Abstract
Recent evidence shows that the auxiliary subunit KChIP2, which assembles with pore-forming Kv4-subunits, represents a new potential regulator of the cardiac calcium-independent transient outward potassium current (Ito) density. In hypertrophy and heart failure, KChIP2 expression has been found to be significantly decreased. Our aim was to examine the role of KChIP2 in cardiac hypertrophy and the effect of restoring its expression on electrical remodeling and cardiac mechanical function using a combination of molecular, biochemical and gene targeting approaches. KChIP2 overexpression through gene transfer of Ad.KChIP2 in neonatal cardiomyocytes resulted in a significant increase in Ito-channel forming Kv4.2 and Kv4.3 protein levels. In vivo gene transfer of KChIP2 in aortic banded adult rats showed that, compared to sham-operated or Ad.β-gal-transduced hearts, KChIP2 significantly attenuated the developed left ventricular hypertrophy, robustly increased Ito densities, shortened action potential duration, and significantly altered myocyte mechanics by shortening contraction amplitudes and maximal rates of contraction and relaxation velocities and decreasing Ca2+ transients. Interestingly, blocking Ito with 4-aminopyridine in KChIP2-overexpressing adult cardiomyocytes significantly increased the Ca2+ transients to control levels. One-day old rat pups intracardially transduced with KChIP2 for two months then subjected to aortic banding for 6–8 weeks (to induce hypertrophy) showed similar echocardiographic, electrical and mechanical remodeling parameters. In addition, in cultured adult cardiomyocytes, KChIP2 overexpression increased the expression of Ca2+-ATPase (SERCA2a) and sodium calcium exchanger but had no effect on ryanodine receptor 2 or phospholamban expression. In neonatal myocytes, KChIP2 notably reversed Ang II-induced hypertrophic changes in protein synthesis and MAP-kinase activation. It also significantly decreased calcineurin expression, NFATc1 expression and nuclear translocation and its downstream target, MCiP1.4. Altogether, these data show that KChIP2 can attenuate cardiac hypertrophy possibly through modulation of intracellular calcium concentration and calcineurin/NFAT pathway.
Keywords: Cardiac hypertrophy, Cardiac contractility, Kv4 channels, KChIP2, Gene transfer
Introduction
Reduction of the calcium-independent transient outward potassium current (Ito) and prolongation of the action potential duration (APD) are consistently observed in experimental animal models of cardiac hypertrophy and failure and human heart failure [1–3]. In hypertrophied myocytes Ito is decreased secondary to reductions in the expression of Kv4.2 and Kv4.3 potassium channel genes [4, 5]. More recently an emerging role for the Ca2+-binding protein Kv Channel-Interacting Proteins (KChIP), which is widely expressed in neuronal and cardiac tissues [6], has been demonstrated. These subunits were found to interact with and modulate the activity of Kv4.2 and Kv4.3 channels [6]. A total of four KChIP isoforms have been discovered with KChIP2 being the only isoform expressed in the heart [6]. KChIP2 has been reported, in multicellular preparations, to increase Ito magnitude more than eight-fold by enhancing the trafficking of channels from the endoplasmic reticulum to the plasma membrane, to shift the voltage dependence of activation to more negative potentials, and to slow the rate of inactivation and speed recovery from inactivation of Kv4x channels [6–12]. Furthermore, KChIP2 has been shown to modulate Kv1.5 expression [13] and cardiac L-type calcium current [14].
The role of KChIP2 in normal development and hypertrophy is emerging. In hypertrophy and heart failure, KChIP2 expression has been found to be significantly decreased [9, 15]. Interestingly, reduced expression of KChIP2 has been reported to be responsible for the reduction in Ito noted in human heart failure and in a rodent model of hypertrophy and heart failure [9, 15]. In fact, KChIP2 was shown to be important for the expression of Ito in the human heart [16] and deficiency of KChIP2 in mice was associated with a complete loss of Ito and increased susceptibility to ventricular arrhythmias [9] although no obvious structural abnormalities were present. Recently, it was reported that KChIP2 modulates the Ca2+ cardiac current [14] and post-transcriptional silencing of KChIP2 decreased Kv and Nav currents [6, 17], suggesting a broad physiological role of KChIP2 in cardiac electrophysiology. We sought in this study to examine the effects of restoration of KChIP2 expression in hypertrophied and failing cardiomyocytes on electrical remodeling, cardiac mechanical function and stress pathways in response to cardiac hypertrophy in vitro and in vivo using a combination of molecular, biochemical and gene transfer approaches.
Materials and Methods
All animal procedures were performed with the approval of IACUC at Mount Sinai School of Medicine and in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals. The methods used in this study are detailed in the online data supplement.
Results
KChIP2 overexpression can quantitatively increase Ito-encoding channel proteins
Before we begin to address the effects of KChIP2 on Kv4 channels, we first examined the protein expression patterns of KChIP2 in cardiomyocytes isolated from neonatal and adult rat hearts. Protein expression of KChIP2, determined by immunoblotting, showed that it is abundantly expressed in adult hearts but expressed at low levels in pooled hearts from one day-old rat pups (Fig.1A). This preferential myocardial expression of KChIP2 in adulthood suggests it may play a physiological role in the myocardium.
Figure 1. KChIP2 increases Kv4.2 and Kv4.3 expression.
Representative immunoblots of individual experiments showing neonatal rat myocytes express far less KChIP2 compared to adult myocytes(A); the expression of Kv4.2 and Kv4.3 is increased following KChIP2 overexpression in neonatal myocytes compared to β-gal (B). Kv4.3 induction shows two bands (66 and ~45/50 kDa). Protein expression was normalized to GAPDH. Bar graphs of fold change in the protein levels are shown in (C). (D) Representative RT-PCR and (E) quantification of mRNA expression of Kv4.2, Kv4.3 and Kv1.4 normalized to GAPDH mRNA (n=4). Data from 3–4 experiments.#, * P< 0.05 Ad.KChIP2 vs. Ad.β-gal.
We next measured channel proteins (Kv4.2, Kv4.3 and Kv1.4) expression by immunoblotting analysis. KChIP2 overexpression through gene transfer of Ad.KChIP2 in neonatal cardiomyocytes for 36 hours resulted in a significant increase in Kv4.2 protein with an apparent mass of 66 kDa (Fig. 1B). The expression of Kv4.3 was also increased, however, two distinct populations of Kv4.3 protein, at 45–50 and 66 kDa, were detected (Fig 1B). The 66 kDa form is similar to the Mr of the native rat heart Kv4.3, however, the nature of the 45–50 kDa band remains unknown. It could be an accelerated protein degradation of Kv4.3 subunit due to failure of trafficking to the cell membrane or may represent an immature form of Kv4.3 since pre-incubation of anti-Kv4.3 antibody with a Kv4.3 peptide significantly eliminated this band (not shown). KChIP2 overexpression, on the other hand, did not affect the expression profile of Kv1.4 (not shown). Quantitative analysis of three to four determinations is depicted in Fig. 1C. Measurement of transcript levels (Fig. 1 D) showed that KChIP2 overexpression, similar to its effect on protein expression, significantly increased the mRNA expression of Kv4.2 and Kv4.3 but had to effect on Kv1.4 mRNA expression (Fig. 1E). These data suggest that KChIP2 might be involved not only in the augmentation of Ito-based channel subunits trafficking to the plasma membrane but also in modulating their protein synthesis.
In vitro KChIP2 overexpression increases Ito current density in neonatal cardiomyocytes
In order to test if the observed increase in Kv4.2/3 protein levels correlates with an elevation in current density, we examined the effect of KChIP2 overexpression on action potentials. Ito densities recorded in isolated myocytes infected with Ad.β-gal (12.9±2.1 pA/pF n=10) were to some extent comparable to control non-infected myocytes (15.6±3.40 pA/pF n=12, P<0.05 at +60 mV). Overexpression of KChIP2 resulted in significantly greater Ito densities (52.3±2.9 pA/pF, n=12) compared to control or myocytes infected with Ad.β-gal.
In Vivo cardiac function following acute overexpression of KChIP2
We evaluated the in vivo effect of KChIP2 on cardiac function in adult rats subjected to severe stenosis of the ascending aorta (~85mm Hg). Banded animals were simultaneously infected with Ad.β-gal or Ad.KChIP2. Eight days after surgery/infection, echocardiography was performed at baseline and following gene transfer of KChIP2. Myocytes were dispersed from the left ventricular wall and mechanical parameters and electrophysiological recordings (i.e. patch-clamp) were determined.
