Normal cardiac physiology relies on the precise localization and function of ion channels, receptors, regulatory molecules, and structural proteins at distinct locations in the cell. Spectrins are actin-associated proteins that provide structural support to the cell membrane and play critical roles in compartmentalization of subcellular microdomains. Importantly, abnormalities in spectrin and spectrin-associated proteins (e.g. ankyrins, obscurin, protein 4.1) have been linked with disease including congenital and acquired arrhythmia syndromes and myopathy [1-10]. The unique structure of spectrin not only allows it to provide mechanical integrity to the cell membrane, but also organize macromolecular complexes at well-defined subcellular domains. Thus, increasing our understanding of spectrin function in the heart may illuminate exciting new pathways for altering cardiac excitability and function.
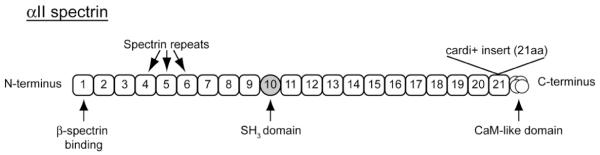
Spectrin is a flexible hetero-tetrameric molecule formed from anti-parallel heterodimers of α- and β-subunits. Originally discovered in the erythrocyte, spectrins are now known to be widely expressed in mammalian tissues. α–spectrin contains 22 functional domains comprised of 21 triple helical spectrin repeats responsible for binding associated proteins, and a C-terminal calmodulin-like domain (Figure 1). β-spectrin consists of an N-terminal actin-binding domain, 17 spectrin repeats, and a C-terminal domain containing a pleckstrin homology domain [11]. α–spectrin binds to β-spectrin at repeat 17 on β–spectrin and repeat one on α–spectrin [12-15]. Importantly, ankyrins bind β–spectrin at repeat 15 [16]. Thus, each spectrin hetero-tetramer contains two binding sites for ankyrin allowing for construction of diverse macromolecular complexes.
Figure 1. Structure of αII-spectrin and location of cardi+ insert.
αII-spectrin is comprised of 21 triple helical spectrin repeats and a C-terminal calmodulin-like domain. Binding to β-spectrin occurs in repeat 1. Repeat 10 contains an SH3 domain commonly found in cell signaling proteins. The newly identified cardi+ splice variant results in a 21 amino acid insert in repeat 21 that affects binding affinity for β-spectrin and may regulate cardiomyocyte growth [28].
To date, two α– isoforms and five β–isoforms have been identified. αI- and βI-spectrin are the predominant isoforms found in erythrocytes while other spectrin gene products are more broadly expressed. In the heart, αII-spectrin is found with βII-spectrin at Z-line and sarcoplasmic reticulum (SR) membranes while both αI- and αII-spectrin are found with βI-spectrin at the plasma membrane [1]. Importantly, alternative splicing of both α– and β– isoforms provides further functional diversity. Specifically, several splicing events have been described for αII-spectrin resulting in small (6-20 residue) insertions in different spectrin repeats [17, 18]. C-terminal regions of βI-, βII-, and βIV-spectrin are also alternatively spliced to produce “long” and “short” isoforms [11, 19, 20]. The “long” β-spectrin isoforms contain a pleckstrin homology (PH) domain, a roughly 100-residue domain with roles in ligand binding and intracellular signaling [11]. Greatly truncated forms of both βII- and βIV-spectrin are also generated through splicing events [19, 21, 22]. While the functional consequences for these splicing events remain unclear, they likely impact the localization and function of spectrin [20].
The importance of spectrin and associated proteins for normal human physiology is illustrated by the strong association between dysfunction in these proteins and human disease. Gene mutations in α- and β- spectrin have been linked to hereditary spherocytosis and hemolytic anemia in humans and mice, although the most common cause of the disease are mutations in ANK1, the gene encoding the spectrin-associated protein ankyrin-R [23, 24]. Spectrin mutations have also been identified as a cause of human spinocerebellar ataxia, a progressive neurological disorder characterized by loss of coordination. Specifically, several loss-of-function mutations in βIII-spectrin have been linked to spinocerebellar ataxia type 5 [25]. Recently, a genome-wide association study identified linkage between variability in the human ANK3 locus (encodes spectrin-associated ankyrin-G) and bipolar disorder [26]. Finally, dysfunction in ankyrins has been associated with potentially lethal cardiac arrhythmia syndromes. Human gene mutations in ANK2 (encodes ankyrin-B) cause a complex arrhythmia syndrome characterized by sinus node dysfunction, atrial fibrillation, ventricular arrhythmias, and increased likelihood for sudden death [3, 5, 7, 8, 27]. Similarly, a human mutation that disrupts ankyrin-G/Nav1.5 interaction results in Brugada Syndrome, characterized by abnormal electrocardiograms and ventricular arrhythmia [4, 9].
