Abstract
Objective
One of the multiple health benefits of soy protein or its isoflavones may be its purported favorable affect on body composition. We examined the effect of isoflavones extracted from soy protein on overall and regional body composition taking into account appetitive hormones as potential mediators, as well as the direct effect on appetitive hormones.
Design
This randomized, double-blind, placebo-controlled multi-center trial included 229 healthy postmenopausal women (45.8 to 65 years, body mass index 24.9±3.0) who consumed placebo or soy isoflavone (80 or 120 mg/day) tablets for 12 months. We used intent-to-treat analysis to examine body composition (whole body lean mass, whole body fat mass, androidal fat mass, androidal-to-gynoidal fat mass ratio) and appetitive hormones (insulin, leptin, ghrelin, adiponectin) in response to treatment.
Results
Repeated measures analysis of variance (ANOVA) indicated that soy isoflavone treatment did not exert a significant effect on body composition measures (P value ranged from 0.36 to 0.79) or appetitive hormone concentrations; the inclusion of covariates in statistical models did not alter these results. Independently of treatment, leptin and ghrelin related inversely to each body composition measure (P values ranged from 0.044 to ≤0.0001). Adiponectin related inversely to all fat measures (P values from 0.0004 to <0.0001). Time since last menstrual period related directly to all fat measures (P values from 0.06 to 0.0055). Dietary fat contributed to whole body (P=0.028) and androidal (P=0.017) fat mass.
Conclusions
Our findings do not support a favorable effect of soy isoflavone tablets on body composition in healthy postmenopausal women.
Keywords: body composition, soy isoflavones, appetitive hormones, postmenopausal women
INTRODUCTION
Soybeans are promoted as a healthy food with multiple health benefits. One purported benefit is that soy protein or its isoflavones may favorably affect body composition. Animal studies have shown that hamsters,1 ovariectomized rats,2 mice,3 and monkeys4 fed a diet containing individual isoflavones (genistein, daidzein, glycitein) or isoflavone-rich soy protein had less body fat compared with control animals. To date, a few small-scale human studies have examined the association between isoflavone intake and body composition. Perimenopausal women who consumed isoflavone-rich (80 mg/day) soy protein for six months had slightly but significantly lower thigh fat mass and greater hip lean mass compared with women who consumed either isoflavone-poor soy protein or whey protein (control) powder.5 Obese sarcopenic postmenopausal women (n=18) who consumed isoflavone supplements (70 mg/day) for six months experienced a significant increase in appendicular leg fat-free mass (FFM) and muscle mass index (appendicular FFM/height2) compared with the placebo group (n=6).6 Nine postmenopausal women who consumed a soy protein shake with 160 mg isoflavones for three months showed smaller increases in total and subcutaneous abdominal fat compared with the isocaloric casein control group (n=6), although visceral fat, total body fat, or lean mass did not differ between groups.7 In a randomized controlled trial, 100 mg/day of isoflavones (supplement form) administered to postmenopausal Japanese women for 24 weeks resulted in a significant reduction in total body fat and prevented an increase in body mass index (BMI) compared with the control group.8
Although the results of human studies vary, in part due to differences in methodology, the above studies suggest some association between isoflavone intake and body composition. Thus, a well-designed, long-term prospective clinical trial should help to clarify whether soy isoflavones indeed exert a physiologically important effect on body composition, particularly central adiposity, in humans. Isoflavones may affect body composition directly, for example via an estrogen receptor-mediated mechanism9 by altering the metabolic activity of adipocytes,3 or indirectly by mediating the action of insulin, leptin, ghrelin, and adiponectin, hormones thought to be involved in the regulation of body composition.
This randomized, placebo-controlled, multi-center clinical trial examined the effects of two doses (80 mg or 120 mg) of soy isoflavones taken for 12 months on overall (whole body fat mass, whole body lean mass) and regional (androidal fat mass, androidal-to-gynoidal fat mass) body composition in healthy postmenopausal women. This study also determined the response to soy isoflavone tablets on these outcomes of interest, but taking into account potential mediators of body composition (insulin, leptin, ghrelin, and adiponectin) and confounding factors (age or time since last menstrual period; aspartate aminotransferase; physical activity; dietary intake of energy, protein, and fat). We also assessed the effect of isoflavone tablets on circulating concentrations of insulin, leptin, ghrelin, and adiponectin.
METHODS
Overall Study Design
This project was ancillary to the Soy Isoflavones for Reducing Bone Loss (SIRBL) study, a randomized, double-blind, placebo-controlled multi-center (Iowa State University [ISU] and University of California at Davis [UCD]) clinical trial. The parent study examined the effect of two doses (80 vs 120 mg/day) of soy protein-derived isoflavones for 36 months on bone loss in healthy postmenopausal women (45 to 65 years of age) who were at-risk for osteoporosis. This ancillary project focused on the effect of soy isoflavone tablets for 12 months on overall body composition and central adiposity and whether appetitive hormones mediated this anticipated effect. The respective Institutional Review Boards (IRB) at ISU (ID# 02-199) and at UCD (ID# 200210884-2) approved our study protocol, consent form, and subject-related materials. Approvals for the DXA procedures were obtained from each institution’s IRB and State Department of Public Health in Iowa and California. We explained the details of the study verbally and in writing to each participant before she signed an informed consent form.
Subject Recruitment, Screening, and Eligibility
Women were recruited (2003 to 2005) throughout the state of Iowa and in the Sacramento and Bay Area regions in California primarily through direct mailing lists, stories in local newspapers, and local/regional radio advertisements. Responders (N=5,255) were initially screened via telephone to identify healthy women (non-osteoporotic, without diseases or conditions, not taking hormones or medications, nonsmokers) ≤65 years who had experienced natural menopause (within 9 months to 10 years), were not experiencing excessive vasomotor symptoms, nonsmokers, and had a BMI ranging from 18.5 through 29.9. The parent SIRBL project established the inclusion/exclusion criteria. We excluded vegans and high alcohol consumers (>7 servings/week), as well as those who were diagnosed with chronic disease, had a first-degree relative with breast cancer, or who chronically used medication (current: cholesterol-lowering and/or anti-hypertensive; past 12 months: oral hormones/estrogen or selective estrogen receptor modulators; past 6 months: estrogen/progestogen creams, calcitonin; ever: bisphosphonates; past 3 months: antibiotics).