We have also performed chronic studies (14–16 weeks) and compared the data obtained to the short-term (acute) findings. These data are detailed in the accompanying online data supplement.
a- In Vivo echocardiographic measurements following KChIP2 overexpression
A pronounced left ventricular hypertrophy developed in banded hearts transduced with Ad.β-gal with a significant increase in heart weight/body weight ratio (Table 1). Left ventricular posterior wall thickness (LVPWd, 2.52±0.41 mm; LVPWs, 4.52±0.37 mm) has increased significantly compared with sham (2.07±0.22 mm; 3.85±0.33 mm; P<0.01, n=8). There were also significant increases in interventricular septal and anterior wall thicknesses (Supplement Table I). However, hypertrophy was not seen in banded hearts transduced with Ad. KChIP2. Left ventricular posterior wall thickness (LVPWd, 2.1±0.2 mm; LVPWs, 3.8±0.39 mm) was significantly restored to normal values compared to Ad.β-gal transduced hearts (P<0.01, n=8) (Supplement Table I). However, KChIP2 did not significantly alter these parameters when transduced into non-banded sham animals (Supplement Table I).
Table 1.
Summary of electrical remodeling parameters in rats subjected to 8 days aortic-banding and simultaneously injected with adenoviruses.
| Sham | Banded+β-gal | Banded+KChlp2 | |
|---|---|---|---|
| HW/BW (mg/g) | 3.33±0.22 (8) | 4.27±0.34 (8)** | 3.51±0.42(8) ^^ |
| Capacitance (pF) | 131.03±9.97(15) | 191.53±23.49(25)** | 136.4±16.53 (15)^^ |
| APD50 (ms) | 14.73±7.97(20) | 24.75±11.49(18)** | 9.76±4.22 (18)* ^^ |
| APD90 (ms) | 46.50±5.9(20) | 67.51±13.97 (18)** | 32.27±8.79 (18)* ^^ |
| Ito density at 60 mV(pA/pF) | 25.0±2.3(15) | 16.1±2.1(25)** | 54.1±6.5(15)* ^^ |
P<0.05 KChIP2 vs Sham;
P<0.01 β-gal vs sham;
P<0.01 KChIP2 vs β-gal.
b- In vivo effect of KChIP2 overexpression on Ito density and action potential duration
Ito density was decreased in myocytes isolated from banded hearts infected with Ad.β-gal (18.9±2.5 pA/pF n=25) compared to myocytes derived from sham hearts (25.6±2.30 pA/pF n=15, P<0.01 at +60 mV) (Fig. 2). Overexpression of KChIP2 produced increased Ito densities (54.1±6.5 pA/pF, P<0.01, n=15) which were significantly larger compared to myocytes from banded hearts infected with Ad.β-gal or sham myocytes (Fig. 2A, B).
Figure 2. In vivo acute KChIP2 overexpression reverses hypertrophy-induced electrical remodeling.
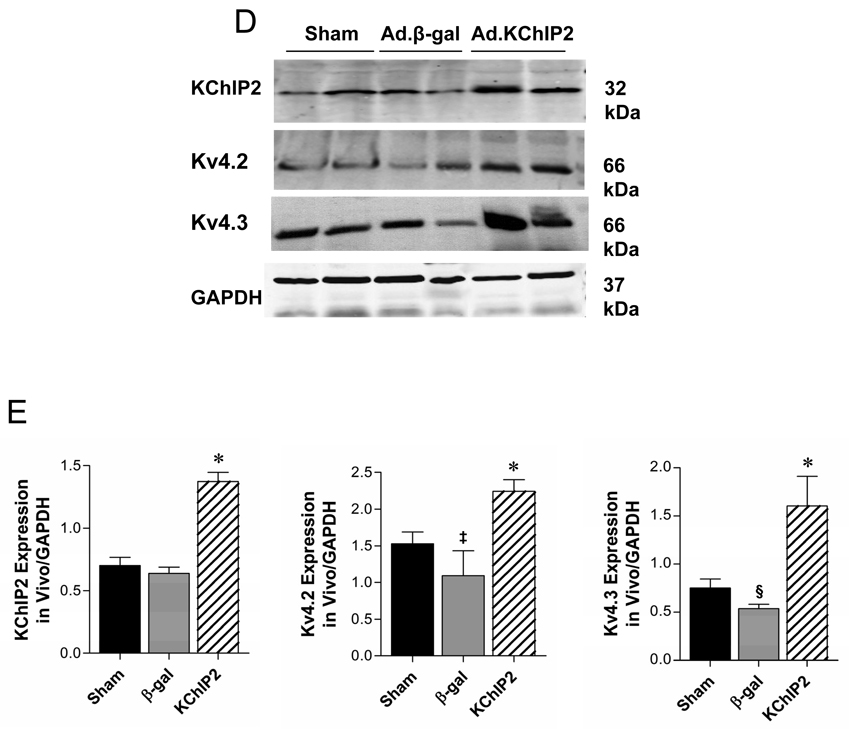
Representative whole-cell outward currents (A) and average current-voltage relationships of Ito (B) recorded from sham or banded hearts infected with β-gal or KChIP2 for 8 days. Data points are means±SEM; Sham vs. Ad.KChIP2, P<0.05; Ad.KChIP2 vs. Ad.β-gal, P<00.1. (C) Representative AP traces from sham or banded hearts infected with β-gal or KChIP2. Zero current potential of single myocytes ranged between −76 to 83mV. There are no significant different among the sham (−79.1+8.4 mV, n=20) β-gal groups (−76.1+6.2mV, n=18) and KChIP2 groups (−77.8+ 4.8 mV, n=18). (D) Representative immunoblots of KChIP2, Kv4.2 and Kv4.3 expression in the above hearts with summarized quantitative data (±SEM) of the relative protein density in arbitrary units (E). GAPDH levels are shown to verify protein loading and used to normalize the expression levels.
We also anticipated that augmentation of Ito density would shorten the APD. Aortic banding caused a significant prolongation of APD50 (50% repolarization) as well as APD90 (90% repolarization) compared to sham-operated controls (Table 1). Expression of KChIP2 significantly shortened APD at 50% and 90% in myocytes from banded hearts infected with KChIP2 compared to myocytes from banded hearts infected with Ad.β-gal (Table 1). Figure 2C shows typical APs recorded in myocytes from sham or banded rats infected with Ad.β-gal or Ad.KChIP2. Interestingly, the increase in Ito density mirrors the increase in KChIP2, Kv4.2 and Kv4.3 protein (Fig. 2D, E) as well as mRNA expression (Supplement Fig. 1) in vivo. These data demonstrate that in vivo adenoviral gene transfer of KChIP2 can specifically increase the expression of Kv4.2/3 proteins and Ito density and shorten APD over and above levels observed in sham-operated animals.
