Abstract
Statins, inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, can have protective effects in various organs. We determined whether application of statins after a detrimental insult protected endothelial cells. Bovine pulmonary arterial endothelial cells (BPAEC) were subjected to a 5-h oxygen-glucose deprivation (OGD) and a 1-h simulated reperfusion. Simvastatin or atorvastatin alone or plus mevalonate (the immediate product of the reaction mediated by HMG-CoA reductase), geranylgeranyl pyrophosphate (GGPP, a product downstream of mevalonate), Ly294002 (a protein kinase B/Akt inhibitor), U0126 [an extracellular signal-regulated kinase (ERK) pathway inhibitor] or diphenyleneiodonium [a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor] were added to cells immediately after the OGD for 1 h. Simvastatin and atorvastatin dose-dependently reduced the OGD and simulated reperfusion-induced lactate dehydrogenase (LDH) release from primary BPAEC and BPAEC between passage 4 and 15. This effect was inhibited by mevalonate, GGPP and Ly294002 and was not affected by U0126. Consistent with those results, simvastatin and atorvastatin increased the expression of phospho-Akt/activated Akt, and did not change the expression of phospho-ERK/activated ERK after the OGD and simulated reperfusion. The OGD and simulated reperfusion-induced LDH release and superoxide production, as measured by the dihydroethidium fluorescent intensity, were inhibited by diphenyleneiodonium. These results suggest that statin post-treatment reduces OGD and simulated reperfusion-induced cell injury. This effect may be mediated by inhibiting HMG-CoA reductase and the subsequent inhibition of small GTPases. GTPase activation depends on GGPP generation and contributes to the formation of NADPH oxidase complex that produces superoxide. The statin post-treatment-induced protection may also involve activated Akt.
Keywords: statin, postconditioning, endothelial cells and protein kinase B
1. Introduction
Statins are inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase. They are widely used in clinic to reduce blood cholesterol levels and, therefore, to decrease risks for cardiovascular diseases. Statins also have pleiotropic effects that are unrelated to its cholesterol lowering results (Davignon, 2004). For example, statins can be used to reduce osteoporosis and tumor growth and to improve ventricular function in patients with heart failure (Davignon, 2004; Miida et al., 2004). Several studies have shown that statins can induce a preconditioning effect (Domoki et al., 2009; Gu et al., 2008; Lazar et al., 2003), a phenomenon in which a prior application of a stimulus or drug reduces injury caused by a subsequent insult. This statin-induced preconditioning effect is inhibited by mevalonate, the immediate product of the reaction mediated by HMG-CoA reductase and the precursor molecule for both sterol (including cholesterol) and nonsterol (isoprenoid) biosynthesis (Domoki et al., 2009).
The term postconditioning is initially used to describe a phenomenon of protection by applying a modified reperfusion sequence [3 to 5 cycles of a brief episode of ischemia (10 – 30 s) and reperfusion (10 – 30 s)] to the tissues or organs that just suffer a prolonged episode of ischemia (Zhao et al., 2003). Subsequently, it has been shown that postconditioning effect can be induced not only by the application of brief episodes of ischemia (ischemic postconditioning) but also by the use of drugs in various organs including brain and heart (Lee et al., 2008; Weihrauch et al., 2005).
Endothelial cells are an important component for all organs and systems. They form a unique barrier to prevent free access of chemicals and cells in the blood to the vascular smooth muscle cells and parenchymal cells of organs. Endothelial cells also release various molecules, such as nitric oxide, to affect the functions of other cells (Nathan and Xie, 1994). Thus, it is critical to maintain the structural and functional integrity of endothelial cells; however, many insults can threaten this integrity. For example, ischemia can injure endothelial cells.
One potential approach that can be used clinically to protect endothelial cells is by a postconditioning/post-treatment mechanism induced by drugs. Thus, we hypothesize that statins can induce a postconditioning effect in endothelial cells. We tested this hypothesis with statins (simvasatin and atorvastatin) in the bovine pulmonary arterial endothelial cells (BPAEC), and used oxygen-glucose deprivation (OGD) to simulate ischemia in vitro. We also determined whether statin-induced postconditioning effects were mediated by inhibiting the HMG-CoA reductase and by activating the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) or extracellular signal-regulated kinase (ERK), two kinases that have been shown to mediate protection induced by various preconditioning and postconditioning stimuli in many organs (Raphael et al., 2006; Zuo et al., 2006).
2. Materials and Methods
2.1. Reagents
Simvastatin and Ly294002 were purchased from Calbiochem (San Diego, CA). Atorvastatin was kindly provided as a gift by Pfizer (New York, NY). Mevalonate, geranylgeranyl pyrophosphate (GGPP), dihydroethidium, U0126, diphenyleneiodonium and the anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (catalogue number: G9545) were obtained from Sigma (St. Louis, MO). The Lactate dehydrogenase (LDH) kit was purchased from Clontech Laboratory (Mountain View, CA). The anti-phospho-ERK antibody (catalogue number: sc-7383) and anti-ERK (catalogue number: sc-93) antibody were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-phospho-Akt antibody (anti-Ser473) (catalogue number: 9271) and the anti-Akt antibody (catalogue number: 9272) were obtained from Cell Signaling Technology, Inc. (Danvers, MA).
2.2. Cell culture
The bovine pulmonary arterial endothelial cell (BPAEC) was isolated and characterized as described earlier (Zuo and Johns, 1997). The cells were cultured in a T75 flask containing 12 ml of culture media composed of Dulbecco’s Modified Eagle’s Medium (DMEM) (containing 1,000 mg/l D-glucose, L-glutamine and pyridoxine HCl), 110 mg/l sodium pyruvate, 10% heat inactivated fetal bovine serum, 90 µg/ml thymidine, 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were kept in a humidified atmosphere of 95% air-5% CO2 at 37°C. The medium was changed three times per week. When the cells were 70 – 80% confluent, they were exposed to 0.05% trypsin-EDTA solution and sub-cultured in a new flask. Experiments were performed on BPAECs at passage 4 to 15.
Primary BPAEC cultures were used in some experiments. These cells were harvested and sorted by flow cytometry as described earlier (Zuo and Johns, 1997). Cultures with > 98% endothelial cells as identified by staining with acetylated low density lipoprotein coupled with 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindo-carbocyanine perchlorate were used in the studies.