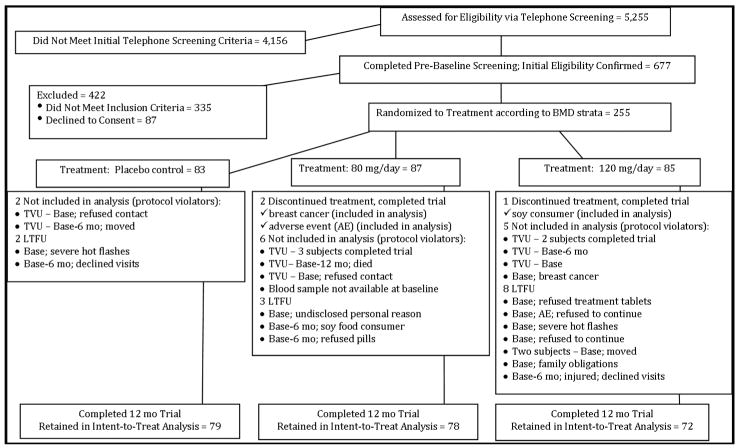
Women who met the initial screening criteria (N=677) were invited to the clinic for further eligibility screening, including BMD assessment using dual-energy X ray absorptiometery (DXA). The SIRBL project focused on disease prevention rather than treatment; thus, we excluded women with BMD lumbar spine and/or proximal femur T-scores that were low (>1.5 SD below young adult mean) or high (>1.0 SD above mean) or with evidence of previous or existing spinal fractures. Once she qualified based on BMD, blood was drawn for a chemistry profile. We excluded women with evidence of diabetes mellitus (fasted glucose ≥6.93 mmol/L [126 mg/dl]); abnormal renal, liver (elevated enzymes), and/or thyroid function; or elevated lipids (LDL-cholesterol >4.10 mmol/L [160 mg/dl]; triacylglycerol >2.25 mmol/L [200 mg/dl]). Based upon our entry criteria, 255 women were randomized to treatment in the parent trial. We excluded 13 women at UCD from this analysis because they did not meet the entry criteria (11 had thickened endometrium, 1 had breast cancer, 1 could not provide a baseline blood sample). We excluded an additional 13 women who did not have body composition data at either 6 or 12 months because they dropped out of the study, resulting in a sample size of 229 eligible women (Figure 1). For this ancillary project, we had 79 women in the placebo group, 78 in the 80 mg/day group, and 72 women in the 120 mg/day group.
FIGURE 1. Participant Screening and Enrollment Flow Chart.
Transvaginal ultrasound (TVU), lost-to-follow-up (LTFU)
We excluded 13 women at UCD from this analysis because they did not meet entry criteria and were deemed protocol violators (11 due to a thickened endometrium, 1 with breast cancer, 1 without a baseline blood sample). In addition, 13 women (11 at UCD, 2 at ISU) had missing body composition data at 6 and/or 12 month (mo) time points because they were lost to follow-up and thus excluded from the analysis. Three women discontinued treatment, but completed the trial; their data were included in the analysis.
Randomization to Treatment and Tablet Formulation
To meet the objectives of the parent project, subjects at each location (ISU, UCD) were stratified according to initial proximal femur BMD (high, medium, low) based upon NHANES III database population values (26): (1) high: > the mean (zero), but ≤ +1.0 SD above the mean; (2) medium: ≤ the mean, but > −0.75 SD below the mean; or (3) low: < −0.75 SD through −1.5 SD below the mean. Once subjects met inclusion/exclusion criteria, they were randomly assigned to one of three treatment groups within each BMD strata at each location: 1) placebo control, 2) 80 mg isoflavones, or 3) 120 mg isoflavones. Placebo material, devoid of isoflavones, consisted of maltodextrin (90%) and caramel color (10%), mixed and spray-dried to produce a brown powder, which mimicked the isoflavone extract. Active tablets contained the same excipients as placebo tablets. All treatment tablets (identical in appearance) contained the same amount of sorbitol, magnesium stearate, and dicalcium phosphate. The ratio of genistein-to-daidzein-to-glycitein (aglycone form) in these tablets was 1.3-to-1-to-0.3, similar to what is found in soybeans. An independent researcher (Patricia Murphy) at ISU confirmed that the actual versus formulated isoflavone doses (mean±SD, mg/d), respectively, were similar to that tested by The Archer Daniels Midland Co. (Decatur, IL): control = 0 vs. 0.3±0.4; 80 mg = 89.5±5.0 vs. 84.3±4.5; 120 mg = 124.0±7.7 vs. 122.5±3.4. Subjects in each group were instructed to take three compressed tablets/d. Bottles did not indicate treatment assignment in order to preserve the double-blind nature of the study.
Data Collected
We have included data that were collected at baseline, 6 month, and 12 month time points for these analyses. The primary outcomes of interest included whole body lean and fat mass, regional (specifically central) fat mass, and fasting concentrations of insulin, leptin, ghrelin, and adiponectin. We obtained anthropometric measurements using trained anthropometrists according to standard protocols. Standing height was taken twice (average value recorded) with a wall-mounted stadiometer (Model S100; Ayrton Corp., Prior Lake, MN) and weight was measured using an ABCO Health-o-Meter scale (Bridgeview, IL). Women changed into hospital scrubs or shorts and a t-shirt, and removed their shoes, belts, watches, and jewelry for the duration of anthropometric and body composition assessment.