c- Effects of KChIP2 on cell contractility and intracellular Ca2+ transients
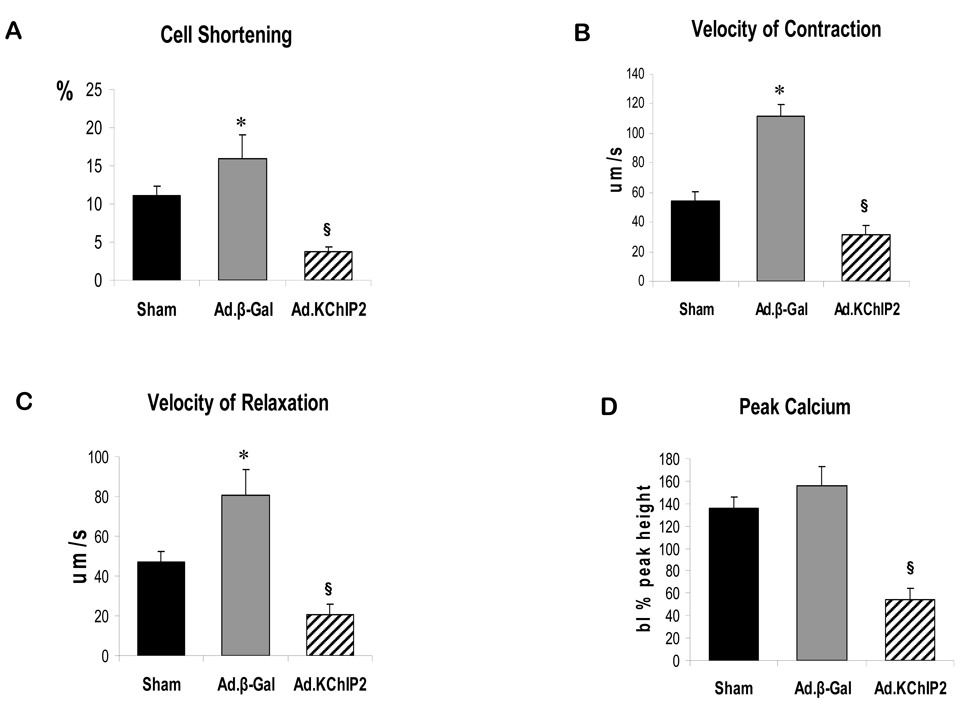
Since impaired contractility has been associated with cardiac hypertrophy and heart failure, we decided to examine whether KChIP2 had any effect on cardiomyocyte contractility and Ca2+ handling. Contractility and Ca2+ transient measurements were obtained in the presence of increasing amount of Ca2+ in cardiomyocytes isolated from hearts infected with Ad.β-gal or Ad.KChIP2. Recordings from cardiomyocytes isolated from banded hearts infected with Ad.β-gal show that aortic banding was associated with increases in peak calcium and cell shortening parameters compared to sham hearts. Overexpression of KChIP2 markedly attenuated the displayed enhancement in contractility (Fig. 3A), rates of cell shortening (Fig. 3B) and relengthening (Fig. 3C), and Ca2+ transients (Fig. 3D) seen with Ad.β-gal-banded hearts.
Figure 3. In vivo KChIP2 expression alters cardiomyocyte mechanics.
KChIP2 effects on cardiomyocyte contractility and Ca2+ transients were determined in cardiomyocytes isolated from banded hearts infected with β-gal or KChIP2 in the presence of 1–10 mM Ca2+ compared to sham hearts., overexpression of KChIP2 significantly decreased cell shortening (A, n=25), velocities of contraction (B, n=25) and relaxation (C, n=25) and induced shorter Ca2+ amplitudes (D, n=21) compared to β-gal (data shown at 4 mM Ca2+). * P< 0.01 Ad.β-gal vs. Sham; § P<0.04, Ad.KChIP2 vs. Ad.β-gal.
Effects of KChIP2 on Ca2+ handling
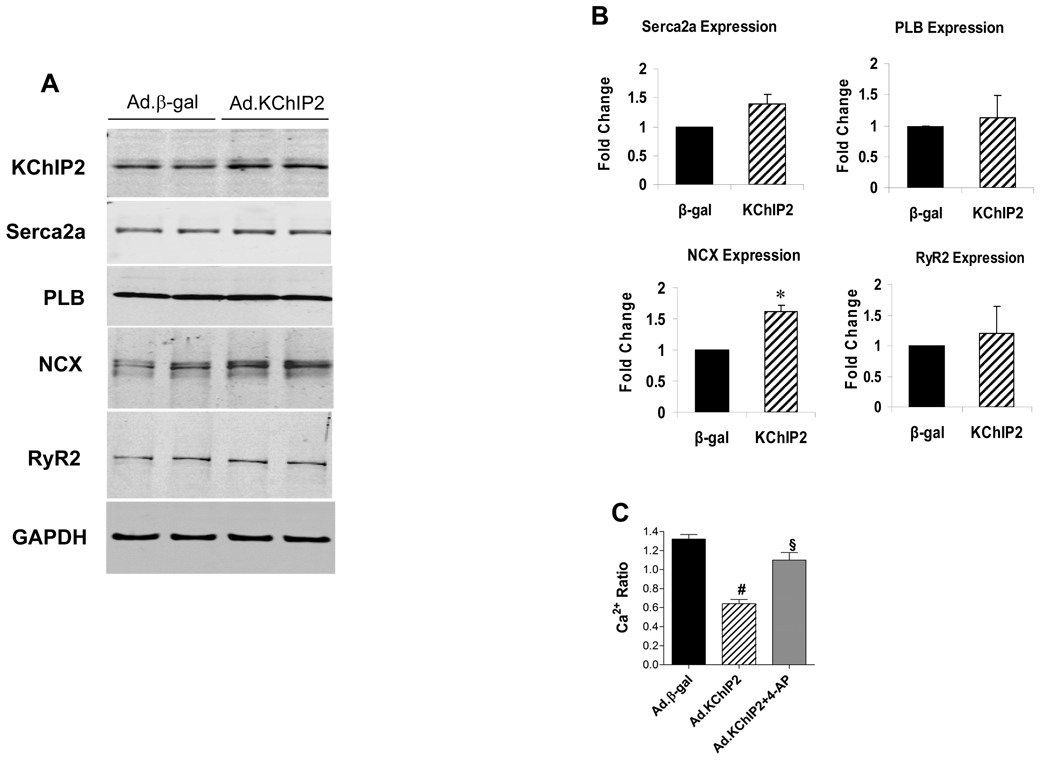
In addition to the observed effect of KChIP2 on APD which has been postulated to significantly determine the amount of inward Ca2+ flux through L-type voltage-dependent Ca2+ channels, we sought to examine its effect on a number of intracellular Ca2+ regulatory proteins. Cultured adult cardiomyocytes infected with Ad.KChIP2 showed increased KChIP2 expression and led to significant increases in SERCA2a and sarcolemmal Na+-Ca2+ exchanger (NCX) protein expression compared with cardiomyocytes expressing β-gal, but had no noticeable effect on the expression of ryanodine receptor 2 (RyR2) or phospholamban (PLB) (Fig. 4A). The quantitative analysis of these determinations is depicted in Fig. 4B. Interestingly, blocking Ito with 4-aminopyridine in KChIP2-overexpressing adult cardiomyocytes significantly increased the Ca2+ transients to approximately control levels (Fig.4C). Our observations are of interest since they show that KChIP2 can modulate the intracellular Ca2+ content by different mechanisms, through the reduction of APD and the regulation of Ca2+ cycling proteins (i.e. NCX and possibly SERCA2a).
Figure 4. KChIP2 regulation of calcium cycling proteins and intracellular Ca2+.
The effect of KChIP2 on the expression of major Ca2+ cycling proteins were examined by immunoblot analysis in isolated adult cardiomyocytes infected with Ad.β-gal or Ad.KChIP2. (A) Western blots of myocyte lysates demonstrating expression of KChIP2, Serca2a, phospholamban (PLB), Na+-Ca2+ exchanger (NCX), and ryanodine receptor 2 (RyR2). GAPDH, an internal loading control, is used to normalize protein expression. (B) Densitometric analysis of mean data (± SEM). (C) Histogram comparing mean (±SEM) Ca2+ ratio determined between β-gal- and KChIP2-infected cardiomyocytes in the absence or presence of 4 mM 4-aminopyridine (4-AP). Blocking Ito with 4-AP in KChIP2-overexpressing adult cardiomyocytes significantly increased the Ca2+ transients. * P< 0.05 Ad.KChIP2 vs. Ad.β-gal; # P< 0.0001 Ad.KChIP2 vs. Ad.β-gal, n=12; § P< 0.001 Ad.KChIP2 vs. Ad.KChIP2+4-AP, n=15.
