Abstract
Extensive circadian clock networks regulate almost every biological process in plants. Clock-controlled physiological responses are coupled with daily oscillations in environmental conditions resulting in enhanced fitness and growth vigor. Identification of core clock components and their associated molecular interactions has established the basic network architecture of plant clocks, which consists of multiple interlocked feedback loops. A hierarchical structure of transcriptional feedback overlaid with regulated protein turnover sets the pace of the clock and ultimately drives all clock-controlled processes. While originally described as linear entities, increasing evidence suggests that many signaling pathways can act as both inputs and outputs within the overall network. Future studies will determine the molecular mechanisms involved in these complex regulatory loops.
The circadian clock
The circadian clock is an endogenous mechanism that allows an organism to coordinate the temporal organization of biological processes to specific times of the day or night. Clock function is widely distributed among species since most organisms adapted their physiology and behavior to the periodic oscillations in external signals that take place throughout each diurnal cycle [1, 2]. These signals entrain or set the pace of the clock, which in turn has the capacity to drive self-sustained oscillations with a period ~ 24 hours [1, 2]. Clock-generated biological rhythms allow an organism to anticipate the onset of environmental changes providing an adaptive advantage that result in increased fitness and survival [3–5].
Because of their non-motile nature, plants strongly rely on the ability to interact with their environment. The circadian clock provides a critical mechanism that integrates multiple external signals and adjusts plant physiological responses accordingly. Indeed, the pervasiveness of the clock extends to nearly every aspect of plant growth and development [6–8]. In this article we review recent progress on the mechanisms underlying clock function in plants. The accumulated data point to an expansive regulatory network that integrates in a reciprocal fashion with many major signaling modules.
Transcriptional circuits within the circadian oscillator
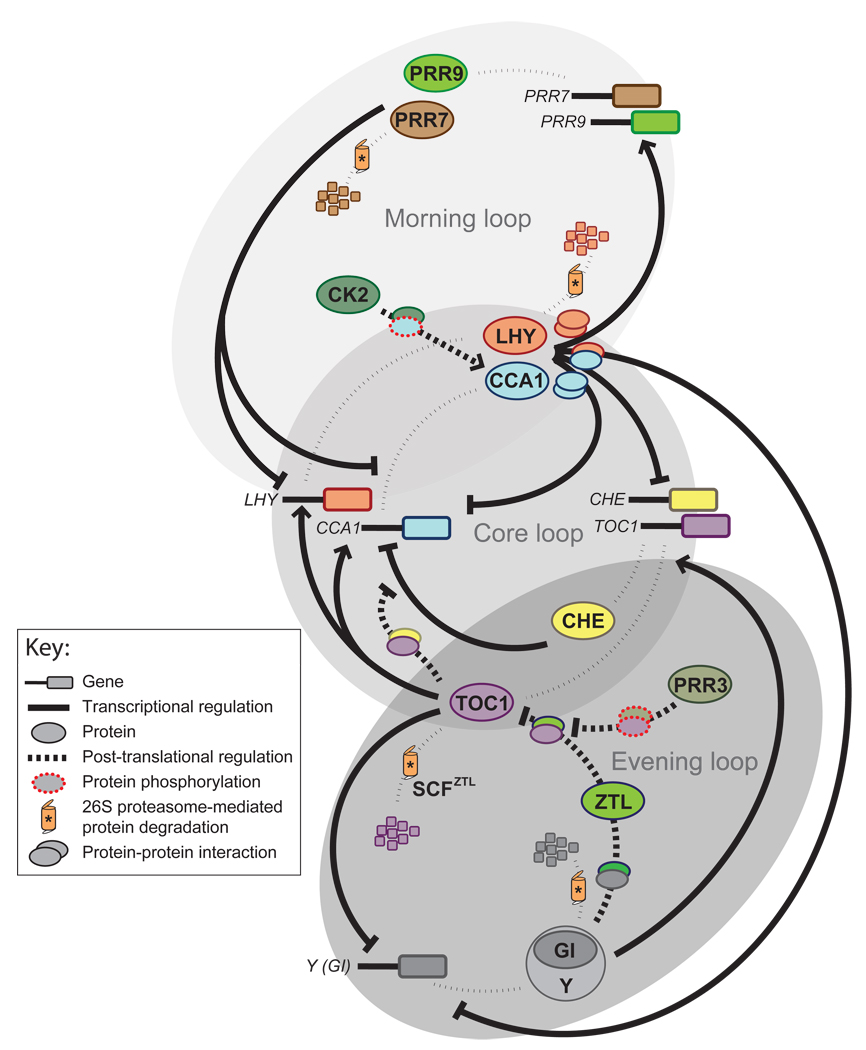
In most organisms studied to date, the circadian clock is proposed to function as a biochemical oscillator built on multiple interlocked regulatory loops [1, 2, 9, 10]. Despite this common architecture, specific clock components and biochemical processes are encountered among different species [1, 11]. The molecular characterization of clock mutants in Arabidopsis (Arabidopsis thaliana) revealed that the circadian oscillator in plants is defined by a core feedback loop that connects morning- and evening-phased circuits (Figure 1). The core feedback mechanism is based on the reciprocal regulation between the two morning-expressed MYB transcription factors CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), and the evening-phased pseudoresponse regulator TIMING OF CAB EXPRESSION 1 (TOC1) [12–15]. CCA1 and LHY are partially redundant [16, 17] and directly repress TOC1 by binding a motif within its promoter region, called the EVENING ELEMENT (EE) [12, 18]. This motif is frequently found in clock-regulated gene promoters and is sufficient to drive robust evening-phased rhythms when multimerized [19]. In addition, it was recently reported that the EE can also mediate cold responses [20]. The mechanism by which overlapping signals converge at the EE possibly involves the binding of alternative transcription factor complexes. This is supported by the ability of CCA1 and LHY to form homo- and hetero-dimers [21, 22], as well as the presence of protein-EE complexes even in the absence of CCA1 and LHY [19].
