Abstract
We have recently shown that a novel endothelial mitogen netrin-1 potently stimulates nitric oxide (NO•) production via a DCC-ERK1/2 dependent mechanism. In view of the well-established cardioprotective role of NO•, the present study investigated whether netrin-1 is cardioprotective via NO• signaling in the heart. Netrin-1 receptor DCC was abundantly expressed in the C57BL/6J mouse hearts. Perfusion of heart with netrin-1 (100 ng/mL) using a Langendorff system significantly increased NO• production. Under ischemia/reperfusion (I/R), netrin-1 induced a substantial reduction in infarct size (21.8±4.9% from 42.5±3.6% in the controls), which was accompanied by an augmented production of NO•. Pre-perfusion with DCC-antibody, U0126 (MEK1/2 inhibitor), L-NAME or PTIO (NO• scavenger) attenuated protective effects of netrin-1 on infarct size and NO• production, indicating upstream roles of DCC and ERK1/2 in NO• production, as well as an essential role of NO• in cardioprotection. Netrin-1 induced reduction in infarct size was significantly attenuated in DCC+/− mice, confirming an intermediate role of DCC. In additional experiments we found netrin-1 increased ERK1/2 and eNOSs1177 phosphorylation, and DCC protein expression, which was diminished by I/R. Furthermore, netrin-1-induced DCC upregulation was NO• and ERK1/2-dependent, implicating a feed-forward mechanism. DAF-AM staining revealed enhanced NO• production in both cardiac endothelial cells (ECs) and myocytes. In primarily isolated cardiomyocytes, netrin-1 also increased NO• production, DCC abundance and ERK1/2 phosphorylation. Of note, cardiac apoptosis was significantly attenuated by netrin-1, which was reversed by DCC-antibody, U0126, L-NAME or PTIO. In summary, our data clearly demonstrate that netrin-1 potently protects the heart from I/R injury by stimulating NO• production from cardiac ECs and myocytes. This potent effect is mediated by a DCC/ERK1/2/eNOSs1177/NO•/DCC feed-forward mechanism in both cell types.
Keywords: Netrin-1, nitric oxide, eNOS, DCC, myocardial infarction, ischemia reperfusion, cardioprotection, ERK1/2, electron spin resonance
Introduction
Netrin-1 is a secreted molecule that is largely known to play a defined role in guiding vertebrate commissural axons in neuronal development [1–3]. Recent studies have further demonstrated a critical role of netrin-1 in endothelial cell proliferation, migration and angiogenic signaling [4–8], in addition to morphogenesis of epithelial cells [9,10]. At least eight netrin receptors have been characterized in neurons, vascular system and other cell types in mammals. These include deleted in colorectal cancer (DCC), UNC5A, B, C, D, neogenin, α6β4 and α3β1 integrins [10–14]. Netrin-1 binding to DCC mediates attractive outgrowth of axons, as well as positive angiogenic signalings in endothelial and vascular smooth muscle cells [4–6]. In contrast, the UNC5B receptor appears repulsive, mediating cellular effects such as filopodial retraction [15,16], particularly in developing capillaries. The overall expression profile of netrin-1 receptors in the heart however, has remained completely unknown.
In a recent study we found netrin-1 induces production of nitric oxide (NO•) to promote aortic endothelial cell migration and proliferation [6]. Uniquely, we found that netrin-1 induced NO• production is DCC-dependent, involving a feed-forward activation of ERK1/2-eNOS [6]. Binding of netrin-1 to DCC leads to an initial activation of ERK1/2, consequent phosphorylation and activation of eNOS as well as production of NO•, which in turn further activates ERK1/2 and more NO• production to prompt endothelial cell growth and migration [6]. In view of the potent cardioprotective effects of NO•, it is logical to speculate that netrin-1 might have beneficial effect in protecting cardiac muscles from ischemia/reperfusion (I/R) insult. It has been established that a deficiency in endothelial nitric oxide synthase (eNOS) exacerbates myocardial I/R injury, whereby eNOS overexpression, NO• donor, or dietary supplementation of nitrite significantly improved cardiac function in models of hypoxia and myocardial I/R injury [17–21]. In addition, netrin-1 has been shown to protect against renal I/R injury in vivo, with unknown molecular/signaling mechanisms [22].
Therefore in the present study we aimed to examine whether and how netrin-1, potentially via production of NO•, is cardioprotective. Three netrin-1 receptors, DCC, neogenin and UNC5B, are present in the endothelial cells (ECs) of various vascular beds, and have been examined to date for their roles in angiogenic signaling. We first found that despite an absence of UNC5B, DCC and neogenin abundantly expressed in C57BL/6J mouse heart, at both mRNA and protein levels. This was consistent with what was found earlier in adult ECs.6 Hearts perfused using a Langendorff system had an excessive myocardial infarction (MI, 42.5±3.6%) after global ischemia (20 min) and reperfusion (60 min), which was dramatically reduced by netrin-1 intervention (100 ng/ml, pre-perfusion 45 min, reperfusion 60 min) to 21.8±4.9%. This finding was accompanied by an augmented NO• production. We then further demonstrated that this cardioprotective effect of netrin-1 was dependent on DCC, and consequent activations of ERK1/2 and eNOSs1177. Both cardiomyocytes and cardiac ECs were responsible for an increase in NO• production, which feed-forwardly upregulated DCC expression. I/R-induced cardiac apoptosis was significantly attenuated by netrin-1. Taken together; these observations clearly demonstrate that netrin-1 potently protects the heart from I/R injury via a DCC/ERK1/2/eNOSs1177/NO•/DCC feed-forward mechanism.
Methods
Materials
Purified mouse netrin-1 was purchased from R&D Systems. Polyclonal antibody for DCC was obtained from EMD Calbiochem. Polyclonal antibodies specific for phosphorylated ERK1/2, eNOSs1177 were obtained from Cell Signaling Technology (CST). Polyclonal antibody specific for VEGFR2 was purchased from AbCAM. Other chemicals were purchased from Sigma in the highest purity.
Animal
Male C57BL/6J mice (6–8 weeks old) were obtained from the Jackson Laboratories (Bar Harbor, ME). DCC+/− mice were obtained from Dr. Marc Tessier-Lavigne from Genentech. Mice were housed under a pathogen-free condition. The use of animals and experimental procedures were approved by the Institutional Animal Care and Usage Committee at the University of California Los Angeles (UCLA).