2.3. Exposure to oxygen-glucose deprivation
The cells were plated into 6-well plates at a density of 5 × 105 cells/ml (2 ml/well) and cultured overnight (about 17 hours). Glucose-free buffer contained 154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 2.3 mM CaCl2, and 5.0 mM HEPES. OGD buffer was prepared by bubbling the glucose-free buffer with 100% N2 for 30 min. Cells in the control group were washed with and incubated in glucose containing buffer (2 g/l glucose) in a humidified atmosphere of 95% air-5% CO2 at 37°C. OGD was applied by washing cells with the OGD buffer three times and then placing the cells in a 2 ml/well of this OGD buffer. These plates were immediately placed in an air-tight chamber (Billups-Rothenberg, CA) gassed with 100% N2 for 10 min. The oxygen content in the outlet of the chamber was monitored with a Datex™ infrared analyzer (Capnomac, Helsinki, Finland) and reached 0% at 3~5 min after the onset of gassing. After closure of the inlet and outlet of the chamber, the chamber was kept at 37°C for 5 h except for the time-course experiment. After confirming that the oxygen content in the chamber was 0% at the end of the OGD period, the chamber was opened and glucose was added to make the final glucose concentration in the buffer at 2 g/l. The plates were then kept for 1 h in a humidified atmosphere of 95% air-5% CO2 at 37°C.
2.4. Application of statins and other chemicals
Simvastatin or atorvastatin was added to the incubation buffer at the beginning of the simulated reperfusion to make the final concentrations from 0.5 to 10 µM in the buffer. In some experiments, diphenyleneiodonium (20 µM), GGPP (10, 20 and 40 µM), mevalonate (200 and 500 µM), Ly294002 (10 µM) or U0126 (1 µM) was added to the incubation buffer at the onset of the simulated reperfusion (the concentrations in the brackets are the final concentrations in the incubation buffer). All of the incubations with these agents were for 1 h.
2.5. Lactate dehydrogenase assay
LDH activity was determined using the LDH cytotoxicity detection kit, as we did before (Kim et al., 2009). Briefly, the incubation solution harvested from the 6-well plates at the end of the experiments was centrifuged at 13,000 rpm for 10 min and 100 µl of the cell-free supernatant was transferred to 96-well plates. The supernatant was incubated with the same amount of reaction mixture from the kit. LDH activity was determined by a colorimetric assay with the absorbance wavelength at 492 nm and the reference wavelength at 655 nm in a spectrophotometry (Bio-Rad Laboratories, Hercules, CA). Background absorbance from the cell-free buffer solution was subtracted from all absorbance measurements. After removal of the buffer from 6-well plates, 1% triton X-100 lysing solution was applied to the remaining cells. The percentage of LDH released to incubation buffer in total LDH was calculated: spontaneously released LDH in the buffer/(spontaneously released LDH in the buffer + intracellular LDH released by triton X-100). The LDH values from various treatment groups were then normalized by the mean values from the corresponding control cells in the same set of experiments.
2.6. Superoxide production assay
Dihydroethidium is a cellular membrane-permeable fluorophore hydroethidine and was used to measure the superoxide production from the BPAEC. The method of measuring superoxide production was similar to a previously described method (Chen et al., 2008) with some modifications. Briefly, the cells were plated into 6-well plates and grew overnight. They were then loaded with 10 µM dihydroethidium in basal DMEM for 30 min. After being washed with phosphate buffered saline, the cells were then subjected to OGD for 5 h and simulated reperfusion for 1 h. Aliquots (1.5 ml) of the incubation buffers were taken for fluorescent intensity measurement on a spectrofluorometer (Instrument Photon Technology International, Birmingham, NJ) with excitation and emission set at 470 and 610 nm, respectively.
2.7. Western blot analysis
The cells were grown in 100-mm culture dishes and subjected to OGD and statin post-treatment as described above. The cells were harvested at 1 h after the OGD and then homogenized in a lysis buffer containing 50 mM Tris (pH 7.4), 140 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 30 µM MG132 and Roche’s complete protease inhibitor mixture (Roche Applied Science, Indianapolis, IN). Homogenates were centrifuged at 4°C for 30 min at 13,000 rpm. The supernatant was used for Western blotting. Thirty microgram proteins per lane were loaded for electrophoresis on a 10% polyacrylamide gel and then blotted onto a polyvinylidene difluoride membrane. After blocking with Tris buffered saline containing 5% (w/v) bovine serum albumin and 0.1% Tween-20, the membranes were incubated with the following primary antibodies: anti-phospho-ERK antibody (1:1000), anti-ERK antibody (1:1000), anti-phospho-Akt antibody (1:1000), anti-Akt antibody (1:1000) or anti-GAPDH antibody (1:5000). Appropriate secondary antibodies were then used. Protein bands were visualized by the enhanced chemiluminescence method and detected and analyzed by Genomic and Proteomic Gel Documentation Systems from Syngene (Frederick, MD). The protein band intensities of ERK and Akt were normalized by the corresponding band intensities of GAPDH. The results from cells under various experimental conditions were then normalized by those of the corresponding control cells.
2.8. Statistical analysis
Data of LDH release and superoxide production are expressed as mean ± S.D. (n ≥ 6 for each experimental condition). Results were analyzed by one-way analysis of variance followed by the Tukey test for post hoc analysis after confirmation of normal distribution of the data or by Kruskal-Wallis analysis of variance on ranks followed by the Dunn’s test when the data are not normally distributed. Western blotting results are also presented as mean ± S.D. (n ≥ 4 for each experimental condition). Results were analyzed by one-way repeated measurement analysis of variance or one-way repeated measurement analysis of variance on ranks followed by the Tukey test for post hoc analysis. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Statins induced a postconditioning effect in the BPAEC
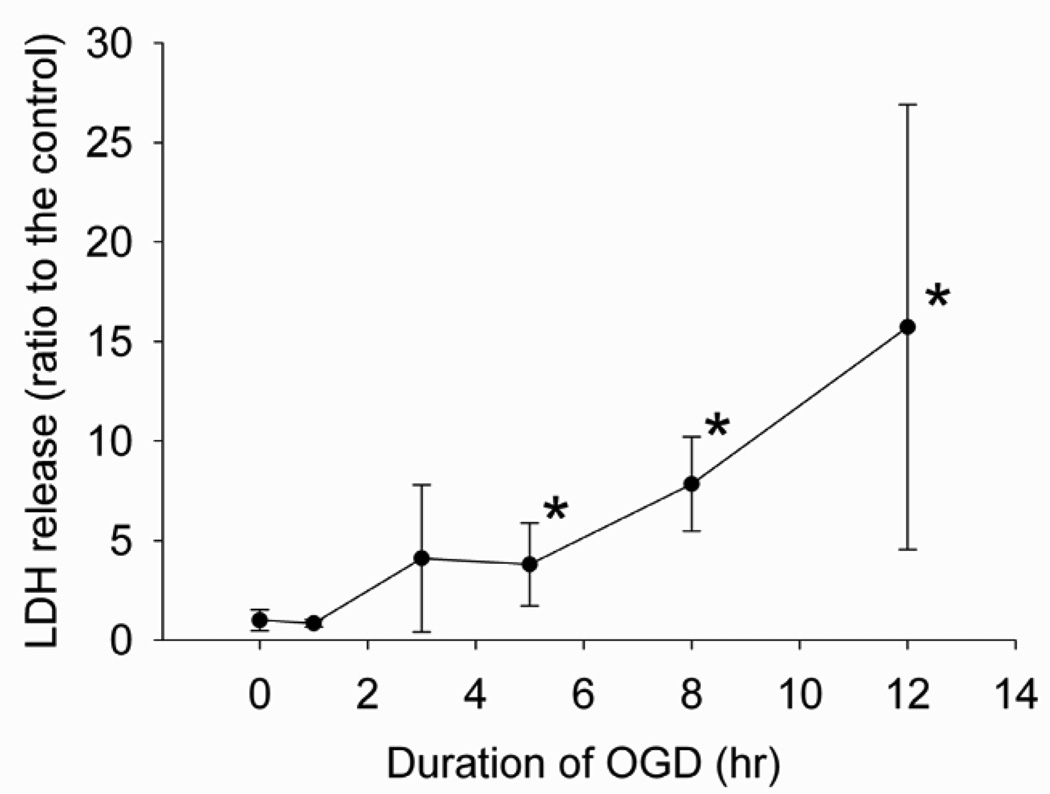
To identify a condition that could cause significant cell injury, we performed a time-course experiment. Different lengths of OGD caused a time-dependent LDH release, a parameter often used to reflect cell injury. Five-hour OGD followed by 1-h simulated reperfusion caused a significant LDH release from cells (Fig. 1). Thus, we chose this condition for other experiments.