Figure 1.
Model of the circadian clock in Arabidopsis. Transcriptional and post-translational mechanisms define the basic architecture of Arabidopsis clock. The core feedback loop consists of two Myb-transcription factors, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), which negatively regulate the expression of TIMING OF CAB EXPRESSION 1 (TOC1). TOC1 been proposed to activate the expression of CCA1 and LHY. An additional module within this loop includes the reciprocal repression between CCA1 and CCA1 HIKING EXPEDITION (CHE). TOC1 likely antagonizes CHE through a direct protein interaction. Two additional phase-specific feedback loops have been proposed. In the morning loop CCA1 and LHY activate the expression of PSEUDORESPONSE REGULATORS 7 and 9 (PRR7 and PRR9), which in turn repress CCA1 and LHY. In the evening loop TOC1 represses an unknown component generically named Y (GIGANTEA (GI) appear to be part of Y), which in turn activates the expression of TOC1. TOC1 levels are controlled by proteasomal degradation mediated by the F-box protein ZEITLUPE (ZTL). This mechanism is modulated by GI and the competitive interaction between ZTL and PSEUDORESPONSE REGULATOR 3 (PRR3) with TOC1. The interaction between TOC1 and PRR3 is likely favored by the phosphorylation of these proteins. For clarity, clock components that cannot be placed with confidence to these feedback loops were omitted. Further details on the regulatory mechanisms shown here can be found throughout the text.
On the other arm of this core feedback loop, TOC1 regulates the expression of both CCA1 and LHY transcripts [12, 23]. While some data suggest that TOC1 functions a transcriptional activator of CCA1 and LHY [12, 24, 25], either reduced or elevated TOC1 levels lead to low CCA1 and LHY mRNA expression [12, 23]. This observation and the lack of known DNA-binding domains in TOC1 protein [14] indicate a complex mechanism for the regulation of CCA1 and LHY by TOC1. Recently, we identified a TCP(TB1, CYC, PCFs) transcription factor [26] called CCA1 HIKING EXPEDITION (CHE) that binds to the CCA1 promoter [27]. Both CHE and CCA1 directly repress each other’s expression, establishing an additional module to the core CCA1-LHY/TOC1 feedback loop [27]. Importantly, we found that CHE and TOC1 proteins directly interact and bind to the same region of the CCA1 promoter, and that the long period caused by increased TOC1 levels is shortened by the overexpression of CHE [27]. Thus, CHE appears to be part of the mechanism by which TOC1 regulates the expression of CCA1. It is possible that TOC1 antagonizes CHE, and probably other transcription factors, to eliminate the repression on CCA1 promoter by the end of the night [28]. As CHE does not appear to bind the LHY promoter, this hypothesis predicts that other TOC1 binding partners might be involved in the regulation of LHY expression. An additional level of regulation within the core feedback loop is provided by the ability of CCA1 and LHY to inhibit their own and each other’s expression [13, 15]. Consistent with the repressor activities of CHE and LHY, we found that they are redundant in the negative regulation of CCA1 [27].
Based on experimental observations and mathematical modeling, two additional phase-specific feedback loops were proposed [25, 29, 30]. One of these circuits is a morning loop where the expression of two TOC1 homologs, PSEUDORESPONSE REGULATOR 7 and 9 (PRR7 and PRR9), is directly activated by CCA1 and LHY [25, 29, 30]. In turn, PRR7 and PRR9 are partially redundant to inhibit the expression of CCA1 and LHY [29]. While the mechanism of this regulation is unknown, extrapolation of the relationship between CHE and TOC1 suggests PRR7 and PRR9 might interact with transcription factors bound to the CCA1 or LHY promoters. The other feedback loop involves the induction of TOC1 by an unknown (generically named Y) evening-phased clock component or function. In this evening loop, Y was also predicted to be light inducible and negatively regulated by TOC1, CCA1 and LHY [24]. These requisites are partially fulfilled by a protein named GIGANTEA (GI), however, additional redundant factors likely contribute to the predicted functions of Y [24, 25].
