1. Introduction
Ca2+-calmodulin-dependent proteinkinase II (CaMKII) is a serine/threonine proteinkinase which phosphorylates several proteins of the excitation-contraction coupling [1–5]. CaMKII has also been shown to be involved in the pathogenesis of hypertrophy and heart failure [6]. Recent experiments from our laboratory revealed a dual role of CaMKII during the injury induced by ischemia/reperfusion (IR). It was shown that CaMKII plays a beneficial role in the reversible IR dysfunction, also known as stunned heart [7–9]. The protective role of CaMKII was mainly mediated by phosphorylation of the Thr17 site of phospholamban (PLN), the sarcoplasmic reticulum (SR) protein that regulates the function of SR-Ca2+-ATPase (SERCA2a). Phosphorylation of Thr17 at the onset of reperfusion was necessary to ameliorate Ca2+ mishandling and mechanical recovery in the stunned heart [8, 9]. However, if the ischemic period was extended, a detrimental effect of CaMKII activation became evident, manifested by an increase in apoptosis/necrosis and an impairment of contractile function, which were abrogated by CaMKII-inhibition [10, 11]. Pioneer studies by Zhu et al., [12] demonstrated that β1 adrenoceptor-induced apoptosis was independent of cAMP and PKA signaling but requires the involvement of a CaMKII-mediated cascade. It was further shown that overexpression of CaMKII results in a profound contractile impairment with major alterations in intracellular Ca2+ handling and pronounced increase in cell death [11, 13, 14]. Although experiments from our laboratory suggested a CaMKII-dependent SR-Ca2+ mishandling involved in the detrimental effect of IR injury [11], these experiments were performed in isolated myocytes subjected to simulated IR injury, an approach that can only partially mimic the IR process in the intact heart. Besides, the signalling pathways by which CaMKII activation produces apoptosis and necrosis during IR, remain unknown. The present experiments were undertaken to gain further insights into the mechanisms of the deleterious effects of CaMKII in the irreversible IR injury. It will be shown that two main players in the signalling cascade by which CaMKII mediates apoptosis and necrosis during IR are the SR and the mitochondria.
2. Materials and Methods
2.1. Animals
Experiments were performed in Wistar male rats (200–300g). A set of the experiments was performed in transgenic mice, (25–30g) expressing four concatenated repeats of the CaMKII autocamtide inhibitory peptide (AIP) selectively at the SR membranes (SR-AIP) [15]. Age-matched wild type mice (WT) served as controls. The mouse transgenic model was used to specifically test the role of CaMKII-dependent phosphorylations at the SR [15]. All animals used were maintained in accordance with the Guide for the Care and Use of Laboratory Animals [NIH Publication No. 85–23, revised 1996].
2.2. Langendorff perfusion and experimental protocol
Isolated hearts were perfused according to the Langendorff technique [2, 8]. Hearts were subjected to 45min of global ischemia followed by 120min of reperfusion. All drugs used were administrated 10min before ischemia and during the first 10min of reperfusion. Details of methods and the effect of the different treatments on basal contractility are provided in the Online Supplementary Data.
2.3. Infarct size
After reperfusion, infarct size was assessed by the triphenyltetrazolium chloride (TTC) technique.
2.4. LDH determination
Cardiac injury was evaluated by LDH released in the perfusion effluent during the first 10min of reperfusion.
2.5. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end (TUNEL) labeling
TUNEL assay was performed on myocardial slices fixed in buffered formalin and processed for histological examination.
2.6. Electrophoresis and Western blot analysis
Homogenates, cytosolic and mitochondrial fractions were prepared from the pulverized ventricular tissue of the perfused hearts [2, 7]. Proteins from cardiac homogenates were probed with antibodies raised against Ser16 and Thr17-phosphorylated PLN, total PLN, Ser2815 and Ser2809-phosphorylated ryanodine receptor (RyR2), total RyR2, active caspase-3, Bcl-2 and Bax. To assess cytochrome c release, mitochondria were separated from cytosol using the Cytochrome c Releasing Apoptotic assay Kit (Biovision Research Products, Mountain View, CA).
2.7. Cytochrome c oxidase activity and mitochondrial swelling
Mitochondrial cytochrome c oxidase activity was assayed with a commercial kit (Cytocox1, Sigma, St. Louis, Mo) according to manufacturer’s instructions. Mitochondrial swelling was determined by light scattering at 520nm in a spectrophotometer.
2.8. Statistical analysis
Data are expressed as mean ± SEM. Unpaired, paired Student t-test or ANOVA followed by Tukey post test were used for statistical comparisons when appropriate. Differences were considered significant at P<0.05.