Fig. 1. Time-course experiment.
Bovine pulmonary arterial endothelial cells were exposed to or not exposed to various lengths of oxygen-glucose deprivation (OGD) and 1-h simulated reperfusion. Results are means ± S.D. (n = 6 – 24). * P < 0.05 compared with control.
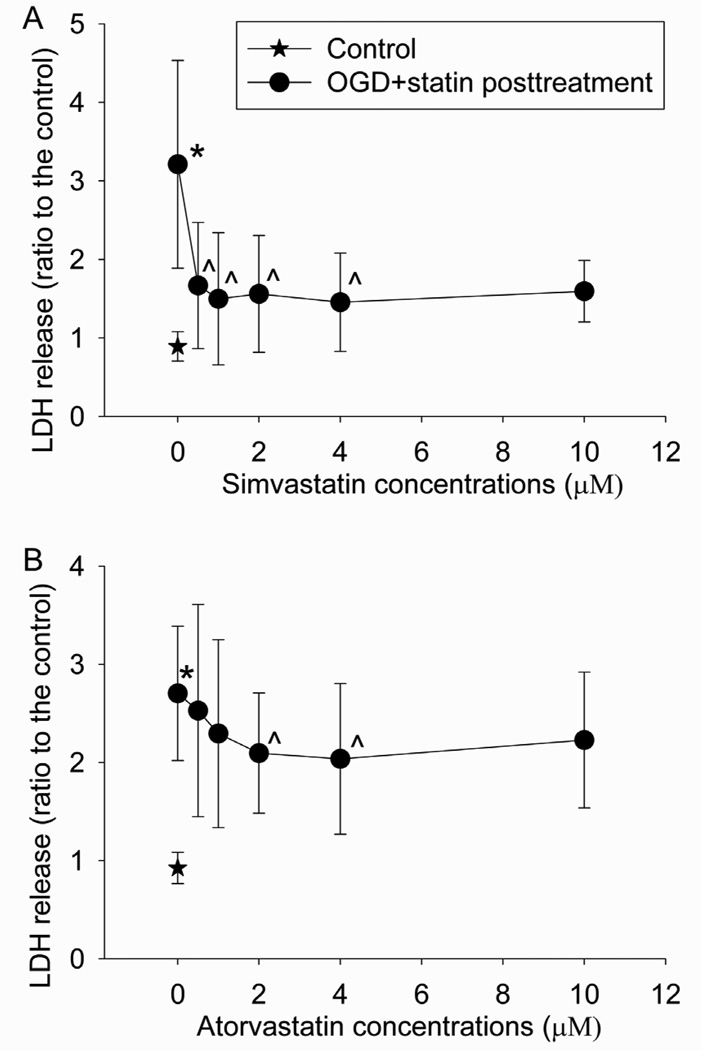
Post-treatment with statins dose-dependently reduced the OGD and simulated reperfusion-induced BPAEC injury (Fig. 2). Post-treatment with 0.5, 1, 2 or 4 µM simvastatin or 2 or 4 µM atorvastatin statistically significantly reduced the OGD- and reperfusion-induced LDH release (Fig. 2).
Fig. 2. Statin postconditioning effect.
Bovine pulmonary arterial endothelial cells were exposed to or not exposed to a 5-h oxygen-glucose deprivation (OGD) and 1-h simulated reperfusion. The cells were posttreated with or without various concentrations of simvastatin or atorvastatin for 1 h immediately after the OGD. Results are means ± S.D. (n = 12 to 41 for panel A and 29 to 45 for panel B). * P < 0.05 compared with control. ^ P < 0.05 compared with OGD only.
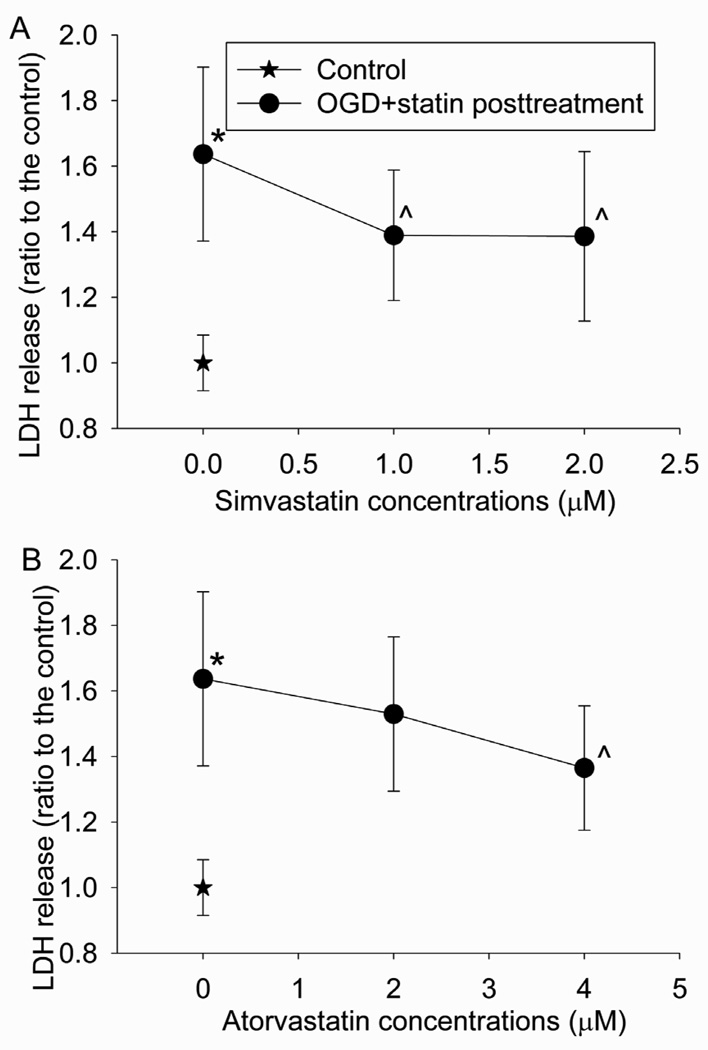
Consistent with the results from BPAEC at passage 4 to 15, OGD and reperfusion also induced cell injury of primary BPAEC cultures and this injury was reduced by simvastatin or atorvastatin post-treatment (Fig. 3).
