Abstract
Background
Exacerbations of childhood asthma and rhinovirus infections both peak during the spring and fall, suggesting that viral infections are major contributors to seasonal asthma morbidity.
Objectives
We sought to evaluate rhinovirus infections during peak seasons in children with asthma and to analyze relationships between viral infection and illness severity.
Methods
Fifty-eight children aged 6 to 8 years with asthma provided 5 consecutive weekly nasal lavage samples during September and April; symptoms, medication use, and peak flow were recorded. Rhinoviruses were identified by using multiplex PCR and partial sequencing of viral genomes.
Results
Viruses were detected in 36% to 50% of the specimens, and 72% to 99% of the viruses were rhinoviruses. There were 52 different strains (including 16 human rhinovirus C) among the 169 rhinovirus isolates; no strains were found in more than 2 collection periods, and all but 2 children had a respiratory tract infection. Virus-positive weeks were associated with greater cold and asthma symptom severity (P < .0001 and P = .0002, respectively). Furthermore, virus-positive illnesses had increased duration and severity of cold and asthma symptoms and more frequent loss of asthma control (47% vs 22%, P = .008). Although allergen-sensitized versus nonsensitized children had the same number of viral infections, the former had 47% more symptomatic viral illnesses (1.19 vs 0.81 per month, P = .03).
Conclusions
Rhinovirus infections are nearly universal in children with asthma during common cold seasons, likely because of a plethora of new strains appearing each season. Illnesses associated with viruses have greater duration and severity. Finally, atopic asthmatic children experienced more frequent and severe virus-induced illnesses.
Key words: Asthma, children, viral respiratory tract infection, human rhinovirus, allergic sensitization, wheezing, cold symptoms, illness
Abbreviations used: HRV, Human rhinovirus; PEF, Peak expiratory flow
There are predictable peaks in asthma exacerbations in the spring and fall in temperate climates.1, 2 For example, regular seasonal cycles of asthma hospitalizations in school-aged children in Canada have been demonstrated over a 15-year period (1990-2004).3, 4 Factors that contribute to the “September epidemic” of asthma morbidity in children are likely to include return to school and concordant exposure to viruses and allergic sensitization together with exposure to relevant airborne allergens.
Despite the close relationships between human rhinovirus (HRV) infections and asthma exacerbations, the relationships between infection and the severity of clinical illness are not completely understood. For example, HRV can cause asymptomatic infections, common colds, or exacerbations of asthma. This gap in knowledge is partly related to difficulties in detecting HRV. Early studies of HRV epidemiology used diagnostics based on tissue culture, which is insensitive. Recent studies using PCR and other molecular techniques indicate that there are new branches on the HRV family tree,5, 6, 7, 8 and one characteristic of the recently detected HRV-C species is that they cannot be detected by using standard tissue culture. New molecular assays make it possible to conduct epidemiologic studies to identify and evaluate the role of specific strains, including HRV-C, in the activity of chronic asthma. For example, a recent study detected HRV-C in almost half of all children who were hospitalized with rhinovirus-associated respiratory tract illnesses.9
In addition to limitations related to viral diagnostics, most previous studies have focused on detection of HRV during periods of illness: there is little information about the true rates of infection, which can be asymptomatic. This lack of data related to mild or asymptomatic illnesses has hampered efforts to identify host and viral factors that contribute to the severity of the illness.
Based on previous findings in children who were hospitalized or seen in acute-care facilities,9, 10, 11 we hypothesized that both viral infections and allergic sensitization contribute to loss of asthma control during peak HRV seasons and that allergic children with HRV infections would be at greatest risk. Our goals for this study were to prospectively evaluate the contribution of HRV to seasonal asthma disease activity and to compare the clinical characteristics of viral and nonviral illnesses. To accomplish these goals, we monitored viral infections in children with asthma during peak common cold seasons, and the virologic information was compared with patterns of upper and lower respiratory tract illnesses, allergic sensitization, and cold and asthma disease activity.
