Abstract
Osteocytes compose 90–95% of all bone cells and are the mechanosensors of bone. In this study, the strain experienced by individual osteocytes resulting from an applied fluid flow shear stress was quantified and correlated to two biological responses measured in real-time within the same individual osteocytes: 1) the upregulation of intracellular calcium and 2) changes in intracellular nitric oxide. Osteocyte-like MLO-Y4 cells were loaded with Fluo-4 AM and DAR-4M and exposed to uniform laminar fluid flow shear stresses of 2, 8, or 16 dynes/cm2. Intracellular calcium and nitric oxide changes were determined by measuring the difference in fluorescence intensity from the cell’s basal level prior to fluid flow and the level immediately following exposure. Individual cell strains were calculated using digital image correlation. MLO-Y4 cells showed a linear increase in cell strain, intracellular calcium concentration, and nitric oxide concentration with an increase in applied fluid flow rate. The increase in intracellular calcium was well correlated to the strain that each cell experienced. This study shows that osteocytes exposed to the same fluid flow experienced a range of individual strains and changes in intracellular calcium and nitric oxide concentrations, and the changes in intracellular calcium were correlated with cell strain. These results are among the first to establish a relationship between the strain experienced by osteocytes in response to fluid flow shear and a biological response at the single cell level. Mechanosensing and chemical signaling in osteocytes has been hypothesized to occur at the single cell level, making it imperative to understand the biological response of the individual cell.
Keywords: osteocytes, calcium, strain, shear stress, fluid flow
Introduction
Bone is known to adapt to its loading conditions via modeling and remolding. Osteocytes comprise 90–95% of all bone cells (Parfitt, A. M. 1977) and function as the mechanosensors of bone (Bonewald, L. F. 2006). They are located in the mineralized bone matrix within cave-like structures called lacunae. Extending from the lacunae is a network of canaliculi, which contain the extensive number of cell processes of the osteocytes. Through the establishment of this complex network of caves and canals osteocytes become ideally situated to sense the presence or absence of bone loading and respond by sending signals to the bone-forming osteoblasts and the bone-absorbing osteoclasts, thereby orchestrating the bone remodeling process. The application of force to the skeletal system produces several potential stimuli for osteocytes. Bone loading induces fluid flow and changes in hydrostatic pressure within the interstitial lacunar-canalicular network, while fluid flow across and around cells induces shear stresses. Bone tissue strain can also be transmitted to embedded cells directly though cellular adhesions and attachments, stretching and deforming the cells. Osteocytes have been shown to respond biologically to both strain via direct mechanical stimulation through membrane/cell stretching, and shear induced by fluid flow (Kamioka, H., Miki, Y., Sumitani, K., Tagami, K., Terai, K., Hosoi, K. and Kawata, T. 1995; Klein-Nulend, J., Semeins, C. M., Ajubi, N. E., Nijweide, P. J. and Burger, E. H. 1995; Klein-Nulend, J., van der Plas, A., Semeins, C. M., Ajubi, N. E., Frangos, J. A., Nijweide, P. J. and Burger, E. H. 1995; Turner, C. H. and Pavalko, F. M. 1998; Burger, E. H. and Klein-Nulend, J. 1999; Nakamura, T. 1999; Cheng, B., Zhao, S., Luo, J., Sprague, E., Bonewald, L. F. and Jiang, J. X. 2001; Bonewald, L. F. 2002; Bonewald, L. F. 2004; Mullender, M., El Haj, A. J., Yang, Y., van Duin, M. A., Burger, E. H. and Klein-Nulend, J. 2004; Kamioka, H., Sugawara, Y., Murshid, S. A., Ishihara, Y., Honjo, T. and Takano-Yamamoto, T. 2006; Rubin, J., Rubin, C. and Jacobs, C. R. 2006; Vatsa, A., Mizuno, D., Smit, T. H., Schmidt, C. F., MacKintosh, F. C. and Klein-Nulend, J. 2006; Vezeridis, P. S., Semeins, C. M., Chen, Q. and Klein-Nulend, J. 2006; Vatsa, A., Smit, T. H. and Klein-Nulend, J. 2007).