Body composition measurements were obtained by certified DXA operators using matching DXA instruments (Delphi W Hologic Inc; Waltham, MA) at each site that were calibrated daily. Cross-training of the operators ensured standardized data collection. To further ensure quality control, one operator assessed overall composition from the whole body DXA scans from both sites. Regional adiposity analysis was performed by one analyzer (LR) who sectioned each whole body DXA scan into waist, hip, and thigh regions based on bone landmarks10 using special software (Discovery Version 12.3:7). The waist region included the first lumbar through the fourth lumbar vertebrae. The hip region began below the fourth lumbar vertebrae and extended to the tip of the greater trochanter of the femur. We calculated androidal fat mass for each subject: waist + hip mass. The thigh region extended superiorly from the greater trochanter to the approximate midpoint between the edge of the thigh region and lateral condyle of the femur. The lateral edge of each region was extended horizontally to encompass all tissue. This DXA analysis provided an estimate of the fat and lean mass within each of these three regions. We calculated the androidal-to-gynoidal fat mass ratio for each subject: waist + hip fat mass/thigh fat mass.
Phlebotomists collected fasted (9 h) blood samples between 7:00 and 8:00 am. We centrifuged blood samples for 15 minutes (4°C) at 1000 × g to separate serum (allowed to clot for 30 min prior to centrifugation) and plasma from whole blood, and stored in aliquots at −80°C until analyses. Certified clinical laboratories (LabCorp; Kansas City, KS at ISU and UCD Medical Center; Sacramento, CA at UCD) analyzed our blood samples for general health markers, including a complete blood count (CBC) with differential, chemistry panel, and thyroid screen. We determined plasma (heparnized) adiponectin, serum leptin, serum insulin, and plasma (EDTA-treated) total ghrelin concentrations in duplicate with radioimmunoassay (RIA) kits (Linco Research; St. Charles, MO) using a Cobra II series auto-gamma counting system (Packard Instrument Company; Meriden, CT). The ISU site performed these hormone analyses using manufacturer-provided quality controls and in-house quality control sera/plasma to calculate inter-assay coefficients of variation (CV); we used duplicate samples to calculate intra-assay CVs. The intra-assay and inter-assay CVs (%), respectively, for adiponectin, leptin, insulin, and ghrelin were 1.5 and 1.7, 2.4 and 2.7, 2.6 and 4.0, and 3.4 and 3.1. Based upon the chemistry profiles of participants at each time point, we included one liver enzyme (aspartate aminotransferase [AST]) that was available for all participants in the regression analyses. Serum AST activity is widely used as reliable surrogate marker of fatty liver.11 We included this liver enzyme because of the relationship between non-alcoholic fatty liver disease and central adiposity, both of which are related to an unfavorable cardio-metabolic profile.12
During the enrollment phase, trained interviewers administered two questionnaires to participants: a nutrition history13 and a health and medical history adapted with modifications.13 We asked subjects to cease taking herbal therapies and/or dietary supplements prior to baseline testing. We assessed dietary intake at each time point using a semi-quantitative food frequency questionnaire from Block Dietary Data Systems (Berkeley, CA). Selected outcomes from the questionnaires that could potentially influence body composition were included in statistical analyses as covariates. We assessed physical activity at each time point using the Paffenbarger physical activity recall14 to obtain activity information from the previous time period, including walking, climbing stairs, and sport and recreational activity. To aid subjects in recalling activities, we provided a list of common recreational and daily (i.e., cleaning, gardening, yard work, farm work) activities categorized by intensity (≤ 5.0, 5.1 to 6.9, or ≥7.0 METS). Each reported recreational or work-related activity was summed using metabolic equivalents of 4 (light), 6 (medium), or 8 (heavy), providing an estimate of weekly energy expenditure.
Statistical Analyses
We performed statistical analyses using SAS (version 9.1; Cary, NC) and considered results statistically significant (two-sided) at P ≤ 0.05. Our analyses included women with complete data at 6 and 12 months beyond the initial baseline value, regardless of treatment compliance (N = 229). We reported descriptive statistics for 229 women using means ± SD for most variables because they were normally distributed. We used median values and range for ghrelin because its data distribution was skewed. We conducted repeated measures ANOVA using the PROC MIXED procedure in SAS to examine the effects of treatment over time for each body composition outcome (whole body lean mass, whole body fat mass, androidal fat mass, and androidal-to-gynoidal fat mass ratio). To account for the variation in different observations of body composition outcomes among women, the response vector for each woman included baseline measurements to examine changes from baseline. We used REML (restricted maximum likelihood) estimation to obtain estimates of variances and correlations between repeated measures (default in SAS for PROC MIXED). For all models but one (whole body fat mass), the most appropriate covariance model for the dependence structure in the repeated measurements was an unstructured (unrestricted) covariance matrix. For whole body fat mass, we determined that a compound symmetry structure (more parsimonious) was more appropriate. These particular model selections were guided by model diagnostic statistics (Akaike’s information criteria [AIC] and Schwarz’s information criterion [BIC]) available in SAS.
Independent variables in modeling the outcomes of interest included those variables that were biologically plausible and/or significantly related to outcome variables using Pearson correlation analysis. These independent variables included: age or time since last menstrual period (TLMP), appetitive hormones (as potential mediators), liver enzymes (aspartate aminotransferase), usual physical activity (kJ/week), energy intake (kJ/day), dietary protein (g/day), and dietary fat (g/day). Each model included site (ISU and UCD) and BMD strata (high, medium, low) within site as obligatory explanatory variables, to account for the randomization of subjects to treatment within each BMD strata at each site.
RESULTS
At baseline, 229 women in this ancillary study ranged from 45.8 to 65.0 years of age and from 0.8 to 10.0 years since menopause. The sample included mainly Caucasian women (92%), three African Americans, three Asians, one Native Hawaiian, one Native American, seven women of mixed race, two of unknown race, and two who chose not to report race. Baseline mean BMI was 24.9±3.0 (range, 17.8 to 32.7); approximately half of the women had a BMI <25.0 kg/m2. The California site enrolled women beyond our BMI inclusion criteria (two women below 18.5 and seven above 29.9).