Methods
Study subjects and study design
The study was approved by the Human Subjects Committee of the School of Medicine and Public Health, University of Wisconsin–Madison. Written informed consent was obtained from the parents before study participation. Fifty-eight children aged 6 to 8 years with asthma were enrolled in this study. Skin prick testing was performed on each subject at the time of enrollment, as well as fluoroenzyme immunoassays with an automated instrument (Unicap 100; Phadia, Uppsala, Sweden) to determine total and allergen-specific IgE levels in plasma. The allergens evaluated by means of skin prick testing were Alternaria species, tree fluid, Cladosporium species, grass mix, Aspergillus species, ragweed, Dermatophagoides farinae, Dermatophagoides pteronyssinus, weed mix, dog dander, cat dander, and cockroach (Greer Laboratories, Lenoir, NC). In addition, allergen-specific IgE levels to D farinae, D pteronyssinus, cat, dog, Alternaria species, ragweed, silver birch, timothy grass, cockroach, egg, and peanut were measured (ImmunoCAP FEIA, Phadia).
In April 2006, nasal samples were collected every other week for a total of 6 weeks. Because of the high frequency of distinct infections, specimen collection was increased to five specimens at weekly intervals during September 2006 and 2007 and April 2007 and 2008. Children and their parents recorded information on daily diary cards. The diary cards consisted of a calendar, and each day had spaces to place a sticker for cold and asthma symptoms, as well as a blank for recording morning peak expiratory flow (PEF) and albuterol use.
All samples were included for the viral analysis. For the comparison of rates of infection with respiratory symptoms, we included only children who collected 5 sequential weekly nasal specimens and were missing less than 1 week of diary card data in any given season. Of the 58 children enrolled, there were 2 children who did not complete any series information. Included in the final analyses were 42 (75%) of the 56 children who completed at least 1 season, of whom 19 (34%) completed 2 seasons.
Collection of nasal samples
At the first study visit, subjects were taught to collect samples of their own nasal mucus using a “nose-blowing” technique.12, 13 Briefly, participants sprayed saline into each nostril, alternately occluded each nare, and blew into a “baggie.” Two milliliters of a solution containing buffered saline (pH 7.4) along with 0.5% gelatin was then added to the baggie, which was then sealed and placed into a container in the freezer. Study materials and diaries were distributed to the homes for biweekly collection. The stability of HRV collected and stored under these conditions was confirmed in preliminary experiments: HRV was detectable even at low concentrations (100 PFU of HRV16 per milliliter), as might be seen in asymptomatic children, and samples were left at room temperature, 4 °C (refrigerated), and −20 °C (home freezer) for up to 5 weeks.
Identification of respiratory viruses
Diagnostic virology was performed for all nasal samples irrespective of whether a child had symptoms. A highly sensitive multiplex PCR-based assay (Respiratory Multicode Assay, EraGen Biosciences, Madison, Wis) was used to test for the following viruses: respiratory syncytial virus (groups A and B), HRV, parainfluenza (1, 2, 3, 4a, and 4b), influenza (A and B), adenovirus (B, C, and E), coronavirus (229E, NL63, OC43, and SARS), enterovirus, and human metapneumovirus.14 Bocavirus detection primers were also added to the Respiratory Multicode Assay mixture for the purposes of this study.