The strain induced upon osteocytes by shear applied via fluid flow has yet to be quantified and associated with any resulting biological responses. However, fluid flow has been shown to rapidly increase intracellular calcium and nitric oxide levels in bone cells (Klein-Nulend, J., Semeins, C. M. et al. 1995; Smalt, R., Mitchell, F. T., Howard, R. L. and Chambers, T. J. 1997; Ajubi, N. E., Klein-Nulend, J., Alblas, M. J., Burger, E. H. and Nijweide, P. J. 1999; Donahue, S. W., Jacobs, C. R. and Donahue, H. J. 2001; Reilly, G. C., Haut, T. R., Yellowley, C. E., Donahue, H. J. and Jacobs, C. R. 2003; Mullender, M., El Haj, A. J. et al. 2004; Bacabac, R. G., Smit, T. H., Mullender, M. G., Van Loon, J. J. and Klein-Nulend, J. 2005; Kamioka, H., Sugawara, Y. et al. 2006; Mullender, M. G., Dijcks, S. J., Bacabac, R. G., Semeins, C. M., Van Loon, J. J. and Klein-Nulend, J. 2006; Vatsa, A., Mizuno, D. et al. 2006; Vezeridis, P. S., Semeins, C. M. et al. 2006; Genetos, D. C., Kephart, C. J., Zhang, Y., Yellowley, C. E. and Donahue, H. J. 2007; Tan, S. D., de Vries, T. J., Kuijpers-Jagtman, A. M., Semeins, C. M., Everts, V. and Klein-Nulend, J. 2007). In a 2006 study by Kamioka et al., it was suggested that the calcium response of bone cells under fluid flow varied in response to the number of cell adhesions. However, this relationship could be more directly related to the actual strains experienced by the individual bone cells as a result of the integrity of these adhesions. It is possible that the more tightly bound a cell is to the substrate, the less strain and deformation the cell will experience in response to fluid shear stress. In this study, the real-time upregulation of intracellular calcium and nitric oxide levels within individual osteocytes in response to an applied fluid flow was examined. The resulting imposed strain of each of the osteocytes was also quantified and correlated to a biological response.
Materials and Methods
Cell culture
Osteocyte-like MLO-Y4 cells were cultured on type I rat tail collagen (Becton, Dickinson and Company, Franklin Lakes, NJ) coated on 100mm dishes in α–minimal essential medium (3MEM) (GIBCO, Grand Island, NY) supplemented with 2.5% fetal bovine serum (FBS) (Summit Biotechnology, Fort Collins, CO), 2.5% calf serum (CS) (HyClone Laboratories, Logan, UT), and 1% penicillin and streptomycin (PS) (Cellgro, Manassas, VA). Cells were maintained at 37°C and 5% CO2 in a humidified incubator and not allowed to exceed 70–80% confluency in order to maintain the dendritic characteristics of the cell line. Forty-eight hours prior to the fluid flow experiment, cells were harvested using 0.25% trypsin (Sigma-Aldrich, St. Louis, MO) and 0.1% ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, St. Louis, MO) in phosphate buffered saline (PBS) (GIBCO, Grand Island, NY) and cultured on type I rat tail collagen-coated 40 mm diameter glass slides at 70–80% confluency (Bioptechs Inc., Butler, PA) as described above.
Intracellular calcium and nitric oxide
To visualize changes in the intracellular calcium concentration, a cell membrane permeable fluorescein dye, Fluo-4 acetoxymethyl ester (Fluo-4 AM; Molecular Probes Inc., Eugene, OR, USA) was used. A cell-permeable diaminorhodamine-4M acetoxymethyl ester dye (DAR-4M AM; EMD Chemicals Inc., San Diego, CA, USA) was used to monitor changes in inracellular nitric oxide concentrations. After rinsing away the culture medium with PBS, slides containing 70–80% confluent MLO-Y4 cells were incubated for 30 minutes at 37°C with 5 μM solutions of Fluo-4 AM ester and DAR-4M AM ester in culture media. After incubation, the cells were washed three times with PBS and incubated for an additional 30 minutes to allow for de-esterification of the intracellular AM esters, rendering the fluorescent dye membrane impermeable. It should be noted that all steps involving the fluorescent dyes were conducted in the dark or with as little exposure to light as possible.
Changes in intracellular calcium and nitric oxide levels were determined by measuring changes in the fluorescent intensity of individual cells loaded with both Fluo-4 AM and DAR-4M AM. The excitation and emission wavelengths of Fluo-4 AM are 494 and 516nm respectively, and a fluorescein isothiocyanate (FITC) filter was used to capture these images. For DAR-4M AM excitation and emission wavelengths were 560 and 575 nm, respectively, and a rhodamine filter set was used. Stabilized basal level intracellular calcium and nitric oxide concentration fluorescence levels were captured as a control immediately before exposure of the cells to fluid flow. Immediately following the application of fluid flow over the cells, a second image was taken. For both the calcium and nitric oxide indicators, the difference between the average fluorescence in regions of interest (ROIs) of the stimulated cell images (F) and the same ROIs in the background fluorescence images (Fo) were calculated. In order to take into account varying basal intracellular calcium and nitric oxide levels, the fluorescence intensity increase was calculated with respect to the individual cell intensities prior to exposure to the fluid flow. Results are presented as a percentage change in fluorescence intensity for each individual cell ((F−Fo)/Fo).