Despite the transcriptional nature of these feedback loops, CHE, CCA1 and LHY are the only transcription factors among the identified core clock components. An additional Myb-transcription factor named LUX ARRHYTHMO (LUX) (also known as PHYTOCLOCK 1 - PCL1) is likely a component of the circadian oscillator [31, 32]. However, because its molecular targets are not yet determined, the exact contribution of LUX within the clock network is unknown. Our ability to identify transcription factors associated to clock gene-promoters seems to be limited not only by the recurrent nature the clock regulatory circuits, but also by the high level of redundancy among plant transcription factors [33, 34]. Consistent with these redundancy issues, while most clock components were identified in loss-of-function genetic screens; CHE, CCA1 and LHY were respectively identified by alternative means like the yeast one-hybrid system [27], hybridization to a phage cDNA library [35] and a gain-of-function mutant screen [13]. CHE belongs to a 13-member subclass of TCP-transcription factors [26, 36] that, because their close homology at the DNA-binding domain, likely share target site specificity. fact, a reduction in CCA1 expression was recently observed in plants overexpressing a translational fusion between TCP20 and the ERF-associated amphiphilic repression (EAR) motif [37], indicating that other TCP-transcription factors might be redundant to CHE on the regulation of CCA1. Also, since TOC1 interacts with the CHE DNA-binding domain [27] it is possible that TCP20 and other TCP-transcription factors participate of the mechanism by which TOC1 regulates the CCA1 promoter activity. [27]. CCA1 and LHY belong to a subfamily of Myb-transcription factors also encompassing REVEILLE 1 to 8 (RVE1 to 8) [38, 39]. All but RVE5 were shown to bind the EE in protein microarrays [40], suggesting they could be redundant to CCA1 and LHY. Recent characterization of RVE1, CIRCADIAN 1 (CIR1/RVE2) and EARLY PHYTOCHROME RESPONSIVE 1 (EPR1/RVE7) revealed that their expression patterns are similar to CCA1 and LHY, peaking close to dawn in subjective days [38, 41, 42]. This circadian expression is dependent on CCA1 and LHY and is likely mediated by EE or EE-like motifs in their promoters. CIR1 and EPR1 were also shown to regulate their own expression [38, 42], but only CIR1 appears to have a role in the core clock [42]. On the other hand, EPR1 and RVE1 have been associated with clock output pathways. While EPR1 was proposed to be part of a slave oscillator, RVE1 was associated to the control of circadian oscillations in auxin levels [38, 41]. Interestingly, the reported phenotypes were observed under the constitutive overexpression of RVE1, CIR1, or EPR1 and only subtle or no phenotypes were observed in the respective single mutants [38, 41, 42]. These observations suggest their activity could be redundant with other transcription factors or, as it seems for RVE1, could be tissue-specific [38, 41, 42].
The lack of phenotypes in single mutants can also be explained by the capacity of several transcription factors to form heterodimers. For example, CHE-related TCP-transcription factors can interact with each other [36] and, as mentioned, CCA1 and LHY can heterodimerize [21, 22]. Since the interaction between CCA1 and LHY appears to occur through their DNA-binding domains, other combinations between homologous Myb-subfamily members might be possible [21, 22]. CCA1 also interacts with the bZIP-transcription factor HY5 [43], suggesting that heterodimerization between transcription factors of different families should be further investigated. While the biological significance of these interactions has not been yet explored, heterodimerization of transcription factors would provide additional regulatory mechanisms to the clock network.
Daily changes in the chromatin structure also regulate the transcription of clock genes. Increased marks for gene activation such as histone acetylation (H3K9Ac and H3K14Ac) and dimethylation (H3K4Me2) at the CCA1, LHY, GI and TOC1 promoters positively correlates with expression levels [18, 44]. In addition, a rhythmic pattern of histone acetylation at the TOC1 promoter precedes the peak of TOC1 expression [18], suggesting the presence of clock-controlled changes in the chromatin structure. Finally, as demonstrated for CCA1 [45], post-transcriptional control of mRNA stability might contribute to the regulation of transcript abundance of other clock genes as well.
Regulated protein turnover within the circadian oscillator
In addition to the transcriptional landscape described above, the current model for Arabidopsis circadian oscillator includes the post-translational regulation of protein levels and function. Stability of many clock components is regulated by proteasomal degradation. In this mechanism, target proteins are recognized and ubiquitinated by E3 ubiquitin-ligases and subsequently degraded by the 26S proteasome complex. Skp/Cullin/F-box (SCF) complexes are a special type of E3 ubiquitin-ligases, whose substrate specificity is defined by distinct F-box proteins [46]. In the circadian clock the proteosomal degradation of TOC1 and its homolog PSEUDORESPONSE REGULATOR 5 (PRR5) is mediated by the interaction with the F-box protein ZEITLUPE (ZTL) [47–51]. Two ZTL homologs, FLAVIN BINDING KELCH F-BOX 1 (FKF1) and LOV KELCH PROTEIN 2 (LKP2), also interact with TOC1 [50]. However, distinct clock phenotypes were observed in ZTL, FKF1 or LKP2 knock-out or overexpression backgrounds [51–54], suggesting that these F-box proteins target alternative clock-specific substrates. ZTL, FKF1, and presumably LKP2, function as blue light photoreceptors [55, 56]. Light dependent interaction between ZTL and GI stabilizes ZTL and TOC1 protein levels throughout the day [55]. TOC1 is further stabilized by PSEUDORESPONSE REGULATOR 3 (PRR3), which directly binds to TOC1 preventing its interaction with ZTL at the beginning of the night [47, 57]. Thus, TOC1 degradation is the result of a complex interplay among TOC1, ZTL, GI and PRR3. Other clock components such as LHY [58], PRR7 [59], PRR9 [60] and GI [61] are also subject to proteasomal degradation. Whereas the stability of GI appears to be regulated by a RING-type E3-ubiquitin-ligase during the night [62], mechanisms for the other proteins are still unknown. Interestingly, as noted for transcription factors, our ability to identify clock-related E3-ubiquitin-ligases is likely limited by genetic and functional redundancies. The F-box protein family is substantially more expanded in plants compared to other eukaryotes (~700 are encoded in Arabidopsis but only ~60 in humans) and; as ZTL, FKF1 and LKP2; many plant F-box protein subfamilies exhibit high domain conservation [63, 64]. Similarly, although not as drastically expanded as F-box-containing proteins, the RING-type E3-ubiquitin-ligase protein family is more populated in Arabidopsis than humans [65].
Protein phosphorylation also regulates activity and abundance of clock components. The overexpression of either CKB3 or CKB4 CASEIN KINASE2 (CK2) β-subunits leads to altered circadian rhythms likely by modifying the phosphorylation status of oscillator components [66–68]. For example, CKB3-mediated phosphorylation of CCA1 modulates its ability to form homodimers and to interact with the DNA [67–69]. A circadian phosphorylation pattern was also observed for TOC1, PRR3, PRR5, PRR7 and PRR9 [47, 59]. Whereas the identity of their respective kinases is unknown, a rhythmic kinase activity is supported by the circadian regulation of CKB4 protein levels [66]. TOC1 and PRR3 phosphorylated forms exhibit enhanced interaction affinity suggesting that the regulation of TOC1 stability by its competitive interaction with PRR3 or ZTL is modulated by their phosphorylation status [47].
