Abstract
Stiff-knee gait is a common walking problem in cerebral palsy characterized by insufficient knee flexion during swing. To identify factors that may limit knee flexion in swing, it is necessary to understand how unimpaired subjects successfully coordinate muscles and passive dynamics (gravity and velocity-related forces) to accelerate the knee into flexion during double support, a critical phase just prior to swing that establishes the conditions for achieving sufficient knee flexion during swing. It is also necessary to understand how contributions to swing initiation change with walking speed, since patients with stiff-knee gait often walk slowly. We analyzed muscle-driven dynamic simulations of eight unimpaired subjects walking at four speeds to quantify the contributions of muscles, gravity, and velocity-related forces (i.e. Coriolis and centrifugal forces) to preswing knee flexion acceleration during double support at each speed. Analysis of the simulations revealed contributions from muscles and passive dynamics varied systematically with walking speed. Preswing knee flexion acceleration was achieved primarily by hip flexor muscles on the preswing leg with assistance from biceps femoris short head. Hip flexors on the preswing leg were primarily responsible for the increase in preswing knee flexion acceleration during double support with faster walking speed. The hip extensors and abductors on the contralateral leg and velocity-related forces opposed preswing knee flexion acceleration during double support.
Introduction
The biomechanical causes of diminished and delayed swing-phase knee flexion, or stiff-knee gait, in children with cerebral palsy are unclear, making it difficult to determine appropriate treatment. Over-activity of the rectus femoris is commonly thought to be the primary cause of stiff-knee gait (Perry, 1987; Sutherland et al., 1990), yet many patients do not improve after rectus femoris transfer (Hadley et al., 1992; Õunpuu et al., 1993a; Rethlefsen et al., 1999; Yngve et al., 2002; Carney and Oeffinger, 2003), a surgery aimed at reducing the muscle's knee extension moment, suggesting that there may be other causes in some cases. Other proposed causes of stiff-knee gait include over-activity of the vasti (Kerrigan et al., 1991; Waters et al., 1979), weakness of the hip flexors (Kerrigan et al., 1998), and weakness of the ankle plantarflexors (Kerrigan and Glenn, 1994). A better understanding of the factors that contribute to stiff-knee gait will allow clinicians to employ treatment strategies that address underlying causes.
To determine the cause of an individual's stiff-knee gait, it is necessary to understand how muscles and passive dynamics (gravity and velocity-related forces) contribute to swing initiation in normal gait. Preswing has been identified as a key portion of the gait cycle affecting swing-phase peak knee flexion because the muscle forces produced during preswing determine the knee flexion velocity at toe-off, which is highly correlated to swing-phase peak knee flexion (Mochan and McMahon, 1980; Piazza and Delp, 1996; Goldberg et al., 2003; Reinbolt et al., 2008). However, a thorough understanding of the biomechanical factors that accelerate the knee during this period does not exist. Understanding these factors can be challenging because muscles that do not cross the knee can still accelerate the knee due to dynamic coupling (Zajac and Gordon, 1989). Modeling and simulation tools are valuable in analyzing gait dynamics because they enable quantification of the effects of muscles, gravity, and velocity-related forces on knee flexion acceleration.
It is necessary to understand how contributions to swing initiation change with walking speed, since patients with stiff-knee gait often walk more slowly than typically developing children. Walking speed affects kinematics, kinetics, and muscle activity during gait (Andriacchi et al., 1977; Murray et al., 1984; Kirtley et al., 1985; Shiavi et al., 1987; Stansfield et al., 2001a, 2001b, 2006; Hof et al., 2002; van der Linden et al., 2002; den Otter et al., 2004; Nymark et al., 2005; Cappellini et al., 2006; Schwartz et al., 2008). The contributions of muscles and passive dynamics to support and progression of the body's mass center (Liu et al., 2008; Neptune et al., 2008) and knee flexion in swing (Arnold et al., 2007b) change with walking speed. Thus, it is essential to understand how contributions of muscles and passive dynamics to swing initiation may change with walking speed. Comparison of contributors to swing initiation between a child with stiff-knee gait and a typically developing child walking at a similar speed will enable discrimination between differences due to pathology or walking speed.
The objectives of this study were to identify the major contributors to preswing knee flexion acceleration during double support and to determine how these contributions change with walking speed.