Fluid flow
For the application of fluid flow to the cells, a closed system, parallel plate, live-cell micro-observation chamber (Focht Chamber System 2, Bioptechs Inc., Butler, PA) was utilized, as it provided a well established laminar flow region (Focht, D. C. 1996) (Figure 1). The chamber was mounted on the stage of an inverted microscope (Nikon Eclipse TE2000-E, Nikon Instruments Inc., Melville, NY) to allow real-time visualization of the cells exposed to flow. The microscope was fully automated with motorized shutters for both transmission illumination and reflected illumination. It had a motorized analyzer and a motorized six-filter cube cassette, which allowed switching between brightfield, DIC, and multi-color fluorescence imaging automatically. The microscope was equipped with a monochrome cooled CCD camera (Coolsnap ES, Photometrics, Tucson, AZ) which was used for the brightfield, DIC, and epi-fluorescence imaging. The glass slides seeded with the Fluo-4 AM and DAR-4M AM-loaded MLO-Y4 cells were placed inside the flow chamber, and a silicon gasket with a 14 mm × 22 mm × 1 mm rectangular cut-out for the region of flow was placed atop the slide. The slide and gasket were covered in flow media, and the chamber was sealed. The flow media consisted of 3MEM supplemented with 1% FBS, 1% CS, and 1% PS. Utilizing a peristaltic pump (Masterflex, Cole-Parmer, Vernon Hills, IL), slides of cells were exposed to laminar fluid flow rates resulting in shear stresses of 2, 8, or 16 dynes/cm2. Each glass slide was exposed to a single fluid flow rate and only one region of cells was imaged per slide. By taking this approach, effects such as cell desensitization to fluid flow were avoided.
Figure 1.
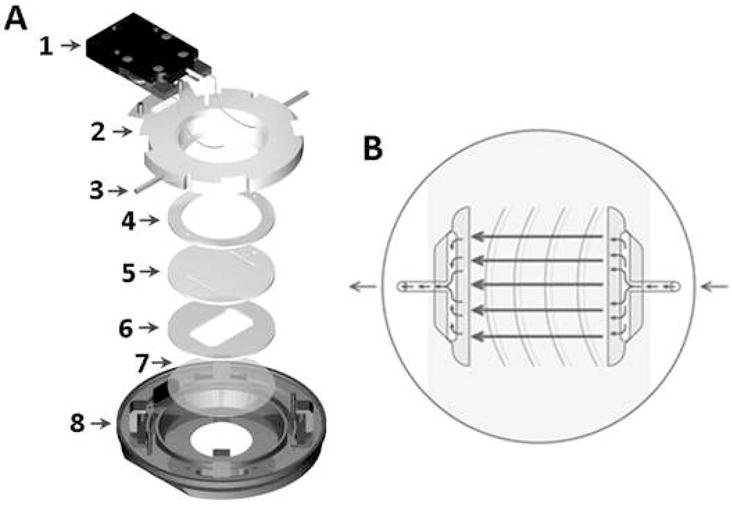
For the application of fluid flow to the cells, a closed system, parallel plate, live-cell micro-observation chamber (Focht Chamber System 2, Bioptechs Inc., Butler, PA) was utilized. (A) Exploded view of the chamber: 1-heater, 2-upper half of the chamber, 3-perfusion tubes, 4-upper gasket, 5-microaqueduct slide, 6-lower gasket, 7-coverslip, 8-lower half of the chamber which locks to the microscope stage. (B) Illustration of the microaqueduct slide perfusion technique with the laminar flow region designated by arrows.
Cell strain
Differential interference contrast (DIC) images were captured prior to and immediately following the application of fluid flow. The strain experienced by the osteocytes was determined by quantifying the deformation of each individual cell utilizing an optical strain measurement method, called microdisplacements by machine vision photogrammetry (DISMAP) (Nicolella, D. P., Nicholls, A. E., Lankford, J. and Davy, D. T. 2001). In this method, digital image correlation is utilized to calculate the strain from the displacement vectors of points selected by the operator. We chose four points per cell body and the strain was calculated as an average strain for each cell body.
Statistical analysis
For statistical comparisons, the Student’s t-test for unpaired samples assuming unequal variances was used. Probability levels of p <0.05 were considered significant. All statistics and additional linear regressions and correlations were performed using statistical analysis software (Statistica, Statsoft, Tulsa, OK).