RT-PCR and Western blot
Total RNA was extracted from mouse hearts using TRIzol (Invitrogen) according to the manufacturer’s instructions. Reverse transcription was performed in standard fashion with iScript cDNA synthesis Kit (Bio-Rad). PCR was performed using the following primer pairs: DCC, fw: CAGCAAAAACTGTGCAAGGA and rev: CGCAAAGTTCAGAATCGTCA; UNC5B, fw: AGTGTAATGGCGAGTGGGTC and rev: CGAAGAGTTCCTCCACTTGC; Neogenin, fw: TGAACCAGTTGTGGGAAACA and rev: GCCACTCATTGGAGGTTTGT; UNC5A, fw: CGTGTCCTGCACTTCAAAGA and rev: CCTGGTAGCTGACAAGGAGC; UNC5C, fw: CACATCTGGAGTGGCTCTCA and rev: GCATAGCTTCTGCCGGATAG; UNC5D, fw: GTAAAGCAGCTCAAGGTGGC and rev: ATGCAGCAGCTTTGGTTCTT; α6, fw: GTGTGTGAACATCAGGTGCC and rev: ATATTCTGAGCAGCAGCGGT; α3, fw: GCTGACCTGATCATCTGCAA and rev: GCAGTAGGACAGGAAGGCAG; β4, fw: GAGAGCGAGAGGGTGTCATC and rev: ATATCTCCATTGGGCCTCCT; PCRs were carried out on an icycler (Bio-Rad) including primers generated for GAPDH, [(95°C/2 min), (95°C/25 sec, 57°C/5 sec, 68°C 5 min) × 35, (72°C/10 min)]. Western blot analysis was performed as previously published [23].
Langendorff perfusion
The mice were anesthetized with intraperitoneal pentobarbitone (60 mg/kg). Hearts were harvested and rapidly transferred into ice-cold Krebs-Henseleit buffer (KHB). The aorta was cannulated with a 20-gauge stainless steel blunt needle and transferred to the Langendorff rig and perfused retrograde instantly with modified Krebs-Henseleit buffer, which contained (in mmol/L): NaCl 118.0, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, D-Glucose 10 at constant pressure (80±1 mmHg) using a peristaltic pump and feedback system.
For no I/R setting, hearts were perfused with KHB or netrin-1 for 120 min. For I/R setting, hearts were subjected to 30 min of perfusion with DCC-antibody (1 μg/ml), U0126 (50 μmol/L), L-NAME (100 μmol/L), PTIO (60 μmol/L) or KHB only, followed by 45 min netrin-1 (100 ng/ml) perfusion. Then I/R injury was consistently produced by subjecting the hearts to 20 min of normo-thermic ischemia, followed by reperfusion for 60 min with netrin-1. These experimental protocols are illustrated in supplemental Figure 1.
Electron spin resonance detection of nitric oxide radical
Bioavailable nitric oxide radical (NO•) from cells or tissues was detected using electron spin resonance (ESR) as described [6,23]. In brief, whole heart homogenates or cardiomyocytes were incubated with equal volume of freshly prepared NO•-specific spin trap Fe2+(DETC)2 colloid (0.5 mmol/L) for 60 min. Gently collected homogenates or cell suspensions were snap-frozen in liquid nitrogen and loaded into a finger Dewar for analysis with an eScan electron spin resonance (ESR) spectrophotometer (Bruker) at the following settings: center field, 3410; field sweep, 100 G; microwave frequency, 9.73 GHz; microwave power, 13.26 mW; modulation amplitude, 9.82 G; 512 points resolution and receiver gain, 356.
Infarct size analysis
Infarct size was determined by triphenyl tetrazolium chloride (TTC) staining. In brief, at the end of the experimental protocol hearts were infused with 1% TTC in phosphate buffered solution (pH 7.4) for 10 min prior to snap freezing at −20°C. While frozen, the hearts were sliced perpendicular to the long-axis of the heart at 1 mm intervals and de-stained in 10% formaldehyde solution to increase contrast between necrotic and viable myocardium. The heart slices were then digitally photographed for planimetry using NIH Image 1.62. Infarct size is expressed as an infarct-to-risk zone ratio (the risk zone is the whole ventricular volume in this global ischemic model).
Creatine kinase (CK) release
The effluent was collected during the entire 60 min of reperfusion. The amount of CK was determined using a CK Reagent Set (Diagnostic Chemicals). CK reagent was reconstituted in 10 ml of buffer provided by the manufacturer. 40 μl of each sample was mixed with 1 ml of reconstituted reagent, incubated for 2 min at 37°C and read at 340 nm by a Microplate Reader (BioTek). The results were normalized by protein concentration, which was determined by DC protein assay (Bio-Rad).
Spatial localization of NO•
In order to visualize NO• and to analyze its spatial localization in heart sections, the level of NO• concentration was monitored using a fluorescent NO• probe, DAF-FM Diacetate. Sections were loaded with DAF-FM Diacetate (10 μmol/L for 1 hr in DMSO), washed to remove excessive probe, and soaked in fresh buffer for an additional 30 min incubation to complete de-esterification of the intracellular diacetates. The sections were then mounted and visualized with a confocal microscope.
Isolation of adult mouse cardiomyocytes
The heart was retrogradely perfused (37°C) at a constant pressure of 80 mm Hg for 5 min with a Ca2+-free Tyrode buffer containing (in mmol/L): NaCl 130, KCl 5.4, MgSO4 1, NaH2PO4 0.6, D-Glucose 10, HEPES 10, which was continuously bubbled with 95% O2 + 5% CO2. The enzymatic digestion was commenced by adding collagenase type II (Worthington, 0.56 mg/ml each) to the perfusion buffer and continued for 20 min. The heart was cut down and placed in the Petri dish with perfusion solution. The digested ventricular tissues were cut into 2–3 mm pieces and gently aspirated with a transfer pipette to facilitate cell dissociation. The myocytes were allowed to sediment by gravity for 8–10 min in the 15-ml Falcon tubes.