The oscillator’s input and output circuits
Outside the oscillator, the circadian system encompasses input pathways that set the pace of the clock and output pathways that regulate the expression of clock-controlled genes. Although originally defined as a unidirectional path, increasing evidence suggests that many inputs are subject to clock regulation and that outputs often feedback to modulate clock function. Light and temperature, the most studied clock-entrainment signals, regulate the abundance of clock components by multiple mechanisms. Phytochrome and cryptocrome photoreceptor signaling pathways mediate the induction of CCA1 [15], LHY [70], PRR9 [25, 29] and GI [24] transcripts [71, 72]. At the post-transcriptional level, light also controls CCA1 mRNA stability [45], LHY translation rate [73] and TOC1 protein abundance [55]. Interestingly, light input pathways are reciprocally regulated by the clock. This phenomenon involves the activity of factors that attenuate light signaling during the day or night, such as EARLY FLOWERING 3 (ELF3) [74, 75], EARLY FLOWERING 4 (ELF4) [76], TIME FOR COFFEE (TIC) [77], LIGHT INSENSITIVE PERIOD 1 (LIP1) [78], FAR-RED ELONGATED HYPOCOTYL 3 (FHY3) [79] and XAP5 CIRCADIAN TIMEKEEPER (XCT) [80]. The circadian regulation of ELF3 and ELF4 gene expression, and likely LIP1 and XCP protein levels [74, 76, 78, 80, 81] evidence that the clock modulates light signaling by transcriptional and post-translational mechanisms. Although neither TIC nor FHY3 oscillate at mRNA or protein levels [79, 82], it is possible the clock directly or indirectly regulate their activity. This is supported the additive phenotypes observed in tic-elf3 double mutant seedlings [77]. Besides their role in setting the pace of the oscillator, these proteins also modulate other light signaling pathways [74, 83] providing a mechanism by which the clock controls different light responses.
Whereas light is a major resetting signal, temperature cycles are sufficient to drive circadian rhythms [84–87]. Core clock components such as CCA1, TOC1, LHY and PRR7 exhibit rhythmic expression after temperature entrainment [86] and altered expression after short or long cold exposures [88–90]. These observations, and the circadian defects encountered after temperature entrainment in loss-of-function alleles of clock genes (lhy-20, ztl-4, prr3-1, prr5-3, prr7-3 and prr9-1) [86], suggest that circadian oscillations after light or temperature cycles are driven by the same clock components. However, distinct mechanisms seem to regulate the expression of clock components by light or temperature signals. Cold treatment induces transcriptional changes largely mediated by the redundant AP2-transcription factors C-REPEAT/DRE BINDING FACTOR 1 to 3 (CBF1, CBF2 and CBF3) [91]. Thus, it is possible that temperature input occurs through the CBF-dependent regulation of core clock-gene transcript levels. Although this is a suitable hypothesis, other mechanisms such as the cold regulation of cytosolic calcium concentration might modulate clock function by temperature as well [92]. Similar to light input signals, responses to low temperature are reciprocally regulated by the circadian clock. Because of this feedback, a large subset of genes is more effectively regulated by low temperatures at the beginning of the day [88, 93]. Although the mechanism is unknown, the induction of CBFs and other transcription factors among these genes, likely trigger a transcriptional regulatory cascade that results in the differential expression of cold-responsive genes [88, 93]. In addition, the clock regulates the circadian expression CBF3 which results in higher transcript levels at the beginning of the day and possibly contributes to the enhanced response to cold temperatures at dawn [7, 94]. Induction of cytosolic calcium by low temperature also exhibits a clock-regulated differential sensitivity throughout the day [95], thus suggesting that multiple mechanisms mediate the clock regulation of cold responses. Consistent with this hypothesis, CBF-dependent and - independent mechanisms regulate the enhanced freezing tolerance in prr5-7-9 triple and gi single mutant seedlings, respectively [89, 96].
By controlling the expression of at least one third of the Arabidopsis transcriptome, the clock appears to regulate circadian rhythms of virtually every plant biological process [6, 7, 97]. Essential mechanisms such as the regulation of seed dormancy and germination [98], hormone responses (reviewed in [99]), hypocotyl growth [100], onset of flowering (reviewed in [101]) and responses to environmental stresses [96, 102] or nutrient availability [103] are regulated by the circadian clock. Intracellular events, such as calcium [104] and small GTPase signaling pathways [78] or chloroplast function [105, 106] also exhibit clock-controlled circadian oscillations. In an interesting turn, recent reports suggest that most of these circadian-regulated mechanisms also feedback on the clock function. As observed for cold treatments, the clock regulates circadian rhythms in the expression of many stress-responsive genes [107]. High tolerance to salt and osmotic stresses was observed in the arrhythmic prr5-7-9 triple mutant [96] and the opposite effect in cca1-lhy double mutant seedlings [102]. While this indicates that salt and osmotic stress responses are clock-outputs, elevated salt concentrations or osmotic pressure induce CCA1 and LHY levels [102, 107], suggesting these signals are clock inputs as well. Clock-regulated gene expression is also encountered in every hormone pathway [6, 7, 108, 109]. Circadian emission of ethylene and abscisic acid- or auxin-regulated responses are modulated by the circadian clock [41, 98, 110–112]. Reciprocally, endogenous reduction in hormone activity or the external application of phytohormones changes period, phase or amplitude of circadian oscillations [111, 113, 114]. Calcium and nitrogen responses also have their own feedback loops [92, 103]. Clock-controlled rhythms in cyclic adenosine diphosphate ribose (cADPR) levels likely drive circadian oscillations of cytosolic calcium; in turn, core clock components (GI, PRR5, PRR7, CCA1 and LHY) are regulated by cADPR [92, 104]. Similarly, whereas transcript levels of nitrogen assimilation genes are directly regulated by CCA1, addition of nitrogen nutrients produce phase shifts in CCA1 expression [103].