Fig. 3. Statin postconditioning effect in primary cultures.
Primary cultures of bovine pulmonary arterial endothelial cells were exposed to or not exposed to a 5-h oxygen-glucose deprivation (OGD) and 1-h simulated reperfusion. The cells were posttreated with or without 1 or 2 µM simvastatin or 2 or 4 µM atorvastatin for 1 h immediately after the OGD. Results are means ± S.D. (n = 18 to 54 for panel A and 18 to 54 for panel B). * P < 0.05 compared with control. ^ P < 0.05 compared with OGD only.
3.2. HMG-CoA reductase inhibition may be involved in the statin-induced postconditioning effect
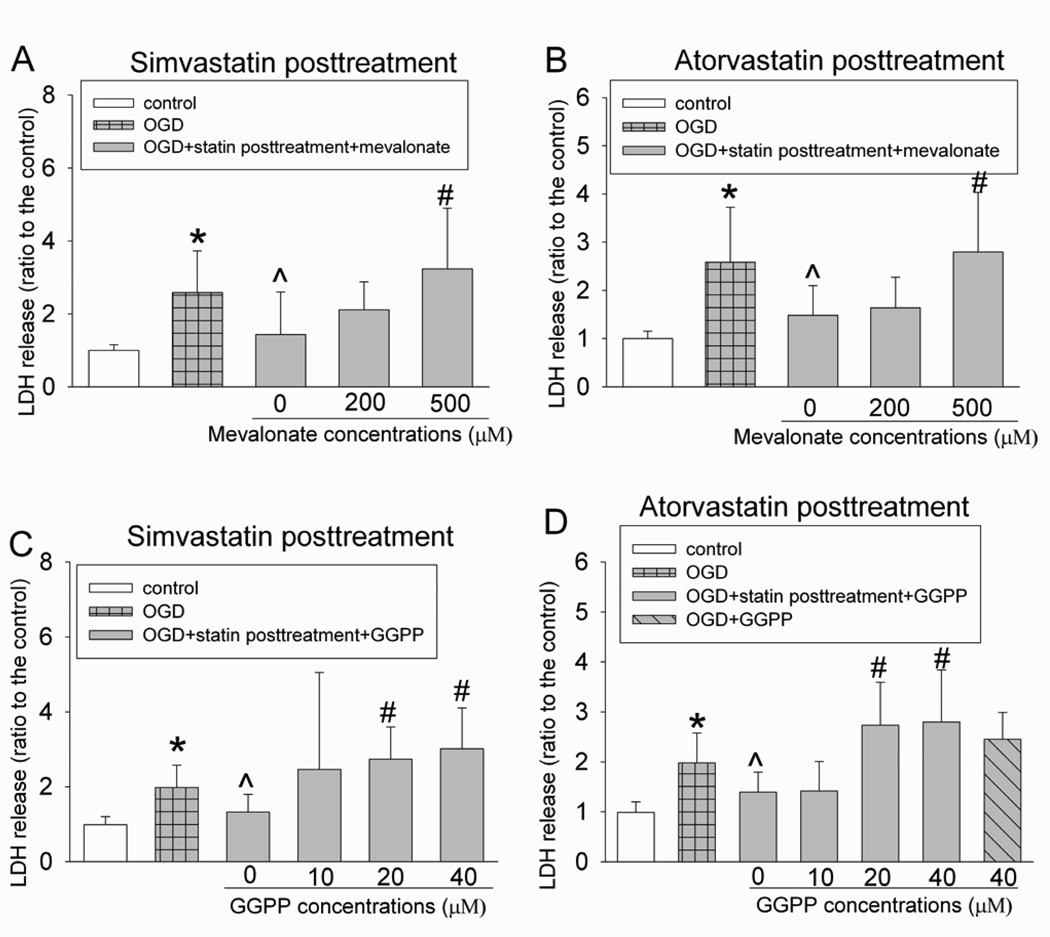
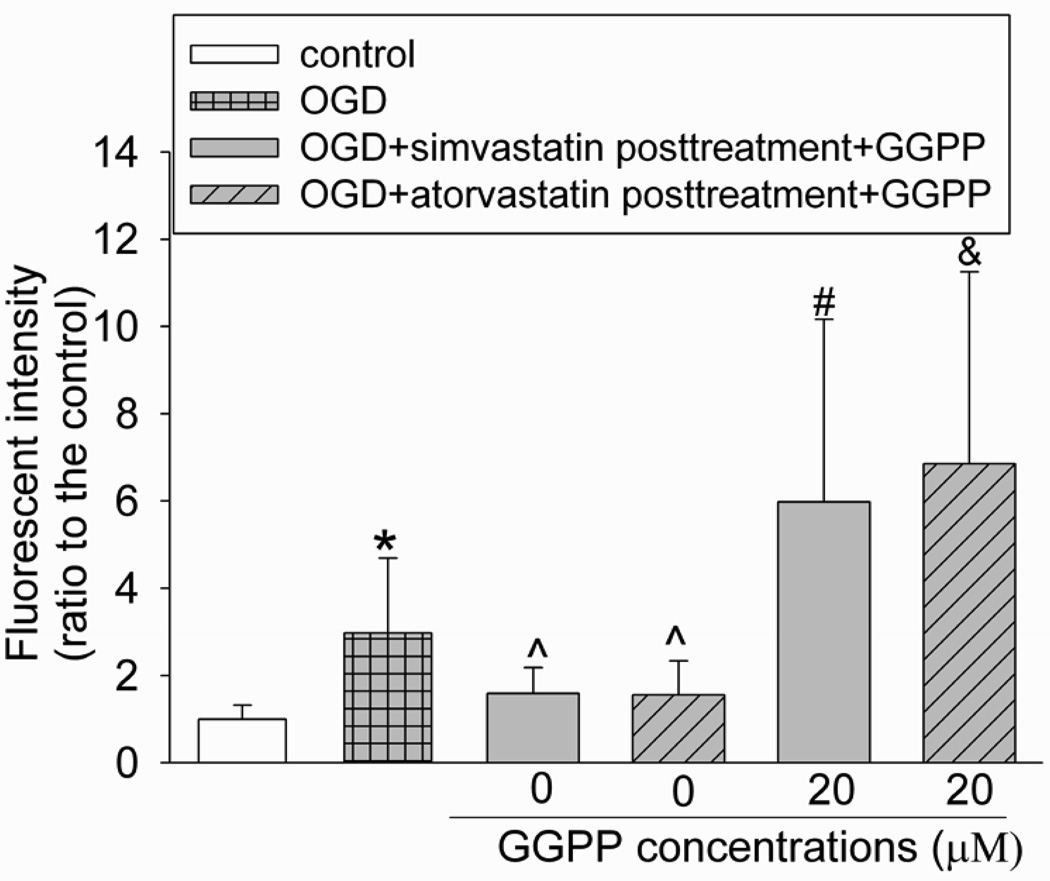
To determine whether the cell protection induced by statins was related to their inhibition of HMG-CoA reductase, we used mevalonate, the immediate product of the reaction mediated by HMG-CoA reductase, and GGPP, an isoprenoid metabolite that is downstream of mevalonate and is necessary for geranylgeranylation/activation of small GTPases (Merla et al., 2007a; Rikitake and Liao, 2005). Mevalonate at 500 µM and GGPP at 20 and 40 µM abolished the protection induced by post-treatment with simvastatin or atorvastatin, while 40 µM GGPP alone did not affect the endothelial cell survival after the OGD and simulated reperfusion (Fig. 4).
