Abstract
Background
Studies of quality of life (QOL) in children with juvenile idiopathic arthritis (JIA) have focused on changes in musculoskeletal function secondary to arthritis. The role of visual functionality as a result of JIA-associated uveitis and its complications has not been examined. We evaluated the individual impact of physical and visual disability on QOL in children with and without uveitis.
Methods
We administered patient-based questionnaires that measured visual function, physical function, and overall QOL to 27 children with JIA or idiopathic uveitis. Demographic data, assessed joint involvement, and reviewed medical records were recorded. Groups with and without uveitis were compared for differences in arthritis and uveitis disease characteristics using the Wilcoxon-Mann-Whitney, χ2, and Fisher exact tests. Associations between physical or visual function and overall QOL were measured using Pearson's correlation coefficient.
Results
Of 27 patients, 85.2% have had arthritis and 51.9% have had uveitis. The group without uveitis had increased morning stiffness (p = 0.036). The uveitis group reported more eye redness (p = 0.033) and photophobia (p = 0.013) than those without uveitis. We observed moderate associations between overall QOL and visual function in the uveitis group (r = −0.579), and overall QOL and physical function in the non-uveitis group (r = −0.562).
Conclusions
This study demonstrates that visual impairment is an important component of QOL in children with uveitis. It suggests that QOL studies should incorporate both visual and physical function measures in their analyses, especially since many children with JIA also suffer from uveitis and visual impairment.
Introduction
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood and is a significant cause of chronic pain, disability, and reduced quality of life (QOL). It is also the most common systemic cause of inflammatory eye disease (ie, uveitis) in children.1 The overall prevalence of JIA-associated uveitis has been reported to be as high as 34% although some reports suggest a decreasing prevalence.2,3 In one study, almost 80% of patients had bilateral involvement.4 At least 20% of individuals with JIA develop severe vision-compromising complications, such as cataracts, band keratopathy, posterior synechiae, hypotony, and glaucoma.4-6 Among all cases of uveitis, reports of complications have been as high as 41%, with 19% of cases having resulted in blindness.7
To better assess vision related QOL, we have developed a self-report questionnaire that assesses the performance of activities in the school and home that rely on vision and are common to children who are 8 to 18 years of age. After initial pilot studies of validity, the questionnaire is currently undergoing further validity and reliability testing.
We hypothesized that among children who have ocular involvement related to JIA-associated uveitis, QOL will be better predicted by accounting for both visual and physical disability. Our objectives were to evaluate visual and physical dysfunction in children with and without uveitis and to determine their contribution to overall QOL.
Subjects and Methods
Design
This exploratory, cross-sectional, observational study was approved by the Institutional Review Board of the Hospital for Special Surgery (HSS) in New York and conformed to the requirements of the United States Health Insurance Portability and Accountability Act. Consent was obtained from the parent/legal guardian, and assent was obtained from the children prior to administration of the instruments.
Subjects
Twenty-seven children with JIA or idiopathic uveitis were recruited from the pediatric rheumatology division outpatient clinics at HSS. Potential subjects were approached consecutively. This study was conducted during the school year (September 2007 to April 2008), and recruitment did not take place during school breaks of more than 3 days duration (eg, spring and winter break). Patient enrollment satisfied the following inclusion criteria: (1) a diagnosis of JIA or idiopathic uveitis (JIA was diagnosed according to the International League of Associations for Rheumatology [ILAR] classification8; children with idiopathic uveitis were included since they have visual compromise similar to children with JIA and uveitis but no musculoskeletal involvement); (2) age 8 to 18 years at time of study and age ≤16 years at time of JIA diagnosis; (3) at least a 3rd grade reading and comprehension level according to school performance; (4) presence of parent or guardian to complete a written demographic questionnaire; and (5) a visit with an ophthalmologist and a rheumatologist within the last 3 months. Patients were excluded from participation if they had any of the following: (1) significant comorbidity unrelated to JIA or uveitis (eg, inflammatory bowel disease, diabetes); (2) major developmental disorders (eg, mental retardation, cerebral palsy); (3) disease diagnosis <1 year (to allow sufficient time for psychological adjustment to a new diagnosis); (4) other rheumatic disease besides JIA (eg, systemic lupus erythematosus); and (5) other systemic disease associated with uveitis.