Detection of apoptosis
Apoptosis was detected by the apoptosis detection kit (Chemicon International) according to the manufacturer’s protocol. At the end of the experimental protocols frozen heart sections (12 μm) were fixed with 1% formalin for 10 min at room temperature (RT) and washed twice in PBS, permeabilized with ethanol/acetic acid (2:1) for 5 min at −20°C, washed twice in PBS, and then incubated for >10 sec with the equilibration buffer. Subsequently, the sections were incubated for 1 hr with DIG-conjugated dUTP and TdT enzymes at 37°C, and then for 10 min in the stop buffer at RT, and finally washed three times in PBS. The sections were then incubated with FITC-conjugated anti-DIG antibody for 30 min at RT, and washed three times in PBS. Coverslipped sections were mounted with medium containing 0.5 μg/ml of Propidium Iodide.
Statistical analysis
All data are presented as mean±SEM from four to six independent experiments (different individual mice or different passage of BAECs used on different days). NO• data have been normalized by protein prior to statistical analysis. ANOVA was used to compare means of different experimental groups. Statistical significance is set as p<0.05.
Results
Netrin-1 stimulates NO• production from mouse heart
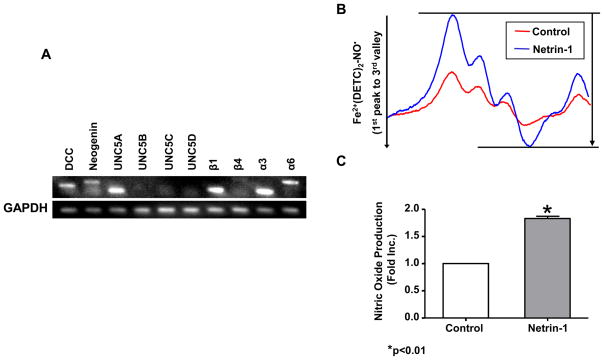
RNA and protein samples were prepared from freshly isolated C57BL/6J mouse hearts. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western blot were used to determine mRNA and protein levels of netrin-1 receptors respectively. Relative abundance of each mRNA expression, indexed by endogenous GAPDH, was used for comparison. Whereas DCC, UNC5A, integrin β1 and α3 were abundantly expressed at mRNA levels, UNC5B, C, D and β4 was undetectable (Figure 1A). Neogenin and 6 were weakly expressed. We have also confirmed DCC and neogenin expression at protein level (data not shown). Given the fact that DCC is present in the mouse heart, we wondered whether netrin-1 stimulates DCC-dependent changes in NO• signaling. Importantly, perfusion of heart with netrin-1 (100 ng/ml, 120 min) resulted in a significant increase in NO• production, as demonstrated by representative ESR spectra and grouped data from six independent experiments (Figure 1B&C). Subsequent experiments indicate that netrin-1 also simulated DCC-dependent NO• production from hearts exposed to I/R injury (see below).
Figure 1. Expression of Netrin-1 receptors in the heart and netrin-1 stimulation of cardiac nitric oxide (NO•) production.
Whole mouse hearts (C57BL/6J) were homogenated for RNA and protein extraction. (A) Expression of DCC, neogenin, UNC5A -D, integrin β1, β4, α3, and α6 mRNA in the mouse heart was measured by RT-PCR and normalized to levels of GAPDH. In additional experiments, mouse hearts were freshly isolated and perfused with netrin-1 (100 ng/ml) on the Langendorff rig for 120 min. Hearts were then homogenated and incubated with the NO•-specific spin trap Fe2+(DETC)2 for 60 min prior to ESR analysis of NO• production. (B) Representative ESR spectra. (C) Grouped data from six independent experiments (Means±SEM, n=6), *p<0.01.
Nitric oxide mediates netrin-1 induced cardiac protection from ischemia/reperfusion injury
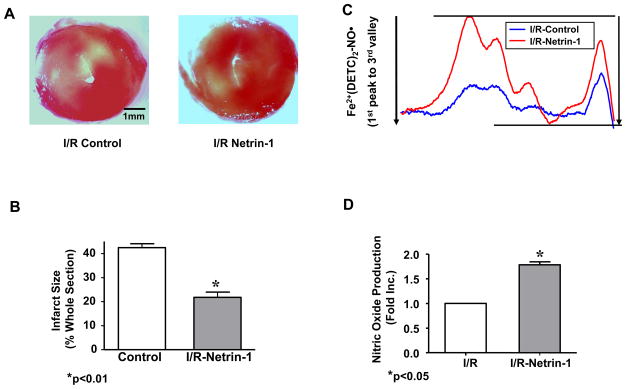
In view of the potent effect of netrin-1 on cardiac NO• production (Figure 1B&C) and the potentially significant role of NO• in mediating cardioprotection, we next examined whether netrin-1 induces cardioprotection during I/R injury. Hearts pre-perfused for 45 min with netrin-1 (100 ng/ml) were subjected to 20 min global ischemia, followed by a 60 min reperfusion with netrin-1. The experimental protocol is illustrated in supplemental Figure 1. Infarct size was quantitated as the area not stained red with tetrazolium red. Netrin-1 treated hearts had a substantial reduction in infarct size compared to untreated controls (21.8±4.9% vs. 42.5±3.6% for netrin-1 treated I/R vs. I/R alone, p<0.01) (Figure 2A&B). This was accompanied by an augmented production of NO• in netrin-1 treated, I/R-ed hearts (1.78±0.12 fold higher than the untreated I/R controls, Figure 2C&D).
Figure 2. Netrin-1 protects heart from ischemia/reperfusion injury.
Hearts were pre-perfused for 45 min with netrin-1 (100 ng/ml) before ischemia/reperfusion (20 min ischemia, 60 min reperfusion with netrin-1). Sections of hearts were stained with 2,3,5-TTC and infarct area calculated as % of risk area. (A) Infarct size was significantly decreased in netrin-1 treated hearts from that of controls (21.8±4.9% vs. 42.5±3.6%, respectively, p<0.01); (B) Infarct size shown in quantitative grouped data (Means±SEM, n=5), * p<0.01. (C) Representative ESR spectra of NO• production from hearts perfused with netrin-1; (D) Grouped data from four independent experiments. * p<0.05;
To determine whether the cardioprotective effect of netrin-1 was dependent on NO•, we subjected mouse hearts to NO• scavenger PTIO (60 μmol/L, 30 min) prior to netrin-1 perfusion and I/R injury. While NO• production was attenuated (Figure 3C&D), the infarct size in PTIO-pre-treated, netrin-1-perfused hearts was also reversed to near control levels of 40.6±4.2% (Figure 3A&B), implicating that the cardioprotective effects of netrin-1 is indeed, NO•-dependent.