Fig. 4. Inhibition of statin post-treatment-induced protection by mevalonate and geranylgeranyl pyrophosphate (GGPP).
Bovine pulmonary arterial endothelial cells were exposed to or not exposed to a 5-h oxygen-glucose deprivation (OGD) and 1-h simulated reperfusion. The cells were posttreated with or without 2 µM simvastatin or 4 µM atorvastatin in the presence or absence of various concentrations of mevalonate or GGPP for 1 h immediately after the OGD. Results are means ± S.D. (n = 15 to 47 for panel A, 18 to 47 for panel B, 12 to 45 for panel C and 12 to 45 for panel D). * P < 0.05 compared with control. ^ P < 0.05 compared with OGD only. # P < 0.05 compared with OGD + statin post-treatment.
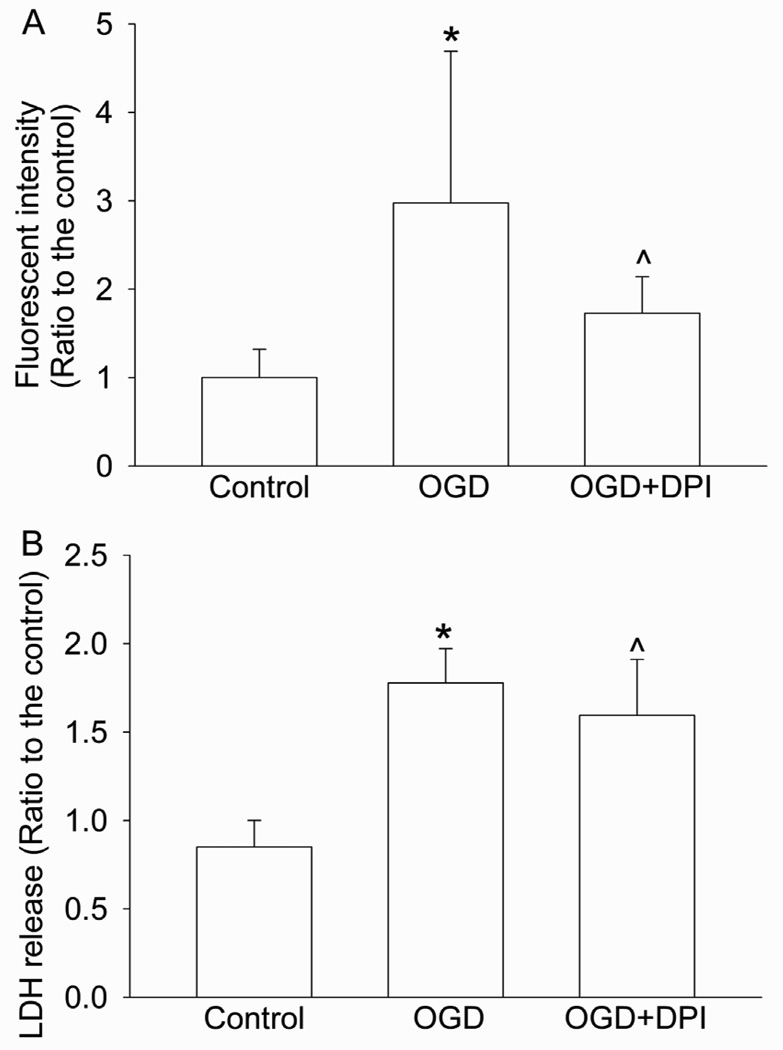
Since overproduction of reactive oxygen species, such as superoxide, is an important mechanism for ischemia-reperfusion injury (Gwag et al., 2001), we determined whether statin post-treatment could reduce superoxide production after OGD. As shown in figure 5, OGD and reperfusion significantly increased superoxide production and this increase was inhibited by post-treatment with simvastatin and atorvastatin. Consistent with the LDH release data, the inhibition of superoxide production by statin postconditioning was abolished by GGPP (Fig. 5). The OGD and reperfusion-induced superoxide production and cell injury were also inhibited by diphenyleneiodonium, an inhibitor of flavoenzymes that include nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Fig. 6).
Fig. 5. Inhibition of superoxide production after oxygen-glucose deprivation (OGD) and simulated reperfusion by statin post-treatment.
Bovine pulmonary arterial endothelial cells were exposed to or not exposed to a 5-h OGD and 1-h simulated reperfusion. The cells were posttreated with or without 2 µM simvastatin or 4 µM atorvastatin in the presence or absence of 20 µM GGPP for 1 h immediately after the OGD. Results are means ± S.D. (n = 18 – 34). * P < 0.05 compared with control. ^ P < 0.05 compared with OGD only. # P < 0.05 compared with OGD + simvastatin post-treatment. & P < 0.05 compared with OGD + atorvastatin post-treatment.
Fig. 6. Inhibition of oxygen-glucose deprivation (OGD) and simulated reperfusion-induced superoxide production and cell injury by diphenyleneiodonium (DPI).
Bovine pulmonary arterial endothelial cells were exposed to or not exposed to a 5-h OGD and 1-h simulated reperfusion. The cells were incubated with or without 20 µM diphenyleneiodonium for 1 h immediately after the OGD. Results are means ± S.D. (n = 19 to 34 for panel A and 11 to 26 for panel B). * P < 0.05 compared with control. ^ P < 0.05 compared with OGD only.