KChIP2 modulation of hypertrophy markers
a. Protein synthesis
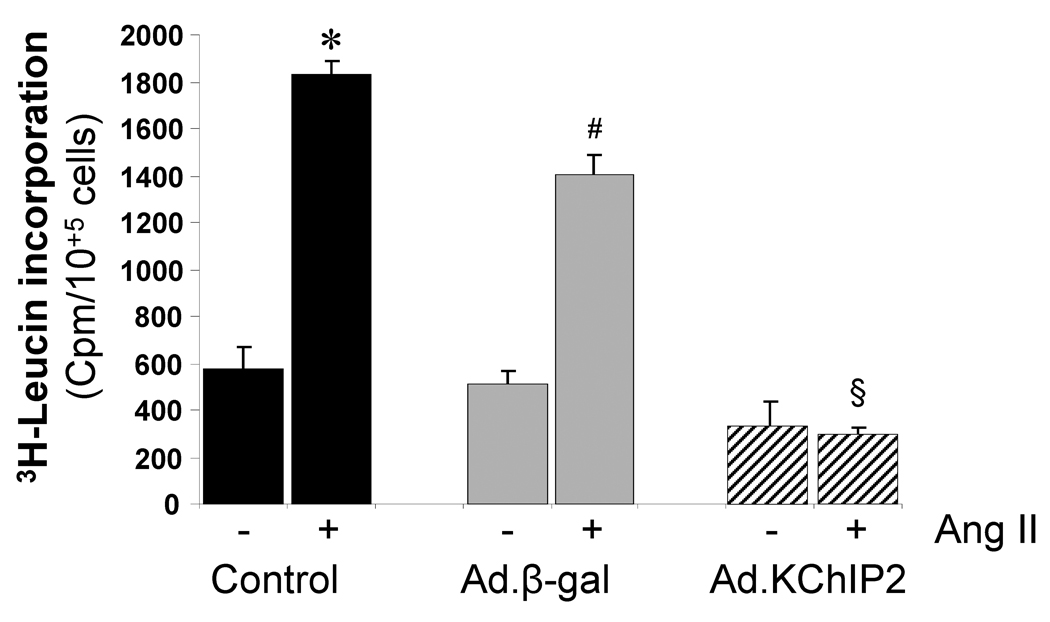
As a major marker of the hypertrophic response, we examined protein synthesis in the presence and absence of the stimulant angiotensin (Ang) II in neonatal rat ventricular myocytes (NRVM). Ang II induced a marked increase in [3H]-Leucine incorporation (4.18 folds, P<0.001) compared to control cells. However, the Ang II-induced increase in [3H]-Leucine incorporation was significantly inhibited (a 7.80 fold decrease compared to control, P<0.0001) in cells infected with Ad.KChIP2 (Fig. 5). Such an effect was not due to infection with the virus alone since infection with Ad.β-gal has slightly blocked Ang II-induced [3H]-Leucine incorporation (Fig. 5).
Figure 5. KChIP2 inhibits protein synthesis.
The effects of KChIP2 on protein synthesis were evaluated by measuring the rate of 3H-Leucine incorporation in neonatal myocytes uninfected (control) or infected with Ad.β-gal or Ad.KChIP2 in the absence (−) or presence (+) of Ang II stimulation (1 µM). KChIP2 overexpression significantly blocked the pronounced increase in Ang II-induced 3H-Leucine incorporation. Data from five individual experiments are shown. * P< 0.001 Cont+AngII vs. Cont; #P<0.01 Ad.β-gal+Ang II vs. Ad.β-gal; §P<0.01 Ad.KChIP2+AngII vs. Ad.β-gal+AngII.
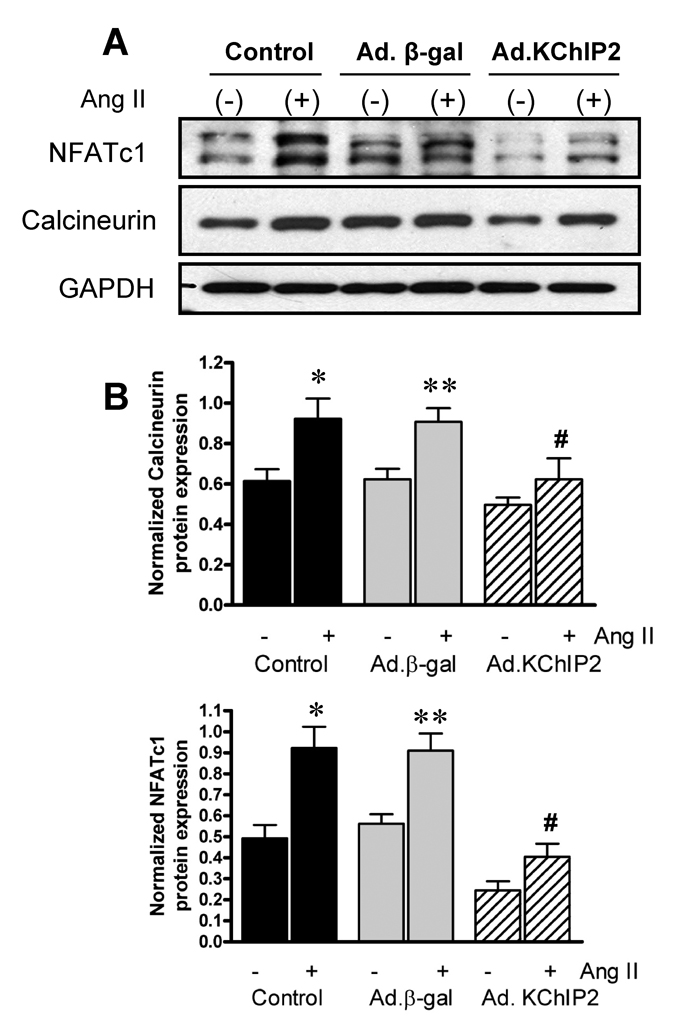
b. Effect of KChIP2 expression on Calcineurin/NFAT pathway
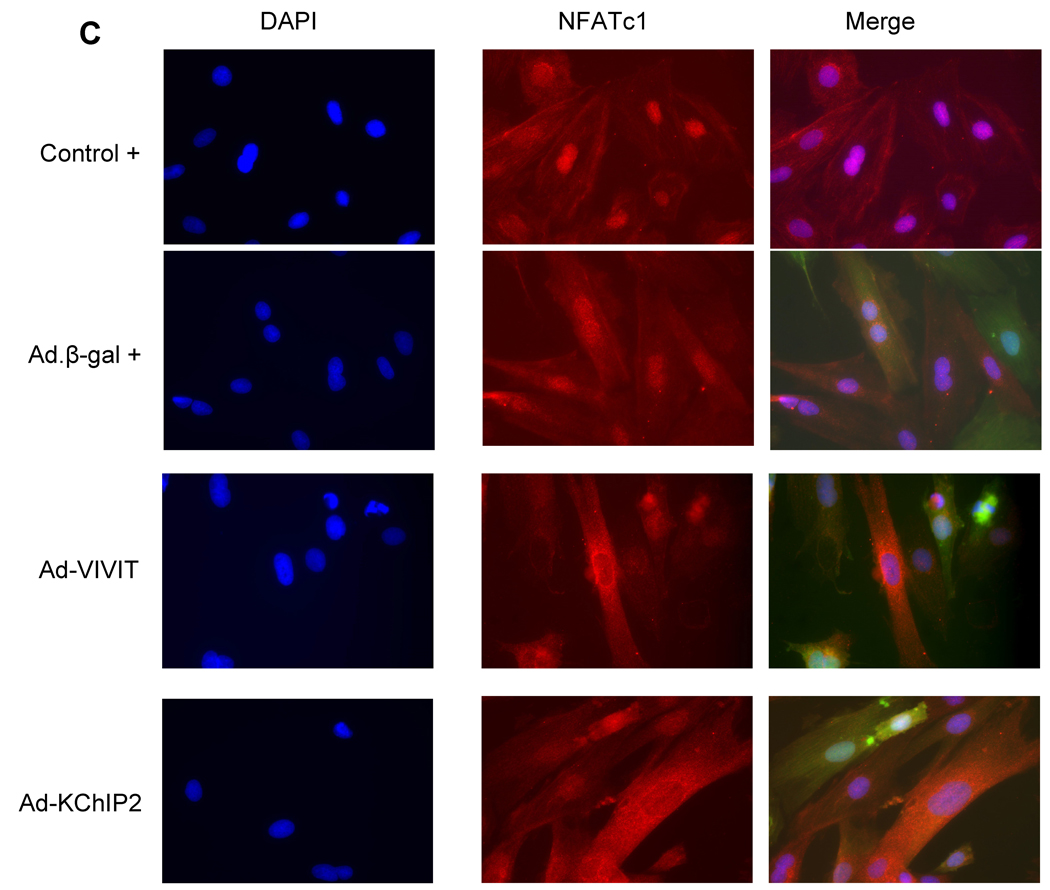
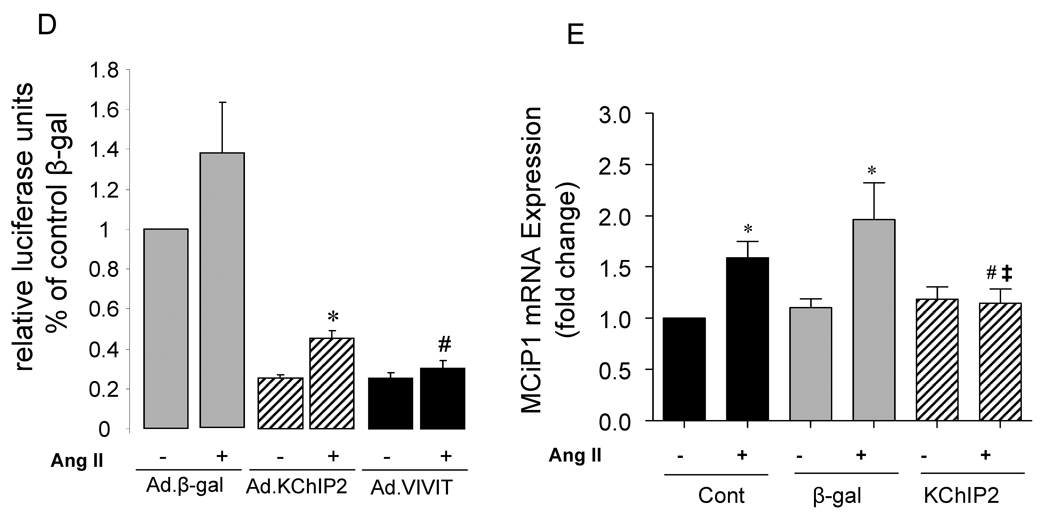
We sought to determine whether the calcineurin-NFAT pathway is involved in the KChIP2-induced abrogation of myocyte hypertrophy since this signaling pathway is well established in the regulation of cardiac hypertrophy. Neonatal rat ventricular myocytes (NRVM) were infected with Ad.KChIP2 or Ad.β-gal in the presence or absence of Ang II. As shown in Fig. 6A, Ang II increased NFATc1 expression in control non-infected or Ad.β-gal–infected NRVM. In contrast, expression of Ad.KChIP2 markedly reduced the observed Ang II-induced NFATc1. Interestingly, even in the absence of Ang II stimulation, KChiP2 substantially reduced the basal level of NFATc1 expression. Likewise, the expression of calcineurin was also significantly decreased by KChIP2 overexpression (Fig. 6A) compared to controls. Quantitative analysis of three to four separate determinations is depicted in Fig. 6B. Furthermore, the abrogation of Ang II-induced NFATc1 activity by KChIP2 transgene was confirmed by examining the nuclear-cytoplasmic translocation of NFATc1 using immunofluorescence, by NFAT-luciferase assay, and by MCiP1.4 expression. Ang II stimulation promoted clear and sustained nuclear localization of NFATc1 (Fig. 6C) in cardiomyocytes, which was substantially blocked by KChIP2 overexpression or by the highly selective inhibitor of calcineurin/NFAT, VIVIT (Fig. 6C). KChIP2 has also significantly reduced NFAT-luciferase (both at base line and following AngII stimulation) (Fig. 6D) and decreased MCiP1.4 expression, a bona fide NFAT downstream target gene (Fig.6E). These data indicate that KChIP2 can target the calcineurin/NFAT signaling and modulate the hypertrophic response through this pathway.
