Capsule Summary
Anti-IgE treatment of intermittent to mild-persistent asthma in a cohort of seven volunteers resulted in improved allergen-induced airway hyperreactivity and a significant reduction in the number of airway myeloid dendritic cells.
Keywords: Anti-IgE treatment, Allergic Asthma, Dendritic cells (DCs), Myeloid DCs (mDCs), Plasmacytoid DCs (pDCs)
To the Editor:
Immunotherapy targeting immunoglobulin E (IgE) reduces aero-allergic hypersensitivity and is an FDA-approved treatment option for moderate to severe allergic asthma.1 Treatment with anti-IgE (Omalizumab, Genentech Inc. and Novartis Corp.) results in a marked reduction in the airway inflammation of asthmatics with a significant decrease in bronchial eosinophils and both CD4+ and CD8+ T cells.2 The primary antigen presenting cells in the lung, dendritic cells (DCs) play a critical role in airway inflammation in asthma, because they constantly sample inhaled allergens/antigens and stimulate antigen-specific T cells.3, 4 It is known that pulmonary inflammation results in an increase in the number of lung DCs.5 Accordingly, steroid therapy of asthmatics that diminished the airway inflammation resulted in a reduction in airway DC numbers.6 Thus, we hypothesized that anti-IgE treatment in asthma volunteers might result in reduced airway DC numbers and decrease their maturation phenotype.
The study included seven intermittent to mild-persistent asthmatics undergoing anti-IgE treatment. Omalizumab (anti-IgE) was administered subcutaneously over 12 wks, based on body weight and serum IgE levels (for details see online repository). All 7 subjects (2 males and 5 females, mean age 30yrs) had positive methacholine challenges and positive allergen skin tests described in detail elsewhere.7 Allergen PC20 values (provocative concentration causing 20% reduction in FEV1 values) were determined both before and after 12 wks of anti-IgE treatment. All subjects showed a significant increase in PC20 values from before to after anti-IgE treatment (p-value = 0.03) except one subject who failed to respond to the tested allergen even before anti-IgE treatment (see Figure 1). Thus, there was an improvement in allergen-specific airway hyperreactivity (AHR) in 6 of 7 subjects following anti-IgE treatment, a finding similar to a previous report.8
Figure 1. Effect of 12 weeks of anti-IgE treatment on the allergen-specific PC20 values of asthma volunteers.
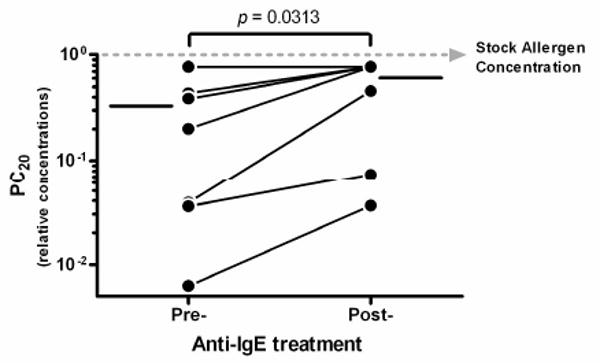
PC20 values or relative allergen concentrations at which the FEV1 values decreased by at least 20% from baseline were determined both before and after 12 wks of anti-IgE treatment. The mean PC20 values are indicated on either side of time-points by solid horizontal lines.
BALF and bronchial biopsy samples obtained at 2 wks before therapy, immediately after 12 wks of therapy and 2 months post-therapy were evaluated for inflammatory cell numbers. Subjects were lavaged using 300ml of saline with a mean BALF recovery of 197.5 ml (range 72-256 ml). There was no significant change in the BALF cells. The biopsy samples were also analyzed semi-quantitatively for intraepithelial and subepithelial inflammatory cell and mucus cell numbers by a pathologist blinded to the timing of the samples. Similar to BALF cellularity, there was no significant change in mucus cells, intraepithelial or subepithelial mononuclear cells, eosinophils or neutrophils.
We next examined DCs in bronchial biopsies following multicolor immunostaining and multispectral confocal imaging techniques (see details on online repository). Airway myeloid DCs (mDCs or conventional DCs) and plasmacytoid DCs (pDCs) were identified, localized and enumerated in these biopsies. For enumeration of DC subsets, the lineage negative (Lin-) cells (cells that did not express specific T cell, B cell, monocyte, neutrophil or NK markers) that expressed the β2-integrin CD11c and HLA-DR were identified as mDCs; whereas Lin- cells expressing the IL3-receptor α-subunit CD123 and HLA-DR were identified as pDCs.3, 4 mDCs were observed in both intraepithelial and subepithelial mucosa, whereas all of the pDCs observed were in the subepithelial region (Figure E1). As depicted in Figure 2A, there was a highly significant (p-value=0.0046) decrease in the number of mDCs immediately after anti-IgE treatment, that persisted for 2 months post-treatment (p-value=0.0205). The average number of mDCs in pre-treatment biopsies was 126/mm2 (range, 28-246) compared to 38/mm2 (range, 10-66) immediately after 12 wks of treatment and 49/mm2 (range, 10-151) 2 months post-treatment. However, the number of pDCs were not significantly changed at either the end of therapy or at 2 months post-therapy (Figure 2B). The average number of pDCs/mm2 in the biopsies was 12/mm2 (range, 0-38) pre-treatment compared to 31/mm2 (range, 0-104) immediately after treatment and 43/mm2 (range, 10-85) 2 months post-treatment. It is possible that the trend towards an increase in pDCs could have become significant with additional numbers of study subjects.