3. RESULTS
3.1. Protective role of CaMKII inhibition in the irreversible IR injury
Figure 1 depicts the mechanical recovery and the degree of necrosis and apoptosis of rat hearts submitted to IR (45/120min) in the presence or absence of 2.5μM of the CaMKII inhibitor KN-93. Figure 1A shows that the contractile recovery significantly increased whereas the infarct size and necrosis, evaluated by TTC staining and LDH release, respectively, significantly decreased in the presence of CaMKII-inhibition. Figures 1B–C show that CaMKII-inhibition also diminished apoptosis, as evidenced by the decrease in TUNEL positive nuclei, the ratio of proapoptotic protein Bax vs. antiapoptotic protein Bcl-2 (Bax/Bcl-2) and the activation of caspase-3, the end effector of apoptosis. The protective action of CaMKII-inhibition was specific, since KN-92, the inactive analogue of KN-93, was unable to prevent the apoptotic effect and to diminish the infarct size produced by IR. In the presence of KN-92, TUNEL positive nuclei and infarct size were 3.45±0.8% of total nuclei number and 56.4±3.72 % of risk area, respectively, being the values in the absence of the drug 3.53±0.59 and 59.4±7.48%, respectively. Moreover, recovery of the contractile function after 120min of reperfusion was similarly depressed and not statistically different from the recovery in the absence of KN-92 (1.8±0.35 and 3.17± 0.51 mmHg, respectively, n=3–7). A direct inhibitory effect of KN-93 on ICa which may contribute to decrease apoptosis and necrosis independently of CaMKII, may be discarded from these results, since this effect of KN-93 on ICa is shared by its inactive analog KN-92 [16]. These results indicate the protective role of CaMKII inhibition in the irreversible IR injury, confirming previous findings from our own laboratory [11].
Figure 1. CaMKII inhibition increased contractile recovery and diminished necrosis and apoptosis in IR.
A. Left panel: Recovery of left ventricular developed pressure (LVDP) in hearts submitted to the IR (45/120min) protocol, in the presence and absence of CaMKII-inhibition (2.5μM KN-93 administrated 10min before ischemia and during the first 10min of reperfusion. n=4 hearts in each group. Right panel: KN significantly reduced infarct size and LDH release in hearts submitted to IR, indicating the prevention of IR-induced necrosis, n= 5–10 experiments. B. 2.5μM KN significantly reduced TUNEL positive cells (brown nuclei with arrow) after IR compared to hearts without KN-93. Bar represents 40μm. The bar graph on the right panel shows pooled data from these experiments, n=3–7. C. CaMKII-inhibition reduced Bax/Bcl-2 ratio and caspase-3 activity supporting the participation of CaMKII in the apoptotic pathway; n=4–5 experiments. *P<0.05 vs. IR in the absence of KN. D. Total PLN and phosphorylation of Thr17 site at 1–3 min of reperfusion; n= 5–9 experiments. *P<0.05 vs. preischemia (preisch).
In additional experiments we studied the time course of the phosphorylation of Thr17 site of PLN, a typical CaMKII substrate. Figure 1D shows that Thr17 phosphorylation increased at 1–3min of reperfusion. Phosphorylation of Thr17 site was transient, returning to control levels at 10min of reperfusion. Total PLN did not significantly change with respect to pre-ischemic values. Moreover, no significant changes in phosphorylation of Ser16 residue of PLN were observed (data not shown). These results are consistent with CaMKII activation at the onset of reperfusion.
3.2. NCX inhibition diminishes the activity of CaMKII and protects against the reperfusion damage
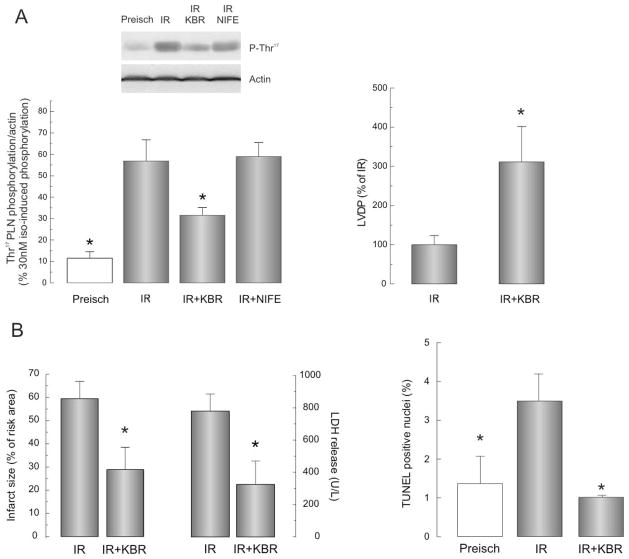
Two findings of the experiments described above revealed that the activity of CaMKII increases during reperfusion: a) There was a significant increase in the phosphorylation of Thr17 of PLN at the beginning of reperfusion; and b) Inhibition of CaMKII ameliorated the deleterious effect of IR. However, the mechanism(s) of CaMKII activation at the onset of reperfusion in irreversible IR injury, is/are not known. Since previous experiments indicated that the influx of Ca2+ during reperfusion or reoxygenation was primarily due to the reverse mode of the NCX [7, 17, 18], we explored the NCX as a possible source of Ca2+ influx involved in CaMKII activation. Figure 2A shows that inhibition of NCX by KBR produced a significant decrease in the phosphorylation of Thr17 of PLN. In contrast, inhibition of L-type Ca2+ channels with nifedipine failed to affect this phosphorylation. The decrease in Thr17 phosphorylation produced by KBR occurred in association with a significant increase in the mechanical recovery after the protocol of IR. Panel B, shows that NCX inhibition also decreased infarct size, LDH release and apoptosis produced by IR injury. Taken together these experiments indicate that the reverse NCX mode is a major pathway of Ca2+ influx upon reperfusion able to activate CaMKII. Thus, the NCX appears as the first step in the CaMKII cascade of events that produced cell death in IR
Figure 2.