We noted wide variability in both overall and regional body fat measures as assessed by DXA (Table 1) among these women. Values for circulating analytes (Table 2) demonstrate that mean or median values were within the range reported in the literature. At baseline, assessment indicated that participants reported consuming a median (range) intake of 6,456 (1,773-19,106) kJ in energy, 61 (15–168) g in protein, and 65 (17–246) g in total fat. Other dietary intakes have been reported elsewhere (10). Physical activity-related energy expenditure (mean ± SD [range]) was 12,768±8,632 (0–51,077) kJ/week. As expected, there were no between-treatment differences in any of the outcomes of interest at baseline. Moreover, we did not find significant changes in the outcomes of interest throughout the study (except for leptin and insulin) or between treatment groups at any time point.
TABLE 1.
Characteristics of Participants at Baseline According to Treatment
| Characteristic | Entire Sample (N=229) | Placebo Control (n=79) | 80 mg/day Isoflavones (n=78) | 120 mg/day Isoflavones (n=72) |
|---|---|---|---|---|
| Age (y) | 54.3±3.3a | 54.0±3.1 | 54.7±3.5 | 54.2±3.5 |
| 45.9–65.0b | 45.8–60.1 | 48.2–65.0 | 46.5–62.0 | |
| Time since last menstrual period (y) | 3.5±2.0 | 3.5±1.9 | 3.6±2.1 | 3.4±2.0 |
| [TLMP] | 0.8–10.0 | 0.8–7.9 | 0.9–10.0 | 1.0–8.0 |
| Body Size | ||||
| Weight (kg) | 67.5±9.3 | 67.2±8.3 | 68.4±10.1 | 66.9±9.5 |
| 43.7–94.5 | 50.4–87.7 | 43.7–94.5 | 46.3–88.5 | |
| Height (cm) | 164.7±6.3 | 165.1±6.1 | 163.9±6.2 | 165.1±6.8 |
| 146.3–182.1 | 152.3–182.1 | 150.6–177.4 | 146.3–177.0 | |
| BMI (kg/m2) | 24.9±3.0 | 24.7±2.9 | 25.4±3.1 | 24.5±3.1 |
| 17.8–32.7 | 19.8–31.7 | 17.8–32.7 | 18.9–31.0 | |
| Overall body compositionc | ||||
| Fat mass (kg) | 23.1±6.3 | 23.1±5.7 | 23.7±6.3 | 22.6±7.0 |
| 8.0–47.8 | 13.6–34.7 | 8.1–42.1 | 8.4–47.8 | |
| Fat mass (%) | 34.4±5.6 | 34.6±5.2 | 34.8±5.1 | 33.7±6.6 |
| 18.1–55.9 | 24.2–44.6 | 18.6–34.9 | 18.1–55.9 | |
| Lean mass (kg) | 43.2±4.5 | 42.9±4.1 | 43.4±4.9 | 43.1±4.5 |
| 29.8–55.4 | 34.7–51.4 | 31.4–55.4 | 29.8–54.7 | |
| Regional body compositionc | ||||
| Waist fat (kg) | 2.3±1.1 | 2.3±1.1 | 2.5±1.1 | 2.3±1.2 |
| 0.4–5.3 | 0.7–5.3 | 0.6–5.2 | 0.4–5.3 | |
| Waist fat (%) | 28.5±8.6 | 28.5±8.6 | 29.2±7.9 | 27.6±9.5 |
| 7.1–52.5 | 11.1–44.0 | 11.2–44.9 | 7.1–52.5 | |
| Hip fat (kg) | 3.4±1.0 | 3.4±1.0 | 3.5±1.0 | 3.2±1.1 |
| 0.7–6.4 | 1.6–6.0 | 0.9–6.2 | 0.7–6.4 | |
| Hip fat (%) | 32.8±5.8 | 33.1±5.4 | 33.1±5.2 | 32.0±6.8 |
| 12.1–51.6 | 18.2–47.2 | 17.7–42.9 | 12.1–51.6 | |
| Thigh fat (kg) | 5.3±1.3 | 5.3±1.5 | 5.3±1.3 | 5.2±1.5 |
| 2.1–10.8 | 3.0–9.0 | 2.1–9.3 | 2.4–10.6 | |
| Thigh fat (%) | 38.7±5.0 | 39.1±4.6 | 38.8±4.7 | 39.1±4.6 |
| 24.2–59.7 | 30.4–51.4 | 24.2–48.2 | 30.4–51.4 | |
| Androidal [waist+hip] fat (kg) | 5.7±2.0 | 5.7±2.0 | 5.9±2.0 | 5.7±2.0 |
| 1.1–11.7 | 2.4–10.7 | 1.7–11.4 | 2.4–10.7 | |
| Androidal-to-gynoidal fat mass ratio | 1.2±0.3 | 1.1±0.3 | 1.1±0.3 | 1.1±0.3 |
| 0.6–2.2 | 0.5–2.2 | 0.6–2.0 | 0.5–2.2 | |
| Waist lean (kg) | 5.5±0.7 | 5.5±0.7 | 5.6±0.8 | 5.5±0.7 |
| 3.5–7.5 | 4.2–7.2 | 4.0–7.1 | 4.2–7.2 | |
| Hip lean (kg) | 6.7±0.9 | 6.7±0.9 | 6.8±1.0 | 6.7±0.9 |
| 4.4–9.3 | 5.0–8.9 | 4.4–9.0 | 5.0–8.9 | |
| Thigh lean (kg) | 8.2±1.0 | 8.1±0.9 | 8.2±1.1 | 8.1±0.9 |
| 5.4–11.4 | 6.5–10.2 | 5.8–11.4 | 6.5–10.2 | |
Mean±SD
Range
Assessed by dual-energy X ray absorptiometery (DXA). These variables were not statistically different at baseline among the three treatment groups. Body composition did not change significantly from baseline to 1 year.