Altogether these data support a robust reciprocal relationship between input- or output-signaling pathways and the circadian oscillator. Little is known about the molecular mechanisms involved in these additional regulatory loops. However, the large proportion of circadian-expressed genes in Arabidopsis [6, 97] and the identification of phase- and response-specific regulatory motifs in clock-controlled promoters [8] suggest that transcriptional regulation plays an important role.
Conclusions and perspectives
The circadian clock provides a robust mechanism to coordinate the delicate interplay between plants and their environment. The core mechanism of this biochemical oscillator composed of interlocked regulatory circuits and functionally overlapping components. molecular wiring is mainly based on the transcriptional regulation of gene expression and post-translational control of protein levels. However, proteins specific to these mechanisms are clearly underrepresented among the clock components identified so far. This is possibly a consequence of the redundant nature of clock mechanisms and the expansion of protein families in plants. Thus, identification of the missing factors will require creative strategies to bypass these limitations. Clock function is mainly driven by light and temperature cycles, but additional environmental, organismal and intracellular clock-input signals have been identified. Single environmental cues possibly trigger the activation of different input pathways to generate a set of overlapping clock-entraining signals that contribute to the fine-tuning and robustness of the circadian clock. The reciprocal regulation between the circadian clock and input-output pathways suggests a heavily integrated system where each signaling pathway could impact the performance of any other through the core clock (Figure 2). Further characterization of the mechanisms underlying these multiple connections will ultimately result in a better understanding of how clock function enhances plant fitness and performance.
Figure 2.
Emerging model of the plant circadian system. Although initially outlined as three modules (clock-input pathways, circadian oscillator, and clock-output pathways) organized in a unidirectional path, accumulated experimental evidence indicates that the circadian system constitutes an expansive regulatory network where the circadian oscillator and major plant signaling modules (red circles) are regulated in a reciprocal fashion (black curved arrows, thicker lines denote the higher importance of these pathways as clock inputs). Main input signals such as light and temperature are also subject to clock regulation and many clock-output pathways often feedback to modulate the function of the circadian oscillator. This mechanism and the multiple interconnections between signaling modules (red dotted arrows) provide an integrated system to sense environmental cues (black dashed frame and arrows) and control plant physiology accordingly.
Acknowledgements
We thank G. Breton, B. Chow, C. Doherty, E. Hamilton, A. Helfer and D.A. Nusinow for critical reading of the manuscript. This work was partly supported by the NIH grants (GM56006 and GM67837). We apologize for not citing all the relevant papers of our colleagues owing to space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell-Pedersen D, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijnen H, Young MW. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet. 2006;40:409–448. doi: 10.1146/annurev.genet.40.110405.090603. [DOI] [PubMed] [Google Scholar]
- 3.Dodd AN, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 4.Green RM, et al. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 2002;129:576–584. doi: 10.1104/pp.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouyang Y, et al. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covington MF, et al. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008;9:R130. doi: 10.1186/gb-2008-9-8-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmer SL, et al. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- 8.Michael TP, et al. Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet. 2008;4:e14. doi: 10.1371/journal.pgen.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton EE, Kay SA. SnapShot: circadian clock proteins. Cell. 2008;135:368–368. doi: 10.1016/j.cell.2008.09.042. e361. [DOI] [PubMed] [Google Scholar]
- 10.Harmer SL. The circadian system in higher plants. Annu Rev Plant Biol. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 11.Wijnen H, et al. Control of daily transcript oscillations in Drosophila by light and the circadian clock. PLoS Genet. 2006;2:e39. doi: 10.1371/journal.pgen.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alabadi D, et al. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 2001;293:880–883. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- 13.Schaffer R, et al. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–1229. doi: 10.1016/s0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- 14.Strayer C, et al. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science. 2000;289:768–771. doi: 10.1126/science.289.5480.768. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZY, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- 16.Alabadi D, et al. Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr Biol. 2002;12:757–761. doi: 10.1016/s0960-9822(02)00815-1. [DOI] [PubMed] [Google Scholar]
- 17.Mizoguchi T, et al. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell. 2002;2:629–641. doi: 10.1016/s1534-5807(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 18.Perales M, Mas P. A functional link between rhythmic changes in chromatin structure and the Arabidopsis biological clock. Plant Cell. 2007;19:2111–2123. doi: 10.1105/tpc.107.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmer SL, Kay SA. Positive and negative factors confer phase-specific circadian regulation of transcription in Arabidopsis. Plant Cell. 2005;17:1926–1940. doi: 10.1105/tpc.105.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikkelsen MD, Thomashow MF. A role for circadian evening elements in cold-regulated gene expression in Arabidopsis. Plant J. 2009;60:328–339. doi: 10.1111/j.1365-313X.2009.03957.x. [DOI] [PubMed] [Google Scholar]