In this issue of Journal of Molecular and Cellular Cardiology, Ursitti and colleagues add to our understanding of the spectrin superfamily by identifying a novel splice variant of αII-spectrin in heart [28]. This new variant, termed cardi+ (named for its predominant expression in heart), accounts for almost 30% of total αII-spectrin in rat hearts at embryonic day 16, but only 6% after three weeks of age suggesting a possible role for αII-spectrin cardi+ in development. The authors also identify two new αII-spectrin isoforms, ∑9 and ∑10, containing the αII-spectrin cardi+ splice variant. Interestingly, the cardi+ insertion decreases the binding affinity of αII-spectrin for β-spectrin. Notably, αII-spectrin cardi+ fusion proteins containing the two terminal spectrin repeats were highly insoluble compared with αII-spectrin cardi-. As noted by the authors, these data suggest that the novel insertion may sequester the αII-spectrin cardi+ population from αII-spectrin cardi- polypeptides. However, the most significant finding from the study is the effect of the cardi+ sequence on cardiomyocyte cellular shape and organization. Specifically, neonatal cardiomyocytes positive for expression of GFP-αII-spectrin cardi+ display aggregates of the fusion protein throughout the cell. In contrast, GFP-αII-spectrin cardi- is diffusely spread throughout the myocyte cytoplasm. Moreover, GFP-αII-spectrin cardi+ expressing myocytes display pronounced processes with significant gaps between the membrane and the myofibrils. These processes are not observed in either control or GFP-αII-spectrin cardi- expressing myocytes.
In summary, the new findings or Ursitti, Bloch, and colleagues add important new understanding regarding the role of the spectrin-associated network in heart. However, these findings also illustrate how little we still understand about these critical molecules. For example, are specific spectrin isoforms restricted to unique excitable cell types (i.e. ventricular, atrial, sinus node)? Moreover, do C-terminal splicing events confer novel protein interaction sites or alter normal regulatory pathways (i.e. add/subtract phosphorylation sites)? Finally, do C-terminal splicing events alter the subcellular distribution and function of specific spectrin gene products? While much remains unknown about the function of different spectrin isoforms in heart, this important new study adds to our growing appreciation for the diversity of spectrin polypeptides in excitable tissues.
Acknowledgments
We acknowledge support from the NIH (HL084583, HL083422 to PJM; HL096805 to TJH) and Pew Scholars Trust (PJM).
Footnotes
Disclosures None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Baines AJ, Pinder JC. The spectrin-associated cytoskeleton in mammalian heart. Front Biosci. 2005;10:3020–33. doi: 10.2741/1759. [DOI] [PubMed] [Google Scholar]
- [2].Arimura T, Matsumoto Y, Okazaki O, Hayashi T, Takahashi M, Inagaki N, et al. Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2007 Oct 19;362(2):281–7. doi: 10.1016/j.bbrc.2007.07.183. [DOI] [PubMed] [Google Scholar]
- [3].Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003 Feb 6;421(6923):634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- [4].Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17533–8. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):9137–42. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hund TJ, Wright PJ, Dun W, Snyder JS, Boyden PA, Mohler PJ. Regulation of the ankyrin-B-based targeting pathway following myocardial infarction. Cardiovasc Res. 2009 Mar 1;81(4):742–9. doi: 10.1093/cvr/cvn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008 Oct 7;105:15617–22. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, et al. Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007 Jan 30;115(4):432–41. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- [9].Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008 Jan 14;180(1):173–86. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stagg MA, Carter E, Sohrabi N, Siedlecka U, Soppa GK, Mead F, et al. Cytoskeletal Protein 4.1R Affects Repolarization and Regulates Calcium Handling in the Heart. Circ Res. 2008 Sep 11; doi: 10.1161/CIRCRESAHA.108.176461. [DOI] [PubMed] [Google Scholar]