TABLE 2.
Circulating Analytes of Participants (N=229) at Baseline, 6 and 12 Month Time Points
| Analytea | Baseline | 6 months | 12 months |
|---|---|---|---|
| Plasma adiponectin (μg/ml) | 17.5±5.7b | 17.3±5.7 | 17.2±5.7 |
| Serum leptinc (ng/ml) | 13.8±6.8 | 14.2±7.2 | 14.7±7.8 |
| Plasma ghrelin (pg/ml) | 1007±390 | 1017±379 | 1023±407 |
| Serum insulinc (μU/ml) | 11.6±5.4 | 11.7±5.6 | 14.3±7.0 |
| Serum aspartate aminotransferase (IU/I) | 22.9±6.0 | 22.9±5.1 | 23.3±6.1 |
Treatment had no effect on these analytes. There were no differences among the groups in circulating analytes at any time point.
Mean±SD; All variables were normally distributed, except for ghrelin (median 959.6; range 345.0–2,382.8).
Significant change (P<0.01) from baseline to 12 months, as determined by paired samples t-test.
Residual analyses for all final models (Table 3) were conducted and did not indicate violations of the assumptions of normality or homogeneity of residual variances. The 12 month treatment with soy isoflavones did not significantly affect any of the body composition measures or appetitive hormone concentrations. Time and the treatment-by-time interaction did not approach significance for any model, but were accounted for in each model. Site was also not a significant contributor in any model, whereas differences among BMD strata were significant for whole body lean mass. The inclusion of covariates in the model did not alter inferences about treatment effects for any of the outcomes. However, several covariates contributed to the body composition outcomes independently of treatment. Leptin and ghrelin (inversely related to outcomes) were the common significant contributors to each of the body composition measures, with leptin exerting a stronger effect than ghrelin. Adiponectin related inversely to all body fat measures. TLMP was associated directly with the fat mass outcomes, indicating that the longer TLMP was related in particular to greater androidal fat mass. The only dietary factor that emerged as significant was dietary fat intake for whole body and androidal fat mass. In addition, we noted significant changes between baseline and 12 months for leptin (increased) and insulin (increased) concentrations, as determined by paired samples t-test (Table 2). However, these changes were unrelated to treatment.
TABLE 3.
Repeated Measures ANOVA: Body Composition Outcomes in Response to Isoflavone Treatment (N=229)
| Parametera | Parameter Estimate (PE) | 95% CI for PE | DF Num/Den | F value | P value |
|---|---|---|---|---|---|
| Whole Body Lean Mass (kg) | |||||
| Site (ISU vs. UCD) | −2018.80 | 1/221 | 1.04 | 0.31b | |
| BMD Strata (within site) | 4/221 | 4.03 | 0.0036b | ||
| Treatment overall | 2/221 | 0.23 | 0.79c | ||
| Time | 2/221 | 0.51 | 0.60d | ||
| Treatment × Time | 4/221 | 0.54 | 0.71e | ||
| Leptin | 55.57 | 31.94 to 79.21 | 1/221 | 21.47 | ≤0.0001b |
| Ghrelin | −0.65 | −1.24 to − 0.059 | 1/221 | 4.71 | 0.031b |
| Whole Body Fat Mass (kg) | |||||
| Site (ISU vs. UCD) | 857.43 | 1/220 | 2.85 | 0.093b | |
| BMD Strata (within site) | 4/220 | 0.71 | 0.58b | ||
| Treatment overall | 2/220 | 0.97 | 0.38c | ||
| Time | 2/446 | 0.52 | 0.60d | ||
| Treatment × Time | 4/446 | 1.32 | 0.26e | ||
| Leptin | 236.44 | 202.83 to 270.06 | 1/446 | 191.1 | ≤0.0001b |
| Adiponectin | −93.98 | −145.40 to −42.56 | 1/446 | 12.9 | 0.0004b |
| Ghrelin | −0.92 | −1.74 to −0.10 | 1/446 | 4.91 | 0.027b |
| Dietary fat (g/d) | 16.87 | 1.83 to 31.91 | 1/446 | 4.86 | 0.028b |
| TLMP | 310.83 | −13.20 to +634.85 | 1/220 | 3.99 | 0.060b |
| Androidal Fat Mass (kg) | |||||
| Site (ISU vs. UCD) | 61.41 | 1/220 | 1.88 | 0.17b | |
| BMD Strata (within site) | 4/220 | 1.22 | 0.304b | ||
| Treatment overall | 2/220 | 1.02 | 0.36c | ||
| Time | 2/220 | 1.35 | 0.26d | ||
| Treatment × Time | 4/220 | 1.06 | 0.38e | ||
| Leptin | 77.82 | 66.34 to 89.30 | 1/220 | 178.38 | ≤0.0001b |
| Adiponectin | −38.88 | −56.29 to −21.48 | 1/220 | 19.39 | ≤0.0001b |
| TLMP | 142.04 | 42.13 to 241.94 | 1/220 | 7.85 | 0.0055b |
| Dietary fat (g/d) | 6.41 | 1.27 to 11.64 | 1/220 | 5.81 | 0.017b |
| Ghrelin | −0.29 | −0.57 to −0.0078 | 1/220 | 4.10 | 0.044b |
| Androidal-to-Gynoidal Fat Mass | |||||
| Site (ISU vs. UCD) | −0.048 | 1/220 | 0.02 | 0.89b | |
| BMD Strata (within site) | 4/220 | 1.24 | 0.296b | ||
| Treatment overall | 2/220 | 1.00 | 0.37c | ||
| Time | 2/220 | 1.24 | 0.29d | ||
| Treatment × Time | 4/220 | 1.59 | 0.18e | ||
| Adiponectin | −0.0061 | −0.0089 to −0.0032 | 1/220 | 17.74 | ≤0.0001b |
| Leptin | 0.0036 | 0.0018 to 0.0055 | 1/220 | 15.05 | ≤0.0001b |
| TLMP | 0.026 | 0.0091 to 0.043 | 1/220 | 9.23 | 0.0027b |
| Ghrelin | −0.00005 | −0.0001 to −9.75E-6 | 1/220 | 5.75 | 0.017b |
Abbreviations: ISU = Iowa State University; UCD = University of California, Davis; BMD = Bone Mineral Density; TLMP = Time since12 Last Menstrual Period.