Results
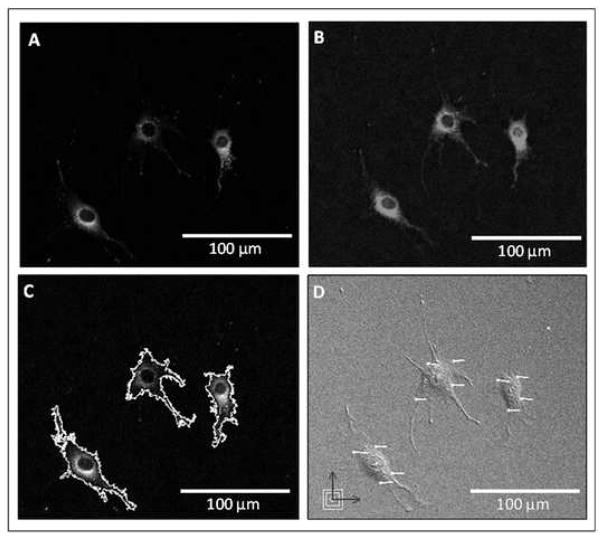
Osteocyte-like MLO-Y4 cells seeded on collagen-coated glass slides were imaged prior to and immediately following exposure to laminar fluid flow resulting in shear stresses of 2, 8, and 16 dynes/cm2. The field of view for each glass slide was randomly selected from the laminar flow region and all viable cells within the field were analyzed. The upregulation of intracellular calcium levels, nitric oxide levels, and average cellular strains were calculated for a total of 96 different individual cells exposed to fluid flow of varying rates (Figure 2). Prior to and following exposure to fluid flow, intracellular calcium and nitric oxide were observed to be localized to both the cell body and cell processes of the MLO-Y4 cells.
Figure 2.
Utilizing fluorescent microscopy, (A) intracellular calcium and (B) nitric oxide levels were imaged in MLO-Y4 cells prior to and then immediately following the initiation of fluid flow over the cells. (C) ROIs were chosen for each cell, and the changes in calcium and nitric oxide levels relative to basal levels were determined. (D) DIC images were also captured before and immediately after the application of fluid flow, and average cell body strains were calculated from strain vectors using DISMAP.
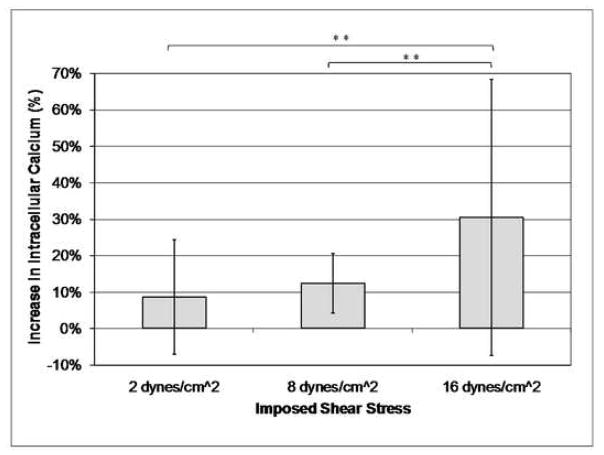
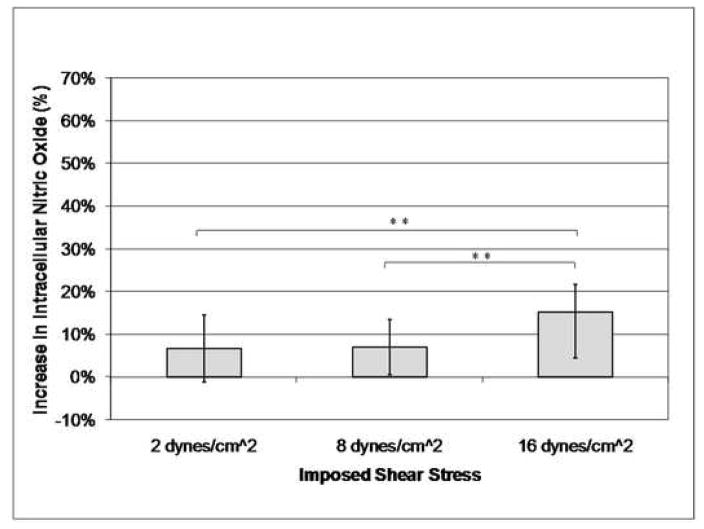
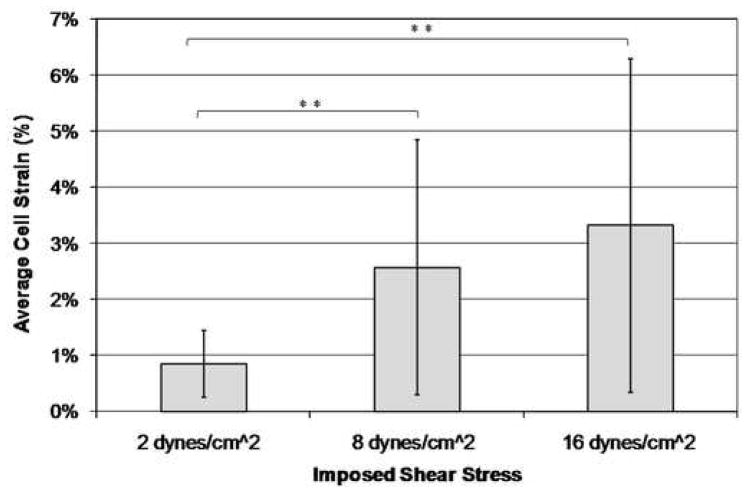
The osteocyte-like MLO-Y4 cells experienced a linear increase in intracellular calcium and nitric oxide concentration with increasing imposed shear stress due to laminar fluid flow exposure (Table 1, Figures 3 and 4). There was also a linear increase in the average strain experienced by the cell body of each cell with increasing imposed shear stress levels (Figure 5). A wide range of strains and changes in intracellular calcium and nitric oxide levels were experienced by the cells, even though the cells were subjected to the same global shear induced strain. However, significant differences between each of the three shear stress flow rates were found for changes in intracellular calcium levels, intracellular nitric oxide levels, and average cell body strain.