Figure 3. Nitric oxide is required for netrin-1 induced cardiac protection from ischemia/reperfusion injury.
Hearts were pre-perfused with PTIO for 30 min prior to netrin-1 perfusion (100 ng/ml, 45 min), followed by ischemia/reperfusion (20 min ischemia, 60 min reperfusion with netrin-1). Sections of hearts were stained with 2,3,5-TTC and infarct area calculated as % of risk area. (A & B) Representative TTC stainings of I/R Netrin-1, I/R PTIO/Netrin-1 and quantitative grouped data (Means±SEM, n=5), *p<0.05 (C) Representative ESR spectra for NO•; (D) Grouped data from three independent experiments, *p<0.001;
DCC/ERK1/2 is required for netrin-1 induced cardioprotection
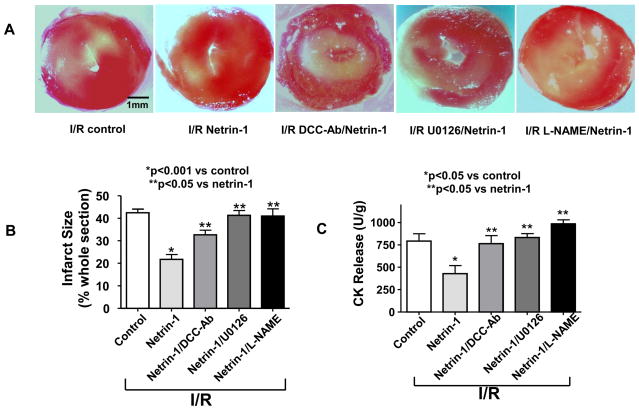
DCC-dependent activation of the mitogen activated protein kinase ERK1/2 has been found a signal transduction pathway for netrin-1 stimulated NO• production in vascular ECs [6]. We next examined whether a similar signaling cascade is the mechanism whereby netrin-1 increases NO• production in the heart. Hearts were pre-perfused with antibody neutralizing DCC, MEK1/2 inhibitor U0126 or NOS inhibitor L-NAME for 30 min prior to netrin-1 perfusion. The apparent cardioprotective effects of netrin-1 were reversed (n=4 for all): the infarct size was increased upon addition of DCC antibody (32.7±4.5%), U0126 (41.3±4.1%) or L-NAME (40.9±7.2%), compared to netrin-1 treatment alone (21.8±4.9%) (Figure 4A&B). In addition, creatine kinase (CK) release, as an index of cardiac muscle damage, showed identical responses (Figure 4C). Concomitantly, increased NO• production by netrin-1 (1.78±0.12 fold vs. untreated I/R controls) was also attenuated by DCC antibody (1.31±0.21 fold vs. untreated I/R controls), U0126 (1.12±0.15 fold vs. untreated I/R controls) or L-NAME (0.90±0.13 fold vs. untreated I/R controls) (Figure 4D&E). Taken together; these results indicate that NO•-dependent cardioprotective effect of netrin-1 is mediated by DCC-dependent activation of ERK1/2 and eNOS.
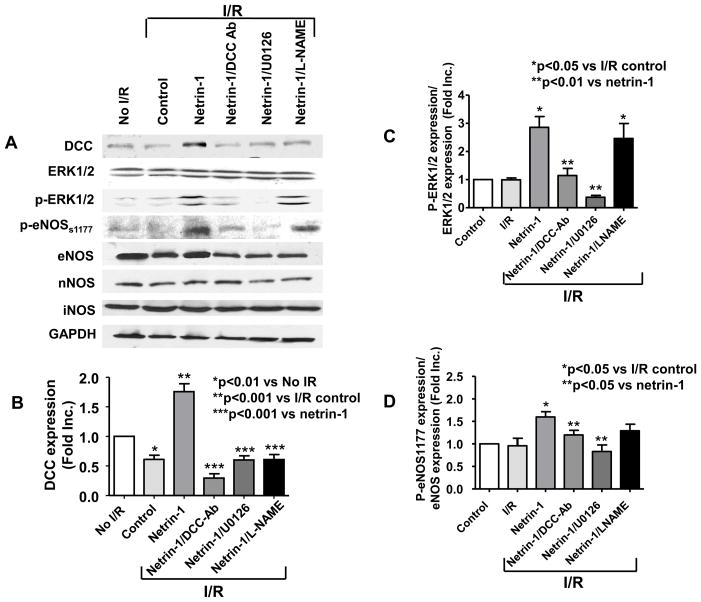
Figure 4. DCC/ERK1/2 activation is required for netrin-1-induced cardioprotection.
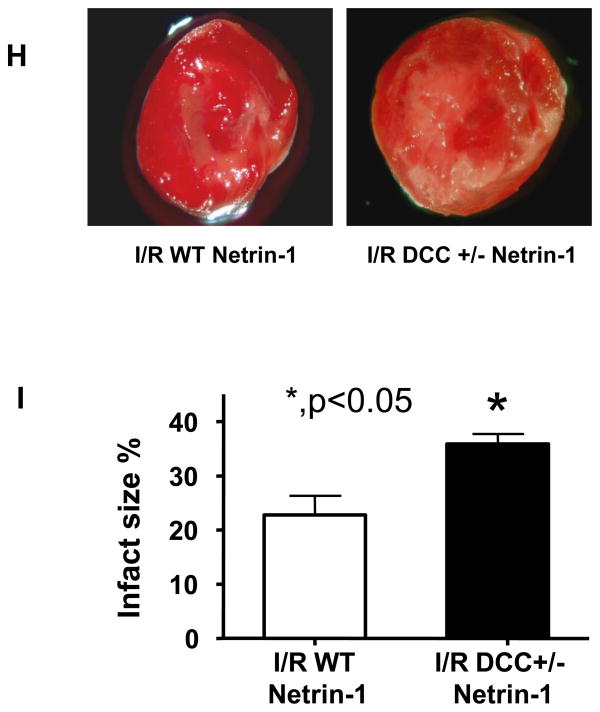
Hearts were perfused with DCC antibody (1 μg/ml), ERK1/2 inhibitor U0126 (50 μmol/L) or L-NAME (100 μmol/L) for 30 min prior to 45 min netrin-1 perfusion. Ischemia/reperfusion injury was consistently produced by subjecting the hearts to 20 min of ischemia, followed by reperfusion for 60 min (with or without netrin-1). Sections of hearts were stained with 2,3,5-TTC and infarct area calculated as % of risk area. (A) Representative TTC stainings of control hearts, and hearts receiving netrin-1, DCC-antibody/netrin-1, U0126/netrin-1 and L-NAME/netrin-1; (B) Infarct size shown in quantitative grouped data of A (Means±SEM, n=5), *p<0.001 vs. control; **p<0.05 vs netrin-1; (C) Creatine kinase (CK) release was measured by collecting effluent during the entire 60 min of reperfusion from control hearts, and hearts receiving netrin-1, DCC-antibody/netrin-1, U0126/netrin-1 and L-NAME/netrin-1 (Means±SEM, n=3). *p<0.05 vs. control; **p<0.05 vs netrin-1; (D) Representative ESR spectra of NO• production of control hearts, and hearts receiving netrin-1, DCC-antibody/netrin-1, U0126/netrin-1 and L-NAME/netrin-1; (E) Grouped data of NO• production of D (Means±SEM, n=5). *p<0.001 vs. control, **p<0.05 vs. netrin-1; (F) Representative ESR spectra of NO• production from IR-ed hearts of WT or DCC+/− mice perfused with netrin-1; (G) Grouped data of NO• production of F (Means±SEM, *p<0.05); (H) Representative TTC stainings of netrin-1 perfused, IR-ed hearts from WT or DCC+/− mice; (I) Infarct size shown in quantitative grouped data of H (Means±SEM, n=3), * p<0.05.