Independent variables for each body composition outcome model included: site (ISU and UCD), BMD strata, age or TLMP, appetitive hormones (adiponectin, leptin, insulin, ghrelin), aspartate aminotransferase, usual physical activity (kJ/week), energy intake (kJ/day), dietary protein (g/day), and dietary fat (g/day). This table shows the results for main effects (treatment, time and treatment-by-time interaction) for each outcome model, as well as covariates that associated significantly (or showed tendency) with each body composition measure. Non-significant covariates have not been reported herein.
P value for F-test to assess association between covariates and outcome variable.
P value for F-test to assess difference in outcome variable between treatments.
P value for F-test to assess a change in outcome variable over time.
P value for F-test to assess treatment-by-time interaction (existence of parallel treatment profiles over time).
DISCUSSION
Several studies have reported that soy isoflavones either from dietary sources or supplements exerted significant, favorable effects on lean mass,6 whole body fat mass,8 regional fat,15–16 or BMI.16 In contrast, our study showed no effect of isoflavone tablets on overall or regional body composition in healthy postmenopausal women. The main strengths of our randomized, placebo-controlled clinical trial compared with previously published reports are that we included a large sample size, used two isoflavone doses (moderate 80 mg and high 120 mg), continued the study for 12 months, and performed a state-of-the-art analysis using DXA to assess overall and regional body composition. In addition, we included appetitive hormones as potential mediators of body composition in our statistical models. However, we must point out that our study sample was rather homogeneous. Thus, our findings should only be extrapolated to a similar group of women. Our participants included healthy women, the vast majority of whom were either in the normal or overweight BMI categories and were within five years of menopause when they enrolled in this study. There is some evidence that the early postmenopausal period is characterized by pronounced changes in body composition, with an increase in both overall and intra-abdominal adiposity.17–19
One possible explanation for the difference between our findings and those of previously published studies is that our treatment consisted of isoflavone extract, whereas a few studies5,7 used soy protein containing isoflavones. A well-designed clinical trial examining soy protein isolate versus isoflavones would help clarify what component might be responsible for the favorable changes in body composition that have been observed in some studies. Another explanation might be that particular isoflavone composition and/or larger doses of isoflavones may be required to significantly influence body composition. Human studies5–8 that have examined the effect of soy isoflavones on body composition tested dosages in the range of 70 mg to 160 mg a day. The composition of soy isoflavone supplements varied among studies. Composition was 50% genistein, 40% daidzein, and 10% glycitein in the Moeller et al. study5 and 63% daidzein, 23% glycitein, and 14% genistein in the Aubertin-Leheudre et al. study.6 The other two studies reported using dosages of 160 mg/d (96 mg were provided as aglycones)7 and 100 mg/d,8 but did not indicate isoflavone profiles or further information about their supplements. Penza et al.20 found that treating mice with genistein affected body composition in a dose-dependent manner, with pharmacological doses of 200,000 μg/kg (vs. nutritional doses of 50,000 μg/kg) inhibiting fat deposition. However, extrapolating from animal models to humans is difficult.
Our study also examined the effect of isoflavone treatment on appetitive hormones. Although research in this area is limited, previous studies showed that a soy protein diet or isoflavone treatment significantly lowered insulin in animals21 and postmenopausal women.22–23 In contrast, the consumption of a soy protein-isoflavone (56 mg or 90 mg) mixture for 6 months had no significant effect on insulin in postmenopausal women after taking into account the baseline insulin concentration.24 Similarly, the consumption of a cereal bar enriched with 50 mg of isolated isoflavones for 8 week did not change insulin or ghrelin concentrations in 34 healthy postmenopausal women in the normal and overweight BMI categories. 25,26 Isoflavone treatment for 93 days did not influence leptin concentration in a small sample of premenopausal and postmenopausal women.27 In contrast, Nikander et al.28 reported that ghrelin concentration differed significantly between non-diabetic postmenopausal women with a history of breast cancer who consumed 114 mg/day of isoflavones for three months compared with those in the placebo control group.28 With respect to adiponectin, there are no long-term human studies, and only one study examined the effect of soy protein isolate on the regulation of adipokines in Wistar rats.29 Their findings showed that a soy protein diet increased the expression and plasma concentration of adiponectin. However, it was unclear which soy compounds influenced the outcome. In summary, there have been a few studies on the effects of soy isoflavones on appetitive hormones with inconsistent findings. To our knowledge, our study is the first large-scale, longitudinal human trial to show that prolonged intake of soy isoflavone tablets does not affect appetitive hormone concentrations in healthy postmenopausal women.
Although isoflavone tablets did not influence major outcomes of interest in this study, a few interesting and significant findings emerged from our analyses. Leptin was positively and ghrelin was negatively associated (consistently) with each of the outcomes of interest, whereas adiponectin was negatively associated with the fat mass outcomes. These results are in agreement with the current knowledge about the relationships between appetitive hormones and body composition.30 Dietary fat intake was positively related to whole body and androidal fat mass, as expected and shown in other studies.31 In contrast, dietary fat intake or other dietary variables did not affect lean mass. In addition, the longer the TLMP, the greater the fat mass in these women, which has also been previously reported in the literature,32 particularly for androidal fat mass. However, as expected, TLMP was not related to lean mass in these women.