Procedures
Demographic/Baseline
Parent and patient-based questionnaires were completed in person and included a child self-report and a parent proxy-report, as indicated. All questionnaires were written in large print, and a member of the research team was available to read the questionnaires to subjects whose visual impairment prevented completion of these instruments on their own. Additional information, such as age of onset, age at visit, gender, ethnicity, and race was collected from the parent/guardian.
Arthritis-related data that were recorded included presence of arthritis, age at arthritis onset, extent and distribution of joint involvement, and morning stiffness. Uveitis-related data included presence of uveitis, age at uveitis onset, eye pain, photophobia, and specific sequelae such as cataracts and glaucoma. All questions related to symptoms were directed to the child and not the parent. A history of past and current medication use, including name, class, duration of use, and mode of administration (oral, intravenous, subcutaneous, intramuscular, and ocular injections), was obtained by self report from the parent and by medical record review.
A systematic medical record review of outpatient and inpatient charts for rheumatology was performed for the interval from the time of diagnosis to the time of interview. These reviews complemented the interviews and confirmed reported information. Additionally, recorded laboratory markers (most recent erythrocyte sedimentation rate test [ESR], antinuclear antibody test [ANA], rheumatoid factor related test [RF]) were noted. The most recent visual acuity assessment by an ophthalmologist within the last three months was recorded.
Assessment of Physical Function
Physical function was measured by the Childhood Health Assessment Questionnaire (CHAQ), which is an instrument that is disease-specific for juvenile arthritis and evaluates functional disability.9 It is adapted from the Stanford Health Assessment Questionnaire (HAQ) and has well-documented reliability and validity. Twenty questions encompass 8 functional components: (1) dressing and grooming, (2) arising, (3) eating, (4) walking, (5) hygiene, (6) reach, (7) grip, and (8) activities. There are three parameters within each area: (1) difficulty in performing daily functions, (2) use of special aids or devices, and (3) activities that require assistance from another person. It requires 10 minutes to complete and scores range from 0 to 3, where higher scores indicate worse physical function. It can be completed by a proxy or self-administered to children of all ages; for this study, only child reports were used.
An assessment of joint involvement was performed at the time of the interview. A clinician trained in joint examination measured joints for tenderness, swelling, and limited range of motion. Specific joints that were evaluated included the cervical spine, temporomandibular joint, lumbar spine, shoulders, wrists, fingers, hips, knees, ankles, and toes. Physician global assessments of disease activity were scored using a Likert scale.
Assessment of Visual Function
Visual function was measured by our visual function questionnaire, Effect of Youngsters' Eyesight in Quality of Life instrument (EYE-Q), a patient-based self-report questionnaire consisting of 13 items that evaluate competence in performing daily tasks that rely on vision in the school and home in children who are 8 to 18 years of age. A 5-point Likert scale response format is used, and scores range from 0 to 5 with higher scores indicating worse visual function. It requires 10 minutes to complete (see e-Supplement 1, available at jaapos.org).
The patients' ophthalmology offices provided medical records to evaluate visual acuity and uveitis activity within the previous 3 months.
Quality of Life Assessment
Health-related QOL was measured by the Pediatric Quality of Life Inventory Version 4.0 (PedsQL 4.0), an instrument for children and adolescents from 2 to 18 years of age and includes 4 core scales: (1) physical functioning (8 items), (2) emotional functioning (5 items), (3) social functioning (5 items), and (4) school functioning (5 items).10 Each item is measured on a 5-point scale, and scores range from 0 to 100 with higher scores indicating better QOL. This study used the child-report forms only.
Statistical Analysis
Initial descriptive analyses of the baseline demographic and clinical variables of children with and without uveitis were performed. Nominal variables were compared using χ2 or Fisher exact test. Ordinal variables and nonparametric continuous variables were compared using Wilcoxon-Mann-Whitney tests. Continuous variables that met the assumption of normality were compared using the independent samples t-test. After applying Bonferroni's adjustment, p-values less than 0.010 were significant for uveitis characteristics, and p-values less than 0.017 were significant for outcome scores. Relationships between physical and visual function and QOL were determined using Pearson's correlation coefficients.