Figure 6. KChIP2 inhibits calcineurin/NFAT pathway.
The effect of KChIP2 on NFATc1 and calcineurin expression (A) was evaluated by Western blotting in neonatal myocytes infected with Ad.β-gal or Ad.KChIP2 (MOI 60) in the presence (+) or absence (−) of Ang II. (B) Quantitative analysis of mean data (± SEM) of 3 to 4 independent experiments. GAPDH, an internal loading control, is used to normalize protein expression. * P< 0.05 Cont+AngII vs. Cont; **P<0.01 Ad.β-gal+Ang II vs. Ad.β-gal; #P<0.01 Ad.KChIP2 vs. Ad.β-gal. (C) Control NRVM non-infected or NRVM infected with Ad.β-gal, Ad.VIVIT (a calcineurin/NFAT-specific inhibitor) or Ad.KChIP2 were stimulated with Ang II (+) for 30 min and analyzed for NFATc1 translocation. Myocyte nuclei are visualized with DAPI staining. (D) NFAT-luciferase activity was measured in NRVM infected with Ad.β-gal, Ad.KChIP2 or Ad.VIVIT in the absence (−) or presence (+) of Ang II (1 µM for 24 hours). Data were normalized to unstimulated β-gal (−AngII). *P=0.021 Ad.KChIP2+AngII vs. Ad.β-gal+AngII; #P=0.014 Ad.VIVIT+AngII vs. Ad.β-gal+AngII. (E) MCiP1.4 mRNA expression was determined by real time-PCR and normalized against GAPDH mRNA in control NRVM and NRVM infected with Ad.β-gal or Ad.KChIP2 and cultured in serum-free medium in the absence (−) or presence (+) of Ang II (1 µM for 24 hours). Density values of corresponding bands from at least six-eight independent experiments were normalized to control unstimulated (−AngII) and are expressed as mean ± SEM. * P< 0.05 Cont+AngII vs. Cont; *P<0.01 Ad.β-gal+AngII vs. Ad.β-gal; #P<0.01 Ad.KChIP2+AngII vs. Ad.β-gal+AngII; ‡P<0.01 Ad.KChIP2+AngII vs. Cont+AngII.
c. Effect of KChIP2 expression on MAP kinases activation
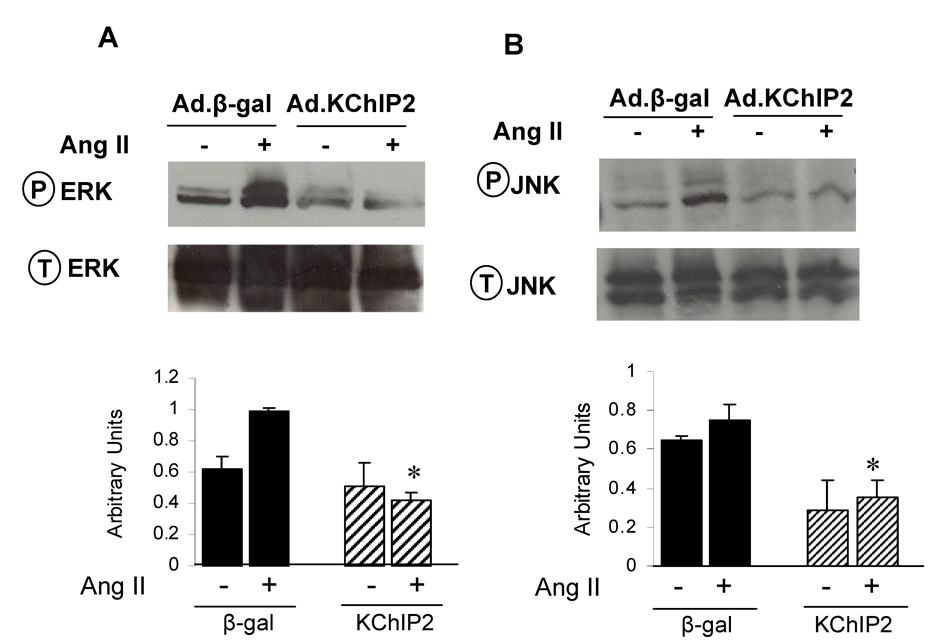
In attempting to determine the mechanism by which KChIP2 regulates Ang II-induced cardiac myocyte hypertrophy, we sought to examine the activities of the stress-activated MAP kinases (p38, JNK and ERK), since they play a central role in the cardiomyocyte growth response. Following Ang II induction of neonatal myocytes, ERK1/2 phosphorylation was increased by 2.5-fold in Ad.β-gal-infected myocytes (n=6, P<0.0001), as identified by quantitative immunoblotting analysis using phospho-specific anti-ERK1/2 (Fig. 7A). In contrast, expression of Ad.KChIP2 markedly reduced Ang II-induced ERK1/2 phosphorylation by 4.5-fold (n=6, P<0.0001) (Fig. 7A). Interestingly, KChIP2 overexpression has also significantly reduced (3-fold, P<0.05) ERK phosphorylation at basal state in the absence of Ang II stimulation.
Figure 7. KChIP2 inhibits MAPkinases activity.