In additional experiments hearts from wild type (WT) and DCC+/− mice were subjected to I/R and netrin-1 perfusion. Netrin-1 provoked NO• production in I/R-ed DCC+/− hearts was significantly reduced (0.66 fold±0.15, p<0.05, Figure 4F&G) comparing to age-matched WT hearts. Netrin-1 induced reduction in infarct size in WT hearts was also markedly attenuated (infarct size: 21.8±4.9% vs. 35.9±3.2% for WT vs. DCC+/− respectively, p<0.05, Figure 4H&I). These data further implicate an intermediate role of DCC in mediating netrin-1 induced cardioprotection.
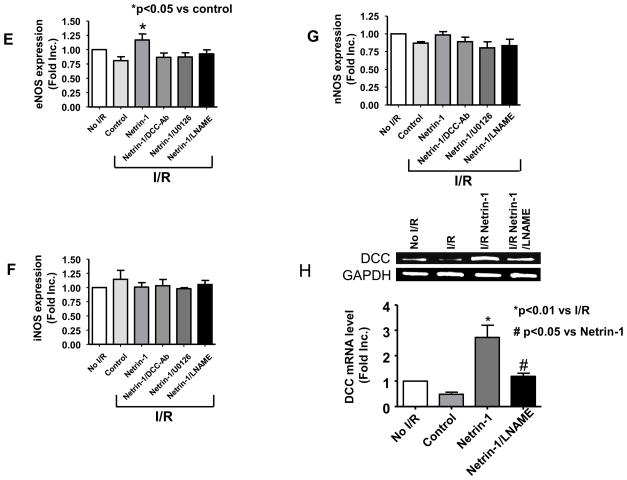
Furthermore, DCC protein abundance, ERK1/2 phosphorylation and eNOSs1179 phosphorylation were all increased, as evidenced by representative Western blots and quantitative data (Figure 5A–D). Interestingly, I/R alone induced a rapid decline in DCC protein and mRNA expression (Figure 5B&H). Netrin-1 perfusion of I/R hearts led to a significant restoration of DCC protein expression, which was abolished by DCC-antibody, U0126, or L-NAME (Figure 5B), indicating a potential feed-forward loop of NO•-ERK1/2-DCC. Bovine aortic endothelial cells (BAEC) and isolated cardiomyocytes were treated with NO• donor MAMANOATE (1 mmol/L, 2 hr) prior to analysis of DCC expression. Of note, NO• donor increased DCC protein expression in both cultured ECs and cardiomyocytes (Figure 5I). In additional experiments hearts were perfused with netrin-1 and harvested at 0, 5, 10 and 30 min. Netrin-1 stimulation resulted in a rapid, time-dependent increase in ERK1/2 and eNOSs1177 phosphorylations (for 0, 5 and 10 min time points), which lasted till up to 30 min (longest time point examined, Figure 5J). Of note, RNA samples extracted from netrin-1-perfused I/R hearts demonstrated an elevation in DCC mRNA levels (Figure 5H) as well, indicating that transcriptional regulation may have occurred to contribute to protein changes.
Figure 5. DCC-ERK1/2-eNOS1177-NO•-DCC feed-forward loop upon netrin-1 activation.
Hearts were perfused as described in Figure 4. All quantitative data are expressed in Means±SEM for statistical analysis. (A) Representative Western blots; (B) Grouped densitometric data of DCC protein expression (n=6), *p<0.01 vs no I/R; **p<0.001 vs I/R control. ***p<0.001 vs netrin-1; (C) Grouped densitometric data of ERK1/2 phosphorylation that is normalized by ERK1/2 (n=3), *p<0.05 vs I/R control, **p<0.01 vs netrin-1; (D) Grouped densitometric data of eNOSs1177 phosphorylation that is normalized by eNOS (n=4), *p<0.01 vs I/R control, **p<0.01 vs netrin-1; (E) Grouped densitometric data of eNOS protein expression (n=5), *p<0.05 vs I/R control; (F) Grouped densitometric data of iNOS protein expression (n=4); (G) Grouped densitometric data of nNOS protein expression (n=3); (H) Representative and grouped mRNA levels of RT-PCR analysis of DCC in post netrin-1-perfused hearts (n=3), *p<0.05; (I) Representative and grouped Western blot data of DCC expression in NO• donor-treated BAECs and cardiomyocytes. (J) Representative and grouped Western blots data of ERK1/2 and eNOSs1177 phosphorylation at different time points. Hearts were perfused with netrin-1 (100 ng/mL) and harvested at 0, 5, 10 and 30 min for Western blot analysis
Moreover, a clear increase in phosphorylated ERK1/2 (Figure 5C) and eNOSs1177 (Figure 5D) was observed with netrin-1 treatment (n=6), which was inhibited by DCC-antibody or U0126, but not by addition of L-NAME. These results seem to indicate that netrin-1 initiates its signaling by binding to DCC, resulting in ERK1/2/eNOS activation to produce NO•, which in turn, upregulates DCC to form a positive feedback loop. Furthermore, protein expression of eNOS was upregulated by netrin-1 perfusion under I/R condition, whereas iNOS and nNOS expression were unaffected (Figure 5A, E-G).