Molecular typing assay
Molecular typing of HRV was performed as previously described.3 Briefly, a 260-bp variable region at the 5′ noncoding region of HRV was amplified from the cDNA from nasal specimens by means of seminested PCR. The 260-bp fragment was cloned and sequenced. The identity of each sequence was verified by means of comparison with the 5′ sequences of the 101 reference HRV serotypes, as well as a number of sequences from newly identified strains.5
Respiratory symptoms and PEF
Parents and children scored cold and asthma symptom severity based on a 4-point scoring system (none, mild, moderate, and severe). Cold symptoms were defined as the following: mild, mild stuffy or runny nose but does not affect daily activities; moderate, moderate stuffy or runny nose and reduced activity but does not affect sleep; and severe, cannot breathe through the nose and not able to sleep well because of symptoms. Asthma symptoms were defined as the following: mild, occasional cough or wheeze but does not affect daily activity; moderate, frequent cough or wheeze with some shortness of breath and reduced activity but not affecting sleep; and severe, not able to sleep well because of symptoms. The personal best PEF for each subject was defined as the average of the 7 highest daily PEF values during the study period. Asthma control was defined by using the current national guidelines15 based on symptoms, PEF, and albuterol use. The criteria for loss of asthma control consisted of at least moderate asthma symptoms and either a decrease in PEF of 20% or more or use of albuterol for more than 2 days per week.
Infection and illness definitions
Two methods were used to analyze the association between infection and illness. First, weeks (Wednesday to the following Tuesday) were designated as virus positive or negative on the basis of viral detection results from nasal mucus samples that were obtained each Saturday. The presence of cold symptoms during each weekly interval was compared with viral detection data.
Second, we identified episodes of infection and illness based on virologic findings and clinical symptoms, respectively, and either could last longer than a single week. Infections were identified by evaluating results of molecular typing; if the same virus was detected in multiple weeks, it was considered a single infection. An episode of respiratory tract illness, which could be viral or nonviral, was defined as at least 2 consecutive days of cold or asthma symptoms rated at least mild in severity by the patient.
Nasal mucus samples were collected once every 7 days; therefore we bisected the weeks and assumed an illness to be associated with a virus if signs of illness were present within 3 days of a specimen showing a positive test result for virus. Associations between viral detection and symptoms were complex in some instances, and the following data-processing rules were established a priori to define a viral illness: (1) symptomatic periods were classified as 2 separate illnesses if there was more than a 3-day interval without symptoms, and (2) if there were different virologic findings within a period of continuous symptoms, this was classified as 2 illnesses.
Statistical analyses
Peak cold and asthma symptom scores were compared by virus recovery with the χ2 test for trend. All other categorical outcomes were analyzed with the Fisher exact test. Differences in continuous outcomes were assessed by using the Wilcoxon rank sum test. A 2-sided 5% level test result was regarded as statistically significant.
Results
Subjects' characteristics
Of the 58 children with asthma enrolled in the study, 42 provided at least 1 complete season of nasal specimens and diary card data and are included in the final illness analyses. The majority (88%) of the subjects had persistent asthma that required a daily controller therapy, and 57% had used an oral corticosteroid to relieve acute asthma symptoms in the year leading up to the study. This was an observational trial, and treatment of asthma was continued as per the child's regular physician. Most of the children were sensitized to at least 1 aeroallergen, as indicated by skin tests (50%), serum allergen-specific IgE measurements (69%), or both. Demographics and baseline FEV1 were similar in those completing and not completing the study procedures (Table I ).
Table I.
Characteristics of subjects (September 2006–April 2008)
| Complete (n = 42) | Incomplete (n = 16) | P value | |
|---|---|---|---|
| Age (y) | 6.5 | 6.6 | .60 |
| Sex (M/F) | 30/12 | 13/3 | .45 |
| ≥1 Positive SPT response | 50% (21/42) | 69% (11/16) | .20 |
| ≥1 Positive FEIA result | 69% (29/42) | 69% (11/16) | .98 |
| Daily asthma controller∗ in last year | 88% (37/42) | 88% (14/16) | .95 |
| Baseline FEV1 (L) | 1.46 ± 0.33 | 1.31 ± 0.28 | .13 |
| Oral corticosteroid in last year | 57% (24/42) | 63% (10/16) | .71 |
| Maternal history | |||
| Asthma | 38% (16/42) | 38% (6/16) | .97 |
| Allergies | 55% (23/42) | 69% (11/16) | .33 |
| Paternal history | |||
| Asthma | 19% (8/42) | 25% (4/16) | .62 |
| Allergies | 40% (17/42) | 50% (8/16) | .51 |
| Total viral infections (per season) | 1.3 ± 0.6 | 1.1 ± 0.6 | .48 |
| Rhinovirus infections (per season) | 1.1 ± 0.7 | 1.0 ± 0.6 | .72 |
| Nonrhinovirus infections (per season) | 0.2 ± 0.4 | 0.1 ± 0.3 | .49 |
| Cold symptoms per season (d) | 12.3 ± 7.5 | 11.1 ± 10.7 | .49 |
| Asthma symptoms per season (d) | 11.1 ± 8.8 | 12.4 ± 13.2 | .71 |
F, Female; FEIA, fluoroenzyme immunoassay; M, male; SPT, skin prick test.