CONCLUSIONS
Findings from our study do not support a claim that intake of soy isoflavone tablets for 12 months favorably affects body composition in healthy postmenopausal women. In addition, soy isoflavone tablets did not influence the appetitive hormones examined in this study. However, we noted the expected relationship between appetitive hormones and body composition (total and regional), as well as between dietary fat intake and fat mass. Body composition – both fat mass and lean mass – are important contributors to disease risk and health outcomes.33 Because our results indicated that extracted soy isoflavones did not influence body composition, we cannot recommend intake of these tablets at this time to modify adiposity in healthy women.
Acknowledgments
Funding: The overall project described was supported by a grant (RO1 AR046922) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The project was also supported by a grant (P01 ES012020) from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), and by a grant (95P50AT004155) from the National Center of Complementary and Alternative Medicine (NCCAM) and ODS of the National Institutes of Health. The project was supported by USDA, ARS, Western Human Nutrition Research Center, and the Clinical & Translational Science Center, Clinical Research Center at the University of California (1M01RR19975-01) and National Center for Medical Research (UL1 RR024146). Archer Daniels Midland Company (Decatur, IL) donated soy isoflavone tablets (Novasoy®) and GlaxoSmithKline (Moon Township, PA) donated calcium and vitamin D supplements (Os-Cal®, distributed through Fisher Clinical Services [Allentown, PA]).
Footnotes
Publisher's Disclaimer: Disclaimer: The content and views expressed in this article are the sole responsibility of the authors and do not necessarily represent the official views of these funding agencies. Funding agencies did not play a role in data collection, management, analysis, or interpretation; or in manuscript preparation or review.
Trial Registration: NIH clinicaltrials.gov Identifier: NCT00043745
Reprints will not be available.
Author Contributions
Study concept and design; secured funding: Alekel, Matvienko
Acquisition of data: Alekel, Matvienko, Ritland, Van Loan
Study supervision: Alekel, Van Loan
Administrative, technical support: Alekel, Van Loan
Statistical analysis and support; writing statistics section: Genschel, Koehler
Interpretation of data: Alekel, Genschel, Matvienko
Drafting of manuscript: Alekel, Matvienko
Critical revision of manuscript for important intellectual content: Alekel, Genschel, Matvienko
Final approval of manuscript: Alekel, Genschel, Koehler, Matvienko, Ritland, Van Loan
Conflict of interest: The authors have no conflicts of interest.
Conflicts of Interest/Financial Disclosures: Coauthors have not reported any conflicts or disclosures.
Additional Contributions
The SIRBL study team would like to thank all of our participants, since without their dedication, this study could not have been completed. We would like to acknowledge our phlebotomists and students (graduate and undergraduate alike) who reported early and steadfastly for testing at our clinic sites. We thank the James R. Randall Research Center, Archer Daniels Midland Company (Decatur, IL) that manufactured and supplied free-of-charge the treatment (Novasoy®) and control tablets, as well as Atrium Biotechnologies Inc. that compressed the ingredients into tablets. We thank GlaxoSmithKline (Moon Township, PA) for donating the calcium and vitamin D supplements (Os-Cal) that were distributed through Fisher Clinical Services (Allentown, PA). We would also like to thank our DSMB and Joan McGowan, PhD, Director, Musculoskeletal Diseases Branch at NIAMS, who provided scrutiny, guidance, and valuable feedback throughout the trial. We are also grateful to the following individuals who worked on various aspects of this project:
Project Coordinators: Laura Hanson, MS, RD (ISU); Oksana Matvienko, PhD (ISU); Allyson Sage, MS (UCD); Carol Chandler, PhD (UCD)
Database Manager/SAS Programmer for statistical analysis for DSMB reports: Kathy Shelley, MS (ISU database manager); C. Ted Peterson, MS (ISU programmer); Christine Chiechi (UCD programmer)
Medical Consultants: Bonnie Beer, MD ( McFarland Clinic, Ames, IA) and Tom Wold, MD (Mather Women’s Health Clinic, Mather, CA), responsible for performing medical procedures as needed
Laboratory Technician: Jeanne W. Stewart, MS (ISU)
DXA Operators: Kathy Hanson, PhD (ISU) analyzed/reviewed DXA scans. Barbara Gale (UCD)
Summary Statement: This study examined the effect of soy isoflavones on overall and regional body composition taking into account appetitive hormones as potential mediators, as well as the direct effect of treatment on appetitive hormones. One year treatment with soy isoflavones did not alter body composition in healthy postmenopausal women.
