Abstract
Background
Functional neuroimaging data from adults have, in general, found frontocerebellar dysfunction associated with acute and chronic marijuana (MJ) use (Loeber & Yurgelun-Todd, 1999). One structural neuroimaging study found reduced cerebellar vermis volume in young adult MJ users with a history of heavy polysubstance use (Aasly et al., 1993). The goal of this study was to characterize cerebellar volume in adolescent chronic MJ users following one month of monitored abstinence.
Method
Participants were MJ users (n=16) and controls (n=16) aged 16-18 years. Extensive exclusionary criteria included history of psychiatric or neurologic disorders. Drug use history, neuropsychological data, and structural brain scans were collected after 28 days of monitored abstinence. Trained research staff defined cerebellar volumes (including three cerebellar vermis lobes and both cerebellar hemispheres) on high-resolution T1-weighted magnetic resonance images.
Results
Adolescent MJ users demonstrated significantly larger inferior posterior (lobules VIII-X) vermis volume (p<.009) than controls, above and beyond effects of lifetime alcohol and other drug use, gender, and intracranial volume. Larger vermis volumes were associated with poorer executive functioning (p’s<.05).
Conclusions
Following one month of abstinence, adolescent MJ users had significantly larger posterior cerebellar vermis volumes than non-using controls. These greater volumes are suggested to be pathological based on linkage to poorer executive functioning. Longitudinal studies are needed to examine typical cerebellar development during adolescence and the influence of marijuana use.
Keywords: Adolescents, MRI, Cerebellum, Vermis, Cannabis, Alcohol, Drug Effects
1. INTRODUCTION
Marijuana (MJ) (active ingredient delta-9-tetrahydrocannabinol or THC) continues to be the most popular illicit drug used among teens, with almost half of 12th graders having tried MJ (Johnston et al., 2008). Early use of MJ, before the age of 15, is particularly problematic, as it increases the risk for developing a substance use disorder (SUD) in the future seven-fold (SAMHSA, 2004). Concomitant alcohol and MJ use is common, as 58% of adolescent drinkers also use MJ (Agosti et al., 2002; Martin et al., 1996). Given the high prevalence of MJ use during adolescence, a time of continued neurodevelopment (e.g., Giedd et al., 1996b; Gogtay et al., 2004; Lenroot and Giedd, 2006; Nagel et al., 2006; Pfefferbaum et al., 1994; Sowell et al., 2004), the influence of chronic use of MJ on brain morphometry among teens is of great interest.
Animal and human studies have suggested that the cerebellum may be vulnerable to the effects of chronic MJ exposure (Chang & Chronicle, 2007; Loeber &Yurgelun-Todd, 1999; Quickfall & Crockford, 2006). THC affects the brain via the cannabinoid receptors (CB1). Animal studies have shown high densities of the CB1 in many brain regions, including the cerebellum, frontal lobes, basal ganglia, limbic forebrain, hypothalamus, and hippocampus (Iverson, 2003; Herkenham et al., 1990; Herkenham et al., 1991a; Herkenham et al., 1991b; Herkenham et al., 1992; Mackie, 2008; Yoshida et al., 2006), although non-human animals have greater cerebellar CB1 density than humans (McPartland et al., 2007; Herkenham et al., 1990). Endogenous cannabinoids have inhibitory effects on both excitatory and inhibitory neurotransmitter release, and play an important role in control of neural circuits, such as in the cerebellum (Childers & Breivogel, 1998; Ghozland et al., 2002; Harkany et al., 2008 Iverson, 2003; Pazos et al., 2005; Pistis et al., 2004; Lau & Schloss, 2008; Suárez et al., 2008). More specifically, animal research has suggested a prominent role of the endogenous cannabinoid system as a retrograde messenger in Purkinje cell synapses, the principal cortical neuron in the cerebellum (Suarez et al., 2008; Yamasaki et al., 2006).
The cerebellum plays a pivotal roll in balance and psychomotor speed, as well as language generation, time estimation, rhythm production, inhibition, attention, and associative memory (Allen & Courchesne, 2003; Courchesne et al., 1994; Ivry & Keele, 1989; Leiner et al., 1993; Luna et al., 2001; Mathew et al., 1998; Timmann et al., 2002; Schmahmann & Sherman, 1998). Consistent with this, exposure to exogenous cannabinoids in animals result in motor abnormalities (Adams & Martin, 1996; Iverson, 2003; Rodriguez de Fonseca et al., 1998; Patel & Hillard, 2001) that are at least partially mediated by CB1 receptor changes in the cerebellum (Casu et al., 2005; Dar, 2000; DeSanty & Dar, 2001).
These animal findings are supported by human neuroimaging studies using functional magnetic resonance imaging (fMRI), positron emission tomography (PET) or single photon emission computed tomography (SPECT). Chronic MJ users have shown abnormal cerebellar or vermis functioning compared to controls (Amen & Waugh, 1998; Block et al., 2000; Block et al., 2002; Bolla et al., 2005; Chang et al., 2006; O’Leary et al., 2000; O’Leary et al., 2002; O’Leary et al., 2003; Sneider et al., 2008; Sneider et al., 2006; Volkow et al., 1996). Behavioral studies on MJ users have also found evidence of cognitive deficits that are thought to be associated with cerebellar functioning. For example, studies have found abnormal time estimation following acute (Hicks et al., 1984; Mathew et al., 1998; McDonald et al., 2003; Lieving et al., 2006) and chronic (Solowij et al., 2002) MJ exposure. Skosnik and colleagues (2008) found abnormalities in classical eyeblink conditioning, which is mediated by the cerebellum, in chronic adolescent and young adult MJ users. With few exceptions (Carlin & Trupin, 1977; Pope et al., 2002; Schaeffer, et al., 1981), studies of adult MJ users have demonstrated deficits in processing speed, attention, and executive functioning (Bolla et al., 2002; Croft et al., 2001; Ehrenreich et al., 1999; Fried et al., 2005; Lyons et al., 2004; Mathew et al., 1998; Messinis et al., 2006; Pope et al., 1997; Pope & Yurgelun-Todd, 1996; Solowij et al., 2002; Varma et al., 1988; Wadsworth et al., 2006; Whitlow et al., 2004).
