Abstract
Bnip3 is a member of the BH3-only subfamily of pro-apoptotic Bcl-2 proteins and is associated with loss of cardiac myocytes after a myocardial infarction. Previous studies have demonstrated that Bnip3 induces mitochondrial dysfunction, but the mechanisms involved in this process remain unknown. In this study, we demonstrate that Bnip3 induces permeabilization of the mitochondria via a novel mechanism that is different from other BH3-only proteins. We found that Bnip3 induced mitochondrial swelling and cytochrome c release in isolated heart mitochondria in vitro. Another BH3-only protein, tBid, also caused release of cytochrome c but failed to induce swelling of mitochondria. Swelling of mitochondria is a characteristic of mitochondrial permeability transition pore (mPTP) opening, but Bnip3-mediated mitochondrial swelling was insensitive to cyclosporine A, an inhibitor of the mPTP and independent of cyclophilin D (cypD), an essential component of the mPTP. Bnip3 also induced permeabilization of the mitochondrial membranes as evident by calcein release from the matrix in both wild type (WT) and cypD deficient mouse embryonic fibroblasts (MEFs). Moreover, Bnip3 induced mitochondrial matrix remodeling and large amplitude swelling of the inner membrane, which led to disassembly of OPA1 complexes and release from the mitochondria. Thus, these studies suggest that Bnip3 mediates mitochondrial permeabilization by a novel mechanism that is different from other BH3-only proteins.
Keywords: Bnip3, Bcl-2, BH3-only proteins, OPA1, mitochondria, cardiac myocytes, apoptosis, necrosis, mitochondrial permeability transition pore, cyclophilin D
Introduction
Mitochondria play critical roles in both the life and death of cells. They are important generators of energy by providing ATP through oxidative phosphorylation, and they are critical regulators of cell death by releasing pro-apoptotic proteins such as cytochrome c, Smac/DIABLO and Omi/HtrA2 [1]. The Bcl-2 family proteins regulate apoptosis mediated by the mitochondria. They monitor changes in the intracellular environment such as the presence of reactive oxygen species (ROS), absence of growth factors and DNA damage and induce permeabilization of the mitochondrial membrane when the conditions are unfavorable for the cell [2]. The major anti-apoptotic family members are Bcl-2 and Bcl-XL, which exert their effect at the mitochondrial outer membrane where they help maintain membrane integrity. In contrast, the two major pro-apoptotic family members Bax and Bak exert their effects by compromising the membrane integrity leading to release of pro-apoptotic proteins such as cytochrome c into the cytosol, resulting in caspase activation and demise of the cell. In addition, the Bcl-2 family includes a large number of so-called pro-apoptotic BH3-only proteins, which include Bid, Bad, Bik, Bim, Noxa, and Puma. These proteins function as stress sensors in the cell and play a major role in transducing signals from the cytosol to the mitochondria where they initiate cell death through the activation of Bax and Bak [2].
Bcl-2 nineteen-kilodalton interacting protein (Bnip3) is a BH3-only protein and has been shown to contribute to cell death in myocardial ischemia/reperfusion (I/R)-injury [3-5] and postinfarct remodeling [6]. Bnip3 is primarily localized in the outer mitochondrial membrane where it induces loss of mitochondrial membrane potential and cell death. However, the exact mechanism by which Bnip3 perturbs mitochondrial function is unclear and somewhat controversial. In contrast to other BH3-only proteins, Bnip3 can induce a form of cell death that shows features of both necrosis and apoptosis [4, 7-10]. For instance, Bnip3 has been reported to induce nucleosomal DNA fragmentation while retaining ATP levels and plasma membrane integrity in neonatal cardiac myocytes, implying apoptotic cell death [7, 10]. In contrast, another study observed that Bnip3 induced vacuolation of the cytoplasm and rapid loss of plasma membrane integrity with minimal nuclear damage in 293T cells, which are features typical of necrotic cell death [9].
Permeabilization of the mitochondrial outer membrane is the commitment step in both apoptosis and necrosis. Two mechanisms, involving opening of two different mitochondrial channels, have been proposed to be responsible for the permeabilization; the Bax/Bak channel and the mitochondrial permeability transition pore (mPTP). In response to a death signal, Bax and Bax undergo conformational changes to form homo- and heterooligomers resulting in pores in the mitochondrial outer membrane large enough to accommodate passage of proteins of the intermembrane space, such as cytochrome c, to the cytosol [1, 11]. In contrast, the mPTP is a non-specific pore in the inner mitochondrial membrane that is permeable to molecules less than 1.5 kDa [12]. Opening of this pore results in massive swelling of the inner membrane and subsequent rupture of the outer mitochondrial membrane, leading to release of all intermembrane space proteins into the cytosol [12, 13]. This channel is thought to mainly play a role in necrosis, whereas the Bax/Bak pore is involved in apoptotic cell death. Interestingly, Bnip3 has been reported to mediate mitochondrial dysfunction via opening of the mPTP [7, 9, 10] and via activation of Bax/Bak [5].
Given the uncertainty concerning the mode of action of Bnip3, we have further investigated the mechanism by which Bnip3 mediates mitochondrial dysfunction. In this study, we report that Bnip3-mediated mitochondrial membrane permeabilization and cell death occurs via a mechanism that is independent of the mPTP. Instead, Bnip3 induces swelling of the inner mitochondrial membrane which is dependent on the presence of Bax/Bak.
Materials and Methods
Animals
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of San Diego State University. Male Sprague-Dawley rats (225-250g) acquired from Harlan were used in this study. CypD−/− mice, which are homozygous for a targeted deletion of the Ppif gene were generated as described previously [14].
Preparation of recombinant proteins
Recombinant Bnip3 was purified as previously described [4]. Briefly, E.coli BL21(DE3) cells transformed with Bnip3 were grown in LB + AMP and expression of Bnip3 was induced with 1 mM IPTG for 4 h. The bacteria were resuspended in Native buffer (150 mM NaCl, 1% Tween-20, 50 mM NaH2PO4, pH 8.0, and complete protease inhibitors (Roche)), followed by sonication on ice. After centrifugation at 20,000 × g for 20 min, the supernatants were added to columns containing Ni-NTA (Qiagen, Inc). The proteins were eluted with 250 mM imidazole in Native buffer, followed by de-salting on PD-10 columns (Amersham-Pharmacia).