Methods
We analyzed simulations created and tested by Liu et al. (2008) using OpenSim software (Delp et al., 2007). The software and simulations are freely available at http://simtk.org. Liu et al. (2008) created these simulations to quantify muscle contributions to support and progression during walking. In this study, we have analyzed the simulations to determine muscular and passive contributions to knee flexion acceleration during double support. The double support period of simulations of eight unimpaired subjects walking at four speeds was analyzed. The subjects' ages ranged from 7 to 18 years with a mean of 12.9 years. Protocols for collection and processing of gait data, including ground reaction forces, kinematics, and electromyographic (EMG) recordings, were reported by Schwartz et al. (2008). Walking trials for each subject were assigned post-hoc to categories of very slow, slow, free, and fast speeds as described by Liu et al. (2008) using a non-dimensionalized walking speed , where v is absolute walking velocity, Lleg is leg length, and g is gravitational acceleration (Hof, 1996). Average walking speeds were 0.54 m/s for very slow, 0.75 m/s for slow, 1.15 m/s for free, and 1.56 m/s for fast.
The musculoskeletal model and procedures for creating and testing the simulations is described in detail elsewhere (Liu et al., 2008). Briefly, a generic musculoskeletal model (Delp et al., 1990; Thelen and Anderson, 2006) with 23 degrees of freedom and 92 muscle-tendon actuators was scaled to match each subject's anthropometry. Subtalar and metatarsophalangeal joints were locked at neutral anatomical angles. Dynamic inconsistency between the measured ground reaction forces and the model kinematics was resolved by applying small external forces and torques (i.e. residuals) to the pelvis and making small adjustments to the model's mass properties and kinematics (Delp et al., 2007). Computed muscle control (Thelen et al., 2003), with constraints on muscle excitations applied as necessary, was used to find a set of actuator excitations that when applied to the model in concert with external ground reaction forces would both track the experimental kinematics and be generally consistent with experimental and literature-reported EMG patterns. We verified that the excitation patterns from the simulations at the different walking speeds generally scaled with speed as reported in the literature (Hof et al., 2002; den Otter et al., 2004; Cappellini et al., 2006; Schwartz et al., 2008).
In each simulation, we quantified the contributions of individual muscles, gravity, and velocity-related forces to preswing knee flexion acceleration using a perturbation analysis (Liu et al., 2006). This analysis independently calculated the contribution from each force (individual muscles, gravity, or velocity-related) and was repeated for all contributors in the simulation at 10 ms intervals throughout double support. For each muscle, we added 1 N to the force produced by an individual muscle in the simulation, integrated forward the equations of motion for a 10 ms period, and observed the resulting change in preswing knee flexion angle. The resulting change in preswing knee flexion angle was used to calculate the preswing knee flexion acceleration generated by 1 N of muscle force, assuming that the acceleration generated by the muscle over the short 10 ms integration period was constant. The preswing knee flexion acceleration generated by 1 N of muscle force was multiplied by the muscle's force in the unperturbed simulation to quantify the muscle's contribution to preswing knee flexion acceleration at the beginning of the 10 ms period. Translational and rotational spring-dampers applied to the center of pressure of each foot accounted for changes in the ground reaction force induced by the muscle perturbation. A similar technique was applied to determine the contribution of gravity to preswing knee flexion acceleration. To quantify contributions from velocity-related forces, the model was set in its original configuration and given its original velocities at the start of every 10 ms period. No muscle, gravity, or ground reaction forces were applied; only reaction forces from the spring-dampers representing foot-floor contact were applied. We integrated forward the equations of motion for a 10 ms period and observed the resulting change in preswing knee flexion angle. The contribution of velocity-related forces to preswing knee flexion acceleration was calculated from this change in preswing knee flexion angle, assuming that the acceleration generated by the velocity-related forces over the short 10 ms integration period was constant. All contributions (muscle, gravity, and velocity-related) were averaged over the period of double support in each simulation.