Results
Difference between All Children with Uveitis and without Uveitis
Characteristics of subjects with uveitis (n = 14) and without uveitis (n = 13) included in this study are shown in Table 1. There was an increased prevalence of a positive ANA in patients with uveitis.
Table 1.
Characteristics of patients with and without uveitis
| Characteristics | Uveitis (N = 14) | No uveitis (N = 13) | p-valuea |
|---|---|---|---|
| Mean age in years (SD) | 14.1 (3.2) | 13.6 (3.2) | |
| Female | 9 (64.3%) | 8 (61.5%) | |
| Arthritis | 10 (71%) | 13 (100%) | |
| ANA (+) | 10 (71.4%) | 5 (38.5%) | |
| Arthritis characteristics | |||
| Median duration in years (range) | 5 (0-15) | 3 (1-8) | |
| AM stiffness | 1 (7.1%) | 7 (53.8%) | |
| Painful joints in last month | 3 (21.4%) | 5 (38.5%) | |
| Painful joints ever | 12 (85.7%) | 13 (100%) | |
| Swollen joints in last month | 2 (14.3%) | 3 (23.1%) | |
| Swollen joints ever | 8 (57.1%) | 10 (76.9%) | |
| Median physician's global assessment, 0-10 (range) | 5 (1-7) | 3 (2-7) | |
| Median ESR at diagnosis (range) | 8.5 (1-63) | 17 (1-87) | |
| Uveitis characteristics | |||
| Median duration uveitis in years (range) | 5 (1-12) | ||
| Eye pain | 5 (36%) | 1 (8%) | 0.165 |
| Eye redness | 7 (50%) | 1 (8%) | 0.033 |
| Photophobia | 8 (57%) | 1 (8%) | 0.013 |
| Cataracts | 4 (29%) | 0 | 0.098 |
| Glaucoma | 3 (21%) | 0 | 0.222 |
| Prior and current medications | |||
| Prednisone | 7 (50%) | 3 (23%) | |
| Methotrexate | 8 (57%) | 8 (62%) | |
| Infliximab | 5 (36%) | 1 (8%) | |
| Etanercept | 4 (29%) | 6 (46%) | |
| Adalimumab | 8 (57%) | 3 (23%) | |
| Daclizumab | 2 (14%) | 0 |
Bonferroni's correction used for multiple comparisons
p < 0.010.
Of the 27 total patients, there were 92.6% Caucasians (26% Hispanics) and 63% females; 55.6% were ANA (+). Mean age at time of study was 13.89 years. Of the total, 85.2% had arthritis (oligo-, 14.8%; poly-, 40.7%; spondylo-, 22.2%; psoriatic, 7.4%), and 51.9% had uveitis. Four patients were diagnosed with idiopathic uveitis and therefore did not have a history of arthritis.
Disease Characteristics of All Children with and without Uveitis
Children without uveitis reported more morning stiffness than those with uveitis. Those with uveitis complained of increased eye redness (p = 0.033) and photophobia (p = 0.013) (Table 1). They suffered from cataracts as a sequelae of their uveitis or corticosteroid treatment. Overall medication use (prior and current) was increased in the uveitis group throughout the course of their disease, which was attributed to the common use of ocular medications among these patients—corticosteroid eye drops (100%), mydriatics (57%), and topical ophthalmic beta-blockers (36%). There did not appear to be a difference in the overall use of nonocular medications.
In general, arthritis disease activity was minimal and the two groups did not appear different with respect to most characteristics such as affected joints. The uveitis groups had almost no cells or protein flare on ophthalmologic examination. There were no differences between the groups with regard to their uveitis characteristics when adjusted for multiple comparisons.
Differences in the Quality of Life Scores in All Children with and without Uveitis
Visual acuity was similar for 21 patients in both groups. In children with uveitis, 82% had normal vision (20/30 or better), 9% had vision loss (20/40-20/200), and 9% had blindness (<20/200). In children without uveitis, 90% had normal vision (20/30 or better), 10% had vision loss (20/40-20/200), and 0% had blindness (<20/200).