Table 1.
Average values of intracellular calcium and nitric oxide increase, and cell body strain for different applied shear rates.
| Average | Standard Deviation | ||
|---|---|---|---|
|
2 dynes/cm2 n=11 |
Increase in Calcium | 9.34% | 15.70% |
| Increase in Nitric Oxide | 8.08% | 7.87% | |
| Average Cell Body Strain | 0.85% | 0.60% | |
|
8 dynes/cm2 n=45 |
Increase in Calcium | 12.18% | 8.23% |
| Increase in Nitric Oxide | 7.46% | 6.53% | |
| Average Cell Body Strain | 2.57% | 2.28% | |
|
16 dynes/cm2 n=40 |
Increase in Calcium | 33.34% | 37.94% |
| Increase in Nitric Oxide | 14.41% | 10.71% | |
| Average Cell Body Strain | 3.32% | 2.97% |
Figure 3.
Increasing imposed shear stress results in an increase in osteocyte intracellular calcium levels (**p < 0.05, error bars show the standard deviation).
Figure 4.
Increasing imposed shear stress results in an increase in osteocyte intracellular nitric oxide levels (**p < 0.05, error bars show the standard deviation).
Figure 5.
Increasing imposed shear stress results in an increase in the average osteocyte cell body strain (**p < 0.05, error bars show the standard deviation).
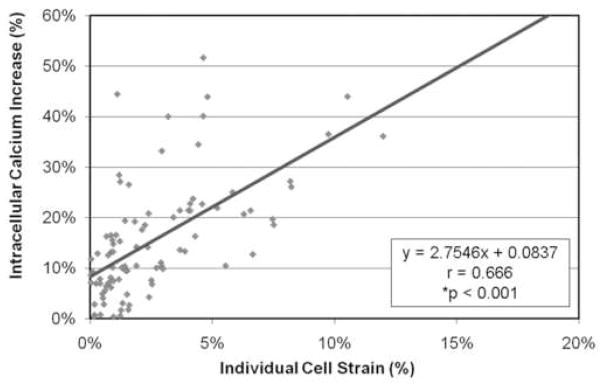
There was a significant correlation between the increase in intracellular calcium concentration and the average osteocyte cell strain in response to fluid flow for each of the imposed shear stress flow rates. With increasing cell strain, there was a related increase in intracellular calcium levels. When the results for the cells of each of the flow rates were combined, the significant correlation remained, regardless of the level of induced shear stress (Figure 6). However, there was not a significant relationship between the increase in intracellular nitric oxide levels and average cell body strain (Figure 7).
Figure 6.
The osteocytes showed an increase in intracellular calcium concentration with an increase in cell strain in response to fluid flow regardless of the induced shear stress.
Figure 7.