Despite behavioral abnormalities implicating the cerebellum, few studies have examined structural brain changes as a result of MJ exposure. One such study found reduced cerebellar vermis volume in young adult MJ users with a history of heavy polysubstance use; however, these findings may have been due to substantial alcohol consumption in the sample (Aasly et al., 1993). Block and colleagues (2000) did not find significant differences in left or right cerebellar volumes in young adult MJ users as compared to non-using controls.
It is important to note that findings from adult studies may not generalize to youth. The endocannabinoid system continues to develop and modulate neurotransmitter systems during adolescent neurodevelopment (Belue et al., 1995; Viveros et al., 2005). Functional MRI studies have demonstrated differences in cerebellar activity across adolescence (e.g., Luna et al., 2001). Although numerous studies have demonstrated adolescent structural brain maturation in the cortex and subcortical regions (e.g., Giedd et al., 1996; Lenroot and Giedd, 2006; Nagel et al., 2006), including pruning of gray matter and myelination of white matter, comparatively few studies have specifically examined cerebellar development. Giedd and colleagues (1996) did not find significant changes in cerebellar size in boys and girls between the ages of 4-18. In contrast, a later report stated that the cerebellum was one of the latest structures to reach peak volume, at approximately age 16, in 36 mostly male healthy adolescents followed longitudinally from ages 8-19 (Mackie et al., 2007). Consistent with this finding, Castellanos et al (2002) found a slight increase in cerebellar volume from ages 10 to 20, although the changes in late adolescence were modest. Hill and colleagues (2007) found reduced gray matter volume in the cerebellum among older adolescents compared to younger adolescents, suggesting cerebellar gray matter pruning.
Clouding the developmental picture, the cerebellum may be particularly vulnerable to environmental impact. Wallace and colleagues (2006) examined the heritability of brain morphometry in several regions among 5 to 18 year-old monozygotic and dyzygotic twin pairs. The most distinct pattern was found in the cerebellum, which had an additive genetic factor of only.49 compared to .77-.89 in other areas of the cortex. Further, unlike the other brain regions, heritability of cerebellar morphometry did not differ according to age. Therefore, cerebellar development may be more environmentally influenced than other brain regions, possibly rendering the cerebellum vulnerable to environmental exposures such as chronic MJ use.
Despite heavy use during adolescence, few studies have examined the effects of chronic MJ use on the cerebellum during this developmental stage. In human adolescents (Medina et al., 2007), chronic MJ exposure has been associated with cerebellar-related cognitive functions, including slower psychomotor processing speed and poorer executive functioning. Our previous investigation indicated moderately larger prefrontal cortex volumes in MJ-using girls compared to non-using females (Medina et al., In Press). Further, our laboratory found that although adolescent MJ users did not significantly differ from controls in hippocampal volume, hippocampal volume was unrelated to verbal memory unlike in normal controls, and increased MJ use was associated with larger left hippocampal volume (Medina et al., 2007). Using voxel-based morphometry, Jarvis and colleagues (2008) found significantly larger gray matter volumes in the cerebellar vermis in 7 adolescents with bipolar disorder and cannabis use disorders compared to 7 with bipolar disorder alone. No studies to date have focused on cerebellar morphometry in adolescent MJ users without psychiatric comorbidities. Therefore, the goal of the current study was to characterize cerebellar morphometry and cerebellar-associated cognitive functioning in 32 adolescents with and without chronic MJ exposure. The secondary goal was to examine whether gender moderated the effects of MJ exposure on cerebellar morphometry, as was found to be the case for the prefrontal cortex (Medina et al., In Press).
2. METHODS
2.1 Participants
As part of an ongoing study, teens were recruited from local schools via flier distribution (e.g., Medina et al., 2007; Tapert et al., 2007). To assess study eligibility, a comprehensive telephone screen was administered to both adolescents and parents/guardians. Inclusion criteria required that youth were 16-18 years old, fluent in English, and had a parent or legal guardian available to consent (for those under 18) and provide historical data. Exclusionary criteria included: history of chronic medical illness, neurological condition, or head trauma with loss of consciousness >2 minutes; history of DSM-IV Axis I disorder (other than substance use disorder) or use of psychoactive medications; significant prenatal alcohol (≥4 drinks in a day or ≥7 drinks in a week) or drug exposure; complicated delivery or premature birth (<33 weeks gestation); learning disability or mental retardation; first-degree relative with history of bipolar I or psychotic disorders; left-handedness; and sensory problems. If at any time during the 28-day abstinence period a participant reported or tested positive for any substance use, he/she was excluded from study.
Teens were classified into two groups based on MJ use: a MJ using (“MJ user”) or a drug-free (“control”) group. Criteria for the MJ user group included >60 lifetime MJ experiences; past month MJ use at time of study enrollment; <25 lifetime uses of any drug other than MJ, alcohol, or nicotine; and not meeting criteria for heavy drinking status (Cahalan et al., 1969). Control group classification criteria were: <5 lifetime experiences with MJ (none in the past month), no previous use of any other drug except nicotine or alcohol, and not meeting criteria for heavy drinking status.
2.2 Measures
2.2.1 Detailed Screening Interview
The computerized DISC Predictive Scales (DPS) (Lucas et al., 2001) was administered to exclude participants with major psychiatric disorders, including DSM-IV Axis I mood, anxiety, attention deficit hyperactivity and conduct disorders. Parallel modules of the computerized Diagnostic Interview Schedule (C-DIS-IV; Robins et al., 1996) were used for 18-year-olds. The Structured Clinical Interview (SCI) measured psychosocial functioning, last menstruation (for females), health history, and handedness.
2.2.2 Parent Interview
If the teen continued to be eligible, their parent or guardian underwent a detailed screening interview using the parent version of the SCI, including information on prenatal, infant, and early childhood development, childhood behavior, medical history, parental socioeconomic status (Hollingshead, 1965), and family history of psychiatric and substance use disorders (Rice et al., 1995). For adolescents younger than 18, the parents/guardians were also administered the parent version of the DPS.