Includes inhaled corticosteroids, montelukast, fluticasone/salmeterol, and cromolyn.
Identification of respiratory viruses
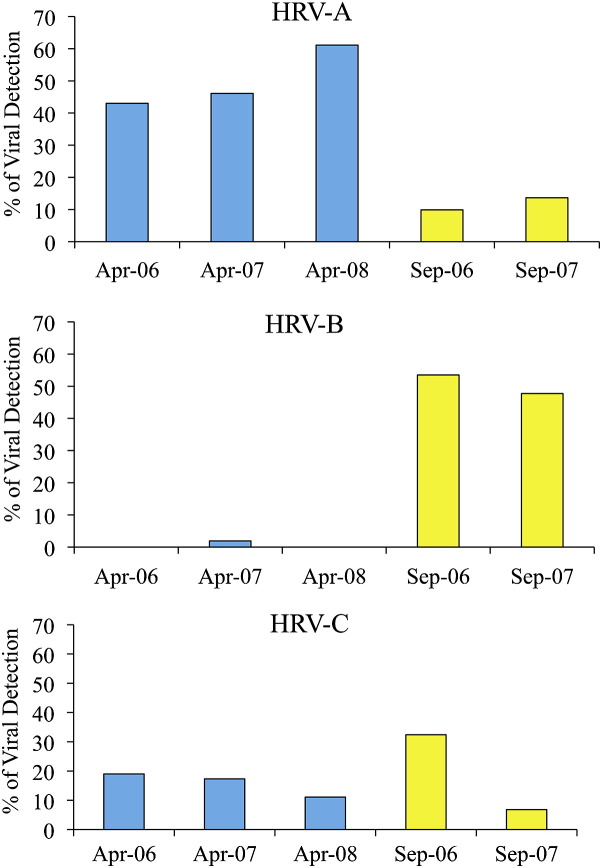
Of the 501 samples collected, 36% to 50% of specimens with positive test results for viruses each season (Fig 1 ), and all but 2 children had a virus detected at some point in time. The 169 samples with positive test results for HRV were found to have 52 different strains (up to 18 strains per season). Of the HRV specimens, 33% were group A, 35% were group B, and 24% were group C, and the remaining could not be typed. There appeared to be seasonal differences in the prevalence of HRV species (Fig 2 ). Overall, 48% of the April viruses were HRV-A, 51% of the September viruses were HRV-B, and similar numbers of HRV-C viruses were detected in April (16%) and September (22%). Other viruses detected included 7 enteroviruses, 6 adenoviruses (4 Adv-C and 2 Adv-B), 5 coronaviruses, 5 influenza viruses, 5 metapneumoviruses, 6 bocaviruses, and 3 parainfluenza viruses.
Fig 1.

Virology of viruses detected during routine monitoring periods.
Fig 2.
Seasonality of HRV species.
We next compared the list of HRV strains from one monitoring period to the next. The percentage of HRV isolates that were carryover strains, either from the previous season or the previous year, ranged from 0% to 28%. There were no strains found in more than 2 collection periods (Table II ).
Table II.