The effect of KChIP2 on ERK (A) and JNK (B) activities was evaluated in neonatal myocytes infected with Ad.β-gal or Ad.KChIP2 in the presence (+) or absence (−) of AngII (1 µM for 30 min). Immunoblots were probed with phospho-specific antibodies against ERK and JNK and then re-probed for the corresponding total protein that confirmed equivalent protein loadings. Shown are representative blots from 4 determinations. * P< 0.01 Ad.KChIP2 vs. Ad.β-gal.
Ang II has also stimulated the activity of JNK as its phosphorylation levels were markedly increase (3-fold, P<0.001) in myocytes infected with Ad.β-gal. Similar to the ERK pathway, we found significant inactivation of JNK after infection with Ad.KChIP2 (3.5-fold decrease compared to Ad.β-gal, P<0.02) (Fig. 7B). In the absence of Ang II, KChIP2 was also able to induce a moderate decrease in JNK activity (1.5-fold compared to Ad.β-gal) (Fig. 7B). KChIP2 induced a minor insignificant change in the activity of p38 MAPkinase (not shown). It is interesting to note that the ERK and JNK signaling pathways modulated by KChIP2 in this study were also found to regulate KChIP2 expression [18].
Discussion
Prolongation of APD is consistently observed in experimental models of cardiac hypertrophy and failure and is attributed to a reduction in Ito [2, 19–21]. Down-regulation of Ito density is also one of the most consistent features in electrophysiological remodeling observed in animal models of hypertrophy and failure as well as in the human heart under pathological conditions [2, 19, 22, 23]. Interestingly, recent findings show that the auxiliary subunit KChIP2 is markedly reduced in human failing hearts [15], and in animal model of cardiac hypertrophy following pressure overload in vivo [9, 18, 24] and phenylephrine-stimulated rat cardiomyocytes in vitro [18]. Therefore, it was postulated that the relative levels of KChIP2 can determine the current density of Ito and as such may significantly contribute to cardiac hypertrophy-associated reduction of Ito [9, 18, 24]. In this study we demonstrate that in vivo gene transfer of KChIP2 can reverse Ito down-regulation and attenuate hypertrophy of the left ventricle under increased loading conditions. We also demonstrate that KChIP2 can target the hypertrophic response through modulation of intracellular Ca2+ concentration and calcineurin/NFAT signaling module.
KChIP2 modulation of LV electrical remodeling, cardiac function and contractility
Since KChIP2 appears to regulate Ito function, it was of special interest to examine the physiological significance of KChIP2 expression normalization on cardiac function in vivo. Echocardiography showed that banded hearts transduced with Ad.KChIP2 demonstrated a considerable decrease in left ventricular walls thicknesses and chamber dimensions and a significant increase in cardiac function. Mechanical properties of myocytes isolated from banded hearts infected with Ad.β-gal showed that aortic banding was associated with increases in peak calcium and cell shortening when compared with sham hearts. However, overexpression of KChIP2 attenuated the enhanced contractile function seen with Ad.β-gal hearts. The induction of hypertrophy in this setting is probably initiated, initially, as an adaptive mechanism to enhance the depressed cardiac function. These electrical and hypertrophic changes produced by KChIP2 in vivo are consistent with our previous data obtained with pore-forming Kv4.3 [25, 26]. Several reports have indicated that impaired Ca2+ homeostasis is a primary stimulus for cardiac hypertrophy and failure [27]. Indeed, in animal models of cardiac hypertrophy and failure, the amplitude of the intracellular Ca2+ transient is increased, in part, as a result of APD prolongation [2, 5], causing positive inotropy. While this event could result from changes in the expression and activity of several calcium cycling proteins, APD prolongation can induce a substantial net Ca2+ influx through L-type Ca2+ channels with each cardiac cycle [2, 28]. This enhanced Ca2+ entry in turn leads to enhanced intracellular Ca2+ transients and increased cardiac contractility, as a result of increased sarcoplasmic reticulum Ca2+ uptake and release [29]. Although this may help to support contraction of the compromised myocardium, it may also harm the myocardium by increasing the propensity to develop arrhythmias [2, 30] and by triggering hypertrophic response. Modulating the action potential magnitude and profile should then have profound effects on Ca2+ transient amplitude and the associated changes. The observation that in vivo cardiac gene transfer of KChIP2 can restore the hypertrophy-associated changes in electrical parameters (i.e. Ito density, APD) and modulate Ca2+ handling proteins (i.e. Serca2a, NCX) may therefore explain the attenuation of the enhanced contractile function and Ca2+ transients seen after pressure overload following KChIP2 and/or Kv4.3 overexpression. Interestingly, recent studies have shown that post-transcriptional gene silencing of KChIP2 in neonatal rat cardiomyocytes suppressed both Ito and INa (sodium current) as well as Na channel α and β1 subunit mRNA expression levels [17]. In addition, despite a decrease in L-type Ca2+ channel current, ventricular myocytes from KChIP2−/− moce were reported to express higher levels of Ca2+ channel protein [31]. Collectively, these data highlight the importance of KChIP2 in the regulation of intracellular Ca2+ concentration.
KChIP2 modulation of cardiac hypertrophy
In hypertrophy and heart failure KChIP2 expression has been found to be significantly decreased [9, 15]. We show here that normalization of KChIP2 expression abrogates the hypertrophic response induced by pressure overload in hypertrophic hearts in vivo and by Ang II in cultured myocytes in vitro. The conclusion that cardiac hypertrophy was decreased is substantiated by the following results that overexpression of KChIP2: 1) significantly decreased the heart weight/body weight ratio, 2) decreased interventricular septal and left ventricular posterior wall thicknesses, 3) decreased cell capacitance, 4) significantly increased Ito density 5), markedly shortened APD, 6) inhibited protein synthesis, 7) inhibited MAPK signaling activity, 8) decreased calcineurin/NFAT expression and blocked NFATc1 nuclear translocation and activity, and 9) downregulated expression of MCiP1.4.
The inhibition of MAPK activity following KChIP2 overexpression is of importance particularly in light of recent studies which showed that MAPK can modulate the expression of KChIP2 [18]. These studies suggest that down-regulation of KChIP2 expression observed in cardiac hypertrophy significantly contributes to the hypertrophy-associated reduction in Ito density. They also indicate that the expression of KChIP2 mRNA is controlled by the 2 branches of MAP-kinase pathways, namely JNKs and MEK-ERK [18]. Hence, the activation of stress-activated MAPkinases could therefore contribute to diminished Ito by transcriptional down-regulation of KChIP2. However, the mechanism of MAPK-induced down-regulation of KChIP2 expression is not clear. In this respect, it is interesting to point out that the KChIP2 promoter region has been reported to bind CREB, a transcription factor that is also a target of ERK phosphorylation [32]. Moreover, ERK has also been suggested to regulate Ito by direct phosphorylation of the pore-forming Kv4.2 subunit [33]. Other mechanisms, including phosphorylation by tyrosine kinases and mRNA degradation of Kv4.3 mRNA by angiotensin II and phenylephrine have also been shown to affect Ito density [34], [35] . So, it appears that factors that regulate Ito-based Kv4-channel genes concomitantly regulate KChIP2, although the precise mechanisms responsible are still poorly understood. Our observation that KChIP2 regulates the activity of the MAPKs coupled with the observation that ERK may also regulate the function of Kv4- channels and KChIP2 raises the potential ability of KChIP2 to play a dual role in regulating Kv4 currents: 1) through direct modulation of Kv4 kinetics and expression; and 2) through a possible feedback mechanism involving the attenuation of the MAPK activation. It will be important to elucidate this feedback mechanism in more detail in order to characterize the link between the signaling molecules responsible for regulation of cardiac growth and electrical remodeling.
The involvement of the calcineurin/NFAT pathway in cardiac growth and hypertrophy is well established [27]. In addition, the activation of calcineurin and the onset of cardiac hypertrophy have been shown to be associated with APD prolongation and to be greatly influenced by increased calcium influx, either directly by increasing the expression of L-type calcium channels or indirectly by reducing the expression of the transient outward K+ channels [36, 37]. We recently reported that down-regulation of Ito and APD prolongation occur early following pressure overload and in vivo cardiac gene transfer of Kv4.3-based Ito can increase Ito density, shorten APD and attenuate the hypertrophic responses via a calcineurin pathway [25]. Similar observations have also been demonstrated earlier in cultured systems using NRVM overexpressiong Kv4.2 [38] or infected with adenoviruses harboring dominant-negative constructs for Kv4.2 [39]. We report in the present study that overexpression of KChIP2 in cultured cardiomyocytes is associated with a marked decrease in the expression of calcineurin and NFAT. The ability of KChIP2 to block the nuclear translocation of NFATc1, as did VIVIT, and to reduce NFAT luciferase activity and down-regulate MCiP1.4 further documents the effect of KChIP2 on calcineurin/NFAT pathway and may provide a potential mechanism through which KChIP2 regulates cardiac hypertrophy.