We also examined differences in the patients' scores on the QOL measures (Table 2). Subjects without uveitis appeared to have worse functional disability evidenced by higher CHAQ scores compared to children with uveitis (p = 0.039). The groups were not significantly different in any of the measures.
Table 2.
Median outcome scores (range)
| Uveitis (N = 14) | No uveitis (N = 13) | p-valuea | |
|---|---|---|---|
| CHAQ (0-3)b | 0.125 (0-2) | 0.75 (0-1.25) | 0.029 |
| EYE-Q (0-5)b | 1.2 (1-1.92) | 1.15 (1-1.4) | 0.153 |
| Peds QL (0-100)c | 79 (60-100) | 80 (51.6-95) | 0.756 |
CHAQ, Childhood Health Assessment Questionnaire; EYE-Q, Effects of Youngsters Eyesight on Quality of life; Peds QL, Pediatric Quality of Life Inventory.
Bonferroni's correction used for multiple comparisons
p< 0.017.
Higher scores indicate worse quality of life.
Higher scores indicate better quality of life.
Visual Function and Quality of Life
We examined the association between the EYE-Q (measure of visual function) and the Peds QL (measure of overall QOL) (Figure 1A). In children with uveitis, there was a moderate correlation between visual function and QOL (r = -0.579, p = 0.038). There was no such correlation in the group without uveitis.
Fig 1.
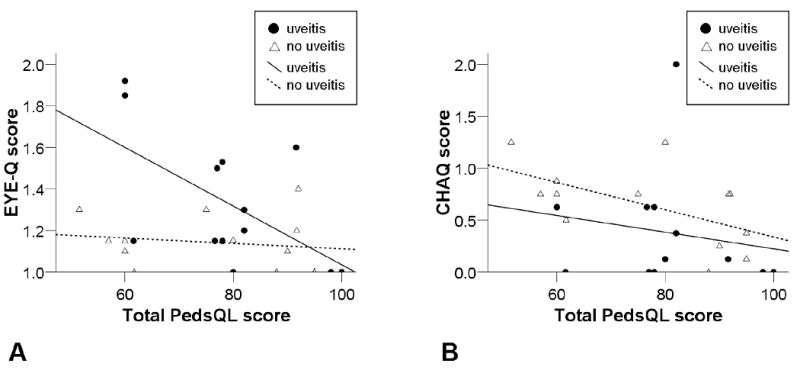
Correlation of Pediatric Quality of Life Inventory score (Total PedsQL score) with (A) Effects of Youngsters' Eyesight on Quality of Life (EYE-Q) and Total PedsQL score saw moderate associations in the uveitis group (r = −0.579, p = 0.038) and (B) Childhood Health Assessment Questionnaire (CHAQ) and Total PedsQL score saw moderate associations in the non-uveitis group (r = −0.562, p = 0.046).
Physical Function and Quality of Life
We examined the association between the CHAQ (measure of physical function) and the Peds QL (measure of overall QOL) in both groups (Figure 1B). In children without uveitis, there was a moderate correlation between physical function and overall QOL (r = −0.562, p = 0.046). There was no such correlation in the group with uveitis.
Discussion
In this study, we evaluated the impact of uveitis on the QOL of children with JIA or idiopathic uveitis and determined that visual function is an important and often overlooked component of QOL. Our results showed a moderate correlation between visual function and QOL in children with uveitis that was not evident in those without uveitis. Likewise, our results suggested that QOL studies should incorporate visual disability in their analysis since many children with JIA also suffer from uveitis.
Our population of patients with uveitis had more ocular symptoms and suffered from greater visual dysfunction as measured by our EYE-Q (p = 0.038). Although the visual acuity of children with and without uveitis was similar, a moderate correlation existed between visual function and QOL in the uveitis group. This suggested that visual acuity alone was not an adequate measure of visual function and that both subjective and objective measures were necessary. Likewise, the inclusion of children with idiopathic uveitis served as a comparison group that emphasized the importance of visual function since these children do not have physical disabilities related to arthritis.