Mitochondrial swelling assay and assessment of cytochrome c release
The isolation of mitochondria was done using a protocol routinely used in our laboratory [15]. Hearts were rapidly excised and ventricles were minced and homogenized by polytron in ice-cold isolation buffer (10 mM MOPS pH 7.4, 250 mM sucrose, 5 mM KH2PO4, 2 mM MgCl2, 1 mM EGTA, 0.1% BSA). Lysates were centrifuged for 10 min at 600 × g to remove unbroken tissue and nuclei, and the supernatants were centrifuged for 10 min at 3,000 × g to pellet mitochondria. The mitochondrial pellet was resuspended in swelling buffer (10 mM MOPS pH 7.4, 250 mM sucrose, 5 mM KH2PO4, 2 mM MgCl2, 5 μM EGTA, 5 mM pyruvate, and 5 mM malate). 60 μg of mitochondria were mixed with 0.1-1 μg recombinant Bnip3 (2.3-230 nM), 1 μg Bnip3ΔTM (278 nM), 100 nM tBid (33.5 nM) or 250 μM Ca2+ in a 96-well plate in a total volume of 200 μl/well. Mitochondrial swelling was monitored by measuring absorbance on a plate reader at 520 nm for 60 min. Shrinking of mitochondria was induced by 5% PEG-3350 (w/v). Mitochondrial swelling curves were graphed using Microsoft Excel. The amplitude of each curve was obtained by subtracting the endpoint absorbance from the initial absorbance obtained from the swelling assay. Vmax was obtained by calculating the slope of the linear segment of the swelling curve. After incubation, the samples were centrifuged for 10 min at 3000 × g to pellet mitochondria. The mitochondria and supernatant were analyzed by Western blotting for release of cytochrome c (BD Biosciences) or OPA1 (BD Biosciences). COX IV (Molecular Probes) was used as a mitochondrial marker. These assays were always done in duplicates and repeated at least 3 times.
Electron microscopy
A suspension of mitochondria was fixed by the addition of an equal volume of 5% glutaraldehyde in 0.075M Na cacodylate buffer pH 7.3 for 1 hr on ice. Following pelleting, the samples were washed in cacodylate buffer, postfixed in 1% osmium tetroxide in 0.15M cacodylate buffer and dehydrated in a graded ethanol series. The pellets were treated with propylene oxide as the transition solvent before embedding in Epon/Araldite (Electron Microscopy Sciences, Hatfield PA). Following initial polymerization to form a small resin button at the bottom of the microfuge tube, the resin pellets were removed from the microfuge tubes and re-embedded in more liquid resin in a regular flat embedding mold (Ted Pella Inc, Redding, CA) and oriented such that transverse sections could be prepared through the depth of the original cell pellet. Thin sections (60 nm) were cut with a diamond knife (Diatome, Hatfield PA), mounted on copper slot grids coated with parlodion and subsequently stained with uranyl acetate and lead citrate for examination on a Philips CM100 electron microscope (FEI, Hillsbrough OR). Images were documented using a Megaview III CCD camera (Olympus Soft Imaging Solutions, Lakewood CO) and then handled in Adobe Photoshop.
Crosslinking assay
For protein crosslinking, mitochondria were treated with DMSO or 10 mM BMH (Pierce) for 30 min at 37°C. Samples were centrifuged for 10 min at 20,000 g at 4°C, and the mitochondrial pellets were resuspended in SDS-PAGE sample loading buffer. DTT in the sample buffer quenched the crosslinking reaction. Proteins were separated on a 4–20% Tris-Glycine gel, transferred onto a nitrocellulose membrane, and probed with an antibody to OPA-1 (BD Transduction Laboratories).
Isolation of Mouse Embryonic Fibroblasts
Mouse embryonic fibroblasts (MEFs) were derived from WT and CypD-/- E13.5 embryos and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. Primary cells were frozen in aliquots after the second passage. MEFs derived from WT and Bax/Bak double knockout (DKO) mice were generously provided by Dr. Craig Thompson (University of Pennsylvania, Philadelphia, PA) [16].
Cell Death assays
WT and cypD-/- MEFs in growth medium without phenol red were infected with β-gal or Bnip3. Cell death was assessed after 24 h by measuring LDH release into the medium using a cytotoxicity detection kit according to the manufacturer's protocol (Roche). The released LDH is expressed as a percentage of total LDH in the well. TUNEL staining was performed using the In Situ Cell Death Detection kit (Roche Applied Science) according to the manufacturer's instructions. Nuclei were counterstained with Hoechst 33342 (Molecular Probes). TUNEL positive nuclei were counted in 10 randomly selected fields of each condition and expressed as a percentage of the total number of nuclei.
Calcein-AM assay
After adenoviral infection (16 h) or 250 μM H2O2 treatment (1 h), MEFs were loaded with 1 μM calcein-AM (Molecular Probes) and 5 mM cobalt chloride for 15 min at 37°C to quench cytosolic calcein fluorescence [17]. The cells were rinsed to remove residual dye and opening of the mPTP was visualized as redistribution of calcein from the mitochondrial matrix into the cytosol. Live cells were observed through a Nikon TE300 fluorescence microscope (Nikon) equipped with a cooled CCD camera (Orca-ER, Hamamatsu). At least 100 to 150 cells were scored from two replicate dishes in three independent experiments.
Statistical Analysis
All values are expressed as means ± Standard Deviation (S.D). ANOVA was performed to identify statistical significance in multiple-group comparisons and Student's t-test was used to evaluate significance between two experimental conditions. A p<0.05 was considered to be statistically significant.