3.3. Akt but not ERK may be involved in the statin-induced postconditioning effect
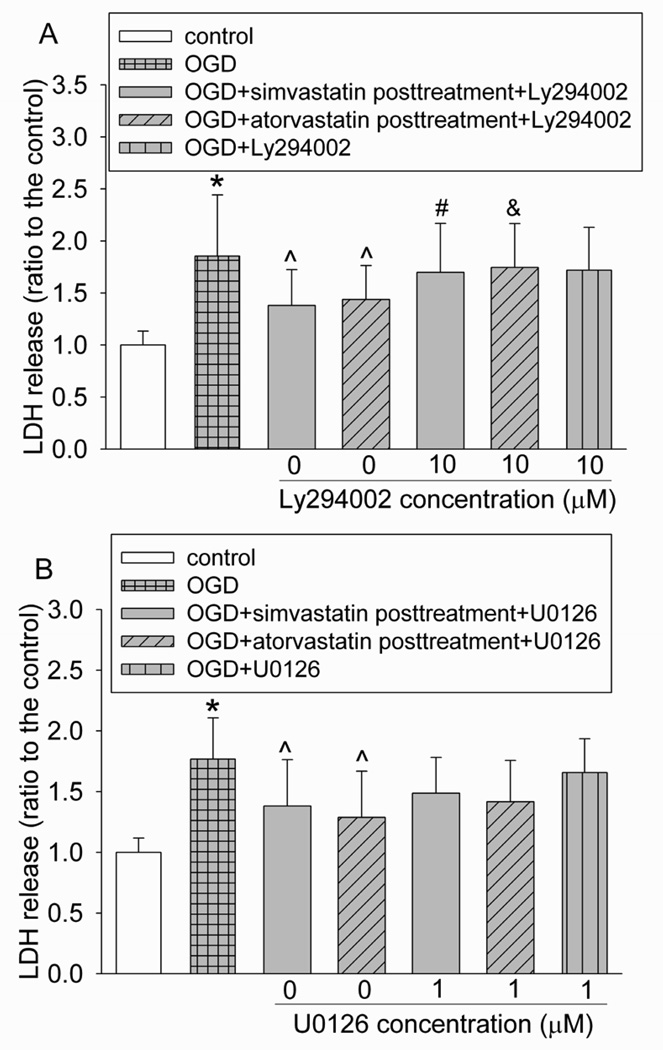
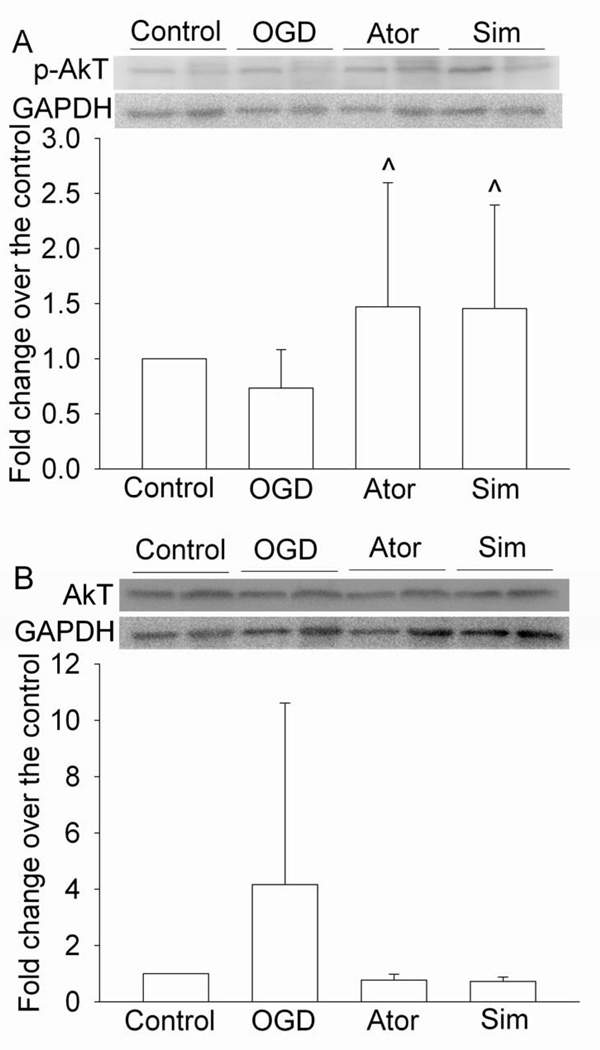
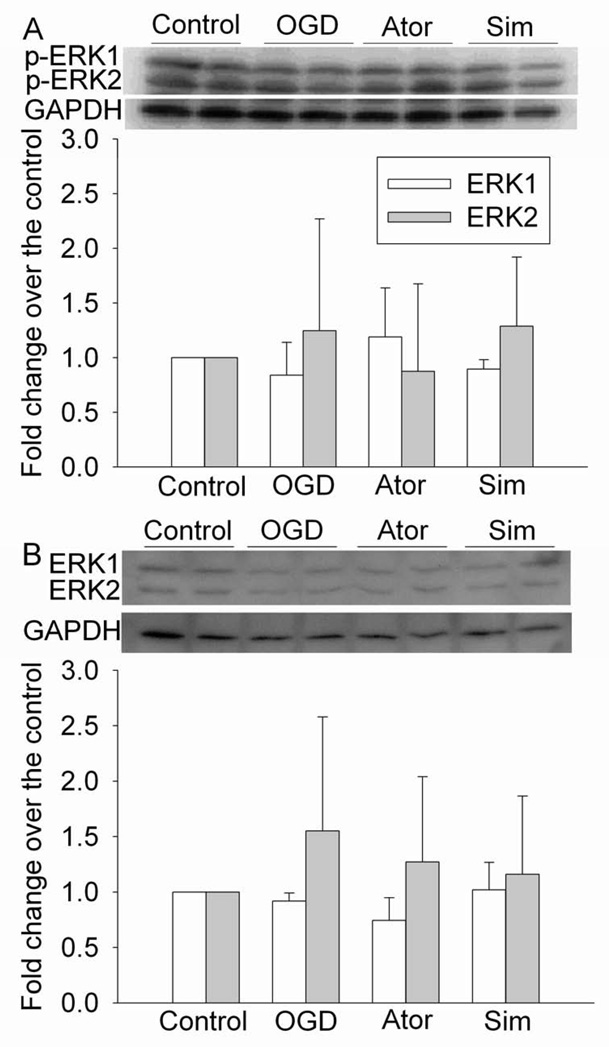
As shown in figure 7, Ly294002, an inhibitor of Akt, attenuated the statin post-treatment-induced protection. However, the ERK inhibitor U0126 did not affect the protective effects of statin post-treatment. Neither Ly294002 nor U0126 affected cell injury in cells subjected to OGD and reperfusion alone (Fig. 7). Consistent with these inhibitor results, simvastatin and atorvastatin increased the expression of phospho-Akt/activated Akt, but did not change the expression of phospho-ERK/activated ERK after the OGD and simulated reperfusion. Simvastatin and atorvastatin did not change the expression of total Akt and ERK (Figs 8 and 9).