A. CaMKII is activated by NCX-induced Ca2+ influx. KBR (5μM), but not nifedipine (0.4μM) prevented the CaMKII-dependent phosphorylation of Thr17 of PLN in hearts submitted to IR (left panel). NCX inhibition also significantly improved LVDP recovery (right panel). B. Inhibition of reverse NCX mode protected hearts submitted to IR from cell death. KBR-treated hearts also showed diminished infarct size and LDH release (left panel) and a decrease in the number of apoptotic cells (right panel). n=4–5 experiments * P<0.05 vs. IR.
3.3. The SR plays a major role in IR injury
To assess the role of the SR in the signalling cascade involved in the IR injury, we repeated the protocol of IR in the presence and absence of thapsigargin, to block SR Ca2+-ATPase (SERCA2a) and dantrolene to inhibit ryanodine and IP3 receptors. Figure 3 shows that both drugs decreased the infarct size (A) and apoptosis assessed by the TUNEL technique (B). These experiments support a crucial role of SR Ca2+ on the deleterious effect of IR, in agreement with previous results [11, 17, 19].
Figure 3. The SR participates in myocyte death induced by IR.
A. Inhibition of SERCA2a with thapsigargin (Thaps) and RyR2 with dantrolene (Dant) diminished the infarct size. Above: typical TTC staining for each situation; below: average of 4–5 experiments. B. Thaps and Dant significantly diminished apoptosis. Representative microphotographies of TUNEL stained sections. Bar represents 40μm. Below to the right: average values of 4–5 experiments. * P<0.05 vs. IR.
3.4. CaMKII-mediated SR phosphorylations are involved in IR injury
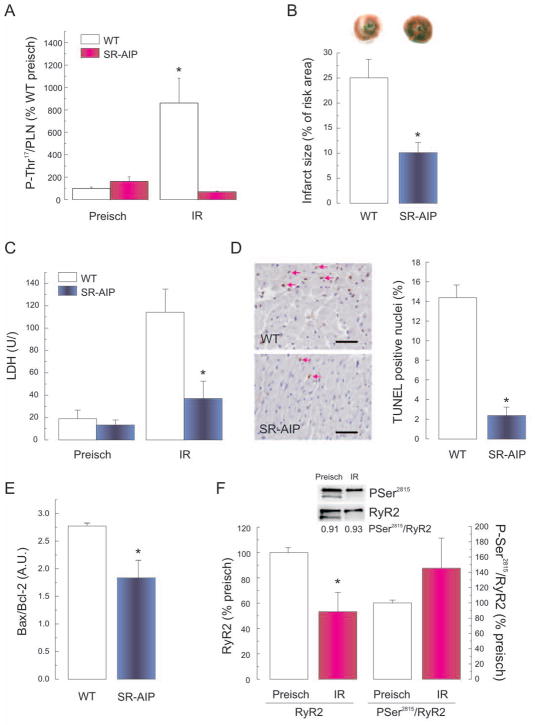
To further investigate whether the deleterious effect of CaMKII was due to a CaMKII-dependent phosphorylation of SR proteins, TG mice with CaMKII inhibition targeted to the SR (SR-AIP mice), were subjected to IR. Figures 4A shows that phosphorylation of Thr17 site of PLN significantly increased in WT mice with respect to pre-ischemic values, but did not change in SR-AIP mice, indicating that CaMKII-Thr17 pathway was highly suppressed. Figure 4B–E show that infarct size and LDH levels as well as TUNEL positive nuclei and Bax/Bcl-2 ratio were significantly lower in SR-AIP mice than in age matched controls. These experiments indicate that CaMKII-dependent phosphorylations at the SR participate in the cascade of events that leads to myocardial damage in IR injury.