Recently, calcineurin/NFAT signaling pathway has been reported to down-regulate the expression of Kv4.2/Kv4.3 channels and KChIP2 in the mouse heart under physiological and pathophysiological conditions [40–42]. Also, raping pacing of adult canine cardiomyocytes deceased Ito density and Kv4.3, but not KChIP2, expression through an increase in calcineurin/NFAT signaling activity [43]. It was further demonstrated that differential calcineurin/NFAT activity contributes to the transmural Ito gradient (produced by Kv4 and KChIP2 expression) across the mouse left ventricular free wall [40]. Given that calcineurin/NFAT signaling causes hypertrophy [27], it was suggested that blocking calcineurin and NFAT activation attenuates left ventricular remodeling in dominant negative Kv4.2 transgenic mice [44] and completely prevents rate-dependent Ito and ICa down-regulation [43, 45]. It is therefore intriguing to speculate that KChIP2, in this study, negatively modulates calcineurin/NFAT pathway in order to preserve the expression of Ito.
Given the preponderance and early time course of electrical remodeling in cardiac hypertrophy and failure, it is possible that the elevations in intracellular calcium associated with APD prolongation may provide an important stimulus for activation of stress pathways including calcineurin and MAPKinases and induction of cardiac hypertrophy. In this context, the observation that KChIP2 abrogates cellular hypertrophy is of interest. Our data indicate that KChIP2 can target cardiac hypertrophy by interfering with the calcineurin/NFAT pathway through a Ca2+-sensitive signaling system involving 1) regulation of Ito density and APD, and 2) modulation of Ca2+ cycling proteins to alter intracellular Ca2+ content. Increasing proteins known to be involved in the sequestration/extrusion of intracellular Ca2+ and shortening the APD, thus limiting Ca2+ influx, generates a lower cytosolic Ca2+ signal that deactivates the calcineurin/NFAT signaling pathway and subsequently attenuates the hypertrophic response.
Causal relationship between the loss of Ito and cardiac hypertrophy
The relationships between reductions of Ito and concomitant APD prolongation and cardiac hypertrophy have been explored but the findings were distinct. Cardiac specific ablation of Ito1 by overexpression of a Kv4.2 channel with a single mis-sense mutation (W362F) did not induce cardiac hypertrophy [46, 47]. However, cardiac specific overexpression of dominant negative Kv4.2 channel (truncated channel:Kv4.2N) resulted in hypertrophy and cardiomyopathy along with a prolonged APD in vivo [28] and in vitro [39]. There are some differences between these two murine models. The W362F mutant subunits can insert into the plasma membrane normally, but the channels are non-conducting and at the same time have a dominant-negative effect when they co-assemble with wild-type subunits [46, 47]. On the other hand Kv4.2N has only dominant negative effects and may also cause toxicity within the cardiac myocytes. In mice overexpressing W362F mutant, there was an increase in Kv1.4 channel expression [46, 47]. However, crossbreeding of Kv1.4−/− and Kv4.2W362F transgenic mice produced offspring that had structurally normal hearts despite significant QT prolongation and frequent ventricular tachycardia [47]. Interestingly, patients with long QT syndrome do not have evidence of hypertrophy at baseline. In addition, there was no evidence of hypertrophy or compromised functioning in Kv4.2−/− [48] or KChIP2−/− hearts [9] [31]; yet, KChIP2−/− mice have decreased total Ito and significantly prolonged APD [9]. However, despite the apparently normal development of KChIP2−/− mice, a potential link between KChIP2 and heart disease was indicated by the finding that left-ventricular hypertrophy induced by pressure overload in mice was associated with a significant decrease in KChIP2 expression [9]. We and others have also shown that hypertrophy-inducing stimuli such as aortic banding can markedly reduce Ito densities and Ito-encoding pore-forming Kv4.3 [25] and auxiliary KChIP2 subunit proteins expression [18]. We also demonstrated that in vivo gene transfer of Kv4.3 can reverse the observed Ito downregulation and attenuate the associated cardiac hypertrophy [25]. Our current findings that KChIP2 restoration in vivo can also attenuate the hypertrophic response to pressure overload argue for a role of these molecules in the remodeling of the myocardium and therefore may play a potential role in the progression of hypertrophy and failure once cardiac hypertrophy is initiated. However, since heart rate, action potential shape, and contractile properties are known to be species dependent, the possibility remains that the role of Ito and its auxiliary subunits in generation of LV pathologies is also species dependent. Although considerable progress has been made, the role of electrical cardiac remodeling associated with the molecular pathogenesis of hypertrophy remains limited and clearly warrants further investigation.
Limitations
In this study we attempted to examine the role of KChIP2 in modulating cardiac contractility and hypertrophy. Although these data are interesting, they may not be extrapolated to the diseased human myocardium because of the presence of comorbid conditions and marked differences in AP profiles. KChIP2 is the predominant isoform of the auxiliary KChIP family to be specifically expressed in the myocardium. Currently, there are about eight KChIP2 isoforms that have been identified and characterized (reviewed in [21]). They appear to exert different regulatory effects on Kv4 function. It remains to be determined whether these isoforms would also produce different effects on cardiac contractility and hypertrophy. Another limitation is the concern that the level of the overexpressed KChIP2 may be higher than the physiological levels since the robust increase in Ito density was greater than in control cardiomyocytes. So, carefully titrating transgene expression will be particularly helpful.
Our findings indicate that normalization of KChIP2 expression in hypertrophied hearts can attenuate the development of cardiac hypertrophic response possibly through restoration of Ito densities and reduction of APD, regulation of intracellular Ca2+ cycling and modulation calcineurin/NFAT pathway.
