41GM079590-03S1, Houghten) for their financial support.
Footnotes
Supporting Information Available: Structures of all the compounds. LC-MS, ES and 1H-NMR of some dithiazoles. LC-MS of all the tetrathiazoles. This information is available free of charge via the Internet at http://pubs.acs.org/.
References and Notes
- 1.Lewis JR. Nat Prod Rep. 1999;16:389–416. [Google Scholar]
- 2.Hargrave KD, Hess FK, Oliver JT. J Med Chem. 1983;26:1158–1163. doi: 10.1021/jm00362a014. [DOI] [PubMed] [Google Scholar]
- 3.(a) Haviv F, Ratajczyk JD, DeNet RW, Kerdesky FA, Walters RL, Schmidt SP, Holms JH, Young PR, Carter GW. J Med Chem. 1988;31:1719–1728. doi: 10.1021/jm00117a010. [DOI] [PubMed] [Google Scholar]; (b) Clemence F, Marter OL, Delevalle F, Benzoni J, Jouanen A, Jouquey S, Mouren M, Deraedt R. J Med Chem. 1988;31:1453–1462. doi: 10.1021/jm00402a034. [DOI] [PubMed] [Google Scholar]
- 4.Jaen JC, Wise LD, Caprathe BW, Tecle H, Bergmeier S, Humblet CC, Heffner TG, Meltzner LT, Pugsley TA. J Med Chem. 1990;33:311–317. doi: 10.1021/jm00163a051. [DOI] [PubMed] [Google Scholar]
- 5.Patt WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG, Jr, Connolly CJC, Doharty AM, Klutchko SR, Sircar I, Steinbaugh BA, Bately BL, Painchand CA, Rapundalo ST, Michniewicz BM, Olzon SCJ. J Med Chem. 1992;35:2562–2572. doi: 10.1021/jm00092a006. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji K, Ishikawa H. Bioorg Med Chem Lett. 1994;4:1601–1606. [Google Scholar]
- 7.Fink BA, Mortensen DS, Stauffer SR, Aron ZD, Katzenellenbogen JA. Chem Biol. 1999;6:205–219. doi: 10.1016/S1074-5521(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 8.Van Muijlwijk-Koezen JE, Timmerman H, Vollinga RC, Von Drabbe Kunzel JF, De Groote M, Visser S, Ijzerman AP. J Med Chem. 2001;44:749–762. doi: 10.1021/jm0003945. [DOI] [PubMed] [Google Scholar]
- 9.Komar Y, Green R, Wise D, Worting LL. J Med Chem. 1988;23:501. [Google Scholar]
- 10.Rosania GR, Chang YT. Expert Opin Ther Patents. 2000;10:215–230. [Google Scholar]
- 11.Dorronsoro I, Castro A, Martinez A. Expert Opin Ther Patents. 2002;12:1527–1536. [Google Scholar]
- 12.Zhang A, Xiong W, Hilbert JE, DeVita EK, Bidlack JM, Neumeyer JL. J Med Chem. 2004;47:1886–1888. doi: 10.1021/jm049978n. [DOI] [PubMed] [Google Scholar]
- 13.Solmaz Ramazan, Kardas Gulfeza. Progress in Organic Coatings. 2009;64(1):81–88. [Google Scholar]
- 14.Garcia-Egido E, Wong SYF, Warrington BH. Lab Chip. 2002;2:31–33. doi: 10.1039/b109360f. [DOI] [PubMed] [Google Scholar]
- 15.Lin PY, Hou RS, Wang HM, Kang IJ, Chen LC. Journal of the Chinese Chemical Society. 2009;56:455–458. [Google Scholar]
- 16.Nefzi A, Ostresh JM, Houghten RA. Parallel solid phase synthesis of tetrasubstituted diethylenetriamines via selective amide alkylation and exhaustive reduction of N-acylated dipeptides. Tetrahedron. 1999;55:335–344. [Google Scholar]
- 17.General procedure for the synthesis of dithiazolo derivatives: 100 mg of MBHA resin (loading: 1.1 mmol/g) was sealed within a polypropylene mesh packet.18 Reactions were carried out in polypropylene bottles. A solution of N-Boc-amino acid (6 equiv, 0.1 M in DMF), HOBt (6 equiv, 0.1 M in DMF), and DIC (6 equiv, 0.1 M in DMF) was added to the reaction vessel. The reaction mixture was shaken at room temperature for 2 h, followed by washing with DMF (2 times) and DCM (2 times). Upon removal of the Boc group with 55% TFA in DCM for 30 min, the resin was washed and neutralized with 5% DIEA in DCM. The resin-bound amine was reacted with carboxylic acid (10 equiv, 0.3 M in DMF), and DIC (10 equiv, 0.3 M in DMF) overnight, followed by washing with DMF (2 times) and DCM (2 times). Air dried resin-bound acylated peptide was reduced using BH3-THF. Typical reaction conditions for the solid-phase reduction of polyamides consist of the treatment of resin-bound peptides with BH3-THF at 65°C for 72 hours. The generated resin-bound borane-amine complexes are then disproportionate following overnight treatment with neat piperidine at 65°C. The reduction is free of racemization. The generated amines were treated with Fmoc-isothiocyanate (6 equiv, 0.3 M in DMF) at room temperature overnight. The Fmoc group was removed with 20% piperidine in DMF (2 times × 10 minutes) followed by the addition of α-halogenoketones (20 equiv, 0.3 M in DMF). The reaction with α–halogenoketones was carried out at 70°C overnight. The cleavage of the product was carried out by the treatment with 100% anhydrous HF at 0°C for 1.5 h, followed by nitrogen gas flow to remove the HF. The product was extracted by 95% acetic acid. After lyophilization, the products were characterized by electrospray LC-MS under ESI conditions and selected compounds by 1H. 5e): 1H-NMR (500 MHz, DMSO-d6): δ (ppm) 7.17–7.34 (m, 5H), 3.95 (m, 1H), 3.25 (m, 2H), 3.40 (m, 2H) 2.87 (dd, J = 5.6 Hz, J = 13.8 Hz, 1H), 2.78 (dd, J = 7.7 Hz, J= 1.4 Hz, 1H), 2.09 (s, 3H), 2.07 (s, 3H), 2.00 (s, 3H), 1.98 (s, 3H). MS (ESI): calcd [MH+] 373.14, found 373.3. (5f): 1H-NMR (500 MHz, DMSO-d6): δ(ppm) 7.16–7.36 (m, 5H), 6.5 (s, 1H), 3.93 (m, 1H), 3.21 (m, 1H), 2.98 (m, 1H), 2.88 (dd, J=5.6 z, J= 14.0 Hz, 1H), 2.78 (dd, J= 7.4 Hz, J= 13.7 Hz, 1H), 2.46 (m, 4H), 2.38 (m, 4H), 1.7 (m, 8H). MS (ESI): calcd [MH+] 425.2, found 425.6. (5p): 1H-NMR (500 MHz, DMSO-d6): δ (ppm) 8.18 (s, 1H), 7.15–7.30 (m, 10 H), 4.18 (m, 1H), 3.62 (m, 2H), 3.31 (m, 2H), 3.10 (m, 1H), 2.90 (dd, J= 6. Hz, J= 13.8 Hz, 1H), 2.75 (m, 1H), 2.63 (m, 1H), 2.49 (s, 3H), 2.07 (s, 3H), 1.97 (s, 3H). MS (ESI): calcd [MH+] 477.2, found 477.7.
- 18.Houghten RA. Proc Natl Acad Sci U S A. 1985;82:5131. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.