QOL has been defined by the World Health Organization as an “individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns.”11 It has been an important outcome and may largely be defined by one's degree of health (ie, health-related QOL). Valid and reliable instruments that estimate the impact of overall health, as well as signs and symptoms of specific conditions have been pertinent to this assessment, especially in disease populations. Standardized measures of health status that encompass a wider concept of QOL have become increasingly important.12
Visual disability has the potential to impact an individual's ability to perform activities of daily living. More specifically, clinically significant impairment would likely lead to difficulty performing tasks that rely on vision. However, there have been no validated instruments to assess visual function in children 8 to 18 years of age, which has led our group to develop our own questionnaire. Objective measures such as visual acuity, visual field, color vision, and contrast sensitivity have been used to determine outcome and visual function in children.13 It has remained uncertain whether these measures alone accurately assess one's degree of visual impairment, and whether they truly represent the patient's perspective of the impact of their disease. Interestingly, both visual functioning and general health status have been considered important measures of QOL and provided complementary information in adult patients with uveitis.14
Health-related QOL in children with arthritis has been thought to be related to various domains such as physical function, pain, ability to cope with disease, psychosocial adjustment, social functioning and impact of disease.15 Although physical function is not the only component of QOL, assessment tools in pediatric rheumatology have been comprised largely of instruments that measure musculoskeletal function, health-related QOL, and overall QOL.16-18 As a result, the impact of uveitis in this population may be underestimated since the effects of visual impairment on QOL have not been examined.
To date, all studies examining QOL in children with JIA have used physical function assessments (ie, Childhood Health Assessment Questionnaire) either as proxies for or predictors of QOL.17,19 This monolithic perspective may result in an underestimation of QOL as its measurement has focused exclusively on musculoskeletal function without considering the potential significance of the disease's ocular manifestations. No studies on visual disability have been conducted in patients with JIA other than studies of visual acuity.5 Likewise, no validated instruments that assess vision related QOL exist to evaluate visual disability as a component of QOL in an 8- to 18-year-old population. In general, physicians rely on objective measures such as visual acuity and contrast sensitivity to determine visual outcome.6,13,20-24 One instrument, the Children's Visual Function Questionnaire, is specific for children ≤7 years of age, and the second, the LV-Prasad Questionnaire, is not culturally relevant.25-27 The adult standardized visual function questionnaires (ie, the National Eye Institute—Visual Function Questionnaire) contain items that are inappropriate for use in children such as driving and shopping.28
Vision related QOL may be assessed by determining the degree of visual impairment in activities of daily living, ie, impaired daily functioning secondary to visual difficulties is a proxy for vision related QOL. To develop our questionnaire (EYE-Q), we selected relevant items from existing instruments and consulted pediatric rheumatologists, pediatric ophthalmology professionals (eg, ophthalmologists, optometrists, clinical research technicians), and children with and without ocular disease. Input from these individuals was used to develop other relevant questions, and refine the phrasing of the items and the response format. This provided us with both face and content-related evidence for validity which primarily depends on the subjective agreement of experts.29 Children who were 8 to 18 years of age then evaluated our instrument for comprehensibility, relevance and ease of format. After this initial assessment, we administered it to children with uveitis as described in this manuscript which further validated our instrument since it was administered to children with and without visual impairment, and was compared to a gold-standard measure of overall QOL. We have since incorporated questions to evaluate photosensitivity, night vision, the use of visual aids (eg, magnifying glass), and the child's perception of overall visual function. We have also created age-appropriate subsections specific for children 8 to 15 and 16 to 18 years of age since cognitive abilities and the importance of performing certain tasks differ with age. This instrument is now undergoing additional validity and test–retest reliability studies.
Our data have been consistent with previous studies showing that children with JIA and uveitis are primarily Caucasian, female, and have (+) ANAs. Their joint involvement was similar to children without uveitis except for increased morning stiffness. This may be a consequence of children with oligoarticular disease having an increased incidence of uveitis but less joint involvement, and children with polyarticular disease having greater joint involvement but less uveitis.