2.2.3 Substance Use Interview
Teens were administered the Customary Drinking and Drug Use Record (CDDR) to assess past 3-month and lifetime substance use, withdrawal symptoms, DSM-IV abuse and dependence criteria, and substance-related life problems (Brown et al., 1998; Stewart and Brown, 1995). The modified Time-Line Followback (TLFB; Sobell and Sobell, 1992) was administered to obtain detailed information regarding type, quantity, and frequency of drug use during the month prior to the monitored abstinence period. Teens were asked frequency of use for each of the following drugs: MJ, alcohol, nicotine, stimulants (cocaine, amphetamine, methamphetamine, ecstasy), opiates (heroin, narcotic pain relievers other than as prescribed), dissociatives/hallucinogens (PCP, mushrooms, LSD, ketamine), sedatives (GHB, barbiturates, benzodiazepines), and misuse of other prescription or over-the-counter medications. Parents were also administered a 28-day TLFB to assess youths’ recent substance use.
2.2.4. Reading Ability
The Wide Range Achievement Test-3rd Edition Reading Subtest was administered to establish reading ability, which is an estimate of premorbid intelligence, which also reflects quality of education (Wilkinson, 1993).
2.2.5 Executive Functioning
As part of the larger study, all teens completed a neuropsychological battery. A composite variable comprised of executive functioning variables was calculated for the entire sample (N=32) using scores from the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) Verbal Fluency total correct, Tower total achievement score, and Tower error scores (Medina et al., 2007a; Medina et al., In Press). A second composite variable reflected psychomotor processing speed, which comprised scores from the D-KEFS Trail Making Test Number Sequencing and Letter Sequencing subtest scores. Finally, a 60-second time estimation task was administered (scored as amount of deviation from 60 seconds).
2.3 Procedures
Eligible adolescents were scheduled to begin a monitored abstinence protocol, followed by neuropsychological testing and an MRI session. As part of the abstinence protocol, teens were monitored with supervised urine and Breathalyzer tests every 3-4 days for a period of 4 weeks. Youths with positive urine samples or breath alcohol concentrations or who appeared intoxicated were offered the option of restarting the abstinence procedure at a later time or to discontinue the study. If toxicology results indicated cessation and maintenance of abstinence, the adolescent completed the research battery. Upon completion of the study, youth and parents/guardians received financial compensation for participation.
High-resolution anatomical magnetic resonance images were collected on a 1.5 Tesla GE Signa LX (Milwaukee, WI) system using a sagittally acquired inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (TR = 2000 ms, TE = 16 ms, FOV = 240 mm, voxel dimensions = 0.9375 × 0.9375 × 1.328 mm, 128 continuous slices, acquisition time = 8:36) (Wong, 2000). Each participant’s high resolution anatomical image was manually AC-PC aligned and skull-stripped using a combination of a hybrid watershed and deformable surface semi-automated skull-stripping program (Segonne et al., 2004), followed by manual editing to calculate intracranial volume (ICV). Cerebellar and vermis regions of interest (ROI) were defined manually (see details below) in AFNI (Cox, 1996) by raters who attained high levels of inter-rater reliability (intraclass correlation coefficients >.95) prior to data collection.
2.4 Data Processing
As stated above, all ROIs were manually delineated on 1-mm sagittal 3D image slices. The cerebellar and vermis ROI protocol was developed by KLM based partly on previously published methods (DelBello et al., 1999; Piguet et al., 2006) and atlas boundaries as defined in Schmahmann and colleagues (1999). To delineate the vermis, tracers identified the midsagittal slice within the cerebellum. Three sections of the vermis were traced on a maximum of three slices in each lateral direction (totaling a maximum of 7 slides for each vermis) (similar to DelBello et al., 1999); if the vermis was no longer distinguishable from the cerebellar hemisphere in these three slices, tracing stopped. The vermis was separated into three sections (DelBello et al., 1999; Piquet et al., 2006; Schmahmann et al., 1999): 1) anterior vermis included lobules I-IV, the 2) superior posterior vermis included lobules VI-VII, and the 3) inferior posterior vermis included lobules VIII-X.
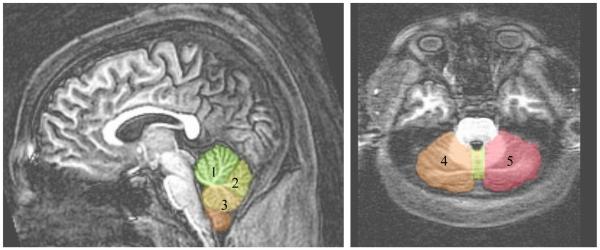
More specifically, the anterior vermis ROI boundaries included the apex of 4th ventricle, anterior limit of primary fissure (including most white matter), and superior/anterior boundaries of the vermis. The superior posterior vermis ROI boundaries included the posterior limit of primary fissure, superior limit of prepyramidal fissure, and the posterior boundary of the vermis. The inferior posterior vermis ROI boundaries included the inferior limit of the prepyramidal fissure to the apex of 4th ventricle and excluded the cerebellar tonsil. Starting at the midsagittal slice, the cerebellar hemispheres were traced laterally; this included the tonsils and excluded the pons and middle cerebellar peduncle. The corpus medullare was included until it clearly became the peduncle. See Figure 1 for midsaggital, axial and coronal slices of these ROI boundaries. All volumes were analyzed as a ratio to overall ICV to control for individual variability in brain size (Giedd et al., 1996b).
Figure 1.
Midsagittal and axial sections of three vermis (1=anterior vermis, 2=superior posterior vermis, 3=inferior posterior vermis) and two cerebellar hemisphere (4=right hemisphere, 5=left hemisphere) boundary delineations.
2.5 Data Analysis
ANOVAs and chi-square tests compared groups on important demographic and drug use variables. Bivariate correlations between drug use variables, executive functioning, psychomotor speed, time estimation and cerebellar volumes were run. Interpretations of statistical significance were made if p<.05.
Because relatively little is known regarding cerebellar development, although substantial neurodevelopment is expected during adolescence, we used polynomial regressions to examine whether linear or quadratic relationships existed between age (centered age entered in block one, and centered age squared entered in block two) and the cerebellar and vermis ROI volumes, after controlling for gender, separately by group. This information was meant to help inform the interpretation of the effects of MJ on cerebellar volume. Due to the cross-sectional nature of the data, these results are viewed as preliminary and need to be replicated in longitudinal studies. Interpretations of statistical significance were made if p<.05.