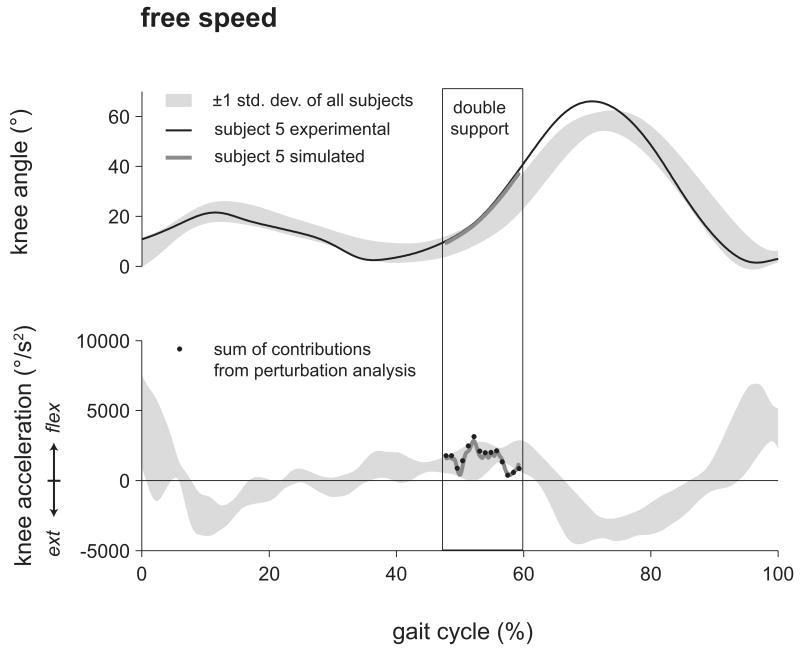
To test the validity of our method, we verified that the sum of all calculated contributions to preswing knee flexion acceleration was in agreement with the unperturbed preswing knee flexion acceleration at each time point of analysis throughout double support (Fig. 1). This suggests that our model of foot-floor contact is a reasonable representation of the constraints on the foot during double support.
Figure 1.
Knee flexion angle and acceleration of subject 5, a representative subject, for the simulation (thick gray line) compared to experimentally measured values (thin black line) during the free speed trial. The simulation closely tracked experimental knee flexion. Shaded region represents ± one standard deviation from the mean of the free speed trials of all eight subjects. Black dots represent the sum of all calculated contributions from the perturbation analysis at each step. Overlap of the black dots with the thick gray line indicates that the sum of contributors to preswing knee flexion acceleration calculated by the perturbation analysis closely approximated the knee flexion acceleration of the simulation.
We performed a one-way repeated measures analysis of variance (SPSS Inc., Chicago, IL) to determine if walking speed had a significant effect on the average contributions of muscles, gravity, and velocity to preswing knee flexion acceleration. For data that violated sphericity assumptions, a Huynh-Feldt epsilon correction was applied. When speed was determined to significantly affect a contributor, we analyzed the within-subject repeated contrasts to determine if there was a significant difference between successive speed pairs (i.e., very slow to slow, slow to free, free to fast). The significance level for all tests was α ≤ 0.05.
Results
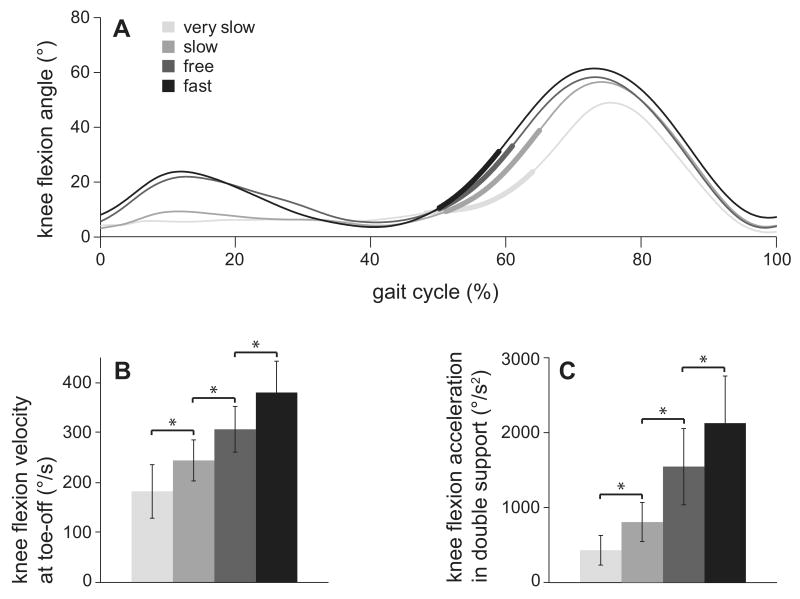
In preparation for swing, the preswing knee is strongly accelerated into flexion during double support. Most of the flexion acceleration occurs before the toe leaves the ground, resulting in a peak knee flexion velocity around toe-off (Fig. 2A). Achieving a sufficient knee flexion velocity at toe-off is crucial to achieving sufficient peak knee flexion in swing. Knee flexion velocity at toe-off increased with walking speed (p < 0.05; Fig. 2B). This was achieved by an increase in average knee flexion acceleration during double support with increased walking speed (p < 0.01; Fig. 2C).