Results
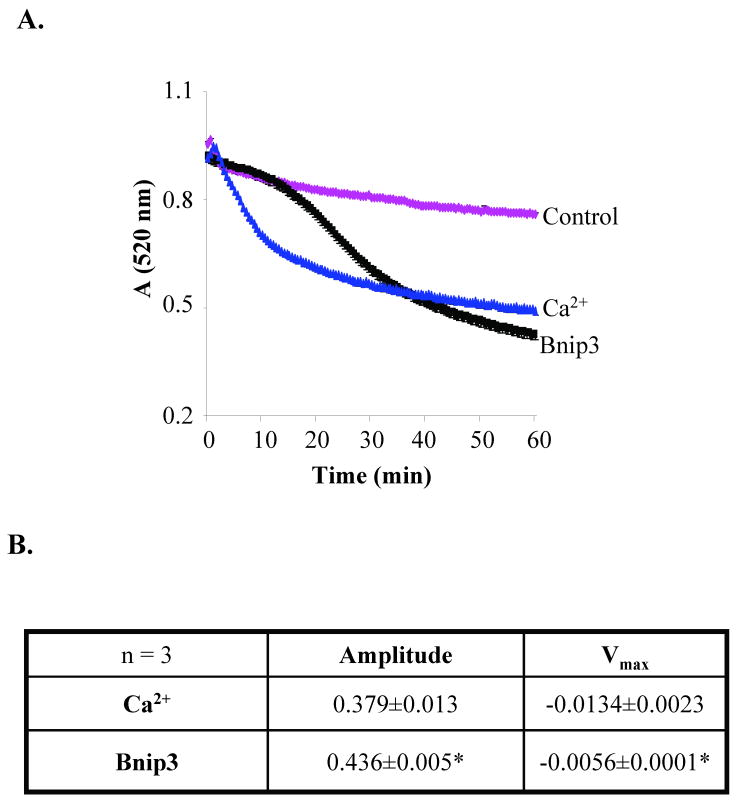
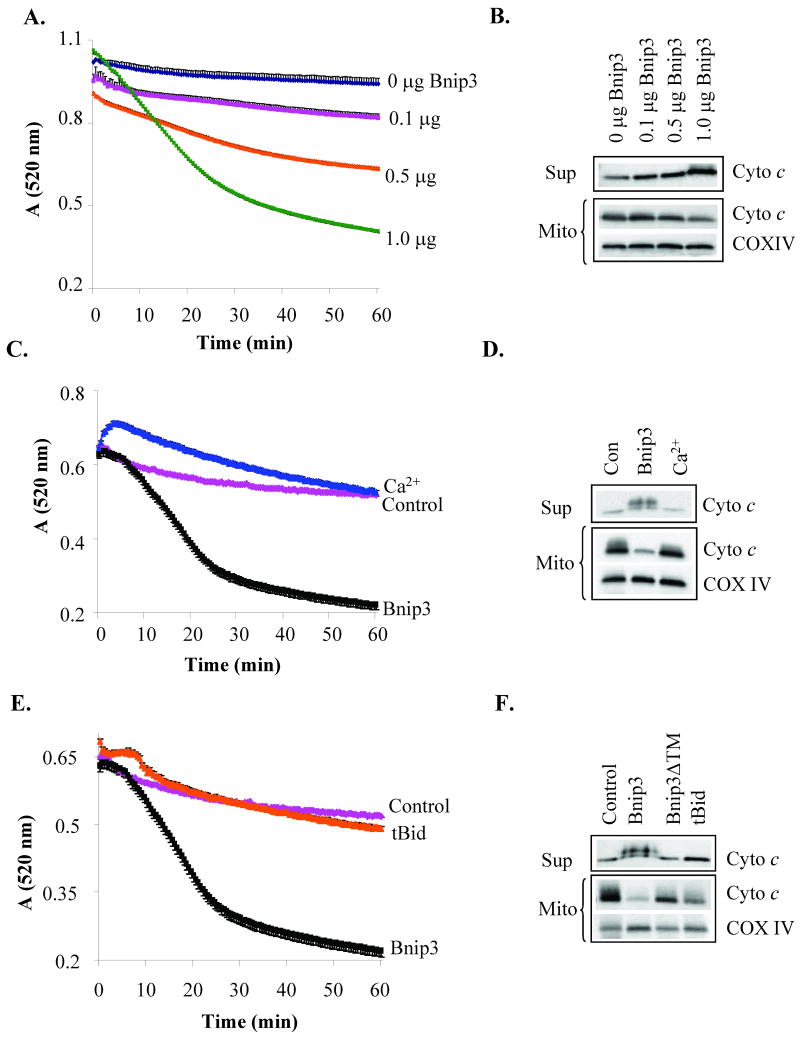
Bnip3 has been reported to mediate cell death via opening of the mPTP in cardiac myocytes [7, 10] and 293T cells [9]. To investigate if Bnip3 could directly induce opening of the mPTP, we performed swelling assays with isolated heart mitochondria to examine the effect of Bnip3 on mPTP opening in vitro. Opening of the mPTP results in mitochondrial swelling, which can be detected as a decrease in absorbance at 520 nm using a spectrophotometer. In the presence of buffer alone, isolated heart mitochondria exhibited little swelling, whereas addition of Ca2+, a known inducer of mPTP opening, caused rapid swelling of mitochondria as shown by the decrease in absorbance (Fig. 1A). Addition of recombinant Bnip3 to the mitochondria also caused a reduction in the absorbance, indicating swelling of mitochondria. However, we noted that Bnip3-mediated swelling occurred at a slower rate than Ca2+, and always to a greater extent (Fig. 1B). Pretreatment of isolated mitochondria with Cyclosporine A (CsA), an inhibitor of the mPTP, prevented Ca2+-mediated swelling (Fig. 2A) and reduced cytochrome c release (Fig. 2C). In contrast, CsA had little effect on Bnip3 mediated-swelling (Fig. 2B) and cytochrome c release (Fig. 2C). We found that mitochondrial swelling and release of cytochrome c occurred in a dose-dependent manner and that CsA was without any effect at all concentrations of Bnip3 (Fig. 2D). Maximal swelling and cytochrome c release occurred at a concentration of 1 μg which is consistent with previous reports where 1 μg of recombinant Bnip3 was required to induce release of pro-apoptotic proteins and loss of mitochondrial membrane potential in isolated mitochondria [4, 18].
Figure 1.
Bnip3 induces swelling of isolated heart mitochondria via a different mechanism than Ca2+. A. Mitochondria isolated from rat hearts were incubated with recombinant Bnip3 (1 μg) or Ca2+ (250 μM) and reduction in absorbance at 520 nm was measured. Swelling assay is representative of three independent experiments. B. The degree of swelling (amplitude) and rate (Vmax) of swelling was significantly different between Ca2+ and Bnip3. Results are means±S.E.M. (n=3,*p<0.05).
Figure 2.
Bnip3-mediated swelling and cytochrome c release are not inhibited by the mPTP inhibitor cyclosporine A. A. Incubation of mitochondria with 1 μM CsA inhibits swelling mediated by 250 μM Ca2+. B. CsA has no effect on swelling mediated by Bnip3 (1 μg). C. Bnip3 (1 μg) and Ca2+ (250 μM) induce release of cytochrome c from isolated mitochondria. CsA (1 μM) reduces Ca2+-mediated cytochrome c release but has no effect on Bnip3-mediated release. Western blot is representative of three independent experiments. D. Isolated mitochondria were treated with 0, 0.1, 0.5, or 1 μg Bnip3 in the presence or absence of 1 μM CsA.