Numbers and strains of HRV detected during peak seasons
| April 2006 (n = 19) | September 2006 (n = 40) | April 2007 (n = 28) | September 2007 (n = 17) | April 2008 (n = 7) |
|---|---|---|---|---|
| 1 R51 | 6 R91 | 1 R91∗ | 2 R17† | 1 R43∗ |
| 2 R65 | 2 R85 | 3 R8 | 3 R6† | 5 R71 |
| 1 R90 | 1 R1B | 2 R85∗ | 2 R47 | 1 R38 |
| 2 R49 | 2 R17 | 2 R11 | 3 R72 | 4 R2 |
| 1 R54 | 3 R6 | 1 R23 | 1 R86† | 1 W10 |
| 1 R81 | 2 R86 | 5 R22 | 3 R61 | 1 W21 |
| 1 R46 | 23 R52 | 3 R53 | 6 R35 | |
| 1 W12 | 1 R97 | 5 R28 | 1 R43 | |
| 1 W36 | 2 R44 | 2 R78 | 1 R83 | |
| 1 W25 | 1 R30 | 1 R34 | 2 R4 | |
| 2 not typed | 1 R27 | 4 W23∗ | 3 R14 | |
| 1 R65∗ | 1 W29 | 2 W8 | ||
| 1 R21 | 1 W38† | 1 W29∗ | ||
| 2 W23 | 1 W20 | |||
| 5 W7 | 1 W12† | |||
| 2 W24 | 1 W45 | |||
| 2 W27 | 4 not typed | |||
| 10 W9 | ||||
| 2 W2 | ||||
| 2 not typed |
R, Classical serotypes; W, new HRV-C strains.
Carryover from previous season.
Carryover from previous year.
Weekly virus detection and respiratory symptoms
We next compared weekly viral detection rates with reported respiratory symptoms. Overall, viruses were detected in 151 (37%) of 404 evaluable weeks. Both cold symptoms (67% vs 31%, P < .001) and asthma symptoms (53% vs 30%, P < .001) were more likely to occur in virus-positive compared with virus-negative weeks (see Table E1 in this article's Online Repository at www.jacionline.org). In addition, virus-positive weeks were associated with greater peak cold severity (P < .0001) and asthma symptom severity (P = .0002, Table III ).
Table III.
Viral detection and severity of respiratory symptoms
| No. of weeks | No. of virus-positive weeks (%) | P value | |
|---|---|---|---|
| Peak cold score | |||
| 0 | 182 | 42 (23) | |
| 1 | 137 | 59 (43) | .0001 |
| 2 | 57 | 37 (65) | |
| 3 | 13 | 8 (62) | |
| Peak asthma score | |||
| 0 | 209 | 57 (27) | |
| 1 | 106 | 49 (46) | .0002 |
| 2 | 69 | 32 (46) | |
| 3 | 19 | 10 (53) |
Illnesses were then classified into one of 4 separate patterns: no symptoms, solitary cold symptoms, solitary asthma symptoms, and combined cold and asthma symptoms. Virus detection rates were increased during weeks with either cold symptoms alone (43%) or the combination of cold and asthma symptoms (58%). When solitary asthma symptoms were reported, virus detection rates were the same (24%) as those in asymptomatic children.
Viral versus nonviral illnesses
The 42 children who completed at least 1 season of data collection had 111 defined illnesses: 66 illnesses were virus positive, and 45 were virus negative. Of the virus-positive illnesses, 27% were associated with isolated cold symptoms, 20% were associated with isolated asthma symptoms, and 53% were associated with both. Overall, there was evidence that illnesses were more severe in the presence of viral infection (Table IV ). For example, in virus-positive illnesses the duration of cold and asthma symptoms was more than twice as long, and loss of control occurred much more frequently (47% vs 22%).
Table IV.