Figure 4. The SR-CaMKII-dependent phosphorylations participate in IR injury.
(A) Increase in phosphorylation of Thr17 site of PLN at the onset of reperfusion (3min) in WT mice. This increase was not apparent in SR-AIP mice with CaMKII inhibition targeted to the SR. SR-AIP showed a significant decrease in infarct size (B), LDH release (C), TUNEL-positive cells (D), and Bax/Bcl-2 ratio (E). Bar in C represents 40μm. (n=3–6, * P<0.05 with respect to WT). F: The significant decrease in the expression of RyR2 at the onset of reperfusion (3min) in rat hearts was associated with a proportional decrease in Ser2815 phosphorylation such that the ratio Ser2815/RyR2 did not change significantly. [n=7–13, * P<0.05 with respect to preischemia (preisch)].
In an attempt to address the possible CaMKII-dependent phosphorylations involved, we explored two main SR proteins implicated in SR Ca2+ handling and substrates of CaMKII, PLN and RyR2. Figures 1D and 2A (rat) and 4A (WT-mice) showed that Thr17 of PLN was phosphorylated at the onset of reperfusion. In contrast, Figure 4F shows immunoblots and overall results indicating that the phosphorylation of Ser2815 of RyR2 significantly decreased at the onset of reperfusion (3min) in association with a proportional decrease in the amount of RyR2. Consequently, the ratio P-Ser2815/RyR2 did not change significantly. No changes in Ser2809/RyR2 phosphorylation were detected (not shown). These results suggested that the phosphorylation of RyR2 is not a good candidate to explain the CaMKII-dependence of the deleterious effects of CaMKII on IR.
3.5. The mitochondria as the final effector of CaMKII-induced apoptosis and necrosis in IR injury
Previous experiments showed a closed coupling between ER/SR Ca2+ release and mitochondrial Ca2+ uptake [20–23]. In the following group of experiments we first investigated the participation of mitochondria on IR injury and then the possible involvement of mitochondria in the CaMKII-mediated cascade of apoptosis/necrosis in IR. To address the first purpose, experiments were performed in the presence and absence of the mitochondrial Ca2+ uniporter inhibitor rutenium red (RR, 5μM) and the more specific one, Ru 360 (RU, 1μM). Figures 5A–B show that both RR and RU decreased the infarct size and the degree of apoptosis induced by IR. Moreover, high mitochondrial Ca2+ has been associated with the release of apoptogenic factors, like cytochrome c, through the opening of the mitochondrial permeability transition pore (mPTP) [24]. We therefore performed experiments in the presence of the mPTP inhibitor, cyclosporine A (0.2μM). Similarly to the inhibition of the mitochondrial Ca2+ uniporter, inhibition of mPTP resulted in amelioration of the infarct size and apoptosis (Figures 5A–B). Taken together, these experiments confirmed previous results regarding the participation of mitochondria in IR injury, delineating a cascade which involves mitochondrial Ca2+ uniporter and the mPTP. To study whether CaMKII was involved in the apoptotic effects of IR mediated by mitochondria, the release of cytochrome c was measured in the absence and presence of CaMKII-inhibition. Figure 5C shows that the release of cytochrome c by the mitochondria was significantly decreased in the presence of KN-93 compared to hearts submitted to IR in the absence of KN-93. These experiments support a key role of mitochondria in the deleterious cascade initiated by CaMKII activation.
Figure 5. Mitochondria is the final effector of CaMKII-dependent cell death.
Both, inhibition of mitochondrial Ca2+ uniporter with ruthenium red (RR) or Ru 360 (RU) and inhibition of the mPTP with cyclosporine A (CsA) diminished infarct size (A) and TUNEL positive cells (B), comparing with hearts without treatment. C. Release of cytochrome c from mitochondria to cytosol was significantly less after 45/120min IR in the presence of CaMKII inhibition with KN-93; n=3–6, *P<0.05 with respect to IR.