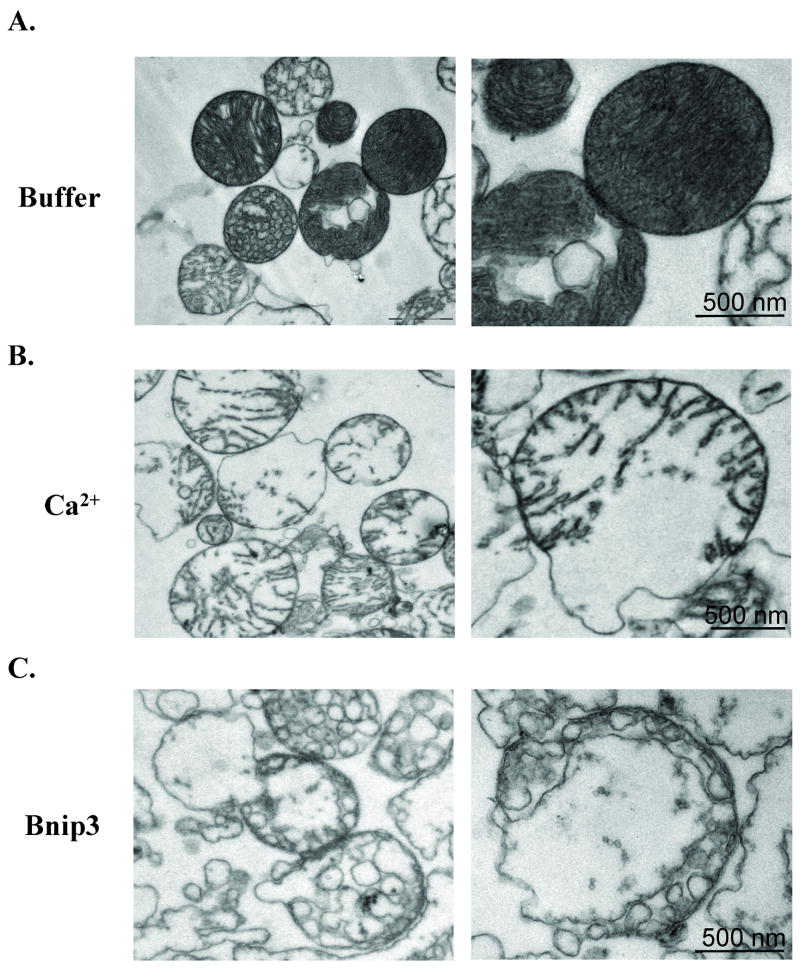
To examine changes in mitochondrial structure after Ca2+ and Bnip3 treatment at the ultrastructural level, we obtained transmission electron micrographs of heart mitochondria that had been incubated with buffer alone, Ca2+ or Bnip3. Healthy mitochondria displayed numerous narrow crested in a continuous electron-dense matrix (Fig. 3A). Isolated mitochondria are unstable in swelling buffer, and the EM showed some mitochondria with signs of matrix remodeling after 45 min incubation incubated in buffer alone. However, freshly isolated mitochondria were intact with good respiration (Supplemental Figure S1). All mitochondria treated with Ca2+ (Fig. 3B) or Bnip3 (Fig. 3C) displayed extensive swelling of inner mitochondrial membrane with ruptured outer membrane. However, there were major differences in the organization of the cristae between Ca2+ and Bnip3-treated mitochondria. Ca2+-treated mitochondria displayed loss of the tubular structures and swelling of the inner membrane, whereas Bnip3-treated mitochondria had complete loss of the tubular cristae and appearance of “vesicle-shaped” matrix.
Figure 3.
Mitochondrial morphological changes and matrix remodeling in Ca2+ and Bnip3-treated mitochondria. Electron microscopy analysis of isolated mitochondria after incubation with (A) buffer, (B) 250 μM Ca2+ or (C) 1 μg Bnip3 for 45 min.
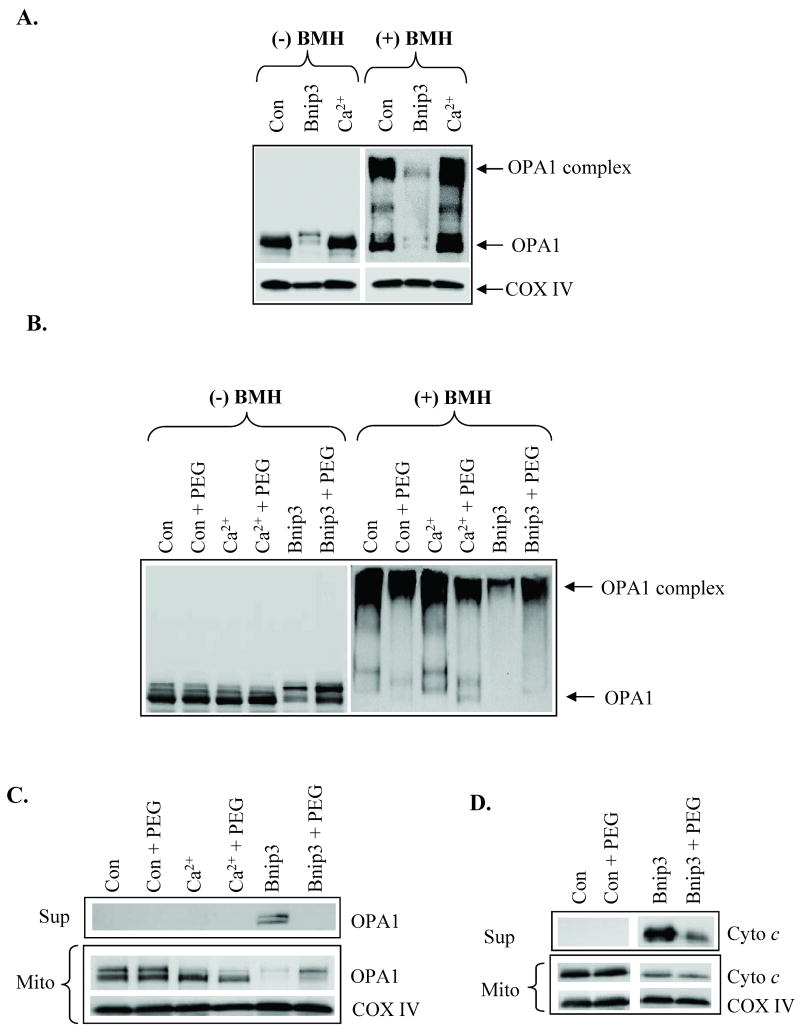
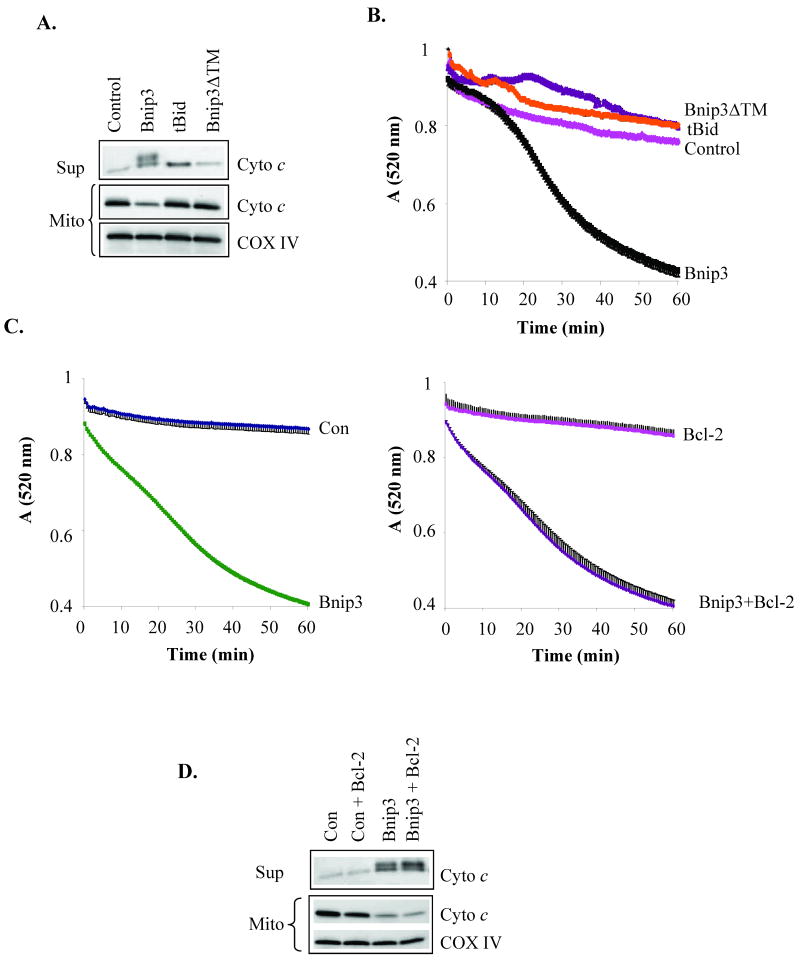
OPA1 is a mitochondrial fusion protein that regulates the shape of the cristae and participates in the formation of narrow tubular crista junctions [19, 20]. OPA1 forms tight crista junctions by forming oligomers [21]. Chemical crosslinking using BMH revealed that OPA1 exists in a ∼290 kDa complex in healthy cardiac mitochondria (Fig. 4A). In contrast, the OPA1 complex was almost completely absent in mitochondria that had been treated with Bnip3. As has been previously reported to occur during apoptosis [22, 23], there was also substantially less OPA1 in the mitochondria, suggesting that the complex has been disrupted and the protein released. Inhibition of swelling by polyethylene glycol (PEG) prevented Bnip3-mediated disruption of the OPA1 complex (Fig. 4B) and release from mitochondria (Fig. 4C). Bnip3-mediated cytochrome c release was mostly prevented