References
- 1.Hanson KB, Song TT, Peterson CT, et al. Daidzein exerts a marked effect on body composition in male hamsters. Presented at the 3rd International Symposium on the Role of Soy in Preventing and Treating Chronic Diseases; October 31–November 3, 1999; Washington DC., USA. [Google Scholar]
- 2.Arjmandi BH, Alekel L, Hollis BW, et al. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996:161–7. doi: 10.1093/jn/126.1.161. [DOI] [PubMed] [Google Scholar]
- 3.Szkudelska K, Nogowski L. Genistein – a dietary compound inducing hormonal and metabolic changes. J Steroid Biochem Mol Biol. 2007;105:37–45. doi: 10.1016/j.jsbmb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Wagner JD, Cefalu WT, Anthony MS, Litwak KN, Zhang L, Clarkson TB. Dietary soy protein and estrogen replacement therapy improve cardiovascular risk factors and decrease aortic cholesterol ester content in ovariectomized cynomologus monkeys. Metabolism. 1997;46:698–705. doi: 10.1016/s0026-0495(97)90016-0. [DOI] [PubMed] [Google Scholar]
- 5.Moeller LE, Peterson CT, Hanson KB, et al. Isofavone-rich soy protein prevents loss of hip lean mass, but does not prevent the shift in regional fat distribution in perimenopausal women. Menopause. 2003;10:322–31. doi: 10.1097/01.GME.0000054763.94658.FD. [DOI] [PubMed] [Google Scholar]
- 6.Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Six months of isoflavone supplement increass fat-free mass in obese-sarcopenic postmenopausal women: a randomized double-blind controlled trial. Eur J Clin Nutr. 2007;61:1442–4. doi: 10.1038/sj.ejcn.1602695. [DOI] [PubMed] [Google Scholar]
- 7.Sites CK, Cooper BC, Toth MJ, Gastaldelli A, Arabshahi A, Barnes S. Effect of daily supplement of soy protein on body composition and insulin secretion in postmenopausal women. Fertil Steril. 2007;88:1609–17. doi: 10.1016/j.fertnstert.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori M, Aizawa T, Tokoro M, Miki T, Yamori Y. Soy isoflavone tablets reduce ostoporosis risk factors and obesity in middle-aged Japanese women. Clin Exp Pharmacol Physiol. 2004;31 (Suppl 2):S39–41. doi: 10.1111/j.1440-1681.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 10.Ritland LM, Alekel DL, Matvienko OA, et al. Centrally located body fat is related to appetitive hormones in healthy postmenopausal women. Eur J Endocrinol. 2008;158:889–97. doi: 10.1530/EJE-07-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–33. viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Oh MK, Winn J, Poordad F. Review article: diagnosis and treatment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:503–22. doi: 10.1111/j.1365-2036.2008.03752.x. [DOI] [PubMed] [Google Scholar]
- 13.Alekel DL, St Germain A, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–52. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- 14.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Oka J, Tabata I, et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: a 1-year randomized placebo-controlled trial. J Bone Miner Res. 2006;21:780–9. doi: 10.1359/jbmr.060208. [DOI] [PubMed] [Google Scholar]
- 16.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;13:1202–6. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 17.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–58. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CJ, Wu CH, Yao WJ, Yang YC, Wu JS, Lu FH. Relationships of age, menopause and central obesity on cardiovascular disease risk factors in Chinese women. Int J Obes Relat Metab Disord. 2000;24:1699–704. doi: 10.1038/sj.ijo.0801457. [DOI] [PubMed] [Google Scholar]
- 19.Ozbey N, Sencer E, Molvalilar S, Orhan Y. Body fat distribution and cardiovascular disease risk factors in pre- and postmenopausal obese women with similar BMI. Endocr J. 2002;49:503–9. doi: 10.1507/endocrj.49.503. [DOI] [PubMed] [Google Scholar]
- 20.Penza M, Montani C, Romani A, et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology. 2006;147:5740–51. doi: 10.1210/en.2006-0365. [DOI] [PubMed] [Google Scholar]
- 21.Ascencio C, Torres N, Isoard-Acosta F, Gómez-Pérez FJ, Hernández-Pando R, Tovar AR. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J Nutr. 2004;134:522–9. doi: 10.1093/jn/134.3.522. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SY, Shaw NS, Tsai KS, Chen CY. The hypoglycemic effects of soy isoflavones on postmenopausal women. Womens Health (Larchmt) 2004;13:1080–6. doi: 10.1089/jwh.2004.13.1080. [DOI] [PubMed] [Google Scholar]
- 23.Atteritano M, Marini H, Minutoli L, et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92:3068–75. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 24.Persky VW, Turyk ME, Wang L, et al. Effect of soy protein on endogenous hormones in postmenopausal women. Am J Clin Nutr. 2002;75:145–53. doi: 10.1093/ajcn/75.1.145. [DOI] [PubMed] [Google Scholar]
- 25.Hall WL, Vafeiadou K, Hallund J, et al. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr. 2006;83:592–600. doi: 10.1093/ajcn.83.3.592. [DOI] [PubMed] [Google Scholar]
- 26.Weickert MO, Reimann M, Otto B, et al. Soy isoflavones increase preprandial peptide YY (PYY), but have no effect on ghrelin and body weight in healthy postmenopausal women. J Negat Results Biomed. 2006;5:11. doi: 10.1186/1477-5751-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phipps WR, Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Kurzer MS. Lack of effect of isoflavonic phytoestrogen intake on leptin concentrations in premenopausal and postmenopausal women. Fertil Steril. 2001;75(6):1059–64. doi: 10.1016/s0015-0282(01)01777-0. [DOI] [PubMed] [Google Scholar]
- 28.Nikander E, Tiitinen A, Laitinen K, Tikkanen M, Ylikorkala O. Effects of isolated isoflavonoids on lipids, lipoproteins, insulin sensitivity, and ghrelin in postmenopausal women. J Clin Endocrinol Metab. 2004;89:3567–72. doi: 10.1210/jc.2003-032229. [DOI] [PubMed] [Google Scholar]
- 29.Nagasawa A, Fukui K, Kojima M, et al. Divergent effects of soy protein diet on the expression of adipocytokines. Biochem Biophys Res Commun. 2003;311:909–14. doi: 10.1016/j.bbrc.2003.10.087. [DOI] [PubMed] [Google Scholar]
- 30.Meier U, Gressner AM. Endocrine Regulation of Energy Metabolism: Review of Pathobiochemical and Clinical Chemical Aspects of Leptin, Ghrelin, Adiponectin, and Resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 31.Astrup A. The role of dietary fat in obesity. Semin Vasc Med. 2005;5:40–7. doi: 10.1055/s-2005-871740. [DOI] [PubMed] [Google Scholar]
- 32.Guthrie JR, Dennerstein L, Taffe JR, et al. Central abdominal fat and endogenous hormones during the menopausal transition. Fertil Steril. 2003;79:1335–40. doi: 10.1016/s0015-0282(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 33.Brown WV, Fujioka K, Wilson PW, Woodworth KA. Obesity: why be concerned? Am J Med. 2009;122(4 Suppl 1):S4–11. doi: 10.1016/j.amjmed.2009.01.002. [DOI] [PubMed] [Google Scholar]