The prevention of both mitochondrial Ca2+ overload and activation of mPTP is critical to preserve mitochondrial integrity and to avoid irreversible cardiomyocyte damage. We therefore performed additional experiments to investigate whether CaMKII inhibition protects from mitochondrial damage following IR by measuring the activity of cytochrome c oxidase in the presence and absence of KN-93. Cytochrome c oxidase is integral to the inner mitochondrial membrane and therefore its activity is undetectable in intact mitochondria unless a detergent, e.g. n-dodecyl-β-maltoside, is added. Figure 6A shows a significant increase in the activity of cytochrome c oxidase unmasked by the detergent, suggesting that a higher number of intact mitochondria were isolated from hearts subjected to IR in the presence of KN-93. Another indicator of mitochondrial integrity is the tolerance to Ca2+-induced swelling due to the opening of mPTP. KN-93 produced a 2.5 fold decrease in the rate of mPTP-related mitochondrial swelling compared to IR in the absence of the inhibitor (Figure 6B). Cyclosporine A (CsA) decreased Ca2+-induced mitochondrial swelling both in the presence and absence of KN-93, indicating that the observed changes resulted from mPTP activity (Figure 6B). KN-93 did not affect the activity of cytochrome c oxidase or mitochondrial swelling in control hearts (data not shown). These results suggest that CaMKII inhibition prevented the assembly of the mPTP and preserved the integrity of mitochondria during IR. Collectively, these findings indicate that mitochondrial Ca2+ overload plays a role in the IR injury observed. More important to the aim of the present study, the results further show that cytochrome c release and Ca2+-induced mitochondrial swelling were both significantly diminished in the presence of CaMKII-inhibition (Figure 5C and 6B), pointing to the mitochondria as the final effector of the CaMKII-dependent signalling pathway leading to irreversible IR injury.
Figure 6. CaMKII-inhibition prevents mitochondria damage in IR.
A. Significant increase in cytochrome c oxidase activity unmasked by the detergent n-Dodecyl β-D-maltoside, indicating that a higher number of intact mitochondria were isolated from hearts subjected to IR in the presence of KN-93. B. Rate constant of mitochondrial swelling calculated from the exponential fitting of light scattering records like those shown in the right panel. (n= 4–6), *P<0.05 vs. IR.
3.6. The death receptor pathways of apoptosis
Caspase-8 activation is thought to be a major step in the extrinsic (death-receptor-dependent) apoptotic pathway [25]. Moreover, recent studies in cultured macrophages suggested a link among different apoptotic pathways evoked by ER-stress, in which CaMKII would play a pivotal role [26]. We therefore studied a possible participation of the extrinsic apoptotic pathway in the CaMKII-dependent-induced apoptosis/necrosis in IR, by exploring the activity of caspase-8 in the presence and absence of CaMKII-inhibition. It was found that the activity of caspase-8 was increased at the end of the reperfusion period. This increase could not be prevented however by KN-93 (IR: 218.1±19.8 vs. IR+KN: 199.1±23.0 expressed as % of the signal obtained in control hearts, not submitted to IR, n=5). These results suggest that the intrinsic mitochondrial pathway appears as the sole route toward apoptosis mediated by CaMKII in cardiac IR.
4. Discussion
The present results describe a cascade of events during IR that involves CaMKII and leads to necrotic and apoptotic cell death. Previous studies, including findings from our own laboratory, have related CaMKII to apoptosis and necrosis in the context of IR injury [10, 11]. However the signalling cascade involved in this effect was not previously assessed. Our results indicate that CaMKII does not participate in the extrinsic cascade of apoptosis but is involved in the intrinsic (mitochondrial) pathway. They further show that this signalling pathway involves sequential activation of the reverse NCX mode, CaMKII and the CaMKII-dependent phosphorylation of SR protein(s), mitochondria Ca2+ overload, cytochrome c release and caspase-3 activation. Interestingly, the cascade of events described mediates not only the programmed cell death known as apoptosis but also a CaMKII-dependent programmed necrosis.
4.1. NCX mediates the increase in CaMKII activity at the onset of reperfusion
The present study showed that in the irreversible IR injury, activation of CaMKII occurred at least in part by Ca2+ influx through the reverse NCX mode. Inhibition of this mode of the exchanger with KBR leads to a decrease in the infarct size, LDH release and the number of apoptotic cells, supporting a major role of the exchanger as a mechanism of cell death in IR. Different reports showed the beneficial effects produced by treatment with NCX inhibitors on ischemic/reperfused hearts [17, 18, 27]. However, the mechanisms underlying this cardioprotective effect remain unclear. It was proposed that during reperfusion, the Ca2+ influx through the NCX induces the release of Ca2+ from an overloaded SR. This effect would mediate the cytosolic Ca2+ oscillations responsible for reperfusion injury in myocytes [17, 27]. The present experiments add to this previous mechanism another deleterious pathway triggered by the NCX, i.e. the increase in the activity of CaMKII, which initiates the sequence of events that produce CaMKII-mediated apoptosis and necrosis in the irreversible IR injury.