in the presence of PEG (Fig. 4D). This suggests that Bnip3-mediated swelling causes the disruption and release of OPA1 and cytochrome c. Truncated Bid (tBid) is a BH3-only protein which has been reported to induce permeabilization of the outer mitochondrial membrane and cytochrome c release [24]. We found that incubation of isolated mitochondria with recombinant tBid induced release of cytochrome c (Fig. 5A) but in the absence of mitochondrial swelling (Fig. 5B). Moreover, Bnip3 contains a transmembrane (TM) domain that is important for its pro-apoptotic activity and deletion of the TM domain blocks the ability of Bnip3 to induce cell death [25, 26]. The inactive Bnip3ΔTM mutant did not induce release of cytochrome c or swelling of mitochondria (Fig. 5A and B). Bcl-2 is an anti-apoptotic protein that can protect cells against death mediated by BH3-only proteins such as tBid and Bad [27]. To investigate if Bcl-2 could prevent Bnip3-mediated swelling and cytochrome c release, mitochondria were incubated with Bnip3 in the presence or absence of recombinant Bcl-2. We found that Bcl-2 had no effect Bnip3-mediated mitochondrial swelling (Fig. 5C) or cytochrome c release (Fig. 5D).
Figure 4.
Bnip3-mediated swelling induces disruption of OPA1 complex. A. After treatment with buffer, Ca2+ (250 μM) or Bnip3 (1 μg) for 30 min, mitochondria were incubated with DMSO or 10 mM BMH for 30 min at 37°C to cross link proteins. Proteins were separated by SDS-PAGE and analyzed by Western blotting for OPA1. B. The presence of PEG prevents Bnip3-mediated disruption of OPA1 complex. C. PEG prevents Bnip3-mediated release of OPA1 from mitochondria. D. PEG partially prevents Bnip3-mediated cytochrome c release. Data shown are representative of three independent experiments.
Figure 5.
The BH3-only protein tBid induces cytochrome c release in the absence of mitochondrial swelling. A. Bnip3 (1 μg) and tBid (100 nM), but not Bnip3ΔTM (1 μg), induce release of cytochrome c release. B. Incubation of heart mitochondria with recombinant tBid (100 ng) or Bnip3ΔTM (1 μg) do not induce swelling of mitochondria. C. Incubation of heart mitochondria with recombinant Bcl-2 (1 μg) does not prevent Bnip3-mediated swelling. D. Bcl-2 does not inhibit cytochrome c release by Bnip3. Data shown are representative of three independent experiments.