Fig. 7. Involvement of Akt in statin postconditioning effect.
Bovine pulmonary arterial endothelial cells were injured by a 5-h oxygen-glucose deprivation (OGD) and 1-h simulated reperfusion. They were posttreated with or without 2 µM simvastatin or 4 µM atorvastatin in the presence or absence of 10 µM Ly294002 or 1 µM U0126 for 1 h immediately after the OGD. Results are mean ± S.D. (n = 28 to 62 for panel A and 10 to 54 for panel B). * P < 0.05 compared with control. ^ P < 0.05 compared with OGD only. # P < 0.05 compared with OGD + simvastatin post-treatment. & P < 0.05 compared with OGD + atorvastatin post-treatment.
Fig. 8. Increase of the expression of phospho-Akt (p-Akt) by statins.
Bovine pulmonary arterial endothelial cells were exposed to a 5-h oxygen-glucose deprivation (OGD) and 1-h simulated reperfusion. They were posttreated with or without 2 µM simvastatin or 4 µM atorvastatin for 1 h immediately after the OGD. Cells were harvested for Western analysis of p-Akt (panel A) and total Akt (pane B). A representative Western blot is shown in the top panel and the graphic presentation of the p-Akt or total Akt protein abundance quantified by integrating the volume of autoradiograms from 8 separate experiments is shown in the bottom panel. Values in graphs are expressed as fold changes over the control and are presented as the means ± S.D. ^ P < 0.05 compared with OGD only. Ator: atorvastatin; Sim: simvastatin.
Fig. 9. No effects on the expression of phospho-extracellular signal-regulated kinase (ERK) (p-ERK) by statins.
Bovine pulmonary arterial endothelial cells were exposed to a 5-h oxygen-glucose deprivation (OGD) and 1-h simulated reperfusion. They were posttreated with or without 2 µM simvastatin or 4 µM atorvastatin for 1 h immediately after the OGD. Cells were harvested for Western analysis of p-ERK (panel A) and total ERK (pane B). A representative Western blot is shown in the top panel and the graphic presentation of the p-ERK or total ERK protein abundance quantified by integrating the volume of autoradiograms from 4 separate experiments is shown in the bottom panel. Values in graphs are expressed as fold changes over the control and are presented as the means ± S.D. ^ P < 0.05 compared with OGD only. Ator: atorvastatin; Sim: simvastatin.
4. Discussion
Statins are a commonly used class of drugs that can reduce blood cholesterol level via inhibition of HMG-CoA reductase. Statins also have many other effects (Davignon, 2004; Miida et al., 2004). For example, statins can induce a preconditioning effect in various organs (Domoki et al., 2009; Gu et al., 2008; Lazar et al., 2003). Recently, it has been shown that application of statins after brain ischemia improves neurological outcome (Prinz et al., 2008; Sugiura et al., 2007). We show here that post-treatment of endothelial cells, a cell type that exists in all organs and systems, with simvastatin and atorvastatin reduces OGD and reperfusion-induced cell injury. These protective effects can be induced at concentrations as low as 0.5 µM simvastatin and 2 µM atorvastatin. Although these concentrations are much higher than the blood concentrations of these statins in healthy volunteers when they take clinical relevant dosages, these concentrations are close to the blood concentrations of these statins (especially in the case of simvastatin) in critical ill patients who take statins and also may be taking drugs that are cytochrome P450 inhibitors (Hermann et al., 2004; Kruger et al., 2009). Because of the concern that cultured cells with multiple passages can have endogenous changes, we confirm the statin post-treatment-induced protection in the primary endothelial cell cultures.
The inhibition of HMG-CoA reductase by statins reduces mevalonate production, which then attenuates cholesterol synthesis via the inhibition of the prenylation pathway. Mevalonate is also a substrate for GGPP production via a series of reactions. GGPP can then be used to geranylgeranylate/activate GTPases, such as Rho A and Rac (Chen et al., 2008; Merla et al., 2007a; Rikitake and Liao, 2005). Thus, statins can lead to the inhibition of GTPase activity. In our study, mevalonate dose-dependently reversed the statin post-treatment–induced protection, suggesting that this protection was mediated by the inhibition of HMG-CoA reductase. Our results also showed that GGPP dose-dependently abolished the statin post-treatment-induced protection, suggesting that this protective effect may not be through the reduction of cholesterol production and may be through the inhibition of geranylgeranylation/activation of small GTPases.
Rac is a small GTPase and is a necessary component of NADPH oxidase complex that facilitates superoxide production (Hordijk, 2006). Our results showed that OGD and reperfusion induced a significant increase of superoxide production. Statin post-treatment attenuated this increased superoxide production. These results, along with the results showing that statin post-treatment reduced OGD and reperfusion-induced cell injury, suggest that the increased superoxide production may participate in the mechanisms of cell injury under this experimental condition. This idea is strongly supported by our results that diphenyleneiodoium, a NADPH oxidase inhibitor, reduced OGD and reperfusion-induced superoxide production and cell injury. Together, these results suggest that statins inhibit HMG-CoA reductase, which then reduces the production of GGPP. The reduced GGPP level decreases the activation of GTPases, which leads to the inhibition of NADPH oxidase activity. This series of reactions ultimately results in a decreased superoxide production and cell protection.
Small GTPases, such as Rho A, can inhibit PI3K/Akt pathway, an intracellular survival pathway (Merla et al., 2007a). Since statins can lead to inhibition of GTPases (Chen et al., 2008; Merla et al., 2007a; Rikitake and Liao, 2005), statins may activate the PI3K/Akt pathway. Consistent with this idea, application of statins has been shown to increase PI3K/Akt activity (Merla et al., 2007b; Nakata et al., 2007) and this effect may be a mechanism for the statin-induced protection. Our study showed that Ly294002, an Akt inhibitor, abolished simvastatin and atorvastatin post-treatment-induced protection in the endothelial cells. Simvastatin and atorvastatin increased the expression of phospho-Akt. These results strongly suggest the involvement of Akt in this protection. However, ERK1/2, protein kinases that may be involved in protection induced by various preconditioning stimuli (Dirnagl et al., 2003; Zuo et al., 2006) and can also be activated by application of statins (Anger et al., 2008; Merla et al., 2007b), may not be involved in the statin post-treatment-induced protection in the endothelial cells because U0126, an ERK1/2 inhibitor, did not inhibit the protective effect of statins, and simvastatin and atorvastatin did not change the expression of phospho-ERK.