Figure 2. Effect of anti-IgE treatment on the numbers of mDCs and pDCs in asthmatic airways (N=7).
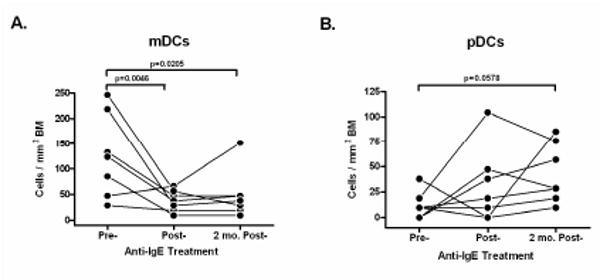
Numbers of mDCs (A) and pDCs (B) were determined by multispectral image analysis of airway biopsies by counting Lin-CD11c+HLA-DR+ cells and Lin-CD123+HLA-DR+ cells per mm2, respectively from separate serial sections. The data from individual subjects is plotted as an average of 4 sections per time-point.
The surface expression of selected function-associated markers on DCs was also analyzed, but there were no significant changes in the percentages of mDCs and pDCs that expressed CD80, CD83 and CD1a, and CD80, CD83 and BDCA2, respectively (Figure E2). The biopsies were also analyzed for changes in CD4+, CD8+ T cells and T regulatory cells, but unlike a previous report,2 we found no effect on the bronchial T cells (Figure E3) following anti-IgE therapy. We believe that this inconsistency could be explained by differences in the severity of asthma in the study subjects. However, we observed a trend toward reduction in the CD4+ T cells, and it is also possible that samples from additional volunteers would have shown a significant decrease.
In summary, in this study of 7 volunteers, we demonstrate that anti-IgE therapy in intermittent to mild persistent asthma resulted in a significant decrease in airway mDCs associated with improved PC20 values. Asthma-associated AHR likely results from chronic inflammation; and allergen-specific T cells play an important role in the maintenance of chronic inflammation.9 DCs are essential for the initiation of primary T cell responses, and they are also very efficient in re-stimulating memory T cells. Thus, we speculate that the decrease in airway mDCs following anti-IgE treatment might contribute in the long term to reduced T cell activity and airway inflammation, and consequently to decreased AHR. We do not believe that the anti-IgE therapy directly reduced mDCs, but rather reduced activation of airway mast cells (due to a decrease in IgE binding to these important effector cells) is most likely the primary effect of therapy. Consistent with this idea, a separate study showed that the same subjects enrolled in this report had a marked reduction in the FcεRI-mediated release of Th2 cytokines and chemokines by peripheral blood basophils following anti-IgE treatment.7 Thus, the diminished release of DC chemotactic chemokines resulting either directly or indirectly from less mast cell activation and secretion could be responsible for the decrease in airway mDC numbers. Strikingly, a decrease in the number of mDCs was detected although the number of other inflammatory cells did not change significantly, which suggests, in view of the clinical response in these patients, that mDC numbers might be a very sensitive indicator of modulation in tissue inflammation.
Acknowledgments
Funds: The study was supported by grants from NHLBI (1-P50-HL56384-06-10 and RO1-HL068111), NIAID (U19-AI57234-01-05 and P01-AI056295-01-05) and NCRR (5M01-RR00997) of National Institutes of Health.
Abbreviations
- DCs
Dendritic cells
- mDCs
myeloid DCs
- pDCs
plasmacytoid DCs
- IgE
immunoglobulin E
- FEV1
forced expiratory volume in 1 second
- PC20
provocative concentration causing 20% reduction in FEV1 values from baseline
Footnotes
Conflict of Interest: None
This article has data in an Online Repository, which is accessible from this issue's table of content online at www.jacionline.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–93. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 3.Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–24. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–32. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moller GM, Overbeek SE, Van Helden-Meeuwsen CG, Van Haarst JM, Prens EP, Mulder PG, et al. Increased numbers of dendritic cells in the bronchial mucosa of atopic asthmatic patients: downregulation by inhaled corticosteroids. Clin Exp Allergy. 1996;26:517–24. [PubMed] [Google Scholar]
- 7.Oliver JM, Tarleton CA, Gilmartin L, Archibeque T, Qualls CR, Diehl L, et al. Reduced FcepsilonRI-Mediated Release of Asthma-Promoting Cytokines and Chemokines from Human Basophils during Omalizumab Therapy. Int Arch Allergy Immunol. 2009;151:275–84. doi: 10.1159/000250436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulet LP, Chapman KR, Cote J, Kalra S, Bhagat R, Swystun VA, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997;155:1835–40. doi: 10.1164/ajrccm.155.6.9196083. [DOI] [PubMed] [Google Scholar]
- 9.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–93. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]