- 21.Lu SX, et al. CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis. Plant Physiol. 2009;150:834–843. doi: 10.1104/pp.108.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yakir E, et al. Posttranslational regulation of CIRCADIAN CLOCK ASSOCIATED1 in the circadian oscillator of Arabidopsis. Plant Physiol. 2009;150:844–857. doi: 10.1104/pp.109.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino S, et al. The APRR1/TOC1 quintet implicated in circadian rhythms of Arabidopsis thaliana: I. Characterization with APRR1-overexpressing plants. Plant Cell Physiol. 2002;43:58–69. doi: 10.1093/pcp/pcf005. [DOI] [PubMed] [Google Scholar]
- 24.Locke JC, et al. Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol Syst Biol. 2005;1 doi: 10.1038/msb4100018. 2005 0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeilinger MN, et al. A novel computational model of the circadian clock in Arabidopsis that incorporates PRR7 and PRR9. Mol Syst Biol. 2006;2:58. doi: 10.1038/msb4100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cubas P, et al. The TCP domain: a motif found in proteins regulating plant growth and development. Plant J. 1999;18:215–222. doi: 10.1046/j.1365-313x.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 27.Pruneda-Paz JL, et al. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science. 2009;323:1481–1485. doi: 10.1126/science.1167206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClung CR. Circadian rhythms. Linking the loops. Science. 2009;323:1440–1441. doi: 10.1126/science.1171418. [DOI] [PubMed] [Google Scholar]
- 29.Farre EM, et al. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol. 2005;15:47–54. doi: 10.1016/j.cub.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 30.Locke JC, et al. Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol Syst Biol. 2006;2:59. doi: 10.1038/msb4100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazen SP, et al. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc Natl Acad Sci U S A. 2005;102:10387–10392. doi: 10.1073/pnas.0503029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onai K, Ishiura M. PHYTOCLOCK 1 encoding a novel GARP protein essential for the Arabidopsis circadian clock. Genes Cells. 2005;10:963–972. doi: 10.1111/j.1365-2443.2005.00892.x. [DOI] [PubMed] [Google Scholar]
- 33.Riechmann JL, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290:2105–2110. doi: 10.1126/science.290.5499.2105. [DOI] [PubMed] [Google Scholar]
- 34.Shiu SH, et al. Transcription factor families have much higher expansion rates in plants than in animals. Plant Physiol. 2005;139:18–26. doi: 10.1104/pp.105.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ZY, et al. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9:491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosugi S, Ohashi Y. DNA binding and dimerization specificity and potential targets for the TCP protein family. Plant J. 2002;30:337–348. doi: 10.1046/j.1365-313x.2002.01294.x. [DOI] [PubMed] [Google Scholar]
- 37.Herve C, et al. In vivo interference with AtTCP20 function induces severe plant growth alterations and deregulates the expression of many genes important for development. Plant Physiol. 2009;149:1462–1477. doi: 10.1104/pp.108.126136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuno N, et al. The novel MYB protein EARLY-PHYTOCHROME-RESPONSIVE1 is a component of a slave circadian oscillator in Arabidopsis. Plant Cell. 2003;15:2476–2488. doi: 10.1105/tpc.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanhui C, et al. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol Biol. 2006;60:107–124. doi: 10.1007/s11103-005-2910-y. [DOI] [PubMed] [Google Scholar]
- 40.Gong W, et al. The Development of Protein Microarrays and Their Applications in DNA-Protein and Protein-Protein Interaction Analyses of Arabidopsis Transcription Factors. Molecular Plant. 2008;1:27–41. doi: 10.1093/mp/ssm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawat R, et al. REVEILLE1, a Myb-like transcription factor, integrates the ciircadian clock and auxin pathways. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813035106. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, et al. Constitutive expression of CIR1 (RVE2) affects several circadian-regulated processes and seed germination in Arabidopsis. Plant J. 2007;51:512–525. doi: 10.1111/j.1365-313X.2007.03156.x. [DOI] [PubMed] [Google Scholar]
- 43.Andronis C, et al. The Clock Protein CCA1 and the bZIP Transcription Factor HY5 Physically Interact to Regulate Gene Expression in Arabidopsis. Molecular Plant. 2008;1:58–67. doi: 10.1093/mp/ssm005. [DOI] [PubMed] [Google Scholar]
- 44.Ni Z, et al. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature. 2009;457:327–331. doi: 10.1038/nature07523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yakir E, et al. CIRCADIAN CLOCK ASSOCIATED1 transcript stability and the entrainment of the circadian clock in Arabidopsis. Plant Physiol. 2007;145:925–932. doi: 10.1104/pp.107.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lechner E, et al. F-box proteins everywhere. Curr Opin Plant Biol. 2006;9:631–638. doi: 10.1016/j.pbi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara S, et al. Post-translational regulation of the Arabidopsis circadian clock through selective proteolysis and phosphorylation of pseudo-response regulator proteins. J Biol Chem. 2008;283:23073–23083. doi: 10.1074/jbc.M803471200. [DOI] [PubMed] [Google Scholar]
- 48.Harmon F, et al. CUL1 regulates TOC1 protein stability in the Arabidopsis circadian clock. Plant J. 2008;55:568–579. doi: 10.1111/j.1365-313X.2008.03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiba T, et al. Targeted degradation of PSEUDO-RESPONSE REGULATOR5 by an SCFZTL complex regulates clock function and photomorphogenesis in Arabidopsis thaliana. Plant Cell. 2007;19:2516–2530. doi: 10.1105/tpc.107.053033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mas P, et al. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature. 2003;426:567–570. doi: 10.1038/nature02163. [DOI] [PubMed] [Google Scholar]
- 51.Somers DE, et al. ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell. 2000;101:319–329. doi: 10.1016/s0092-8674(00)80841-7. [DOI] [PubMed] [Google Scholar]