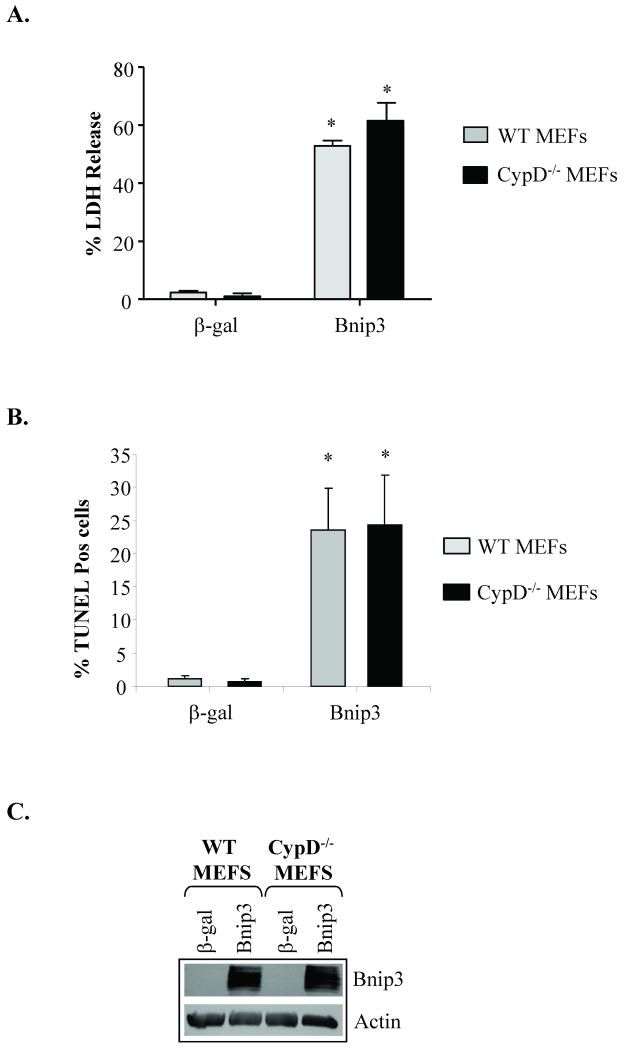
Cyclophilin D is an important component of the mPTP and mitochondria lacking cypD are resistant to mPTP opening [14]. To further investigate whether Bnip3 induced swelling and cytochrome c release independent of the mPTP, we incubated recombinant Bnip3 with mitochondria isolated from hearts of cypD-/- mice. Similar to mitochondria isolated from the rat heart, we found that Bnip3 induced dose-dependent mitochondrial swelling (Fig. 6A) and release of cytochrome c (Fig. 6B) in cypD deficient mitochondria. As previously reported by Baines et al. [14] cypD deficient mitochondria were resistant to swelling and cytochrome c release induced by Ca2+ (Fig. 6C and D). In contrast, Bnip3 induced both swelling and cytochrome c release in cypD-/- mitochondria. The BH3-only protein tBid induced cytochrome c release in the absence of mitochondrial swelling in cypD deficient mitochondria (Fig. 6E and F). To further investigate the role of the mPTP in Bnip3-mediated mitochondrial dysfunction and cell death, primary mouse embryonic fibroblasts (MEFs) were isolated from WT and cypD-/- mice and then infected with an adenovirus encoding β-galactosidase (β-gal) or Bnip3. After 24 h, cell death was determined by measuring release of LDH into the cell culture media or by staining cells for the presence of fragmented DNA. As shown in Figure 7, Bnip3 induced significant cell death in WT and cypD-/- MEFs as measured by LDH release (Fig. 7A) and TUNEL staining (Fig. 7B). Consistent with experiments in vitro, we found that Bnip3 induced cytochrome c release in WT and cypD-/- MEFs (Supplemental Fig. S2) and that reconstituting cyclophilin D into cypD-/- MEFs had no effect on Bnip3-mediated cytochrome c release (Supplemental Fig. S3). Analysis of Bnip3 protein levels in infected cells confirmed similar levels of Bnip3 overexpression in WT and cypD-/- MEFs (Fig. 7C). These data suggest that Bnip3 can mediate cell death even in the absence of a functional mPTP.
Figure 6.
Heart mitochondria isolated from cypD deficient mice are resistant to Ca2+-mediated swelling but not to Bnip3. A. Mitochondrial swelling of cypD deficient mitochondria treated with 0, 0.1, 0.5, or 1 μg Bnip3. B. Cytochrome c release in cypD deficient mitochondria by Bnip3._C. Mitochondria lacking cypD were incubated with 250 μM Ca2+ or 1 μg Bnip3 and swelling was assessed by measuring a decrease in absorbance. D. Western blot analysis of cypD deficient mitochondria for release of cytochrome c. E. Swelling of cypD deficient mitochondria was determined by measuring a decrease in absorbance. F. CypD deficient mitochondria were incubated with Bnip3 (1 μg), Bnip3ΔTM (1 μg), or tBid (100 ng) and analyzed for release of cytochrome c by Western blotting. Data shown are representative of three independent experiments.
Figure 7.
Bnip3 induces cell death in both wild type (WT) and cypD-/- MEFs. A. Cells were infected with an adenovirus encoding β-gal or Bnip3 for 24 h. Cell death was determined by (A) assessing release of LDH into the culture media or (B) TUNEL assay. Results are means±S.E.M. (n=3, p<0.001). C. Expression of Bnip3 in WT and cypD-/- MEFs infected with an adenovirus encoding β-gal or Bnip3 for 24 h was determined by Western blot analysis.
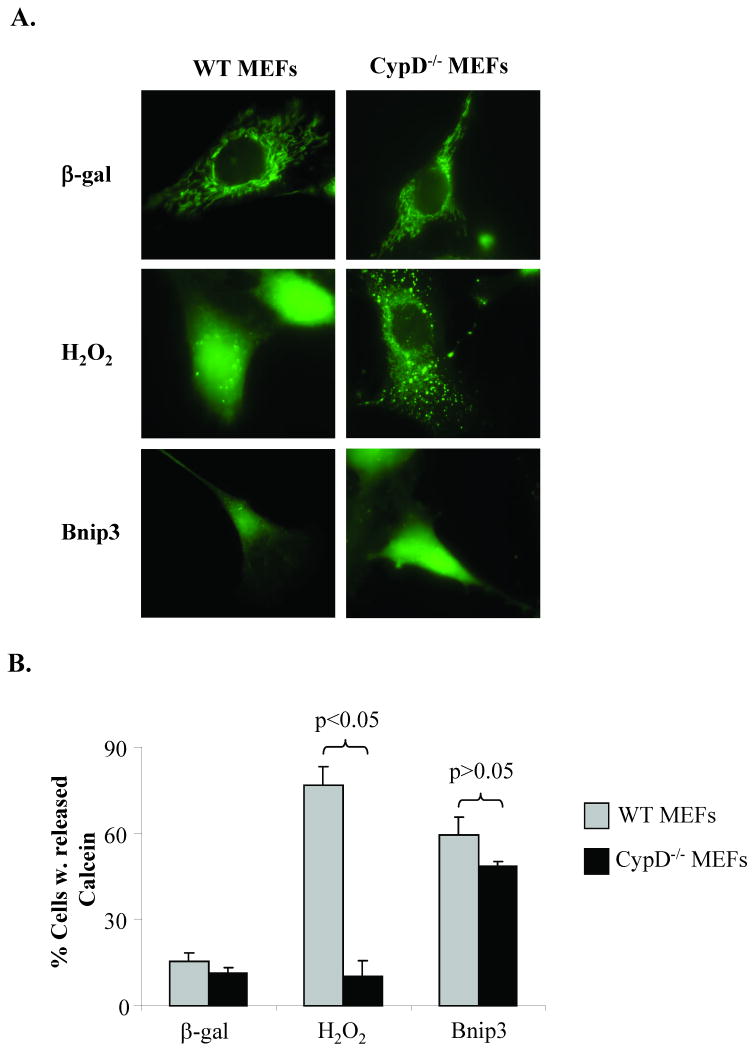
In addition, opening of the mPTP can be determined in cells using the membrane-permeating fluorescent probe calcein-AM, which freely enters the mitochondrial matrix but cannot exit except through an open pore due to processing by cellular esterases. The release of calcein from mitochondria was analyzed by fluorescence microscopy as described by Bernardi et al. [17]. WT and cypD-/- MEFs infected with an adenovirus expressing β-gal and loaded with calcein-AM showed calcein retention in mitochondria. Treatment of WT MEFs with 250 μM H2O2, a concentration that has been demonstrated to induce mPTP opening [14], caused a reduction in mitochondrial calcein fluorescence and an increase in cytosolic staining, whereas calcein fluorescence was retained within the mitochondria of cypD-/- MEFs (Fig. 8A and B). In contrast, overexpression of Bnip3 induced release of calcein from both WT and cypD-/- MEFs, confirming that Bnip3 does not require cypD, which is considered essential for opening of the conventional mPTP, to induce permeabilization of the inner mitochondrial membrane [14, 28, 29]. Re-introduction of cyclophilin D in cypD-/- MEFS had no effect on Bnip3-mediated calcein release but restored the ability of H2O2 to induce release of calcein (Supplemental Figure S4).