Cardiomyoctyes and ECs-derived NO• production in response to netrin-1
The NO•-specific fluorescent probe DAF-AM was used to estimate changes in NO• production in left ventricle. In netrin-1 perfused, post-I/R heart NO• staining was clearly increased (supplemental Figure 2A, D, G, J). As shown in supplemental Figure 2C, F, I, L, some of the increase in NO• staining was detected specifically in the ECs that line cardiac microvessels, which also labeled positive for VEGFR2 fluorescent antibody (supplemental Figure 2B, E, H, K). The rest of the NO• staining seemed to come primarily from cardiomyocytes, and showed an increase in netrin-1 treated myocardium.
To further investigate a specific role of cardiomyocytes in netrin-1 signaling in the heart, cardiomyocytes were freshly isolated from hearts and treated with netrin-1 (30 min or 60 min) in Tyrode’s solution. There was a significant increase in NO• production from cardiomyocytes treated with netrin-1, as demonstrated by representative ESR spectra and grouped data from three independent experiments (supplemental Figure 3A–D). Protein abundance of DCC and ERK1/2 phosphorylation were also significantly elevated in netrin-1-treated cardiomyocytes (supplemental Figure 3E), echoing results found in lysed cardiac tissues (Figure 5B). These results seem to suggest that netrin-1-stimulated NO• production from cardiomyocytes is also DCC-dependent, and requires activation of ERK1/2 and eNOS.
Netrin-1 protects cardiac cells from apoptosis
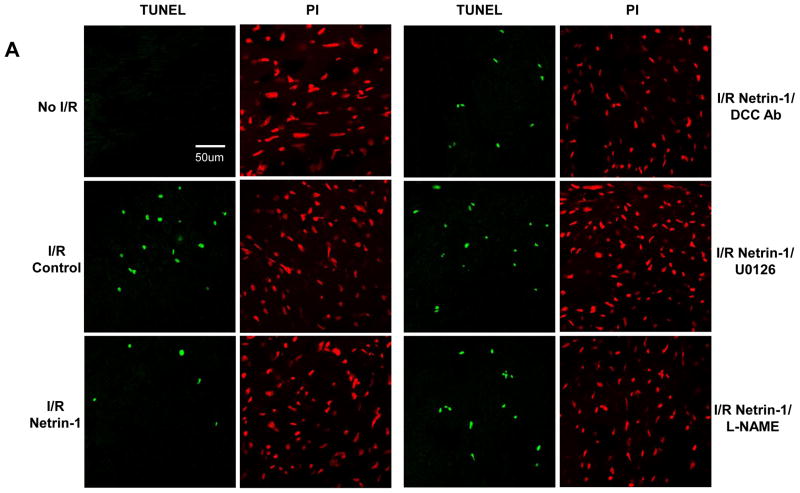
NO• protects ECs and cardiomyocytes from apoptosis induced by oxidant stress, inflammatory cytokines and chemotherapeutic agents [24–27]. TUNEL assay was performed to determine whether netrin-1 inhibits apoptotic cell death induced by I/R. Left ventricular sections were incubated with FITC-conjugated anti-DIG antibody and coverslipped with the mounting medium containing 0.5 μg/ml of Propidium Iodide (PI). Representative TUNEL (green) and PI (red) stained sections and quantitative analysis are shown in Figure 6A&B. I/R injury led to a significant increase in the percentage of TUNEL-positive cells compared to the non-I/R sections (18.2±1.45% vs. 0.33±0.5%). The heart pre-treated with netrin-1 exhibited a significant decrease in TUNEL positive cells (6.78±0.93%). The decrease in apoptosis was completely attenuated by DCC-antibody (17.83±2.87%), U0126 (19.24±2.4%) or L-NAME (17.82±2.19%), again consistently indicating intermediate roles of DCC, ERK1/2 and NO•.
Figure 6. Netrin-1 reduces cardiomyocyte apoptosis in post-I/R hearts.
Hearts perfused as described in Fig 4. (A) Representative detection of I/R-induced apoptotic cells by TUNEL (green), and of all the cells by Propidium Iodide (PI, red), in heart sections of the following conditions: control, ischemia/reperfusion (I/R), netrin-1 treated I/R, netrin-1/DCC I/R, netrin-1/U0126 I/R or Netrin-1/L-NAME I/R; (B) Grouped data from three independent experiments. *p<0.001 vs I/R control, **p<0.05 vs netrin-1.
Discussion
The most significant finding of the study is the innovative identification of a DCC/ERK1/2/eNOSs1177/NO•/DCC/NO• pathway that protects the heart from I/R induced apoptotic cell death and infarction. NO• is produced following a DCC-dependent ERK1/2-eNOSs1177 activation in both cardiomyocytes and cardiac ECs, and that elevation in DCC expression was abolished by inhibition of ERK1/2 or NO•, revealing a feed-forward loop of DCC-ERK1/2-NO•-DCC. I/R induced a rapid loss in DCC protein and mRNA expression, which was recovered by netrin-1 to a level that was two fold of the control. Exogenous NO• donor also upregulated DCC expression in both cultured myocytes and cardiac ECs.
Despite having been well characterized for its role in regulating axonal guidance [1–3], any potential effects of circulating netrin-1 on the pathophysiology of the cardiovascular system remain largely elusive. While recent studies identified an angiogenic role of netrin-1 [4–8], whether it affects cardiac function is completely unknown. In the present study for the first time we delineated effects of netrin-1 perfusion on cardiac protection. Also for the first time we examined expressional profiles in the heart of the eight known netrin-1 receptors. As demonstrated in Figure 1, DCC, neogenin, UNC5A, integerin β1 and α3 were found highly expressed in C57BL/6J mouse hearts (mRNA detected by RT-PCR). Among these DCC and neogenin are attractive receptors that mediate axonal outgrowth, and both expressed in cultured endothelial cells; however only DCC mediates netrin-1 stimulated NO• production and endothelial cell growth and migration [6]. The repulsive receptor UNC5B however was not expressed in mouse heart (Figure 1), although found in endothelial cells previously [15,27]. The mouse hearts we used for these analysis were rinsed out of blood completely using Krebs-Henseleit buffer, so that the mRNA extracted was not contaminated by those of blood cells. If using uncleaned hearts or monocytes, abundant UNC5B mRNA was detected (data not shown), which is consistent to previous findings that monocytes express large amount of UNC5B [28].