- 52.Nelson DC, et al. FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell. 2000;101:331–340. doi: 10.1016/s0092-8674(00)80842-9. [DOI] [PubMed] [Google Scholar]
- 53.Schultz TF, et al. A role for LKP2 in the circadian clock of Arabidopsis. Plant Cell. 2001;13:2659–2670. doi: 10.1105/tpc.010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somers DE, et al. The F-box protein ZEITLUPE confers dosage-dependent control on the circadian clock, photomorphogenesis, and flowering time. Plant Cell. 2004;16:769–782. doi: 10.1105/tpc.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim WY, et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature. 2007;449:356–360. doi: 10.1038/nature06132. [DOI] [PubMed] [Google Scholar]
- 56.Sawa M, et al. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–265. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Para A, et al. PRR3 Is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell. 2007;19:3462–3473. doi: 10.1105/tpc.107.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song HR, Carre IA. DET1 regulates the proteasomal degradation of LHY, a component of the Arabidopsis circadian clock. Plant Mol Biol. 2005;57:761–771. doi: 10.1007/s11103-005-3096-z. [DOI] [PubMed] [Google Scholar]
- 59.Farre EM, Kay SA. PRR7 protein levels are regulated by light and the circadian clock in Arabidopsis. Plant J. 2007;52:548–560. doi: 10.1111/j.1365-313X.2007.03258.x. [DOI] [PubMed] [Google Scholar]
- 60.Ito S, et al. Rhythmic and light-inducible appearance of clock-associated pseudo-response regulator protein PRR9 through programmed degradation in the dark in Arabidopsis thaliana. Plant Cell Physiol. 2007;48:1644–1651. doi: 10.1093/pcp/pcm122. [DOI] [PubMed] [Google Scholar]
- 61.David KM, et al. Arabidopsis GIGANTEA protein is post-transcriptionally regulated by light and darkc. FEBS Lett. 2006;580:1193–1197. doi: 10.1016/j.febslet.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 62.Yu JW, et al. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell. 2008;32:617–630. doi: 10.1016/j.molcel.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gagne JM, et al. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci U S A. 2002;99:11519–11524. doi: 10.1073/pnas.162339999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu G, et al. Evolution of F-box genes in plants: different modes of sequence divergence and their relationships with functional diversification. Proc Natl Acad Sci U S A. 2009;106:835–840. doi: 10.1073/pnas.0812043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone SL, et al. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 2005;137:13–30. doi: 10.1104/pp.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perales M, et al. The proteasome-dependent degradation of CKB4 is regulated by the Arabidopsis biological clock. Plant J. 2006;46:849–860. doi: 10.1111/j.1365-313X.2006.02744.x. [DOI] [PubMed] [Google Scholar]
- 67.Sugano S, et al. Protein kinase CK2 interacts with and phosphorylates the Arabidopsis circadian clock-associated 1 protein. Proc Natl Acad Sci U S A. 1998;95:11020–11025. doi: 10.1073/pnas.95.18.11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugano S, et al. The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc Natl Acad Sci U S A. 1999;96:12362–12366. doi: 10.1073/pnas.96.22.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daniel X, et al. CK2 phosphorylation of CCA1 is necessary for its circadian oscillator function in Arabidopsis. Proc Natl Acad Sci U S A. 2004;101:3292–3297. doi: 10.1073/pnas.0400163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez-Garcia JF, et al. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 2000;288:859–863. doi: 10.1126/science.288.5467.859. [DOI] [PubMed] [Google Scholar]
- 71.Devlin PF, Kay SA. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell. 2000;12:2499–2510. doi: 10.1105/tpc.12.12.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Somers DE, et al. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science. 1998;282:1488–1490. doi: 10.1126/science.282.5393.1488. [DOI] [PubMed] [Google Scholar]
- 73.Kim JY, et al. Light-regulated translation mediates gated induction of the Arabidopsis clock protein LHY. EMBO J. 2003;22:935–944. doi: 10.1093/emboj/cdg075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Covington MF, et al. ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell. 2001;13:1305–1315. doi: 10.1105/tpc.13.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McWatters HG, et al. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature. 2000;408:716–720. doi: 10.1038/35047079. [DOI] [PubMed] [Google Scholar]
- 76.Doyle MR, et al. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature. 2002;419:74–77. doi: 10.1038/nature00954. [DOI] [PubMed] [Google Scholar]
- 77.Hall A, et al. The TIME FOR COFFEE gene maintains the amplitude and timing of Arabidopsis circadian clocks. Plant Cell. 2003;15:2719–2729. doi: 10.1105/tpc.013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kevei E, et al. Arabidopsis thaliana circadian clock is regulated by the small GTPase LIP1. Curr Biol. 2007;17:1456–1464. doi: 10.1016/j.cub.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Allen T, et al. Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock. Plant Cell. 2006;18:2506–2516. doi: 10.1105/tpc.105.037358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin-Tryon EL, Harmer SL. XAP5 CIRCADIAN TIMEKEEPER coordinates light signals for proper timing of photomorphogenesis and the circadian clock in Arabidopsis. Plant Cell. 2008;20:1244–1259. doi: 10.1105/tpc.107.056655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hicks KA, et al. EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell. 2001;13:1281–1292. doi: 10.1105/tpc.13.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding Z, et al. TIME FOR COFFEE encodes a nuclear regulator in the Arabidopsis thaliana circadian clock. Plant Cell. 2007;19:1522–1536. doi: 10.1105/tpc.106.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson SL, et al. Attenuation of phytochrome A and B signaling pathways by the Arabidopsis circadian clock. Plant Cell. 1997;9:1727–1743. doi: 10.1105/tpc.9.10.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heintzen C, et al. A light- and temperature-entrained circadian clock controls expression of transcripts encoding nuclear proteins with homology to RNA-binding proteins in meristematic tissue. Plant J. 1994;5:799–813. doi: 10.1046/j.1365-313x.1994.5060799.x. [DOI] [PubMed] [Google Scholar]