4.2. CaMKII-dependent phosphorylations of SR are central to the mechanism of necrosis/apoptosis in IR injury
Our results showed the participation of the SR and CaMKII-dependent SR phosphorylations in the CaMKII-dependent IR-induced apoptotic and necrotic pathway, by two different and complementary approaches: 1. Pharmacological inhibition of SERCA2a (SR Ca2+ load) and RyR2 (SR Ca2+ release); and 2. IR protocols on transgenic mice with inhibition of CaMKII, targeted to the SR. The first approach points to the SR as a central player in the myocyte death pathway due to IR, in agreement with previous results that indicate that the SR is an integral component of inducible apoptosis in different pathological situations [28]. The second approach allows us to conclude that amelioration of the deleterious effect of IR is at least in part due to CaMKII-dependent phosphorylation of SR proteins. To the best of our knowledge, this is the first report clearly showing the importance of CaMKII-dependent phosphorylation of SR proteins not only in the apoptotic but also in the necrotic myocyte death of IR hearts.
Inhibition of CaMKII in the SR-AIP mice was targeted to the SR. However, it was found that these mice also present an inhibition of ICa facilitation [29], which may contribute to the decrease in apoptosis/necrosis observed in SR-AIP animals. Although the present findings cannot completely rule out a CaMKII-dependent increase in Ca2+ entry via ICa at the onset of reperfusion, this possibility would not oppose to the to the main role of CaMKII and the SR in the apoptotic and necrotic pathway of IR described in the present experiment. Moreover, a possible increase in ICa, although may favour an increase in SR Ca2+ load at the onset of reflow, would not contribute to CaMKII activation, since blockade of the L-type Ca2+ channels with nifedipine failed to prevent the increase in CaMKII activity which, in contrast, was prevented by KBR (Fig 2), at a concentration that does not affect basal myocardial contractility (See table 1 in supplement data).
4.2.a PLN as a possible participant in the deleterious effect mediated by CaMKII in IR
Although in the present study Thr17 site phosphorylation was used as a tool to examine CaMKII activation, owing to the fact the PLN is one of the major SR-Ca2+ handling proteins, it is tempting to speculate that phosphorylation of PLN might contribute to the deleterious effect of CaMKII in IR. Indeed, previous experiments support the relevance of CaMKII-dependent PLN phosphorylation in the damage caused by ischemia: AC3 transgenic mice expressing a CaMKII inhibitory peptide and submitted to myocardial infarction, showed a decreased number of apoptotic cells. This protection was absent in interbred AC3 mice with phospholamban-knockout (PLN−/−) mice [10]. Moreover, recent experiments performed in transiently transfected HEK 293 cells described that the antiapoptotic effect of the protein HAX-1 would be due to modulation of SERCA2a expression and ER Ca2+ levels [30]. Other studies, however, point to a beneficial rather than detrimental effect of PLN, particularly Thr17 site, in IR. As mentioned above, previous studies of our own laboratory have clearly demonstrated the importance of Thr17 phosphorylation on amelioration of Ca2+ mishandling during reperfusion after a short ischemic period [9]. It has also been shown that PKG-dependent PLN phosphorylation was cardioprotective in simulated IR in isolated myocytes [31]. More recent results further reveal that the beneficial effect of inhibitor-1 on IR injury occurs through the increase in Thr17 site of PLN [32]. Thus, the role of PLN phosphorylation on IR remains elusive. These controversial results seem not to arise from species differences, since most of the experiments mentioned above referred to rodents. As previously suggested, the final beneficial or detrimental outcome of PLN phosphorylation might tightly depend on the extent of Ca2+ uptake and SR Ca2+ load achieved during ischemia and at the onset of reperfusion. For instance, moderate increases in SR Ca2+ content have been associated with beneficial effects [32], whereas more important increases, as those expected in PLN−/− mice, were associated with detrimental actions [10].
4.2.b Possible role of RyR2 in the deleterious effect mediated by CaMKII in IR
RyR2 is also a substrate of CaMKII at Ser2815 residue. This phosphorylation has been associated with an increase in Ca2+ leak from the SR [33], which may favour mitochondrial Ca2+ overload (See below). Experiments wherein hearts were frozen at 1–3min of reperfusion, -the moment at which CaMKII appears to be active-, failed to show any significant increase in the phosphorylation of Ser2815 site of RyR2. Thus a role of phosphorylation of RyR2 in the CaMKII-cascade of necrosis and apoptosis in IR, is not supported by the present experiments. It should be noted, however, that RyR2 may contribute to this deleterious cascade by alternative mechanisms, unrelated to phosphorylation. For instance, earlier experiments described a decrease in RyR2 expression during cardiac ischemia, -similar to that observed by us at the onset of reperfusion-, associated with an increase in the rate of SR Ca2+ release. This paradoxical result was explained by an ischemic damage of RyR2 leading to an increase in the open probability and/or conductance of Ca2+-release channels [34], which might favour diastolic Ca2+ leak. Redox alterations at the onset of reperfusion might also influence the activity of RyR2 and SR Ca2+ leak [35]. Moreover, since acidosis is a main component of ischemia, an increase in SR Ca2+ leak at the beginning of reperfusion might also occur due to the relief of RyR2 from the previous inhibition exerted by acidosis [36]. The possible role of these putative mechanisms remains to be explored.