Supplementary Material
Acknowledgements
This study was supported in part by grants from NIH (DL and RJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Nabauer M, Kaab S. Potassium channel down-regulation in heart failure. Cardiovascular research. 1998 Feb;37(2):324–334. doi: 10.1016/s0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- 2.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovascular research. 1999 May;42(2):270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 3.Wickenden AD, Kaprielian R, Kassiri Z, Tsoporis JN, Tsushima R, Fishman GI, et al. The role of action potential prolongation and altered intracellular calcium handling in the pathogenesis of heart failure. Cardiovascular research. 1998 Feb;37(2):312–323. doi: 10.1016/s0008-6363(97)00256-3. [DOI] [PubMed] [Google Scholar]
- 4.Gidh-Jain M, Huang B, Jain P, el-Sherif N. Differential expression of voltage-gated K+ channel genes in left ventricular remodeled myocardium after experimental myocardial infarction. Circulation research. 1996 Oct;79(4):669–675. doi: 10.1161/01.res.79.4.669. [DOI] [PubMed] [Google Scholar]
- 5.Kaprielian R, Wickenden AD, Kassiri Z, Parker TG, Liu PP, Backx PH. Relationship between K+ channel down-regulation and [Ca2+]i in rat ventricular myocytes following myocardial infarction. The Journal of physiology. 1999 May 15;517(Pt 1):229–245. doi: 10.1111/j.1469-7793.1999.0229z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000 Feb 3;403(6769):553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 7.Bahring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. The Journal of biological chemistry. 2001;276(26):23888–23894. doi: 10.1074/jbc.M101320200. [DOI] [PubMed] [Google Scholar]
- 8.Decher N, Barth AS, Gonzalez T, Steinmeyer K, Sanguinetti MC. Novel KChIP2 isoforms increase functional diversity of transient outward potassium currents. The Journal of physiology. 2004 Jun 15;557(Pt 3):761–772. doi: 10.1113/jphysiol.2004.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001 Dec 14;107(6):801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 10.Patel SP, Parai R, Parai R, Campbell DL. Regulation of Kv4.3 voltage-dependent gating kinetics by KChIP2 isoforms. The Journal of physiology. 2004 May 15;557(Pt 1):19–41. doi: 10.1113/jphysiol.2003.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosati B, Grau F, Rodriguez S, Li H, Nerbonne JM, McKinnon D. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. The Journal of physiology. 2003 May 1;548(Pt 3):815–822. doi: 10.1113/jphysiol.2002.033704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, et al. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. The Journal of physiology. 2001 May 15;533(Pt 1):119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Guo W, Mellor RL, Nerbonne JM. KChIP2 modulates the cell surface expression of Kv 1.5-encoded K(+) channels. Journal of molecular and cellular cardiology. 2005 Jul;39(1):121–132. doi: 10.1016/j.yjmcc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen MB, Wang C, Ozgen N, Wang HG, Rosen MR, Pitt GS. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circulation research. 2009 Jun 19;104(12):1382–1389. doi: 10.1161/CIRCRESAHA.109.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radicke S, Cotella D, Graf EM, Banse U, Jost N, Varro A, et al. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovascular research. 2006 Sep 1;71(4):695–703. doi: 10.1016/j.cardiores.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Deschenes I, DiSilvestre D, Juang GJ, Wu RC, An WF, Tomaselli GF. Regulation of Kv4.3 current by KChIP2 splice variants: a component of native cardiac I(to)? Circulation. 2002 Jul 23;106(4):423–429. doi: 10.1161/01.cir.0000025417.65658.b6. [DOI] [PubMed] [Google Scholar]
- 17.Deschenes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. Journal of molecular and cellular cardiology. 2008;45:336–346. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia Y, Takimoto K. Mitogen-activated protein kinases control cardiac KChIP2 gene expression. Circulation research. 2006 Feb 17;98(3):386–393. doi: 10.1161/01.RES.0000201956.86258.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiological reviews. 2005 Oct;85(4):1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 20.Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward potassium current (I(to)) in normal and diseased myocardium. Journal of molecular and cellular cardiology. 2001 May;33(5):851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- 21.Patel SP, Campbell DL. Transient outward potassium current, 'Ito', phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. The Journal of physiology. 2005 Nov 15;569(Pt 1):7–39. doi: 10.1113/jphysiol.2005.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circulation research. 1993 Aug;73(2):379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 23.Tomita F, Bassett AL, Myerburg RJ, Kimura S. Diminished transient outward currents in rat hypertrophied ventricular myocytes. Circulation research. 1994 Aug;75(2):296–303. doi: 10.1161/01.res.75.2.296. [DOI] [PubMed] [Google Scholar]
- 24.Sanguinetti MC. When the KChIPs are down. Nature medicine. 2002 Jan;8(1):18–19. doi: 10.1038/nm0102-18. [DOI] [PubMed] [Google Scholar]
- 25.Lebeche D, Kaprielian R, del Monte F, Tomaselli G, Gwathmey JK, Schwartz A, et al. In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation. 2004 Nov 30;110(22):3435–3443. doi: 10.1161/01.CIR.0000148176.33730.3F. [DOI] [PubMed] [Google Scholar]
- 26.Lebeche D, Kaprielian R, Hajjar R. Modulation of action potential duration on myocyte hypertrophic pathways. Journal of molecular and cellular cardiology. 2006;40(5):725–735. doi: 10.1016/j.yjmcc.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annual review of physiology. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 28.Wickenden AD, Lee P, Sah R, Huang Q, Fishman GI, Backx PH. Targeted expression of a dominant-negative K(v)4.2 K(+) channel subunit in the mouse heart. Circulation research. 1999 Nov 26;85(11):1067–1076. doi: 10.1161/01.res.85.11.1067. [DOI] [PubMed] [Google Scholar]
- 29.Shorofsky SR, Aggarwal R, Corretti M, Baffa JM, Strum JM, Al-Seikhan BA, et al. Cellular mechanisms of altered contractility in the hypertrophied heart: big hearts, big sparks. Circulation research. 1999 Mar 5;84(4):424–434. doi: 10.1161/01.res.84.4.424. [DOI] [PubMed] [Google Scholar]
- 30.Sanguinetti MC. Reduced transient outward K+ current and cardiac hypertrophy: causal relationship or epiphenomenon? Circulation research. 2002 Mar 22;90(5):497–499. doi: 10.1161/01.res.0000014284.39141.8c. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen MB, Sosunov EA, Anyukhovsky EP, Ozgen N, Boyden PA, Rosen MR. Deleting the accessory subunit KChIP2 results in loss of I(to,f) and increased I(K,slow) that maintains normal action potential configuration. Heart Rhythm. 2009 Mar;6(3):370–377. doi: 10.1016/j.hrthm.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patberg KW, Obreztchikova MN, Giardina SF, Symes AJ, Plotnikov AN, Qu J, et al. The cAMP response element binding protein modulates expression of the transient outward current: implications for cardiac memory. Cardiovascular research. 2005;68(2):259–267. doi: 10.1016/j.cardiores.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, et al. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. American journal of physiology. 2006 Mar;290(3):C852–C861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang TT, Takimoto K, Stewart AF, Zhu C, Levitan ES. Independent regulation of cardiac Kv4.3 potassium channel expression by angiotensin II and phenylephrine. Circulation research. 2001 Mar 16;88(5):476–482. doi: 10.1161/01.res.88.5.476. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Kumar R, Wagner MB, Cheng J, Mishra M, Joyner RW. Regulation of transient outward current in human atrial myocytes by protein tyrosine kinase pathway. Journal of cardiovascular electrophysiology. 2002 Sep;13(9):927–935. doi: 10.1046/j.1540-8167.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 36.Petrashevskaya NN, Bodi I, Rubio M, Molkentin JD, Schwartz A. Cardiac function and electrical remodeling of the calcineurin-overexpressed transgenic mouse. Cardiovascular research. 2002 Apr;54(1):117–132. doi: 10.1016/s0008-6363(02)00241-9. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Kutschke W, Richardson KE, Karimi M, Hill JA. Electrical remodeling in pressure-overload cardiac hypertrophy: role of calcineurin. Circulation. 2001 Oct 2;104(14):1657–1663. doi: 10.1161/hc3901.095766. [DOI] [PubMed] [Google Scholar]
- 38.Zobel C, Kassiri Z, Nguyen TT, Meng Y, Backx PH. Prevention of hypertrophy by overexpression of Kv4.2 in cultured neonatal cardiomyocytes. Circulation. 2002 Oct 29;106(18):2385–2391. doi: 10.1161/01.cir.0000033970.22130.93. [DOI] [PubMed] [Google Scholar]
- 39.Kassiri Z, Zobel C, Nguyen TT, Molkentin JD, Backx PH. Reduction of I(to) causes hypertrophy in neonatal rat ventricular myocytes. Circulation research. 2002;90(5):578–585. doi: 10.1161/01.res.0000012223.86441.a1. [DOI] [PubMed] [Google Scholar]
- 40.Rossow CF, Dilly KW, Santana LF. Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circulation research. 2006;98(10):1306–1313. doi: 10.1161/01.RES.0000222028.92993.10. [DOI] [PubMed] [Google Scholar]
- 41.Rossow CF, Dilly KW, Yuan C, Nieves-Cintron M, Cabarrus JL, Santana LF. NFATc3-dependent loss of I(to) gradient across the left ventricular wall during chronic beta adrenergic stimulation. Journal of molecular and cellular cardiology. 2009;46(2):249–256. doi: 10.1016/j.yjmcc.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. NFATc3-induced reductions in voltage-gated K+ currents after myocardial infarction. Circulation research. 2004 May 28;94(10):1340–1350. doi: 10.1161/01.RES.0000128406.08418.34. [DOI] [PubMed] [Google Scholar]
- 43.Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, et al. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circulation research. 2008 Sep 26;103(7):733–742. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- 44.Sah R, Oudit GY, Nguyen TT, Lim HW, Wickenden AD, Wilson GJ, et al. Inhibition of calcineurin and sarcolemmal Ca2+ influx protects cardiac morphology and ventricular function in K(v)4.2N transgenic mice. Circulation. 2002 Apr 16;105(15):1850–1856. doi: 10.1161/01.cir.0000014211.47830.4d. [DOI] [PubMed] [Google Scholar]
- 45.Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, et al. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circulation research. 2008 Oct 10;103(8):845–854. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- 46.Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Circulation research. 1998 Sep 7;83(5):560–567. doi: 10.1161/01.res.83.5.560. [DOI] [PubMed] [Google Scholar]
- 47.Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circulation research. 2000 Jul 7;87(1):73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- 48.Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, et al. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circulation research. 2005 Dec 9;97(12):1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.