- 85.Michael TP, McClung CR. Phase-specific circadian clock regulatory elements in Arabidopsis. Plant Physiol. 2002;130:627–638. doi: 10.1104/pp.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salome PA, McClung CR. PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell. 2005;17:791–803. doi: 10.1105/tpc.104.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Somers DE, et al. The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development. 1998;125:485–494. doi: 10.1242/dev.125.3.485. [DOI] [PubMed] [Google Scholar]
- 88.Bieniawska Z, et al. Disruption of the Arabidopsis circadian clock is responsible for extensive variation in the cold-responsive transcriptome. Plant Physiol. 2008;147:263–279. doi: 10.1104/pp.108.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao S, et al. Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis. Plant Cell Rep. 2005;24:683–690. doi: 10.1007/s00299-005-0061-x. [DOI] [PubMed] [Google Scholar]
- 90.Vogel JT, et al. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005;41:195–211. doi: 10.1111/j.1365-313X.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- 91.Gilmour SJ, et al. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol. 2004;54:767–781. doi: 10.1023/B:PLAN.0000040902.06881.d4. [DOI] [PubMed] [Google Scholar]
- 92.Dodd AN, et al. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science. 2007;318:1789–1792. doi: 10.1126/science.1146757. [DOI] [PubMed] [Google Scholar]
- 93.Fowler SG, et al. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 2005;137:961–968. doi: 10.1104/pp.104.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edwards KD, et al. FLOWERING LOCUS C mediates natural variation in the high-temperature response of the Arabidopsis circadian clock. Plant Cell. 2006;18:639–650. doi: 10.1105/tpc.105.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dodd AN, et al. Time of day modulates low-temperature Ca signals in Arabidopsis. Plant J. 2006;48:962–973. doi: 10.1111/j.1365-313X.2006.02933.x. [DOI] [PubMed] [Google Scholar]
- 96.Nakamichi N, et al. Transcript profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR arrhythmic triple mutant reveals a role for the circadian clock in cold stress response. Plant Cell Physiol. 2009;50:447–462. doi: 10.1093/pcp/pcp004. [DOI] [PubMed] [Google Scholar]
- 97.Michael TP, McClung CR. Enhancer trapping reveals widespread circadian clock transcriptional control in Arabidopsis. Plant Physiol. 2003;132:629–639. doi: 10.1104/pp.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Penfield S, Hall A. A role for multiple circadian clock genes in the response to signals that break seed dormancy in Arabidopsis. Plant Cell. 2009;21:1722–1732. doi: 10.1105/tpc.108.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robertson FC, et al. Interactions between circadian and hormonal signalling in plants. Plant Mol Biol. 2009;69:419–427. doi: 10.1007/s11103-008-9407-4. [DOI] [PubMed] [Google Scholar]
- 100.Nozue K, et al. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 101.Imaizumi T. Arabidopsis circadian clock and photoperiodism: time to think about location. Curr Opin Plant Biol. 2010;13:83–89. doi: 10.1016/j.pbi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kant P, et al. Functional-genomics-based identification of genes that regulate Arabidopsis responses to multiple abiotic stresses. Plant Cell Environ. 2008;31:697–714. doi: 10.1111/j.1365-3040.2008.01779.x. [DOI] [PubMed] [Google Scholar]
- 103.Gutierrez RA, et al. Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc Natl Acad Sci U S A. 2008;105:4939–4944. doi: 10.1073/pnas.0800211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu X, et al. Distinct light and clock modulation of cytosolic free Ca2+ oscillations and rhythmic CHLOROPHYLL A/B BINDING PROTEIN2 promoter activity in Arabidopsis. Plant Cell. 2007;19:3474–3490. doi: 10.1105/tpc.106.046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hassidim M, et al. Mutations in CHLOROPLAST RNA BINDING provide evidence for the involvement of the chloroplast in the regulation of the circadian clock in Arabidopsis. Plant J. 2007;51:551–562. doi: 10.1111/j.1365-313X.2007.03160.x. [DOI] [PubMed] [Google Scholar]
- 106.Stephenson PG, et al. PIF3 is a repressor of chloroplast development. Proc Natl Acad Sci U S A. 2009;106:7654–7659. doi: 10.1073/pnas.0811684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kreps JA, et al. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Michael TP, et al. A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biol. 2008;6:e225. doi: 10.1371/journal.pbio.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mizuno T, Yamashino T. Comparative transcriptome of diurnally oscillating genes and hormone-responsive genes in Arabidopsis thaliana: insight into circadian clock-controlled daily responses to common ambient stresses in plants. Plant Cell Physiol. 2008;49:481–487. doi: 10.1093/pcp/pcn008. [DOI] [PubMed] [Google Scholar]
- 110.Covington MF, Harmer SL. The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol. 2007;5:e222. doi: 10.1371/journal.pbio.0050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Legnaioli T, et al. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 2009;28:3745–3757. doi: 10.1038/emboj.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thain SC, et al. Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol. 2004;136:3751–3761. doi: 10.1104/pp.104.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanano S, et al. Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells. 2006;11:1381–1392. doi: 10.1111/j.1365-2443.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 114.Salome PA, et al. Arabidopsis response regulators ARR3 and ARR4 play cytokinin-independent roles in the control of circadian period. Plant Cell. 2006;18:55–69. doi: 10.1105/tpc.105.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]