Figure 8.
Bnip3, but not Ca2+, induces release of calcein from mitochondrial matrix in CypD-/- MEFs. A. Cells infected with β-gal or Bnip3, or treated with 250 μM H2O2 were loaded with 1 μM calcein-AM and 5 mM CoCl2. B. Quantitation of cells with cytosolic calcein. Results are means±S.E.M. (n=3, p<0.05).
Since Bax and Bak are downstream targets of BH3-only proteins and we have previously shown that Bnip3 mediates cell death via Bax/Bak [5], we investigated whether Bnip3 required Bax/Bak to induce release of calcein from the mitochondria. Treatment of WT and Bax/Bak DKO MEFs with H2O2 caused release of calcein from the mitochondria (Fig. 9A and B). However, Bax/Bak DKO MEFs overexpressing Bnip3 were significantly resistant to release of calcein, suggesting that Bax/Bak are required for permeabilization of the mitochondrial inner membrane by Bnip3.
Figure 9.
H2O2, but not Bnip3, promotes release of calcein from mitochondria in Bax/Bak DKO MEFs. A. Cells infected with β-gal or Bnip3, or treated with 250 μM H2O2 were loaded with 1 μM calcein-AM and 5 mM CoCl2. B. Quantitation of cells with cytosolic calcein. Results are means±S.E.M. (n=3, p<0.05).
Discussion
Bnip3 is known to localize to and exert its action at the mitochondria. Since inhibitors of the mPTP have been reported to partially protect against Bnip3-mediated cell death [5, 9], it seemed likely that Bnip3 would initiate cell death via opening of the mPTP. However, our data clearly demonstrate that a functional mPTP is not required for Bnip3-mediated mitochondrial dysfunction. First, swelling assays and ultrastructural analysis of mitochondria showed that the mechanism of Bnip3-mediated swelling of mitochondria and release of cytochrome c were different from Ca2+, a known mediator of mPTP opening. Second, overexpression of Bnip3 caused rapid permeabilization of the mitochondrial membrane and cell death in both WT and cypD-/- MEFs.
There are several reports that Bnip3 mediates cell death via opening of the mPTP [7, 9, 10]. However, it is clear from our study and others that while Bnip3 can induce opening of the mPTP, the mPTP is not required for Bnip3-mediated cell death. Vande et al. initially reported that Bnip3 induced rapid opening of the mPTP and cell death in 293T cells which was reduced in the presence of CsA or bongkrekic (BA), two different inhibitors of the mPTP [9]. Interestingly, they noted that maximum suppression of mitochondrial dysfunction and cell death was only 50% with either inhibitor, suggesting that there was an alternative mechanism by which Bnip3 mediated cell death. Similarly, we found that treatment of cells with CsA or BA only partially protected against Bnip3-mediated mitochondrial dysfunction in MEFs [5]. We also found that the mPTP inhibitors had no effect on Bnip3-mediated activation of pro-apoptotic Bax and Bak, suggesting that opening of the mPTP occurs downstream of Bax/Bak activation or possibly by a separate pathway activated by Bnip3. In this study, we found that Bax/Bak DKO MEFs were resistant to Bnip3-mediated calcein release from mitochondria. However, we noted that ∼20% of Bax/Bak DKO MEFs consistently released calcein in response to Bnip3, suggesting that Bnip3 can open the mPTP via a separate pathway (Fig. 8B). Our finding that mPTP inhibitors provides ∼50% reduction in Bnip3-mediated cell death, suggests that Bnip3 also promotes opening of the mPTP via the Bax/Bak pathway (possibly through ROS). The observation that Bnip3 activates two separate pathways of cell death would explain the atypical programmed cell death pathway resembling both necrosis and apoptosis that many investigators have described with Bnip3.
BH3-only proteins are known to promote outer membrane permeabilization via Bax/Bak [16, 30]. The fact that Bax/Bak DKO MEFs were resistant to Bnip3-mediated calcein release, suggests that Bax/Bak are required for Bnip3-mediated mitochondrial inner membrane permeabilization. These results are consistent with the notion that all BH3-only proteins, including Bnip3, require Bax and/or Bak as downstream effectors to initiate mitochondrial dysfunction. BH3-only proteins activate Bax/Bak which forms a pore in the outer mitochondrial membrane [1]. However, our results suggest that Bnip3 is capable of disrupting the integrity of both inner and outer mitochondrial membranes, suggesting that the mechanism of mitochondrial membrane permeabilization is different from other BH3-only proteins such as tBid. For instance, we found that Bnip3 promoted both mitochondrial swelling and cytochrome c release, whereas tBid induced cytochrome c release in the absence of swelling. This is consistent with previous studies which have reported that tBid induces cytochrome c release independently of the mPTP [14, 31, 32]. Also, Bcl-2 is known to protect against tBid-mediated cytochrome c release and cell death [27, 33], but we found that Bcl-2 had no effect on Bnip3-mediated swelling and cytochrome c release. This finding is consistent with the previous report that Bcl-2 only partially protects against Bnip3 and that Bnip3 later overcomes Bcl-2 mediated protection [25]. Moreover, the release of calcein, trapped in the matrix of mitochondria in healthy cells, requires disruption of both inner and outer membranes. We found that Bnip3 induced release of calcein in cells with a dysfunctional mPTP, but not in Bax/Bak deficient cells. In contrast, it has been reported that the Bax/Bak pore opening in response to tBid is insufficient for calcein release [34]. Clearly, Bnip3 mediates permeabilization via a mechanism that is different from tBid but still requires the presence of Bax/Bak.