To assess relationships between group status, gender-by-group interactions, and cerebellar volumes, ordinary least squares multiple regressions were run with each of the five cerebellar variables (left hemisphere, right hemisphere, anterior vermis, superior posterior vermis, inferior posterior vermis). The first block entered the following independent variables: group status (MJ-user vs. control), gender, lifetime alcohol use, and lifetime other drug use (any drugs besides alcohol, nicotine or MJ). A group x gender interaction term was entered in the second block. If the interaction term did not significantly contribute to the model, only results from the first block were reported. As a follow-up, regression analyses were run to assess the relationship between cerebellar volumes, group, and cerebellar-by-group interactions in predicting executive functioning, psychomotor processing speed, and time estimation after controlling for gender, lifetime alcohol use, and lifetime other drug use.
3. RESULTS
3.1 Descriptive Comparisons
ANOVAs and chi-squares assessed whether the MJ users and controls differed demographically (n=16/group). The MJ users and controls did not differ on age [average=18.0 years for both groups; F(1,31)=.12, p=.74]; reading ability [F(1,31)=.14, p=.71], gender [MJ users: 4 females, 12 males; controls: 6 females, 10 males; x2(1)=.52, p=.45], family history of substance use disorders (SUD) [x2(2)=3.62, p=.16], or parental total household income [F(1,31)=.02, p=.90], or ICV [F(1,31)=.15, p=.71]. While 75% of MJ users and 63% of controls were Caucasian, minority MJ users were 13% multiple ethnicities, 6% Pacific Islander, and 6% “other,” while minority controls were 37% Asian-American [x2(4)=10.18, p=.04] (see Table 1).
Table 1. Demographic, substance use, and cerebellar volume variables by group.
| MJ Users (n=16) % or M±SD |
Controls (n=16) % or M±SD |
|
|---|---|---|
| Gender (male) | 75% | 63% |
| Ethnicity (Caucasian) * | 75% | 63% |
| Age | 18±0.7 | 18±0.9 |
| Reading standard score (WRAT-3) | 107±6 | 106±9 |
| Lifetime alcohol use (episodes) * | 195±137 | 23±47 |
| Lifetime marijuana use (episodes) * | 476±269 | 1±1 |
| Lifetime other drug use (episodes)* | 7±9 | 0±0 |
| Intracranial volume (cc3) | 1492.88 ± 108.39 | 1476.22±137.50 |
| Right cerebellar hemisphere (cc3) | 48.7±3 | 46.86±2.83 |
| Left cerebellar hemisphere (cc3) | 48.9±3 | 47.27±2.97 |
| Anterior vermis (cc3) | 2.34±0.25 | 2.50±0.37 |
| Posterior superior vermis (cc3) | 1.37±0.27 | 1.37±0.33 |
| Posterior inferior vermis (cc3) | 1.72±0.15 | 1.63±0.22 |
p<.05.
Abstinence was monitored with urine toxicology screens and Breathalyzer tests, and participants were abstinent from all drugs for at least 30 days prior to scanning. Light to moderate alcohol use was permitted, but participants with self-reported binge drinking (≥4 drinks for females or ≥5 drinks for males within a day) or biological evidence of alcohol use during this time were excluded. On average, MJ users had used marijuana for 3.4 years (±1.7, range= 0.8-6.7), and had greater lifetime MJ [F(1,31)=50.0, p=.0001] and alcohol use than controls [F(1,31)=22.6, p=.0001]. No control had used any drug besides alcohol or marijuana, but MJ users had used other drugs an average of 7 times in their lives [F(1,31)=10.42, p=.003] (see Table 1). The average abstinence from any alcohol use for MJ users was 44 days (±61, range=9-270 days) and 132 days (±130, range=30-365 days) for controls. Average other drug abstinence for the MJ users was 107 days (±33, range=30-300 days).
3.2 Cerebellar Morphometry
Among controls, girls had larger right [beta = −.58, p < .02] and total [beta = −.51, p < .04] cerebellar hemisphere volumes than boys after controlling for ICV, although it is important to note that there were only 4 girls in the sample. After controlling for gender, there was a significant quadratic [beta = .76, p < .02] relationship between age and right hemisphere cerebellar volume and significant linear [beta = .75, p < .02] and quadratic [beta = .76, p < .02] associations between age and left hemisphere cerebellar volume. Total cerebellar volume was significantly predicted by both linear [beta = .67, p < .03] and quadratic [beta = .78, p < .02] age terms. Age did not predict vermal volumes in the controls. Among MJ users, there were no significant linear or quadratic relationships between age and any of the regional cerebellar volumes. As seen in Figure 2; cerebellar volumes peaked around age 17.5-18.0.
Figure 2.
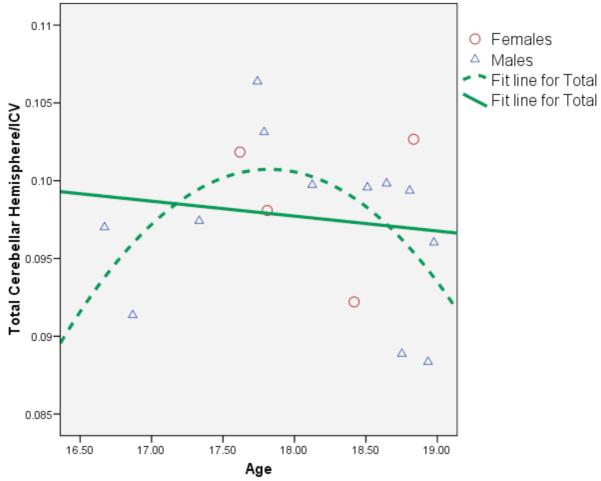
Scatterplot demonstrating significant linear (p<.03) and quadratic (p<.02) relationships between age and cerebellar hemisphere volumes in the healthy controls (n=16).
3.3 Primary Findings: Predicting Cerebellar Volumes
After controlling for gender, lifetime alcohol and other drug use, group status (MJ users vs. controls) was associated with larger posterior inferior vermal volumes [beta = .66, p < .009], but not any other cerebellar or vermis region of interest (p’s >.05) (see Figure 3). In contrast, increased alcohol use was associated with smaller posterior inferior vermal volumes [beta = −.51, p < .04]. (See Figure 4 for bivariate relationships between alcohol use and inferior posterior vermis volume by group.) There were no significant group-by-gender interactions in predicting cerebellar volumes (p’s >.05). Gender significantly predicted anterior vermis [beta = −.46, p < .02], posterior superior vermis [beta = −.46, p < .02], and posterior inferior vermis [beta = −.47, p < .02]; in all cases being female was associated with larger vermal volumes. Other drug use was not associated with cerebellar volumes in this sample (p’s >.05).