Figure 2.
(A) Knee flexion angle over the gait cycle averaged over all eight subjects for each speed with the period of double support highlighted by the thick regions. The slope of this curve represents knee flexion velocity, which peaks near toe-off. (B) Knee flexion velocity at toe-off averaged over all eight subjects increased with walking speed. (C) Knee flexion acceleration averaged over double support and across all eight subjects increased with walking speed. * denotes significant (p < 0.05) difference between successive speeds.
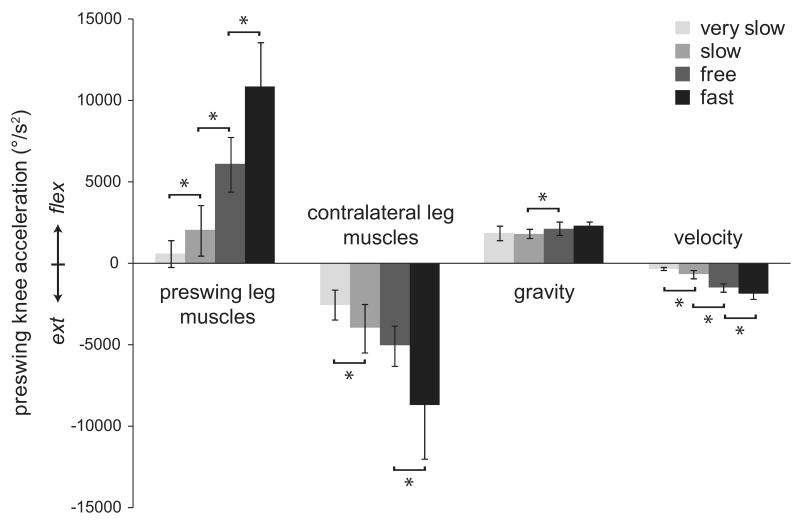
Muscles on both legs contributed to acceleration of the preswing knee (Fig. 3). The net effect of muscles on the preswing leg was to accelerate the knee into flexion during double support. This was accomplished primarily by the hip flexors (mainly iliacus and psoas) with assistance from biceps femoris short head (BFSH) (Fig. 4). With faster walking speed, the hip flexors contributed more to knee flexion acceleration (slow to free, p < 0.01; free to fast, p < 0.01). Other preswing leg muscles, including gastrocnemius and the ankle dorsiflexors (DF), also contributed to preswing knee flexion acceleration, but made small contributions relative to the preswing hip flexors. Some muscles on the preswing leg decelerated knee flexion, including the uniarticular plantarflexors (UPF) (mainly soleus), vasti, rectus femoris, and the hip extensors and abductors. The net effect of muscles on the contralateral leg at all speeds was to decelerate preswing knee flexion. This was primarily due to hip extensors and abductors on the contralateral leg. Hip flexors on the contralateral leg opposed the extension effect, but to a lesser extent. Contributions from back muscles and residual forces and torques varied across subjects, but were small on average. Average residual contributions to knee flexion acceleration across subjects at each speed were -417 °/s2 for very slow, -170 °/s2 for slow, -1646 °/s2 for free, and -939 °/s2 for fast.
Figure 3.
Net contributions of preswing leg muscles, contralateral leg muscles, velocity-related forces, and gravity to preswing knee flexion acceleration averaged over double support at four walking speeds. Bars represent mean contributions during double support across all eight subjects. Error bars represent ± one standard deviation. * denotes significant (p < 0.05) difference between successive speeds.
Figure 4.