There were a number of limitations to this study. The study design was observational and cross-sectional with a limited recruitment period of 6 months. This prevented the determination of the effects of disease flare on QOL since most of the subjects were not in flare during the time of analysis. The population was homogenous and consisted primarily of Caucasian children with sufficient disease control wherein they had minimal joint and ocular involvement. Inclusion of a larger number of children with idiopathic uveitis or the different JIA subtypes (psoriasis, poly, and oligo) with more varied disease activity may allow us to better detect differences in QOL. The size of our cohort also precluded the performance of multiple linear regressions to test for possible predictors of overall QOL.
This study provided evidence of the importance of all components of QOL. The results reported suggested that our visual function questionnaire has clinical utility as an outcome measure. We are currently conducting further validity and reliability studies in a population of children with a wide range of vision. We plan a larger cross-sectional study that examines the effects of both visual and physical disability on a more diverse JIA and uveitis population. Likewise, a longitudinal study would enable us to determine the overall impact of visual impairment in children with JIA and uveitis long-term and the effects of disease flares, medication use, and other QOL-related variables on overall QOL.
In conclusion, current QOL studies have focused on the physical component of JIA regardless of the extent of musculoskeletal involvement. It is likely that both overall disability and QOL have been underestimated among children with uveitis as the instruments used in these studies do not take into account the ophthalmologic diagnoses or consequences. Knowledge of the impact of inflammatory eye involvement on visual function and QOL of all patients will lead to the development of better interventions to ensure these children have the best opportunities to lead normal lives as children and adults. Only by understanding how and to what extent QOL is negatively impacted by visual disability will we be able to identify these children's needs and provide requisite assistance to maintain function and sustain optimal levels of QOL.
Supplementary Material
Acknowledgments
Grant Support: Arthritis Foundation—New York Chapter; WCMC CTSC, UL1RR024996
The authors thank Julie Cherian, MD, for her assistance in data collection.
Footnotes
Institution at which the study was conducted: Hospital for Special Surgery
Some material was presented as a poster at the American College of Rheumatology in 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunningham ET, Jr, Suhler EB. Childhood uveitis—young patients, old problems, new perspectives. J AAPOS. 2008;12:537–8. doi: 10.1016/j.jaapos.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy J, Kivlin J, Lindsley C, Nocton J. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 2006;117:1843–5. doi: 10.1542/peds.2006-0421. [DOI] [PubMed] [Google Scholar]
- 3.Oren B, Sehgal A, Simon JW, et al. The prevalence of uveitis in juvenile rheumatoid arthritis. J AAPOS. 2001;5:2–4. doi: 10.1067/mpa.2001.111017. [DOI] [PubMed] [Google Scholar]
- 4.Paroli MP, Speranza S, Marino M, Pirraglia MP, Pivetti-Pezzi P. Prognosis of juvenile rheumatoid arthritis-associated uveitis. Eur J Ophthalmol. 2003;13:616–21. doi: 10.1177/112067210301300704. [DOI] [PubMed] [Google Scholar]
- 5.Ozdal PC, Vianna RN, Deschenes J. Visual outcome of juvenile rheumatoid arthritis-associated uveitis in adults. Ocul Immunol Inflamm. 2005;13:33–8. doi: 10.1080/09273940590909220. [DOI] [PubMed] [Google Scholar]
- 6.Sabri K, Saurenmann RK, Silverman ED, Levin AV. Course, complications, and outcome of juvenile arthritis-related uveitis. J AAPOS. 2008;12:539–45. doi: 10.1016/j.jaapos.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Edelsten C, Lee V, Bentley CR, Kanski JJ, Graham EM. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol. 2002;86:51–6. doi: 10.1136/bjo.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–92. [PubMed] [Google Scholar]