- [11].Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001 Jul;81(3):1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- [12].Cherry L, Menhart N, Fung LW. Interactions of the alpha-spectrin N-terminal region with beta-spectrin. Implications for the spectrin tetramerization reaction. J Biol Chem. 1999 Jan 22;274(4):2077–84. doi: 10.1074/jbc.274.4.2077. [DOI] [PubMed] [Google Scholar]
- [13].Kennedy SP, Weed SA, Forget BG, Morrow JS. A partial structural repeat forms the heterodimer self-association site of all beta-spectrins. J Biol Chem. 1994 Apr 15;269(15):11400–8. [PubMed] [Google Scholar]
- [14].Kotula L, DeSilva TM, Speicher DW, Curtis PJ. Functional characterization of recombinant human red cell alpha-spectrin polypeptides containing the tetramer binding site. J Biol Chem. 1993 Jul 15;268(20):14788–93. [PubMed] [Google Scholar]
- [15].Bignone PA, Baines AJ. Spectrin alpha II and beta II isoforms interact with high affinity at the tetramerization site. Biochem J. 2003 Sep 15;374(Pt 3):613–24. doi: 10.1042/BJ20030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991 Oct;115(1):267–77. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cianci CD, Zhang Z, Pradhan D, Morrow JS. Brain and muscle express a unique alternative transcript of alphaII spectrin. Biochemistry. 1999 Nov 30;38(48):15721–30. doi: 10.1021/bi991458k. [DOI] [PubMed] [Google Scholar]
- [18].Moon RT, McMahon AP. Generation of diversity in nonerythroid spectrins. Multiple polypeptides are predicted by sequence analysis of cDNAs encompassing the coding region of human nonerythroid alpha-spectrin. J Biol Chem. 1990 Mar 15;265(8):4427–33. [PubMed] [Google Scholar]
- [19].Berghs S, Aggujaro D, Dirkx R, Jr., Maksimova E, Stabach P, Hermel JM, et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000 Nov 27;151(5):985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hayes NV, Scott C, Heerkens E, Ohanian V, Maggs AM, Pinder JC, et al. Identification of a novel C-terminal variant of beta II spectrin: two isoforms of beta II spectrin have distinct intracellular locations and activities. J Cell Sci. 2000 Jun;113(Pt 11):2023–34. doi: 10.1242/jcs.113.11.2023. [DOI] [PubMed] [Google Scholar]
- [21].Mishra L, Cai T, Yu P, Monga SP, Mishra B. Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene. 1999 Jan 14;18(2):353–64. doi: 10.1038/sj.onc.1202313. [DOI] [PubMed] [Google Scholar]
- [22].Tse WT, Tang J, Jin O, Korsgren C, John KM, Kung AL, et al. A new spectrin, beta IV, has a major truncated isoform that associates with promyelocytic leukemia protein nuclear bodies and the nuclear matrix. J Biol Chem. 2001 Jun 29;276(26):23974–85. doi: 10.1074/jbc.M009307200. [DOI] [PubMed] [Google Scholar]
- [23].Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008 Jan;14(1):28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- [24].Agre P, Casella JF, Zinkham WH, McMillan C, Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. Nature. 1985 Mar 28 3;Apr 28 3;314(6009):380–3. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- [25].Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006 Feb;38(2):184–90. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- [26].Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008 Aug 17; doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mohler PJ, Hoffman JA, Davis JQ, Abdi KM, Kim CR, Jones SK, et al. Isoform specificity among ankyrins. An amphipathic alpha-helix in the divergent regulatory domain of ankyrin-B interacts with the molecular co-chaperone Hdj1/Hsp40. J Biol Chem. 2004 Jun 11;279(24):25798–804. doi: 10.1074/jbc.M401296200. [DOI] [PubMed] [Google Scholar]
- [28].Zhang Y, Resneck WG, Lee PC, Randall WR, Bloch RJ, Ursitti JA. Characterization and expression of a heart-selective alternatively spliced variant of alphaII-spectrin, cardi+, during development in rat. Journal of Molecular and Cellular Cardiology. 2010 doi: 10.1016/j.yjmcc.2010.01.001. x: xxx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]