Figure 3.
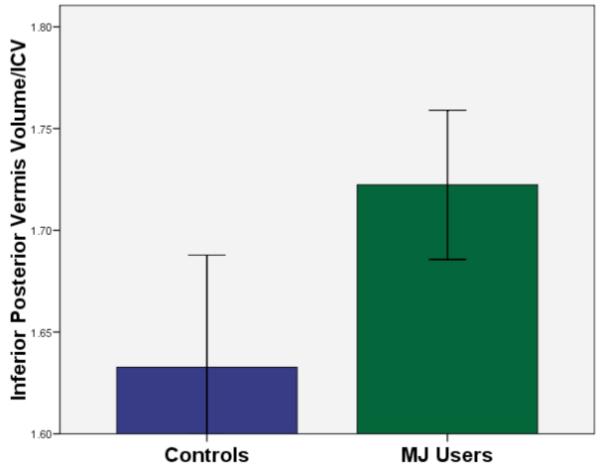
Mean (+/− 1 SE) inferior posterior vermis volume/ICV by group (MJ group status significantly predicted inferior posterior vermis volume/ICV after controlling for gender, alcohol and other drug use; N=32, p<.009).
Figure 4.
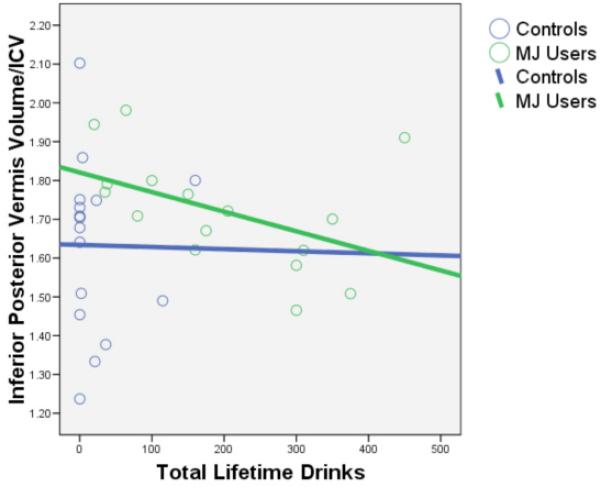
Bivariate scatterplot between the inferior posterior vermis volume/ICV and total lifetime alcohol drinks by group. Lifetime alcohol use significantly predicted inferior posterior vermis volume/ICV after controlling for MJ use, other drug use, and gender (N=32, p<.04).
3.4 Brain-Behavior Relationships
3.4.1 Executive Functioning
Independent of group status, gender, alcohol and other drug use, smaller anterior [beta = −.39, p < .04] and posterior inferior [beta = −.60, p < .001] vermis volumes were associated with superior executive functioning in this sample of adolescents; there were no significant group-by-cerebellar volume interactions in predicting executive functioning (see Figure 5).
Figure 5.
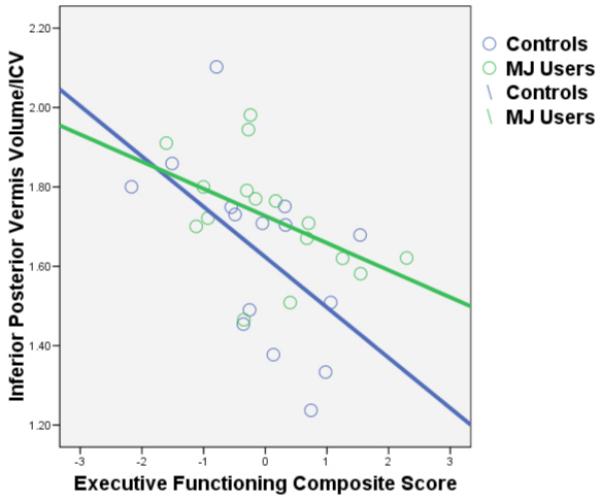
Bivariate scatterplot between the inferior posterior vermis volume/ICV and executive functioning composite score by group. Executive functioning predicted inferior posterior vermis volume after controlling for MJ group status, gender, alcohol and other drug use (N=32, p<.001).
3.4.2 Psychomotor Speed
No significant relationships were seen between cerebellar volume and psychomotor speed. Group-by-cerebellar volume interactions were also non-significant.
3.4.3 Time Estimation
No main or interactive effects for cerebellar volumes were seen in predicting time estimation performance.
4. DISCUSSION
Marijuana (MJ) is the most commonly used drug during adolescence (Johnston et al., 2008). Chronic MJ exposure during this stage of ongoing neuromaturation (e.g., Giedd et al., 1996b; Gogtay et al., 2004; Lenroot and Giedd, 2006; Sowell et al., 2004) may result in abnormal brain structure. In human adolescents, chronic MJ exposure has been associated with slower psychomotor processing speed and poorer executive functioning (Medina et al., 2007), two areas of cognition associated with cerebellar function. This is the first study to date to examine manually defined cerebellar regions of interest in adolescent MJ users and controls without comorbid psychiatric disorders. After one month of abstinence, adolescent MJ users demonstrated significantly larger inferior posterior (lobules VIII-X) vermis volumes than non-using controls. Larger vermal volumes were associated with poorer executive functioning, suggesting that the larger volumes seen in the MJ users may be an indicator of pathology. Gender did not moderate the effects of MJ use on cerebellar structure, although the girls generally demonstrated relatively larger cerebellar volumes compared to the boys.
These findings are consistent with Jarvis and colleagues (2008), who found increased gray matter vermis volumes in adolescents with comorbid bipolar disorder and cannabis use disorders compared to bipolar alone patients. Abnormalities in inferior posterior vermis have also been found in patients with multiple episodes of depression compared to first episode patients and healthy volunteers (DelBello et al., 1999) as well as in adolescents with attention deficit hyperactivity disorder (Berquin et al., 1998; Bussing et al., 2002; Hill et al., 2003; Mostofsky et al., 1998). Other studies have also linked the vermis lobes to affect regulation and attention (see Schahmann et al., 2000 for review). Given the increased sub-clinical depressive symptoms (Medina et al., 2007b) and poor complex attention (Medina et al., 2007a) in adolescent MJ users, future studies are needed to assess the role of the vermis on affective processing and attention in adolescent MJ users.