Characteristics of viral versus nonviral illnesses
| Virus negative (n = 45) | Virus positive (n = 66) | P value | |
|---|---|---|---|
| Maximum decrease in PEF (%) | 25 ± 14 | 29 ± 15 | .10 |
| Albuterol use (d) | 1.9 ± 3.7 | 3.7 ± 5.7 | .16 |
| Duration of cold symptoms (d) | 3.0 ± 3.3 | 8.1 ± 5.6 | <.0001 |
| Duration of asthma symptoms (d) | 3.2 ± 4.6 | 7.2 ± 7.8 | .0008 |
| Loss of asthma control | 10 (22%) | 31 (47%) | .008 |
| Moderate or severe cold symptoms | 5 (11%) | 28 (42%) | .0004 |
| Moderate or severe asthma symptoms | 13 (29%) | 34 (52%) | .02 |
Allergic sensitization, frequency of infection, and severity of illness
Finally, we compared rates of infection (virus-positive with or without symptoms) and illness (defined by the presence of clinical symptoms) in children who were sensitized to at least 1 allergen versus those who were not. Of the 42 children who completed at least 1 season, 29 (69%) were sensitized, and 13 (31%) were nonsensitized. Rates of infections, total illnesses, and numbers of nonviral illnesses per month (April and September) were similar between the 2 groups. In contrast, the sensitized group had 47% more virus-associated illnesses per season (1.19 vs 0.81, P = .03, Table V ).
Table V.
Effect of sensitization on rates of illness and infection
| Nonsensitized (n = 13) | Sensitized (n = 29) | P value | |
|---|---|---|---|
| Infections per season | 1.27 ± 0.83 | 1.31 ± 0.56 | .85 |
| Illnesses per season | 1.65 ± 0.59 | 1.95 ± 0.62 | .16 |
| Nonviral illnesses per season | 0.85 ± 0.85 | 0.76 ± 0.65 | .72 |
| Viral illnesses per season | 0.81 ± 0.56 | 1.19 ± 0.51 | .03 |
| Asymptomatic infections per season | 0.54 ± 0.75 | 0.14 ± 0.30 | .02 |
| Mild viral illnesses per season | 0.38 ± 0.46 | 0.45 ± 0.52 | .79 |
| Moderate-severe viral illnesses per season | 0.42 ± 0.49 | 0.74 ± 0.54 | .09 |
We next tested for association between sensitization and the severity of symptoms associated with documented viral infections. The nonsensitized children most commonly reported none or mild cold symptoms with their viral infections, whereas almost half of the viral infections in the sensitized children resulted in moderate or severe cold symptoms (P = .01 for trend). Similarly, almost half of the viral infections in the sensitized children resulted in moderate or severe asthma symptoms, and more than half of the viral infections in the nonsensitized children were reported as asymptomatic (P = .02 for trend, Fig 3 ).
Fig 3.
Effect of sensitization on the severity of cold and asthma symptoms associated with viral infection.
Discussion
During peak HRV seasons, respiratory viruses were detected in up to half of weekly samples obtained from school-aged children with asthma, and viral infection was nearly universal each April and each September. Although other viruses were detected, HRV made up the vast majority of viruses detected during April and September, and up to 18 viral strains were detected in a single month in the Madison, Wisconsin, area. The specific strains of HRV differed dramatically from season to season and year to year, with relatively few strains carried over. This study adds new information about patterns of the different species of rhinovirus, including the newly defined HRV-C. One of the unique features of this study is that we evaluated children with asthma during peak cold seasons irrespective of whether they were having asthma symptoms, cold symptoms, or both. As expected, HRV infections were major contributors to cold and asthma symptoms; however, correlations between viral infections and illness were not absolute. Clearly, nonviral factors also contributed to upper and lower airway symptoms in these children, and many infections (24%) were asymptomatic. Comparison of viral with nonviral illnesses demonstrated that viral illnesses lasted longer, were more severe, and were more likely to be associated with loss of asthma control. Finally, children with allergic sensitization had similar rates of viral infections but significantly increased rates and severity of viral illnesses compared with children who were not sensitized.