- 9.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761–9. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 10.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 11.WHOQOL Group. The development of the World Health Organization Quality of Life assessment instrument (the WHOQOL) Berlin: Springer-Verlag; 1994. [Google Scholar]
- 12.Tucker LB. Outcome measures in childhood rheumatic diseases. Curr Rheumatol Rep. 2000;2:349–54. doi: 10.1007/s11926-000-0074-y. [DOI] [PubMed] [Google Scholar]
- 13.Murphy CC, Hughes EH, Frost NA, Dick AD. Quality of life and visual function in patients with intermediate uveitis. Br J Ophthalmol. 2005;89:1161–5. doi: 10.1136/bjo.2005.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman RM, Jacobsen G, Whitcup SM. Visual functioning and general health status in patients with uveitis. Arch Ophthalmol. 2001;119:841–9. doi: 10.1001/archopht.119.6.841. [DOI] [PubMed] [Google Scholar]
- 15.Miller ML, LeBovidge J, Feldman B. Health-related quality of life in children with arthritis. Rheum Dis Clin North Am. 2002;28:493–501. vi. doi: 10.1016/s0889-857x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 16.Duffy CM. Measurement of health status, functional status, and quality of life in children with juvenile idiopathic arthritis: Clinical science for the pediatrician. Rheum Dis Clin North Am. 2007;33:389–402. doi: 10.1016/j.rdc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Moorthy LN, Peterson MG, Harrison MJ, Onel KB, Lehman TJ. Physical function assessment tools in pediatric rheumatology. Pediatr Rheumatol Online J. 2008;6:9. doi: 10.1186/1546-0096-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solari N, Viola S, Pistorio A, et al. Assessing current outcomes of juvenile idiopathic arthritis: A cross-sectional study in a tertiary center sample. Arthritis Rheum. 2008;59:1571–9. doi: 10.1002/art.24202. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez-Suarez R, Pistorio A, Cespedes Cruz A, et al. Health-related quality of life of patients with juvenile idiopathic arthritis coming from 3 different geographic areas. The PRINTO multinational quality of life cohort study. Rheumatology (Oxford) 2007;46:314–20. doi: 10.1093/rheumatology/kel218. [DOI] [PubMed] [Google Scholar]
- 20.Yang LL, Lambert SR, Drews-Botsch C, Stulting RD. Long-term visual outcome of penetrating keratoplasty in infants and children with Peters anomaly. J AAPOS. 2009;13:175–80. doi: 10.1016/j.jaapos.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Scott WE, Kutschke PJ, Keech RV, Pfeifer WL, Nichols B, Zhang L. Amblyopia treatment outcomes. J AAPOS. 2005;9:107–11. doi: 10.1016/j.jaapos.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Hall LS, Ceisler E, Abramson DH. Visual outcomes in children with bilateral retinoblastoma. J AAPOS. 1999;3:138–42. doi: 10.1016/s1091-8531(99)70058-3. [DOI] [PubMed] [Google Scholar]
- 23.Ledoux DM, Trivedi RH, Wilson ME, Jr, Payne JF. Pediatric cataract extraction with intraocular lens implantation: Visual acuity outcome when measured at age four years and older. J AAPOS. 2007;11:218–24. doi: 10.1016/j.jaapos.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Friling R, Kramer M, Snir M, Axer-Siegel R, Weinberger D, Mukamel M. Clinical course and outcome of uveitis in children. J AAPOS. 2005;9:379–82. doi: 10.1016/j.jaapos.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Felius J, Stager DR, Sr, Berry PM, et al. Development of an instrument to assess vision-related quality of life in young children. Am J Ophthalmol. 2004;138:362–72. doi: 10.1016/j.ajo.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Gothwal VK, Lovie-Kitchin JE, Nutheti R. The development of the LV Prasad-Functional Vision Questionnaire: A measure of functional vision performance of visually impaired children. Invest Ophthalmol Vis Sci. 2003;44:4131–9. doi: 10.1167/iovs.02-1238. [DOI] [PubMed] [Google Scholar]
- 27.Birch EE, Cheng CS, Felius J. Validity and reliability of the Children's Visual Function Questionnaire (CVFQ) J AAPOS. 2007;11:473–9. doi: 10.1016/j.jaapos.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–58. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 29.Morgan GA, Gliner JA, Harmon RJ. Measurement validity. J Am Acad Child Adolesc Psychiatry. 2001;40:729–31. doi: 10.1097/00004583-200106000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