Within the controls, we found significant linear and quadratic relationships between age and total cerebellar hemisphere volumes in this cross-sectional sample. Younger teens had larger cerebellar volumes than older teens, suggesting a late peak (approximately 17.5 years old) in cerebellar volumes with pruning in the latter teen years. However, our sample included more older than younger teens. This finding is consistent with prior research suggesting slight increases in cerebellar volume until the later teen years followed by pruning in late adolescence/early adulthood (Castellanos et al., 2002; Hill et al., 2007; Mackie et al., 2007). Consistent with Giedd and colleagues (1996), we did not find a relationship between age and vermal volumes, which may be because the cerebellum appears particularly vulnerable to environmental impact (Wallace et al., 2006); therefore, individual differences in vermis volume during the adolescent stage may be more due to lifestyle (i.e., smoking MJ or using other drugs such as alcohol) than innate programmatic neurodevelopment. It is notable that findings were primarily driven by the boys in this cross-sectional sample and need to be replicated in larger, longitudinal studies including both males and females.
Given the possibility of ongoing pruning in the cerebellum during late adolescence, one possible interpretation of increased inferior posterior vermal volumes in the MJ users is that chronic MJ exposure interrupts healthy gray matter pruning process. This would be consistent with our other structural findings indicating that increased MJ use is associated with larger left hippocampal volumes, greater left vs. right hippocampal asymmetry (Medina et al., 2007c), and increased PFC volumes in females (Medina et al., in press). Although the mechanism underlying healthy gray matter pruning is unknown, endogenous endocannabinoids have a role in regulating synaptic activity and neuronal plasticity (e.g., affecting brain derived neurotrophic factor, TrkB signaling, Wnt ligands, and glutamate release) (see Harkany et al., 2008a; 2008b). Further, recent findings suggest astrocytes mediate neuronal release of the C1q protein, which in turn tags neurons with weak synaptic connections (Stevens et al., 2007) and chronic cannabinoid exposure is associated with astrocytic dysfunction (Bindukumar et al., 2008). Therefore, chronic exposure to MJ may disrupt the endogenous cannabinoid system (Casu et al., 2005; Dar, 2000; DeSanty & Dar, 2001), interrupting its role in synaptic pruning and leading to poorer executive functioning in adolescent MJ users (Medina et al., 2007a). Other possible mechanisms include alterations of other neurotransmitter systems (Childers & Breivogel, 1998; Ghozland et al., 2002; Harkany et al., 2008 Iverson, 2003; Pazos et al., 2005; Pistis et al., 2004; Lau & Schloss, 2008; Suárez et al., 2008), changed cerebral blood flow in the cerebellum (Block et al., 2000; Herning et al., 2005; Mathew et al., 1998; O’Leary et al., 2003; O’Leary et al., 2007; Sneider et al., 2008; Volkow et al., 1996) or vasoconstriction (Herning et al., 2001), which may lead to morphological alterations.
In this sample of adolescents, significant relationships were observed between cerebellar vermis volumes and executive functioning, which is consistent with diffusion tensor imaging and functional connectivity/fMRI studies demonstrating connectivity between the cerebellum and the PFC (Allen et al., 2005; Leh et al., 2007; Rubia et al., 2007; Luna et al., 2001; Neufang et al., 2008). Although psychomotor differences were seen in a larger sample of adolescent MJ users (Medina et al., 2007), a lack of relationship between psychomotor processing speed and cerebellar volumes may indicate that psychomotor speed in these adolescents is mediated primarily by the basal ganglia (Egerton et al., 2005; Iverson 2003; Rodriguez de Fonseca et al., 1998), a brain region that has greater CB1 receptor binding compared to the cerebellum in humans (Burns et al., 2007). Inconsistent with prior adult research (Solowij et al., 2002), we did not observe time estimation differences in this sample of adolescent MJ users. This may be due to differences in methodology; Solowij and colleagues utilized a time estimation method where participants had to estimate how long it took to complete a task. In contrast, we had the adolescents estimate when 60 seconds have passed. Therefore, differences in time estimation may not be seen on this relatively easy time estimation procedure.
Consistent with prior adult (e.g., Sullivan & Pfefferbaum, 2009) and prenatal alcohol exposure (O’Hare et al., 2005) findings, increased alcohol use was associated with smaller vermis volumes. However, this is in contrast to DeBellis and colleagues (2005), who did not find vermis volume differences in adolescents with and without alcohol use disorders. This may be due to differences in defining the vermal ROIs; DeBellis et al. (2005) combined all lobes of the vermis while the current study differentiated three distinct regions and only found differences in the inferior posterior vermis. It is notable that MJ and alcohol effects were in the opposite directions, which we also found in the hippocampus (Medina et al., 2007) and PFC (Medina et al., in press) in adolescent MJ users. Further, nicotine use in combination with alcohol use has been associated with vermal metabolite levels (Durazzo et al., 2004). Therefore, future research is needed to examine the effects of combined use of alcohol, nicotine, and MJ use on the cerebellum. For example, animal research has shown that a CB1 agonist (WIN-55212) decrease GABA release in cerebellar purkinje neurons and Win-5512 administration blocked ethanol-induced GABA release (Kelm et al., 2008). In contrast, the cannabinoid antagonist AM-251 did not reduce ethanol-induced GABA release (Kelm et al., 2007).
Limitations of this study are important to consider. It is difficult to disentangle possible preexisting differences from specific drug effects (Aytaclar et al., 1999; Nigg et al., 2004; Ridenour et al., in press). The current sample had strong internal reliability as it excluded individuals with Axis I comorbid disorders such as conduct disorder and attention-deficit hyperactivity disorder, and groups were well-matched on family history of SUD, education, parental income status, and reading ability. Still, subclinical attentional and mood symptoms may predate substance use. Further, as previously mentioned, the current sample was relatively small and primarily male. Finally, due to unreliable parcellation of white matter and CSF from gray matter with these T1 MRI images, we were not able to confirm that vermis volume differences were primarily driven by the gray matter; additional research segmenting gray from white matter is needed to examine the effects of MJ use on gray matter pruning and white matter integrity. The latter could be examined further with diffusion tensor imaging.