Remodeling of the inner mitochondrial membrane during apoptosis is characterized by fusion of individual crista and widening of crista junctions which ensures complete release of cytochrome c and mitochondrial dysfunction [35-37]. In our studies, we found that Bnip3 induced loss of the tubular cristae and appearance of “vesicle-shape” matrix. This type of shape has previously been described by Scorrano et al. [37] in mitochondria treated with tBid. However, tBid treatment did not result in rupture of the outer mitochondrial membrane. This is consistent with our finding that Bnip3, but not tBid, induced swelling of isolated mitochondria. The dynamin-related GTPase OPA1 is a mitochondrial fusion protein that mediates fusion of the inner membrane [19, 20], and controls cristae remodeling during apoptosis [21]. OPA1 maintains tight crista junctions by forming oligomers in the inner mitochondrial membrane. Upon induction of apoptosis, the OPA1 complex is destroyed [21] and OPA1 is released into the cytosol [22, 23]. In the present study, we found that Bnip3 treatment caused disruption of OPA1 oligomers and subsequent release from mitochondria. In contrast, Ca2+ treated mitochondria did not display similar cristae remodeling and release of OPA1. Interestingly, Bnip3-mediated disruption and release of OPA1 was dependent on mitochondrial swelling suggesting that Bnip3 does not directly induce disruption of OPA1. It is not known why swelling induced by Ca2+ failed to disrupt and release OPA1.
We found that cytochrome c often appeared as a doublet on Western blots in Bnip3 treated samples, suggesting that Bnip3 induces a currently unknown modification of cytochrome c. We could find no evidence in the literature that cytochrome c can be modified by other BH3-only proteins. However, cytochrome c has been reported to be sensitive to oxidative modification. For instance, cytochrome c has been reported to be modified by 4-hydroxy-2-nonenal, a secondary product of lipid peroxidation [38], as well as by hydrogen peroxide [39]. Since Bnip3 has been reported to induce oxidative stress [9], it will be interesting to investigate if Bnip3 treatment induces oxidative modification of cytochrome c.
In this study, we have identified a novel mechanism by which the pro-apoptotic protein Bnip3 mediates permeabilization of the mitochondrial membranes. Since Bnip3 has been implicated in the pathogenesis of various diseases including heart failure and cancer, Bnip3 or one of its downstream effectors represents an important potential therapeutic target for treatment or prevention of disease.
Supplementary Material
Acknowledgments
MEFs derived from WT and Bax/Bak double knockout (DKO) mice were generously provided by Dr. Craig Thompson (University of Pennsylvania, Philadelphia, PA). This work was supported by NIH grants HL060590 (RAG) and HL087023 (ÅBG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007 Jan;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson AB, Gottlieb RA. Bcl-2 Family Members and Apoptosis, Taken to Heart. Am J Physiol Cell Physiol. 2006 Aug 30; doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 3.Graham RM, Thompson JW, Wei J, Bishopric NH, Webster KA. Regulation of bnip3 death pathways by calcium, phosphorylation, and hypoxia-reoxygenation. Antioxid Redox Signal. 2007 Sep;9(9):1309–16. doi: 10.1089/ars.2007.1726. [DOI] [PubMed] [Google Scholar]
- 4.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007 Jan;14(1):146–57. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 5.Kubli DA, Ycaza JE, Gustafsson AB. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J. 2007 Aug 1;405(3):407–15. doi: 10.1042/BJ20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007 Oct;117(10):2825–33. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA. 2002;99(20):12825–30. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 Functions as a Mitochondrial Sensor of Oxidative Stress during Myocardial Ischemia and Reperfusion. Am J Physiol Heart Circ Physiol. 2008 Sep 12; doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vande VC, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. MolCell Biol. 2000;20(15):5454–68. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91(3):226–31. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 11.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002 Nov 1;111(3):331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 12.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004 Feb 15;61(3):372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 13.Korman EF, Blondin GA, Vail WJ, Green DE. The mechanism of mitochondrial swelling. VII. The constant topology of the mitochondrial inner membrane during swelling. J Bioenerg. 1970 Oct;1(4):379–86. doi: 10.1007/BF01654575. [DOI] [PubMed] [Google Scholar]
- 14.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson AB, Tsai JG, Logue SE, Crow MT, Gottlieb RA. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J Biol Chem. 2004;279(20):21233–8. doi: 10.1074/jbc.M400695200. [DOI] [PubMed] [Google Scholar]
- 16.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001 Jun 15;15(12):1481–6. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem. 1999 Sep;264(3):687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Cho JJ, Ha J, Park JH. The carboxy terminal C-tail of BNip3 is crucial in induction of mitochondrial permeability transition in isolated mitochondria. Arch Biochem Biophys. 2002 Feb 15;398(2):147–52. doi: 10.1006/abbi.2001.2673. [DOI] [PubMed] [Google Scholar]
- 19.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, et al. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006 Oct 20;127(2):383–95. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006 Jul 14;126(1):177–89. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005 Oct 21;280(42):35742–50. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- 23.Ju WK, Lindsey JD, Angert M, Patel A, Weinreb RN. Glutamate receptor activation triggers OPA1 release and induces apoptotic cell death in ischemic rat retina. Mol Vis. 2008;14:2629–38. [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998 Aug 21;94(4):481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Ray R, Dubik D, Shi L, Cizeau J, Bleackley RC, et al. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. JExpMed. 1997;186(12):1975–83. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray R, Chen G, Vande VC, Cizeau J, Park JH, Reed JC, et al. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. JBiolChem. 2000;275(2):1439–48. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 27.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. MolCell. 2001;8(3):705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–8. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 29.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005 May 13;280(19):18558–61. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 30.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. Embo J. 1998 Jan 2;17(1):37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol. 2000 Sep 4;150(5):1027–36. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi X, Yin XM, Dong Z. Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem. 2003 May 9;278(19):16992–9. doi: 10.1074/jbc.M300039200. [DOI] [PubMed] [Google Scholar]
- 34.Wolff S, Erster S, Palacios G, Moll UM. p53's mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008 Jul;18(7):733–44. doi: 10.1038/cr.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001 Oct;1(4):515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 36.Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. Embo J. 2005 Apr 20;24(8):1546–56. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002 Jan;2(1):55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 38.Isom AL, Barnes S, Wilson L, Kirk M, Coward L, Darley-Usmar V. Modification of Cytochrome c by 4-hydroxy- 2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J Am Soc Mass Spectrom. 2004 Aug;15(8):1136–47. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Kim NH, Jeong MS, Choi SY, Kang JH. Oxidative modification of cytochrome c by hydrogen peroxide. Mol Cells. 2006 Oct 31;22(2):220–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.