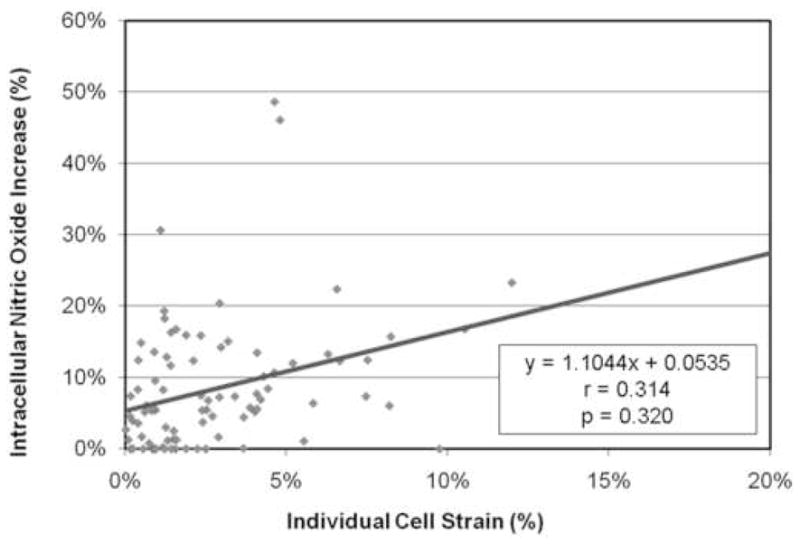
The osteocytes showed a slight increase in intracellular nitric oxide concentration with increasing cell strain, however there was not a significant correlation between the two.
Discussion
The purpose of this study was to measure both the real-time changes in intracellular calcium and nitric oxide levels and the mechanical strain in individual osteocyte-like MLO-Y4 cells exposed to a laminar fluid flow field. Interestingly, cells exposed to the same fluid flow experienced a wide range of strains and changes in intracellular calcium and nitric oxide concentrations, suggesting that strain at the cell level is influenced by more than just the globally applied shear rate. This finding highlights the importance of knowing the strain experienced by a single cell when trying to predict or elicit a strain-sensitive biologic response as each cell will respond differently based upon differences in the actual strain they perceive in response to the same globally applied force. Mechanosensing and chemical signaling in osteocytes has been hypothesized to occur at the single cell level, making it imperative to understand the biological response of the individual cell (Burger, E. H. and Klein-Nulend, J. 1999).
A limitation of this study was the fidelity of the captured images of the cell populations, and the resulting ability to analyze the cellular strains. Because of the desire to capture the responses of a population of cells to the imposed fluid flow rates, and therefore have a higher sample size, the ability to calculate the strains within the cell were limited to that of the average strain experienced by the cell body. It has been hypothesized that it is the cell processes of the osteocyte that may be responsible for its mechanotransduction capabilities (Aonuma, Y., Adachi, T., et al. 2007). Future studies are planned to analyze the strain and calcium and nitric oxide levels in the cell processes as well using higher resolution images.
Similar to other studies in the literature, the osteocyte-like MLO-Y4 cells in this study were found to increase their intracellular calcium levels in response to shear stress induced via fluid flow (Ajubi, N. E., Klein-Nulend, J. et al. 1999; Reilly, G. C., Haut, T. R. et al. 2003); however, this increase was not uniform across all cells in a given experiment. A significant correlation was found between cell strain and changes in intracellular calcium levels, with larger cell strains or deformations leading to increased intracellular calcium levels. These results may indicate that intracellular calcium levels and downstream signaling pathways such as prostaglandin E2 (PGE2) release are strain dependent, and do not rely simply upon the sensation of fluid flow to elicit a biologic response.
Utilizing the significant positive linear relationship between increasing cell strain and the resulting increase in intracellular calcium, more accurate predictions can made with regard to the biological response of osteocytes to imposed mechanical stimuli. Fluid flow imposed shear stress results in a range of strains and biological responses for the perturbed population of osteocytes. If the resulting individual cell strain is known, the biological response can be more closely predicted.