In conclusion, after one month of abstinence, MJ users demonstrated significantly larger inferior posterior vermis volumes compared to controls. Given that larger vermis volumes were associated with poorer executive functioning, this is preliminary evidence of a pathological process affecting vermis morphometry in MJ users. Longitudinal research is needed to explore both normal cerebellar development during adolescence and the influence of chronic early MJ use on adolescent neurodevelopment.
ACKNOWLEDGEMENTS
This work was supported by grants R01 AA13419 and R01 DA021182 (Tapert). Dr. Medina was supported by F32 DA020206 during portions of this manuscript. Dr. Nagel is supported by K08 NS52147. Portions of this study were presented by the first author at the 2008 meeting of the Society for Neuroscience in San Diego, CA. Gratitude is expressed to the staff of the Adolescent Brain Imaging Project, to the participants, and to their families.
REFERENCES
- Aasly J, Storsaeter O, Nilsen G, Smevik O, Rinck P. Minor structural brain changes in young drug abusers. A magnetic resonance study. Acta Neurologica Scandinavica. 1993;87:210–4. doi: 10.1111/j.1600-0404.1993.tb04103.x. [DOI] [PubMed] [Google Scholar]
- Adams IB, Martin BR. Cannabis: Pharmacology and toxicology in animals and humans. Addiction. 1996;91(11):1585–1614. [PubMed] [Google Scholar]
- Amen DG, Waugh M. High resolution brain SPECT imaging of marijuana smokers with AD/HD. Journal of Psychoactive Drugs. 1998;30:209–14. doi: 10.1080/02791072.1998.10399692. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. British Medical Journal. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, et al. The cerebellum in attention-deficit/hyperactivity disorder: a morphometric study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sastre M, Zimmer A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci U S A. 2005;102:15670–5. doi: 10.1073/pnas.0504640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–6. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology, Biochemistry and Behavior. 2002;72:237–50. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology. 1998;51:1532–1537. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- Bovasso G. Cannabis abuse as risk factor for depressive symptoms. American Journal of Psychiatry. 2001;158:2033–2037. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR. The effects of delta9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur J Pharmacol. 2003;459:139–50. doi: 10.1016/s0014-2999(02)02854-6. [DOI] [PubMed] [Google Scholar]
- Brook D, Brook J, Zhang C, Cohen P, Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Archives of General Psychiatry. 2002;59:1093–1044. doi: 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Bussing R, Grudnik J, Mason D, Wasiak M, Leonard C. ADHD and conduct disorder: an MRI study in a community sample. World J Biol Psychiatry. 2002;3:216–220. doi: 10.3109/15622970209150624. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. Rutgers Center of Alcohol Studies; New Brunswick, NJ: 1969. [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on bold (blood oxygen level dependent) response to spatial working memory. Alcohol and Alcoholism. 2005;40(3):194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Casu MA, Pisu C, Sanna A, Tambaro S, Spada GP, Mongeau R, Pani L. Effect of delta9-tetrahydrocannabinol on phosphorylated CREB in rat cerebellum: an immunohistochemical study. Brain Res. 2005;1048(1-2):41–7. doi: 10.1016/j.brainres.2005.04.053. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:448–55. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug Alcohol Depend. 1998;51(1-2):173–87. doi: 10.1016/s0376-8716(98)00075-1. [DOI] [PubMed] [Google Scholar]
- Coddington E, Lewis C, Rose JD, Moore FL. Endocannabinoids mediate the effects of acute stress and corticosterone on sex behavior. Endocrinology. 2007;148:493–500. doi: 10.1210/en.2006-0740. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dar MS. Cerebellar CB(1) receptor mediation of Delta(9)-THC-induced motor incoordination and its potentiation by ethanol and modulation by the cerebellar adenosinergic A(1) receptor in the mouse. Brain Res. 2000;864(2):186–94. doi: 10.1016/s0006-8993(00)02103-x. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI Analysis of the Cerebellum in Bipolar Disorder: A Pilot Study. Neuropsychopharmacology. 1999;21(1):63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Manual for the Delis-Kaplan Executive Function System (D-KEFS) Psychological Corp; San Antonio, TX: 2001. [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSanty KP, Dar MS. Cannabinoid-induced motor incoordination through the cerebellar CB(1) receptor in mice. Pharmacol Biochem Behav. 2001;69(1-2):251–9. doi: 10.1016/s0091-3057(01)00539-1. [DOI] [PubMed] [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Clinical signs of cerebellar dysfunction in schizophrenia, alcoholism, and their comorbidity. Schizophr Res. 2002;57:281–91. doi: 10.1016/s0920-9964(01)00300-0. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998a;10:2825–30. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci U S A. 1998b;95:10269–73. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28(12):1849–60. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–95. doi: 10.1016/j.neubiorev.2005.12.002. Epub 2006 Mar 29. [DOI] [PubMed] [Google Scholar]
- Egerton A, Brett RR, Pratt JA. Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–91. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Kirsteins L, Emanuele MA. Effect of chronic ethanol exposure on female rat reproductive cyclicity and hormone secretion. Alcohol Clin Exp Res. 2001;25:1025–9. [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell M. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- Fride E. The endocannabinoid-CB receptor system: Importance for development and in pediatric disease. Neuro Endocrinol Lett. 2004;25:24–30. [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss YC, Hamburger SD, Rapoport JL. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Res Dev Brain Res. 1996a;91:274–80. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996b;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996c;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;17:17. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN, Frackowiak RJ. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46.65. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Green BE, Ritter C. Marijuana use and depression. Journal of Health and Social Behavior. 2000;41:40–49. [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Research Cognitive Brain Research. 2005;23:107–18. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, Barabás K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. 2008b;286(1-2 Suppl 1):S84–90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Harkany T, Mackie K, Doherty P. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008a;18(3):338–45. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Groen BG, Lynn AB, De Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res. 1991b;552(2):301–10. doi: 10.1016/0006-8993(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991a;11(2):563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann N Y Acad Sci. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–9. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Marijuana abusers are at increased risk for stroke. Preliminary evidence from cerebrovascular perfusion data. Ann N Y Acad Sci. 2001;939:413–5. doi: 10.1111/j.1749-6632.2001.tb03652.x. [DOI] [PubMed] [Google Scholar]
- Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61(1):41–7. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–70. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Annals of New York Academy of Sciences. 2004;1021:384–90. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–97. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Andrusiak E, Tran A, Bowers MB, Jr., Roth RH. Delta 9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997b;16:426–32. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Verrico CD, Le D, Roth RH. Repeated exposure to delta 9-tetrahydrocannabinol reduces prefrontal cortical dopamine metabolism in the rat. Neurosci Lett. 1998;246:169–72. doi: 10.1016/s0304-3940(98)00254-7. [DOI] [PubMed] [Google Scholar]