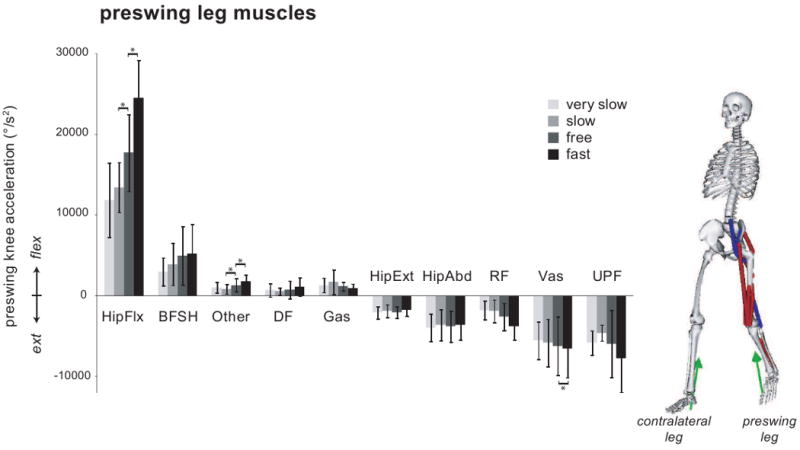
Contributions from muscle groups, grouped by functional action, on the preswing leg to preswing knee flexion acceleration during double support averaged across all subjects at each speed. Error bars represent ± one standard deviation. * denotes p < 0.05 for within-subjects repeated contrasts analyses. Model depicts major contributors to flexion as blue line muscles and major contributors to extension as red line muscles. Green arrows represent the ground reaction forces. HipFlx, the hip flexors, includes iliacus, psoas, tensor fasciae latae, and sartorius. BFSH is the biceps femoris short head. DF, the ankle dorsiflexors, includes tibialis anterior, extensor digitorum longus, extensor hallucis longus, and peroneus tertius. Gas includes medial and lateral gastrocnemius. HipExt, the hip extensors, includes gluteus maximus, adductor magnus, biceps femoris long head, semimembranosus, and semitendinosus. HipAbd, the hip abductors, includes gluteus medius and gluteus minimus. RF is the rectus femoris. VAS includes vastus medialis, vastus intermedius, and vastus lateralis. UPF, the uniarticular plantarflexors, includes soleus, tibialis posterior, flexor digitorum longus, flexor hallucis longus, peroneus longus, and peroneus brevis. Other includes all of the other muscles of the preswing leg in the model.
The contributions of passive dynamics during double support varied systematically with walking speed (Fig. 3). Gravity accelerated the knee into flexion (Movie 1) with a relatively constant magnitude across speeds, though slightly greater at free and fast speeds (p < 0.01). Velocity-related (Coriolis and centrifugal) forces mildly decelerated preswing knee flexion with an increasing effect with faster walking speed (very slow to slow, p < 0.001; slow to free, p < 0.001; free to fast, p = 0.012).
Discussion
Our simulations showed that the hip flexors, iliacus and psoas, on the preswing limb were primarily responsible for accelerating the knee into flexion during double support. This is consistent with previous simulation studies (Yamaguchi and Zajac,1990; Goldberg et al., 2004). The increase in knee flexion acceleration during double support with faster walking speed was primarily due to increased force generated by the hip flexors. Neptune et al. (2008) also found a dramatic increase in iliopsoas muscle work to accelerate the preswing leg at faster walking speeds. In our subjects, peak hip flexion moment during double support increased three-fold between very slow and fast speeds, a larger increase than either the knee or ankle moments. Large increases in hip moment during double support with increasing speed were also observed in other studies (Schwartz et al., 2008; van der Linden et al., 2002; Stansfield et al., 2001b). Additionally, we found the vasti, rectus femoris, and hip abductors and extensors decelerate knee flexion during double support, consistent with other studies (Neptune et al., 2001, Fig. 8; Goldberg et al., 2004).