4.3. The mitochondria as the end effector of the CaMKII-dependent necrotic and apoptotic pathway
The results of the present experiments indicate in the first place that mitochondria, possibly by an increase in mitochondrial Ca2+ overload, are involved in the pathways of necrosis/apoptosis produced by IR. Secondly, and more important to the aim of the present manuscript, that mitochondria are involved in the CaMKII-dependent programmed pathway of cell death produced by IR. We showed that CaMKII-inhibition by KN-93 diminished: a. the release of cytochrome c, a mediator of the intrinsic (mitochondrial) apoptotic pathway, b. Ca2+-induced mitochondria swelling and c. LDH release, a marker of necrotic death. Reduction of SR Ca2+ loading with thapsigargin or of SR Ca2+ release with dantrolene, or inhibition of CaMKII-dependent phosphorylations at the SR level, also prevented myocyte death. The results thus suggest a close coupling between SR Ca2+ release and mitochondrial Ca2+ uptake in the CaMKII mediated apoptotic/necrotic pathway. The interplay between SR and mitochondria under different stimuli has been known for many years to be pivotal in triggering apoptotic signals [20, 37]. In line with this idea, a recent publication described a direct effect of SR on mPTP and cell death in intact cardiac myocytes in the context of IR [23].
Whereas both necrosis and apoptosis have been shown to contribute to cell death induced by myocardial IR, necrosis appears to be the main component of cell death at least immediately after reperfusion [38]. Our results showing a decrease in infarct size and LDH release in the presence of thapsigargin or dantrolene or in the AIP-transgenic mice indicated that similar conclusions to those discussed for the apoptotic cascade can be drawn for the necrotic pathway, underscoring the fact that the necrotic cell death is also mediated by CaMKII and includes the mitochondria as the final step. This striking finding is quite remarkable and is in line with recent reports challenging the concept of necrotic death as a chaotic unregulated process [39]. Finally, our results demonstrated that although activation of caspase-8 occurred during IR, CaMKII was not involved in the extrinsic apoptotic pathway.
4.4 Limitations of the study
The present experiments proposed alterations in Ca2+ handling at different intracellular levels. These conclusions were based on specific inhibition of the main proteins responsible for Ca2+ movements at these particular levels and not on direct measurements of intracellular Ca2+. Alterations of Ca2+ handling in line with those suggested in the present manuscript, have been previously documented [11, 17, 23]. The main objective of the present manuscript was to describe the signalling cascade that involves CaMKII activation and leads to apoptosis/necrosis, during IR.
Finally, this study was performed in the intact heart in ex vivo conditions. These conditions are more suitable to reproduce in vivo IR injury than isolated myocytes; we are aware however that they do not completely reproduce the in vivo situation. Nonetheless, it is expected that identification of a previously undefined cascade by which CaMKII leads to apoptosis and necrosis in IR injury under the controlled conditions of the present experiments, gives strong support to future research in vivo and in the clinical setting.
Supplementary Material
Acknowledgments
This work was supported by PICT 26117 (FONCyT), PIP 5300 and 02139 (CONICET) and FIRCA grant 5R03TW007713-02 to AM; NIH, HL26057, HL64018, LEDUCQ Foundation to EGK, Fondecyt 1080497 and 1080481 to PD and GS. The authors are grateful to Mónica Rando, Omar Castillo and Inés Vera for their skilled technical assistance.