The concept that NO• is a powerful cardioprotectant against I/R injury has become well accepted [17,20,29]. Whereas in eNOS deficient mice the infarct size is significantly augmented following I/R injury [30], overexpression of eNOS is associated with reduced infarct size [17,18,31]. Nitrite supplementation results in a 48% reduction in infarct size post I/R insult [32–35]. Indeed, NO• has been shown to mediate cardioprotective effects of estrogen [36], statins [37], moderate alcohol [38], peroxisome proliferator-activated receptor-alpha [39] and sildenafil [40]. In our study, netrin-1 induced an approximate 2-fold increase in cardiac NO• production, which was associated with a 49% decrease in infarct size following I/R injury. The specific, NO•-mediated cardioprotective effect of netrin-1 was abolished by NO• scavenger PTIO (Figure 3) and NOS inhibitor L-NAME (Figure 4). Taken together; our data further establishes a cardioprotective role of NO• and reveals the mechanisms whereby netrin-1 exhibits cardioprotection.
Our findings support an essential role of DCC in mediating netrin-1 induction of NO• in cardiac ECs and myocytes. DCC-antibody abolishes netrin-1 induction of NO• production in I/R-ed hearts. This finding shares similarities with our previous observation that netrin-1 stimulates NO• production in mature ECs in a DCC-dependent manner [6]. DCC protein abundance and mRNA expression were increased in netrin-1-perfused, I/R-ed hearts, although I/R alone induced a decline in DCC protein and mRNA expression (Figure 5A&B&I). NO• donor also upregulated DCC protein expression in both cultured ECs and cardiomyocytes (Figure 5H). Taken together; these data reveal a feed-forward loop of DCC-NO•-DCC, and a predominant role of DCC in mediating netrin-1-dependent cardioprotection. Though the expression of neogenin was abundant, neogenin-antibody had no effect on netrin-1 stimulated NO• production or cardioprotection (data not shown).
Netrin-1 perfusion resulted in marked increases in ERK1/2 (Figure 5C) and eNOSs1177 (Figure 5D) phosphorylations, which were inhibited by DCC-antibody or U0126, but not by addition of L-NAME. These seem to suggest that netrin-1/DCC/ERK1/2/eNOS pathway is turned on first, as inhibition of NO• production was ineffective in preventing DCC/ERK activation, whereas, the secondary feed-forward mechanism of NO•/DCC/ERK1/2 occurs later, as DCC-antibody and U0126 blocked eNOS phosphorylation. The role of ERK1/2 in mediating netrin-1 activation of eNOS is similar to previous observations that ERK1/2 is involved in transient activation of eNOS by ROS [41,42]. It is interesting to speculate that this may share similarities with ROS-dependent preconditioning. Recent studies by Das et al demonstrated that ERK1/2 phosphorylation mediates sildenafil-induced myocardial protection against I/R injury in mice [43]. Previous studies by Li et al also demonstrated that ERK1/2 mediates NO• donor stimulated apoptotic reduction during I/R.44 Transgenic mice with activated MEK1/ERK2 have protected myocardium when exposed to I/R insult in vivo [45]. Confirming a cardioprotective role of ERK1/2, our data also neatly identifies a netrin-1/DCC/ERK1/2/eNOS/NO•/DCC feed-forward loop that mediates the cardioprotective effects of netrin-1.
We have also further evaluated a potential role of PI3K/AKT pathway in mediating netrin-1 induced cardioprotection. Following the same I/R protocol outlined in suppl. Fig. 1, we found that after the entire I/R procedure, AKT phosphorylation was minimal and unaffected by DCC-Ab, U0126 or L-NAME (data not shown), although our data do not rule out the possibility that AKT was more active at earlier time points. LY294002 pretreatment also failed to reduced netrin-1 stimulated NO• production (data not shown), implicating that PI3K/AKT pathway is not required for netrin-1 stimulation of NO• production in this particular Langendorff perfusion model.
Netrin-1 induced reduction in myocardial injury, is at least in part, mediated by NO•-dependent decrease in myocardial apoptosis. We observed that TUNEL-positive cardiomyocytes were decreased by 62.7% in netrin-1 treated hearts than that of controls. Apoptosis has been observed previously in hearts subjected to either continuous ischemia or ischemia followed by reperfusion [46,47]. One of the major protective mechanisms of NO• has been shown to be prevention of myocardial apoptosis/death [31,48]. Indeed, myocardial apoptosis was significantly increased in eNOS-deficient mice during fetal and neonatal heart development, implicating that basal NO• release from eNOS protects cardiomyocytes from apoptosis [49]. Weiland et. al. have also shown that inhibition of endogenous eNOS potentiates I/R induced myocardial apoptosis via caspase 3 pathway [50]. Consistent to these observations, our data demonstrated that inhibition of netrin-1 signaling to attenuate NO• production resulted in loss of the cardioprotective effects of netrin-1.
In summary, our innovative findings characterized a signaling mechanism whereby netrin-1 exerts its powerful cardioprotective effect during I/R injury. Upon netrin-1 perfusion, its attractive receptor DCC is activated, resulting in ERK1/2/eNOSs1177 activation, which in turn, produces NO• to upregulate DCC expression, forming a feed-forward loop to maintain DCC activity and additional NO• production. A persistent supply of NO• may thus underlie the marked reductions in infarct size and cardiac apoptosis. Only previously known as a neuronal developmental protein and a regulator of angiogenesis, netrin-1, here gains a new role as a potent cardioprotective agent. Additional investigations focusing on the therapeutic potential of netrin-1 in managing ischemic heart disease may prove highly rewarding.
Supplementary Material
Acknowledgments
The authors’ work has been supported by National Heart, Lung and Blood Institute (NHLBI) Grant HL077440 (HC), HL057244 (PLL and HC), HL080111 (PPP and HC), an American Diabetes Association Award 7-08-RA-18 (HC), and a Start-up Fund from the University of California Los Angeles (HC).