- Jentsch J, Andrusiak E, Tran A, Bowers M, Jr., Roth R. Delta9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: Blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997a;16:426–432. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman HG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2004. National Institute on Drug Abuse; Bethesda, MD: 2005. [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology. 2004;176:239–47. doi: 10.1007/s00213-004-1885-8. Epub 2004 Jun 16. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323(1):356–64. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J Neurophysiol. 2008;100(6):3417–28. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim HJ, Noh HS, Roh GS, Kang SS, Cho GJ, Park SK, Lee BJ, Choi WS. Suppression by ethanol of male reproductive activity. Brain Res. 2003;989:91–8. doi: 10.1016/s0006-8993(03)03372-9. [DOI] [PubMed] [Google Scholar]
- Lau T, Schloss P. The cannabinoid CB1 receptor is expressed on serotonergic and dopaminergic neurons. Eur J Pharmacol. 2008;578(2-3):137–41. doi: 10.1016/j.ejphar.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Yurgelun-Todd DA. Human neuroimaging of acute and chronic marijuana use: implications for frontocerebellar dysfunction. Human Psychopharmacology: Clinical & Experimental. 1999;14:291–304. [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40(4):443–9. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicology and Teratology. 2001;23:437–43. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–4. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Verslype M, Preud’homme X, Vanderhaeghen J. Activation of multiple transcription factor genes by tetraydrocannabinol in rat forebrain. Neuroreport. 1994;5:1265–1268. doi: 10.1097/00001756-199406020-00028. [DOI] [PubMed] [Google Scholar]
- Mani SK, Mitchell A, O’Malley BW. progesterone receptor and dopamine receptors are required in Delta 9-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proc Natl Acad Sci. 2001;98:1249–1254. doi: 10.1073/pnas.031563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–93. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007a;13:807–20. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008a;32:386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007b;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007c;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Reiss AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17:1427–31. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16(12):1285–90. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid CB(1) receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther. 2001;297(2):629–37. [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin MB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: Cohort study. British Medical Journal. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos MR, Núñez E, Benito C, Tolón RM, Romero J. Functional neuroanatomy of the endocannabinoid system. Pharmacol Biochem Behav. 2005;81(2):239–47. doi: 10.1016/j.pbb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Piguet O, Cramsie J, Bennette HP, et al. Contributions of age and alcohol consumption to cerebellar integrity, gait and cognition in non-demented very old individuals. Eur Arch Psychitry Clin Neurosci. 2006;256:504–511. doi: 10.1007/s00406-006-0671-5. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Research. 2002;948:155–8. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biological Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychotherapy & Psychosomatics. 1997;66:179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Porcella A, Gessa GLP, Pani L. 9-Tetrahydrocannabinol increases sequence-specific AP-1 DNA-binding activity and Fos-related antigens in the rat brain. Eur. J. Neurosci. 1998;10:1743–1751. doi: 10.1046/j.1460-9568.1998.00175.x. [DOI] [PubMed] [Google Scholar]
- Price J, Shear PK, Medina KL. Ecstasy Consumption and Verbal Memory Functioning: Gender Effects; Poster session presented at the annual meeting for the International Neuropsychological Society in Honolulu; HI. 2008, February. [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version 4.0. (DIS 4.0) 1996. [Google Scholar]
- Rodríguez de Fonseca F, Del Arco I, Martín-Calderón JL, Gorriti MA, Navarro M. Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis. 1998;5(6 Pt B):483–501. doi: 10.1006/nbdi.1998.0217. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–23. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia L, Fernandez-Ruiz JJ, Cebeira M, Ramos JA. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacology, Biochemistry and Behavior. 1995;51:731–7. doi: 10.1016/0091-3057(95)00023-p. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Donyon J, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–9. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–10. doi: 10.1016/j.drugalcdep.2005.01.009. Epub 2005 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Raye Z, Litten JPA, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press, Inc; Totowa, NJ, US: 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Learning impairment in the radial-arm maze following prolonged cannabis treatment in rats. Psychopharmacology (Berl) 1982;77:117–23. doi: 10.1007/BF00431932. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology (Berl) 1985;85:436–9. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- Suárez J, Bermúdez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509(4):400–21. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Suárez J, Bermúdez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509(4):400–21. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44(2):155–65. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunving K. Psychiatric effects of cannabis use. Acta Psychiatrica Scandinavica. 1985;72:209–17. doi: 10.1111/j.1600-0447.1985.tb02597.x. [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Jr., Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. The American Journal on Addictions. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, Bormans G. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [(18)F]MK-9470 PET. Neuroimage. 2008a;39:1533–41. doi: 10.1016/j.neuroimage.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Koole M, Sanabria Bohorquez SM, Goffin K, Guenther I, Belanger MJ, Cote J, Rothenberg P, De Lepeleire I, Grachev ID, Hargreaves RJ, Bormans G, Burns HD. Whole-body biodistribution and radiation dosimetry of the human cannabinoid type-1 receptor ligand 18F-MK-9470 in healthy subjects. J Nucl Med. 2008b;49:439–45. doi: 10.2967/jnumed.107.047290. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Roth RH. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse. 2003;49:61–6. doi: 10.1002/syn.10215. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Research. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Ali SF, Paule MG, Scallet AC, Slikker W., Jr. Chronic exposure to delta 9-tetrahydrocannabinol fails to irreversibly alter brain cannabinoid receptors. Brain Res. 1991;544:145–9. doi: 10.1016/0006-8993(91)90897-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test, 3rd Edition (WRAT-3) Manual. Wide Range, Inc.; Wilmington, DE: 1993. [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test-3 Administration Manual. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. Journal of Addictive Diseases. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank RL. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society of Magnetic Resonance Medicine. 2000;8:683. [Google Scholar]
- Yamasaki M, Hashimoto K, Kano M. Miniature synaptic events elicited by presynaptic Ca2 rise are selectively suppressed by cannabinoid receptor activation in cerebellar Purkinje cells. J Neurosci. 2006;26:86–95. doi: 10.1523/JNEUROSCI.2258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]