Our results showed the two major plantarflexors, soleus and gastrocnemius, had opposite effects on preswing knee acceleration. The knee extension acceleration of soleus is consistent with other studies (Goldberg et al., 2004; Neptune et al., 2001, Fig. 8). The mild knee flexion acceleration of gastrocnemius that we found contrasts with reports that the gastrocnemius has a large flexion effect (Yamaguchi and Zajac, 1990; Goldberg et al., 2004) or an extension effect (Neptune et al., 2001, Fig. 8) on the preswing knee. The gastrocnemius generates a plantarflexion moment that induces knee extension acceleration and a knee flexion moment that induces knee flexion acceleration; thus, its action is sensitive to the muscle's ankle and knee moment arms, body position, and foot contact model, which varied among the studies. Although the net effect of the plantarflexors may be to extend the knee, studies have suggested they play a role in swing initiation since it has been observed that hip flexors compensate during preswing when plantarflexors are weak (Nadeau et al., 1999) or absent (Zmitrewicz, et al., 2007). Neptune et al. (2008) suggested that gastrocnemius contributes to swing initiation by delivering energy to the preswing leg.
The roles of hip flexion and ankle plantarflexion moments in swing initiation have been demonstrated in dynamic walking models. Kuo et al (2002) reported that an increased hip torque produces increased step frequency, while an increased toe-off impulse produces longer steps at an approximately constant step frequency; they suggested that a combination of hip work and toe-off impulse may improve walking energetics. Other studies have demonstrated a torque applied at the hip and/or a push-off impulse applied to the foot can produce stable gait on level ground (Collins et al., 2005).
In our simulations, the contralateral leg muscles contributed an extension acceleration to the preswing knee at all speeds through their action on the pelvis. Muscles on the contralateral leg including hip extensors, posterior hip abductors (which have hip extension moment arms), and vasti extended the knee and hip of the contralateral leg. In the body configuration of double support, this caused the pelvis to tilt posteriorly, list upward on the side of preswing leg, and rise slightly. As a result, the hip joint on the preswing leg was pushed upward (superiorly) and forward (anteriorly). Reaction forces at the preswing hip joint extended the preswing knee since the foot of the preswing leg remained on the ground during double support (Movie 2). At faster walking speeds, the contralateral leg muscles had a stronger deceleration effect on preswing knee flexion (Fig. 3).
Classic texts and studies of passive dynamic walkers have suggested that muscle activity during preswing sets the initial conditions for passive knee motion during swing (Boakes and Rab, 2006; Gage, 2004; Perry, 1992; McGeer, 1990; Mochon and McMahon, 1980). However, muscles are active during swing, and studies muscle-actuated models have found that this muscle activity contributes during both preswing and early swing to achieve appropriate knee motion before and during swing. Although this study focused on double support, muscular or passive forces during early swing phase (the period of swing before peak knee flexion) may also affect peak knee flexion during swing. The knee undergoes a large flexion acceleration during double support to prepare for toe-off (Fig. 2C). After toe-off the knee undergoes an extension acceleration throughout early swing (Arnold et al., 2007a). During double support or early swing, forces causing inadequate knee flexion acceleration or excessive knee extension acceleration may limit peak knee flexion during swing, resulting in stiff-knee gait. In this study we found that during double support the preswing knee is accelerated into flexion mainly by swing leg muscles (primarily the hip flexors and biceps femoris) and gravity at all speeds. Arnold et al. (2007a; 2007b) found that during early swing the swing knee is extended by stance leg muscles (mainly vasti and uniarticular plantarflexors) and velocity-related forces at all speeds. It is necessary to analyze muscle contributions during both preswing and early swing to investigate possible causes of stiff-knee gait.
Our results should be interpreted in light of several limitations of this study. First, our estimates of muscle contributions to knee flexion acceleration were dependent on the force produced by each muscle during the simulation. Although experimental joint moments and EMG were generally consistent with simulated values, it was not feasible to compare simulated muscle forces to experimentally measured forces. Secondly, residual actuators in the model, which applied external forces and torques to the pelvis, contributed to knee flexion acceleration with a magnitude comparable to net muscle contribution in some trials. These residual actuators do not represent real physical forces, but instead characterize errors in kinematic and kinetic measurements and deficiencies in the model, such as the lack of arms and joint simplifications. We chose a method to reduce rather than eliminate these residual forces, as the latter can result in implausible motions of the back. In these trials, net muscle contributions were affected, but the relative magnitude of individual muscle contributions was consistent with trials in which residuals did not contribute substantially to knee flexion acceleration. Thirdly, although care was taken to validate simulated muscle activations with experimental EMG, rectus femoris and gastrocnemius activations for some subjects during double support did not increase with speed as expected (Hof et al., 2002; den Otter et al., 2004; Cappellini et al., 2006; Schwartz et al., 2008). If activations and forces in the gastrocnemius and rectus femoris were greater, we would have observed slightly larger contributions to knee acceleration.