Footnotes
DISCLOSURE
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–35. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 2.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–24. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 3.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 4.Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101:16210–5. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A. 2006;103:6530–5. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–4. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navankasattusas S, Whitehead KJ, Suli A, Sorensen LK, Lim AH, Zhao J, et al. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development. 2008;135:659–67. doi: 10.1242/dev.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, et al. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolopoulos SN, Giancotti FG. Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle. 2005;4:e131–5. [PubMed] [Google Scholar]
- 11.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 12.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–63. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 13.Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 14.Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 16.Larrivee B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–47. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elrod JW, Greer JJ, Bryan NS, Langston W, Szot JF, Gebregzlabher H, et al. Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:1517–23. doi: 10.1161/01.ATV.0000224324.52466.e6. [DOI] [PubMed] [Google Scholar]
- 18.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, et al. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–82. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 19.Pabla R, Buda AJ, Flynn DM, Blesse SA, Shin AM, Curtis MJ, et al. Nitric oxide attenuates neutrophil-mediated myocardial contractile dysfunction after ischemia and reperfusion. Circ Res. 1996;78:65–72. doi: 10.1161/01.res.78.1.65. [DOI] [PubMed] [Google Scholar]
- 20.Siegfried MR, Carey C, Ma XL, Lefer AM. Beneficial effects of SPM-5185, a cysteine-containing NO donor in myocardial ischemia-reperfusion. Am J Physiol. 1992;263:H771–7. doi: 10.1152/ajpheart.1992.263.3.H771. [DOI] [PubMed] [Google Scholar]
- 21.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H379–84. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Reeves WB, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol. 2008;294(4):F739–47. doi: 10.1152/ajprenal.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn JY, Wang T, Cai H. An Ezrin/Calpain/PI3K/AMPK/AKT/eNOSs1179 Signaling Cascade Mediating VEGF-dependent Endothelial Nitric Oxide Production. Circ Res. 2009;104:50–59. doi: 10.1161/CIRCRESAHA.108.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemost. 2003;1:2112–8. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 25.Chang J, Rao NV, Markewitz BA, Hoidal JR, Michael JR. Nitric oxide donor prevents hydrogen peroxide-mediated endothelial cell injury. Am J Physiol. 1996;270:L931–40. doi: 10.1152/ajplung.1996.270.6.L931. [DOI] [PubMed] [Google Scholar]
- 26.Monastyrskaya E, Folarin N, Malyshev I, Green C, Andreeva L. Application of the nitric oxide donor SNAP to cardiomyocytes in culture provides protection against oxidative stress. Nitric Oxide. 2002;7:127–31. doi: 10.1016/s1089-8603(02)00107-6. [DOI] [PubMed] [Google Scholar]
- 27.Hida A, Kawakami A, Miyashita T, Yamasaki S, Nakashima K, Tanaka F, et al. Nitric oxide acts on the mitochondria and protects human endothelial cells from apoptosis. J Lab Clin Med. 2004;144:148–55. doi: 10.1016/j.lab.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102:14729–34. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd’Heuil D, et al. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol. 1999;276:H1567–73. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 31.Brunner F, Maier R, Andrew P, Wolkart G, Zechner R, Mayer B. Attenuation of myocardial ischemia/reperfusion injury in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Cardiovasc Res. 2003;57:55–62. doi: 10.1016/s0008-6363(02)00649-1. [DOI] [PubMed] [Google Scholar]
- 32.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–9. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–61. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moens AL, Champion HC, Claeys MJ, Tavazzi B, Kaminski PM, Wolin MS, et al. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation. 2008;117:1810–9. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser H, Davidge ST, Clanachan AS. Activation of Ca(2+)-independent nitric oxide synthase by 17beta-estradiol in post-ischemic rat heart. Cardiovasc Res. 2000;46:111–8. doi: 10.1016/s0008-6363(99)00424-1. [DOI] [PubMed] [Google Scholar]
- 37.Di Napoli P, Antonio Taccardi A, Grilli A, Spina R, Felaco M, Barsotti A, et al. Simvastatin reduces reperfusion injury by modulating nitric oxide synthase expression: an ex vivo study in isolated working rat hearts. Cardiovasc Res. 2001;51:283–93. doi: 10.1016/s0008-6363(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 38.Abou-Agag LH, Khoo NK, Binsack R, White CR, Darley-Usmar V, Grenett HE, et al. Evidence of cardiovascular protection by moderate alcohol: role of nitric oxide. Free Radic Biol Med. 2005;39:540–8. doi: 10.1016/j.freeradbiomed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Bulhak AA, Sjoquist PO, Xu CB, Edvinsson L, Pernow J. Protection against myocardial ischaemia/reperfusion injury by PPAR-alpha activation is related to production of nitric oxide and endothelin-1. Basic Res Cardiol. 2006;101:244–52. doi: 10.1007/s00395-005-0580-1. [DOI] [PubMed] [Google Scholar]
- 40.Elrod JW, Greer JJ, Lefer DJ. Sildenafil-mediated acute cardioprotection is independent of the NO/cGMP pathway. Am J Physiol Heart Circ Physiol. 2007;292:H342–7. doi: 10.1152/ajpheart.00306.2006. [DOI] [PubMed] [Google Scholar]
- 41.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol. 2003;63:325–31. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 42.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–25. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 43.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2009;296:H1236–43. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li DY, Tao L, Liu H, Christopher TA, Lopez BL, Ma XL. Role of ERK1/2 in the anti-apoptotic and cardioprotective effects of nitric oxide after myocardial ischemia and reperfusion. Apoptosis. 2006;11:923–30. doi: 10.1007/s10495-006-6305-6. [DOI] [PubMed] [Google Scholar]
- 45.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–41. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 46.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–8. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci U S A. 1995;92:8031–5. doi: 10.1073/pnas.92.17.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, et al. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. 2007;50:808–17. doi: 10.1016/j.jacc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 49.Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T, et al. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation. 2002;106:873–9. doi: 10.1161/01.cir.0000024114.82981.ea. [DOI] [PubMed] [Google Scholar]
- 50.Weiland U, Haendeler J, Ihling C, Albus U, Scholz W, Ruetten H, et al. Inhibition of endogenous nitric oxide synthase potentiates ischemia-reperfusion-induced myocardial apoptosis via a caspase-3 dependent pathway. Cardiovasc Res. 2000;45:671–8. doi: 10.1016/s0008-6363(99)00347-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.