A possible explanation for the range of strains and intracellular calcium responses experienced by the osteocytes, other than general biological variation, includes a varying number of integrin-based cell adhesions between the osteocytes and the collagen-coated glass slide substrate. Cells with more attachments to the substrate are likely more tightly bound to the surface and therefore will likely have a more stable base from which to resist the surface shear stresses created by the fluid flow. If a cell has fewer attachments and is less tightly adhered to the substrate, the cell will be more ‘fluid-like’ and will deform to a larger degree in response to the applied shear via the fluid flow. Thus, we predict that among cells exposed to a given shear stress, those that are most tightly bound to the substrate will experience the least amount of cell strain and the smallest strain-sensitive biological response, while those least tightly adhered would experience the greatest amount of strain and the largest strain-sensitive biological response. This implicates the cell’s interaction with its environment as an integral component of its ability to sense and respond to strain. The cell must be firmly attached or embedded enough to be anchored and able to be deformed, but not so well attached/embedded that deformation is not possible. It follows that an environment and or interaction that allows for more deformation will result in greater mechanosensitivity. In the case of the osteocyte, adhesions between the cell and both the lacunae and canaliculi as well as the material properties of the perilacunar matrix are likely to modulate the ability of the cell to be deformed and thus its ability to respond to strain and function as mechanosensors (Bonivtch, A. R., Bonewald, L. F. and Nicolella, D. P. 2007). The strength of osteocyte adhesion could be related to the number and/or type of integrin-mediated adhesions to the lacunae and canaliculi. If the number of cellular adhesions and integrins are changed in a disease state or during the aging process, for example, such changes in how the osteocyte interacts with its environment, might explain a loss or gain in mechanosensitivity and a subsequent change in bone remodeling or a changing of the set point for maintaining bone homeostasis.
In this study, nitric oxide production in MLO-Y4 cells, a parameter for bone cell activation, was found to be dependent on the fluid shear stress rate. These results are consistent with in vitro studies previously published in the literature. Bacabac et al. illustrated using the MC3T3-E1 cell line that nitric oxide levels in osteoblastic cells are shear stress dependent (Bacabac, R. G., Smit, T. H., Mullender, M. G., Dijcks, S. J., Van Loon, J. J. and Klein-Nulend, J. 2004; Bacabac, R. G., Smit, T. H. et al. 2005). These results were further confirmed in the same cell line using pulsatile fluid flow by Mullender et al. (Mullender, M. G., Dijcks, S. J. et al. 2006). Primary osteocytes, isolated from fetal chicken calvariae, were also shown to be responsive to pulsating fluid flow shear stress, upregulating nitric oxide production and the downstream inhibition of osteoclast formation and bone resorption (Klein-Nulend, J., Semeins, C. M. et al. 1995; Vezeridis, P. S., Semeins, C. M. et al. 2006; Tan, S. D., de Vries, T. J. et al. 2007).
In the present study, nitric oxide was not found to be cell strain dependent. Others have published similar findings showing an increase in nitric oxide in response to shear stress induced via fluid flow in vitro but no nitric oxide response when subjected to unidirectional linear substrate strains in osteoblastic rat calvarial and long bone cells, MC3T3-E1, UMR-106-01, and ROS 17/2.8 cells (Smalt, R., Mitchell, F. T., Howard, R. L. and Chambers, T. J. 1997; Smalt, R., Mitchell, F. T. et al. 1997). More recently, MLO-Y4 cells have been shown to increase their nitric oxide production in response to perturbations with a microneedle (Vatsa, A., Mizuno, D. et al. 2006; Vatsa, A., Smit, T. H. et al. 2007). This type of stimulation is different than cell deformation induced by fluid flow as thedeformation is concentrated in a single point and location within the cell and may result in the excitation of distinct signaling cascades due to local cytoskeletal strain concentrations. Additional studies investigating the real-time nitric oxide response of individual MLO-Y4 cells to global cellular deformation via substrate stretching are planned.
In summary, we have measured real-time biological responses of individual osteocyte-like cells to the shear stress induced by uniform laminar flow and correlated these responses to the amount of strain specifically experienced by each cell. Intracellular calcium, nitric oxide, and strain were all found to increase when cells were exposed to varying flow rates resulting in increasing shear stress. Furthermore, an increase in cellular strain was shown to be significantly correlated to an increase in intracellular calcium levels of single osteocytes, thereby providing the first evidence that some biological responses elicited by fluid flow are due, in part, to sensation of cell strain rather than solely the sensation of fluid flow.
Acknowledgments
This research was funded by NIH/NIAMS P01 AR046798.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amber L. Rath, Email: arath@wfubmc.edu.
Lynda F. Bonewald, Email: bonewaldl@umkc.edu.
Jian Ling, Email: jian.ling@swri.org.
Jean X. Jiang, Email: jiangj@uthscsa.edu.
Mark E. Van Dyke, Email: mavandyk@wfubmc.edu.
Daniel P. Nicolella, Email: daniel.nicolella@swri.org.