Rates of viral infections differ based on age, season, and the presence of respiratory symptoms. In previous studies of patients with asthma and respiratory symptoms, viral detection was as high as 62% to 88%,1, 3, 10, 11 and during asymptomatic periods, detection ranges from 12% to 44%.1, 10 The relatively high viral detection rate (36% to 50%) in our study is likely a result of our sampling both symptomatic and asymptomatic patients during periods of peak HRV prevalence. It is of particular interest that there was an extensive diversity of HRV strains detected in one community over the 3-year study period. Almost all of the strains detected each season were new (72% to 100%), and no one strain was detected in more than 2 collection periods. These findings are in substantial agreement with early studies of HRV epidemiology that used culture alone to detect HRV strains16, 17 and add new information about patterns of newly defined HRV species. Although the strains varied from season to season and year to year, the species clustered into a pattern: HRV-A in the spring, HRV-B in the fall, and HRV-C in both seasons. This pattern is different than what has been reported in a previous study of hospitalized children with asthma in which HRV-C appeared to predominate in the fall.9 Given the high rate of change, a longer period of evaluation will be needed before any firm conclusions can be reached regarding the seasonality of HRV species.
The high rate of viral detection during peak HRV seasons suggests that caution is needed in assigning causality between HRV detection and patterns of respiratory tract illness. In fact, viral detection was not always associated with cold symptoms, asthma symptoms, or both; both symptoms were also found during virus-negative weeks. On the other hand, there was a positive correlation between viral infection and asthma symptom severity. In addition, we found that viral illnesses were more severe, with longer durations of cold and asthma symptoms and more loss of asthma control compared with that seen with nonviral illnesses. These findings suggest that viral infections are closely associated with more significant cold and asthma symptoms and that a combination of viral and nonviral factors might be necessary to produce more severe symptoms.
There is great interest in defining potential interactions between allergy and viral infection that promote acute respiratory symptoms. More than two thirds of our subjects were sensitized to at least 1 allergen tested; those who were sensitized had the same number of infections but experienced 47% more viral respiratory tract illnesses with increased severity of both cold and asthma symptoms compared with the nonsensitized children. Furthermore, the nonsensitized children were more likely to experience asymptomatic infections. This would not have been apparent without routine sampling of nasal secretions. The results are consistent with observations in previous studies that have suggested that sensitization to aeroallergens is a significant risk factor and might be critical for severe viral illnesses.10, 11, 18, 19 Furthermore, it has also been suggested that sensitization and allergen exposure or viral infection alone do not act as independent risk factors for an asthma hospitalization; rather, they only increase the risk in combination.18 Because there are currently no effective antiviral treatments for common cold viruses, there is a need for clinical studies to determine whether controlling allergic inflammation is an effective strategy for reducing the risk of severe virus-induced asthma symptoms in allergic subjects.
The strengths of current study included the prospective study design; weekly sampling of nasal mucus regardless of symptoms; use of sensitive molecular-based viral diagnostics, including molecular typing; and comparison of viral versus nonviral illnesses. Limitations of the study include the absence of children without asthma and the modest sample size. Based on our findings in this study, we are currently conducting a larger study that will include both children with and without asthma and will focus on identifying additional host and viral characteristics that are associated with illness severity.
In conclusion, this study has shown that nearly all children with asthma are infected with viruses during the peak common cold seasons. Of the many HRV strains moving through a community in any given month, many strains were associated with loss of asthma control, including the newly discovered HRV-C species viruses. Additional studies are needed to determine whether there are specific characteristics of these viruses that promote wheezing and acute asthma. These data also show that respiratory tract illnesses associated with viruses are more severe and of longer duration and provide further evidence of interactions between allergy and virus-induced respiratory morbidity. Understanding the mechanisms for these interactions might be the key to designing novel and more effective treatments or preventive strategies for virus-induced exacerbations of asthma.
Clinical implications.
The combination of viral infection and allergy increases the morbidity of respiratory tract illnesses in children with asthma.
Footnotes
Supported by National Institutes of Health grant R01 HL080072.