Footnotes
Disclosure Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994;5:854–61. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 2.Mundiña-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem. 1996;271:33561–7. doi: 10.1074/jbc.271.52.33561. [DOI] [PubMed] [Google Scholar]
- 3.Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919–39. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- 4.Ferrero P, Said M, Sánchez G, Vittone L, Valverde C, Donoso P, et al. Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation. J Mol Cell Cardiol. 2007;43:281–91. doi: 10.1016/j.yjmcc.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonnell SM, García-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, et al. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102:e65–72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476–86. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Vittone L, Mundiña-Weilenmann C, Said M, Ferrero P, Mattiazzi A. Time course and mechanisms of phosphorylation of phospholamban residues in ischemia-reperfused rat hearts. Dissociation of phospholamban phosphorylation pathways. J Mol Cell Cardiol. 2002;34:39–50. doi: 10.1006/jmcc.2001.1488. [DOI] [PubMed] [Google Scholar]
- 8.Said M, Vittone L, Mundina-Weilenmann C, Ferrero P, Kranias EG, Mattiazzi A. Role of dual-site phospholamban phosphorylation in the stunned heart: insights from phospholamban site-specific mutants. Am J Physiol. 2003;285:H1198–205. doi: 10.1152/ajpheart.00209.2003. [DOI] [PubMed] [Google Scholar]
- 9.Valverde CA, Mundiña-Weilenmann C, Reyes M, Kranias EG, Escobar AL, Mattiazzi A. Phospholamban phosphorylation sites are necessary for the recovery of intracellular Ca2+ in the stunned heart of rodents. Cardiovasc Res. 2006;70:335–45. doi: 10.1016/j.cardiores.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Zhu WZ, Joiner ML, Zhang R, Oddis CV, Hou Y, et al. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am J Physiol. 2006;291:H3065–75. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 11.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, et al. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73:689–98. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:597–600. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKII deltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–11. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J Biol Chem. 2007;282:10833–9. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]
- 15.Ji Y, Li B, Reed TD, Lorenz JN, Kaetzel MA, Dedman JR. Targeted inhibition of Ca2+/calmodulin-dependent proteinkinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J Biol Chem. 2003;278:25063–71. doi: 10.1074/jbc.M302193200. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Blair LAC, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: Reversible inhibition of L-type calcium channels. Biochem Biophys Res Com. 2006;345:1606–10. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 17.Schäfer C, Ladilov Y, Inserte J, Schäfer M, Haffner S, Garcia-Dorado D, et al. Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovasc Res. 2001;51:241–50. doi: 10.1016/s0008-6363(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 18.Eigel BN, Gursahani H, Hadley RW. Na+/Ca2+ exchanger plays a key role in inducing apoptosis after hypoxia in cultured guinea pig ventricular myocytes. Am J Physiol. 2004;287:H1466–75. doi: 10.1152/ajpheart.00874.2003. [DOI] [PubMed] [Google Scholar]
- 19.Siegmund B, Schlack W, Ladilov JV, Balser C, Piper MH. Halothane Protects Cardiomyocytes Against Reoxygenation-Induced Hypercontracture. Circulation. 1997;96:4372–79. doi: 10.1161/01.cir.96.12.4372. [DOI] [PubMed] [Google Scholar]
- 20.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–6. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 21.García-Pérez C, Hajnóczky G, Csordás G. Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. J Biol Chem. 2008;283:32771–80. doi: 10.1074/jbc.M803385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, et al. Ca(2+) transfer from the ER to mitochondria: When, how and why. Biochim Biophys Acta. 2009;1787:1342–51. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz-Meana M, Abellán A, Miró-Casas E, Agulló E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. Am J Physiol Heart Circ Physiol. 2009;297:H1281–9. doi: 10.1152/ajpheart.00435.2009. [DOI] [PubMed] [Google Scholar]
- 24.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 25.Kruidering M, Evan GI. Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life. 2000;50:85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- 26.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–41. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inserte J, Garcia-Dorado D, Ruiz-Meana M, Padilla F, Barrabés JA, Pina P, et al. Effect of inhibition of Na(+)/Ca(2+) exchanger at the time of myocardial reperfusion on hypercontracture and cell death. Cardiovasc Res. 2002;55:739–48. doi: 10.1016/s0008-6363(02)00461-3. [DOI] [PubMed] [Google Scholar]
- 28.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–80. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 29.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007;42:196–205. doi: 10.1016/j.yjmcc.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vafiadaki E, Arvanitis DA, Pagakis SN, Papalouka V, Sanoudou D, Kontrogianni, et al. The anti-apoptotic protein HAX-1 interacts with SERCA2 and regulates its protein levels to promote cell survival. Mol Biol Cell. 2009;20:306–18. doi: 10.1091/mbc.E08-06-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdallah Y, Gkatzoflia A, Pieper H, Zoga E, Walther S, Kasseckert S, et al. Mechanism of cGMP-mediated protection in a cellular model of myocardial reperfusion injury. Cardiovasc Res. 2005;66:123–31. doi: 10.1016/j.cardiores.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–20. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, et al. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res. 2006;98:235–44. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 34.Domenech RJ, Sánchez G, Donoso P, Parra V, Macho P. Effect of tachycardia on myocardial sarcoplasmic reticulum and Ca2+ dynamics: a mechanism for preconditioning? J Mol Cell Cardiol. 2003;35:1429–37. doi: 10.1016/j.yjmcc.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo C, Bull R, Behrens MI, Donoso P. Redox regulation of RyR-mediated Ca2+ release in muscle and neurons. Biol Res. 2004;37:539–52. doi: 10.4067/s0716-97602004000400007. [DOI] [PubMed] [Google Scholar]
- 36.Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, et al. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent proteinkinase II. Am J Physiol. 2008;295:H1669–83. doi: 10.1152/ajpheart.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–17. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 38.Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 39.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.