References
- Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiol. 1999;276(1 Pt 1):E171–8. doi: 10.1152/ajpendo.1999.276.1.E171. [DOI] [PubMed] [Google Scholar]
- Aonuma Y, Adachi T, Tanaka M, Hojo M, Takano-Yamamoto T, Kamioka H. Mechanosensitivity of a single osteocyte: Difference in cell process and cell body. Journal of Biomechanical Science and Engineering. 2007;2(S1):S165. [Google Scholar]
- Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJ, Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun. 2004;315(4):823–9. doi: 10.1016/j.bbrc.2004.01.138. [DOI] [PubMed] [Google Scholar]
- Bacabac RG, Smit TH, Mullender MG, Van Loon JJ, Klein-Nulend J. Initial stress-kick is required for fluid shear stress-induced rate dependent activation of bone cells. Ann Biomed Eng. 2005;33(1):104–10. doi: 10.1007/s10439-005-8968-5. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. Osteocytes: a proposed multifunctional bone cell. J Musculoskelet Neuronal Interact. 2002;2(3):239–41. [PubMed] [Google Scholar]
- Bonewald LF. Osteocyte biology: its implications for osteoporosis. J Musculoskelet Neuronal Interact. 2004;4(1):101–4. [PubMed] [Google Scholar]
- Bonewald LF. Mechanosensation and Transduction in Osteocytes. Bonekey Osteovision. 2006;3(10):7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonivtch AR, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech. 2007;40(10):2199–206. doi: 10.1016/j.jbiomech.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. Faseb J. 1999;13(Suppl):S101–12. [PubMed] [Google Scholar]
- Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16(2):249–59. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol. 2001;281(5):C1635–41. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- Focht DC. Live-cell microscopy: environmental control for mammalian specimens. Nat Biotechnol. 1996;14(3):361–2. doi: 10.1038/nbt0396-361. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212(1):207–14. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamioka H, Miki Y, Sumitani K, Tagami K, Terai K, Hosoi K, Kawata T. Extracellular calcium causes the release of calcium from intracellular stores in chick osteocytes. Biochem Biophys Res Commun. 1995;212(2):692–6. doi: 10.1006/bbrc.1995.2024. [DOI] [PubMed] [Google Scholar]
- Kamioka H, Sugawara Y, Murshid SA, Ishihara Y, Honjo T, Takano-Yamamoto T. Fluid shear stress induces less calcium response in a single primary osteocyte than in a single osteoblast: implication of different focal adhesion formation. J Bone Miner Res. 2006;21(7):1012–21. doi: 10.1359/jbmr.060408. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts--correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217(2):640–8. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. Faseb J. 1995;9(5):441–5. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- Mullender M, El Haj AJ, Yang Y, van Duin MA, Burger EH, Klein-Nulend J. Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput. 2004;42(1):14–21. doi: 10.1007/BF02351006. [DOI] [PubMed] [Google Scholar]
- Mullender MG, Dijcks SJ, Bacabac RG, Semeins CM, Van Loon JJ, Klein-Nulend J. Release of nitric oxide, but not prostaglandin E2, by bone cells depends on fluid flow frequency. J Orthop Res. 2006;24(6):1170–7. doi: 10.1002/jor.20179. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Mechanical Stress and Osteocytes. J Bone Miner Metab. 1999;17:56. [Google Scholar]
- Nicolella DP, Nicholls AE, Lankford J, Davy DT. Machine vision photogrammetry: a technique for measurement of microstructural strain in cortical bone. J Biomech. 2001;34(1):135–9. doi: 10.1016/s0021-9290(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption--bone flow theory. Clin Orthop Relat Res. 1977;(127):236–47. [PubMed] [Google Scholar]
- Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology. 2003;40(6):591–603. [PubMed] [Google Scholar]
- Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalt R, Mitchell FT, Howard RL, Chambers TJ. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol. 1997;273(4 Pt 1):E751–8. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- Smalt R, Mitchell FT, Howard RL, Chambers TJ. Mechanotransduction in bone cells: induction of nitric oxide and prostaglandin synthesis by fluid shear stress, but not by mechanical strain. Adv Exp Med Biol. 1997;433:311–4. doi: 10.1007/978-1-4899-1810-9_66. [DOI] [PubMed] [Google Scholar]
- Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41(5):745–51. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci. 1998;3(6):346–55. doi: 10.1007/s007760050064. [DOI] [PubMed] [Google Scholar]
- Vatsa A, Mizuno D, Smit TH, Schmidt CF, MacKintosh FC, Klein-Nulend J. Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res. 2006;21(11):1722–8. doi: 10.1359/jbmr.060720. [DOI] [PubMed] [Google Scholar]
- Vatsa A, Smit TH, Klein-Nulend J. Extracellular NO signalling from a mechanically stimulated osteocyte. J Biomech. 2007;40:S89–S95. doi: 10.1016/j.jbiomech.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2006;348(3):1082–8. doi: 10.1016/j.bbrc.2006.07.146. [DOI] [PubMed] [Google Scholar]