Disclosure of potential conflict of interest: R. F. Lemanske, Jr, has given lectures for Merck and AstraZeneca and has consulted for AstraZeneca, Map Pharmaceuticals, Gray Consulting, Smith Research, Merck Childhood Asthma Network, Novartis, Quintiles/Innovax, RC Horowitz & Co, International Meetings and Science, and Scienomics. J. E. Gern has stock options in EraGen Biosciences and 3V Biosciences, has consulted for 3V Biosciences and Synairgen, and has received research support from AstraZeneca and Merck. The rest of the authors have declared that they have no conflict of interest.
Table E1.
Virus detection and individual symptoms
| Weeks | Virus-negative weeks | Virus-positive weeks | P value |
|---|---|---|---|
| Cold negative | 174 (69%) | 49 (33%) | <.0001 |
| Cold positive | 79 (31%) | 102 (67%) | |
| Total | 253 | 151 | |
| Asthma negative | 177 (70%) | 71 (47%) | <.0001 |
| Asthma positive | 76 (30%) | 80 (53%) | |
| Total | 253 | 151 |
References
- 1.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Campbell M.J., Josephs L.K. The relationship between upper respiratory infections and hospital admissions for asthma: a time trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 3.Johnston N.W., Johnston S.L., Duncan J.M., Greene J.M., Kebadze T., Keith P.K. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115:132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston N.W., Johnston S.L., Norman G.R., Dai J., Sears M.R. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Lee W.M., Kiesner C., Pappas T., Lee I., Grindle K., Jartti T. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McErlean P., Shackelton L.A., Lambert S.B., Nissen M.D., Sloots T.P., Mackay I.M. Characterization of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol. 2007;39:67–75. doi: 10.1016/j.jcv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kistler A.L., Webster D.R., Rouskin S., Magrini V., Credle J.J., Schnurr D.P. Genome-wide diversity and selective pressure in the human rhinovirus. Virol J. 2007;4:40. doi: 10.1186/1743-422X-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmenberg A.C., Spiro D., Kuzmickas R., Wang S., Djikeng A., Rathe J.A. Sequencing and analyses of all known human rhinovirus genomes reveals structure and evolution. Science. 2009;324:55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller E.K., Edwards K.M., Weinberg G.A., Iwane M.K., Griffin M.R., Hall C.B. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakes G.P., Arruda E., Ingram J.M., Hoover G.E., Zambrano J.C., Hayden F.G. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 11.Heymann P.W., Carper H.T., Murphy D.D., Platts-Mills T.A., Patrie J., McLaughlin A.P. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell K.R., Shorr R., Cherry J.D., Hendley J.O. Improved method for collection of nasal mucus. J Infect Dis. 1977;136:109–111. doi: 10.1093/infdis/136.1.109. [DOI] [PubMed] [Google Scholar]
- 13.Hayden F.G., Herrington D.T., Coats T.L., Kim K., Cooper E.C., Villano S.A. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36:1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W.M., Grindle K.A., Pappas T.E., Marshall D., Moser M., Beaty E. A high-throughput, sensitive and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Expert panel report 3: guidelines for the diagnosis and management of asthma. US Department of Health and Human Services; Bethesda: 2007. National Asthma Education and Prevention Program; National Heart, Lung, and Blood Institute; National Institutes of Health. [Google Scholar]
- 16.Monto A.S., Bryan E.R., Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J Infect Dis. 1987;156:43–49. doi: 10.1093/infdis/156.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Fox J.P., Cooney M.K., Hall C.E., Foy H.M. Rhinoviruses in Seattle families, 1975-1979. Am J Epidemiol. 1985;122:830–846. doi: 10.1093/oxfordjournals.aje.a114166. [DOI] [PubMed] [Google Scholar]
- 18.Murray C.S., Poletti G., Kebadze T., Morris J., Woodcock A., Johnston S.L. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–382. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sly P.D., Boner A.L., Bjorksten B., Bush A., Custovic A., Eigenmann P.A. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]