Our findings may have a broad implication. Post-treatment/postconditioning may be more clinically practicable than preconditioning because post-treatment eliminates the need to predicate the occurrence of detrimental insults, such as ischemia, for its application. Endothelial cells are ubiquitous in all organs and tissues and are a necessary component as a physical barrier to prevent free access of chemicals and cells in the blood to the vascular smooth muscle cells and parenchymal cells of organs. Thus, it is critically important to maintain the functional and structural integrity of endothelial cells under various physiological and pathophysiological conditions.
In summary, we have shown for the first time that post-treatment with simvastatin and atorvastatin induces protection against OGD and simulated reperfusion in endothelial cells. This effect may be mediated by inhibition of HMG-CoA reductase and the production of GGPP, which leads to inhibition of GTPases and decreased superoxide production. PI3K/Akt, but not ERk1/2, may also be involved in the statin post-treatment-induced protection in the endothelial cells.
Acknowledgement
This study was supported by grants (R01 GM065211 and R01 NS045983 to Z Zuo) from the National Institute of Health, Bethesda, MD, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, OH, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (0755450U to Z Zuo), Baltimore, MD, and the Department of Anesthesiology, University of Virginia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anger T, El-Chafchak J, Habib A, Stumpf C, Weyand M, Daniel WG, Hombach V, Hoeher M, Garlichs CD. Statins stimulate RGS-regulated ERK 1/2 activation in human calcified and stenotic aortic valves. Exp. Mol. Pathol. 2008;85:101–111. doi: 10.1016/j.yexmp.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L575–L583. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Domoki F, Kis B, Gaspar T, Snipes JA, Parks JS, Bari F, Busija DW. Rosuvastatin induces delayed preconditioning against oxygen-glucose deprivation in cultured cortical neurons. Am. J. Physiol. Cell Physiol. 2009;296:C97–C105. doi: 10.1152/ajpcell.00366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Kehl F, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Simvastatin restores ischemic preconditioning in the presence of hyperglycemia through a nitric oxide-mediated mechanism. Anesthesiology. 2008;108:634–642. doi: 10.1097/ALN.0b013e3181672590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwag BJ, Won SJ, Kim DY. Excitotoxicity, oxidative stress, and apoptosis in ischemic neuronal death. In: Lin CS, editor. New Concepts in Cerebral Ischemia. New York: CRC Press; 2001. pp. 79–112. [Google Scholar]
- Hermann M, Asberg A, Christensen H, Holdaas H, Hartmann A, Reubsaet JL. Substantially elevated levels of atorvastatin and metabolites in cyclosporine-treated renal transplant recipients. Clin. Pharmacol. Ther. 2004;76:388–391. doi: 10.1016/j.clpt.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ. Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- Kim JA, Li L, Zuo Z. Isoflurane induces a postconditioning effect on bovine pulmonary arterial endothelial cells exposed to oxygen-glucose deprivation. Eur. J. Pharmacol. 2009;615:144–149. doi: 10.1016/j.ejphar.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger PS, Freir NM, Venkatesh B, Robertson TA, Roberts MS, Jones M. A preliminary study of atorvastatin plasma concentrations in critically ill patients with sepsis. Intensive Care Med. 2009;35:717–721. doi: 10.1007/s00134-008-1358-3. [DOI] [PubMed] [Google Scholar]
- Lazar HL, Bao Y, Zhang Y, Bernard SA. Pretreatment with statins enhances myocardial protection during coronary revascularization. J. Thorac. Cardiovasc. Surg. 2003;125:1037–1042. doi: 10.1067/mtc.2003.177. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Li L, Jung H-H, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla R, Daher IN, Ye Y, Uretsky BF, Birnbaum Y. Pretreatment with statins may reduce cardiovascular morbidity and mortality after elective surgery and percutaneous coronary intervention: clinical evidence and possible underlying mechanisms. Am. Heart J. 2007a;154:391–402. doi: 10.1016/j.ahj.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Merla R, Ye Y, Lin Y, Manickavasagam S, Huang MH, Perez-Polo RJ, Uretsky BF, Birnbaum Y. The central role of adenosine in statin-induced ERK1/2, Akt, and eNOS phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 2007b;293:H1918–H1928. doi: 10.1152/ajpheart.00416.2007. [DOI] [PubMed] [Google Scholar]
- Miida T, Hirayama S, Nakamura Y. Cholesterol-independent effects of statins and new therapeutic targets: ischemic stroke and dementia. J. Atheroscler. Thromb. 2004;11:253–264. doi: 10.5551/jat.11.253. [DOI] [PubMed] [Google Scholar]
- Nakata S, Tsutsui M, Shimokawa H, Yamashita T, Tanimoto A, Tasaki H, Ozumi K, Sabanai K, Morishita T, Suda O, Hirano H, Sasaguri Y, Nakashima Y, Yanagihara N. Statin treatment upregulates vascular neuronal nitric oxide synthase through Akt/NF-kappaB pathway. Arterioscler. Thromb. Vasc. Biol. 2007;27:92–98. doi: 10.1161/01.ATV.0000251615.61858.33. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Prinz V, Laufs U, Gertz K, Kronenberg G, Balkaya M, Leithner C, Lindauer U, Endres M. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39:433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zuo Z, Gozal Y. Volatile Anesthetic Preconditioning Attenuates Myocardial Apoptosis in Rabbits after Regional Ischemia and Reperfusion via Akt Signaling and Modulation of Bcl-2 Family Proteins. J. Pharmacol. Exp. Ther. 2006;318:186–194. doi: 10.1124/jpet.105.100537. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ. Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura S, Yagita Y, Sasaki T, Todo K, Terasaki Y, Ohyama N, Hori M, Kitagawa K. Postischemic administration of HMG CoA reductase inhibitor inhibits infarct expansion after transient middle cerebral artery occlusion. Brain Res. 2007;1181:125–129. doi: 10.1016/j.brainres.2007.08.069. [DOI] [PubMed] [Google Scholar]
- Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth. Analg. 2005;101:942–949. doi: 10.1213/01.ane.0000171931.08371.a2. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Johns RA. Inhalational anesthetics up-regulate constitutive and lipopolysaccharide-induced inducible nitric oxide synthase expression and activity. Mol. Pharmacol. 1997;52:606–612. doi: 10.1124/mol.52.4.606. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Wang Y, Huang Y. Isoflurane preconditioning protects human neuroblastoma SH-SY5Y cells against in vitro simulated ischemia-reperfusion through the activation of extracellular signal-regulated kinases pathway. Eur. J. Pharmacol. 2006;542:84–91. doi: 10.1016/j.ejphar.2006.05.027. [DOI] [PubMed] [Google Scholar]