This study identifies the factors that contribute to knee flexion acceleration during double support and provides a framework for future studies to investigate the muscular and passive contributions to knee flexion acceleration in subjects with stiff-knee gait. Further studies may further elucidate the role of the ankle plantarflexors in other aspects of swing initiation, such as forward propulsion of the swing leg.
Supplementary Material
Movie 1. Gravity accelerates the preswing knee into flexion during double support.
Movie 2. Contralateral muscles contribute an extension accelerations to the preswing knee during double support.
Acknowledgments
The authors gratefully acknowledge May Liu, Clay Anderson, and Michael Schwartz for generating the simulations analyzed in this study, Chand John for assisting in the recreation of the simulations in OpenSim v1.5, and Edith Arnold, Samuel Hamner, Jennifer Hicks, Chand John, and Katherine Steele for helpful comments on an earlier version of the manuscript. This project was supported by NIH award numbers R01 HD046814, T32GM063495, and NIH Roadmap for Medical Research U54 GM072970. We also acknowledge NSF award CNS-0619926 for computer resources.
Footnotes
Conflict of interest: None of the authors has a conflict of interest regarding this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andriacchi TP, Ogle JA, Galante JO. Walking speed as a basis for normal and abnormal gait measurements. Journal of Biomechanics. 1977;10:261–268. doi: 10.1016/0021-9290(77)90049-5. [DOI] [PubMed] [Google Scholar]
- Arnold AS, Thelen DG, Schwartz MH, Anderson FC, Delp SL. Muscular coordination of knee motion during the terminal-swing phase of normal gait. Journal of Biomechanics. 2007a;40:3314– 3324. doi: 10.1016/j.jbiomech.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AS, Schwartz MH, Thelen DG, Delp SL. Contributions of muscles to terminal-swing knee motions vary with walking speed. Journal of Biomechanics. 2007b;40:3660–3671. doi: 10.1016/j.jbiomech.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes JL, Rab GT. Muscle activity during walking. In: Rose J, Gamble JG, editors. Human Walking. Lippincott Williams and Wilkins; Baltimore: 2006. [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. Journal of Neurophysiology. 2006;95:3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- Carney BT, Oeffinger D. Sagittal knee kinematics following combined hamstring lengthening and rectus femoris transfer. Journal of the Southern Orthopaedic Association. 2003;12:149–153. [PubMed] [Google Scholar]
- Collins S, Ruina A, Tedrake R, Wisse M. Efficient bipedal robots based on passive-dynamic walkers. Science. 2005;307:1082–1085. doi: 10.1126/science.1107799. [DOI] [PubMed] [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Transactions on Biomedical Engineering. 2007;54:1940–1950. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- den Otter AR, Geurts ACH, Mulder T, Duysens J. Speed related changes in muscle activity from normal to very slow walking speeds. Gait and Posture. 2004;19:270–278. doi: 10.1016/S0966-6362(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Gage JR. A qualitative description of normal gait. In: Gage JR, editor. The Treatment of Gait Problems in Cerebral Palsy. Mac Keith Press; London: 2004. pp. 42–70. [Google Scholar]
- Goldberg SR, Anderson FC, Pandy MG, Delp SL. Muscles that influence knee flexion velocity in double support: implications for stiff-knee gait. Journal of Biomechanics. 2004;37:1189–1196. doi: 10.1016/j.jbiomech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Õunpuu S, Delp SL. The importance of swing-phase initial conditions in stiff-knee gait. Journal of Biomechanics. 2003;36:1111–1116. doi: 10.1016/s0021-9290(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Hadley N, Chambers C, Scarborough N, Cain T, Rossi D. Knee motion following multiple soft-tissue releases in ambulatory patients with cerebral palsy. Journal of Pediatric Orthopaedics. 1992;12:324–328. doi: 10.1097/01241398-199205000-00008. [DOI] [PubMed] [Google Scholar]
- Hof AL. Scaling gait data to body size. Gait and Posture. 1996;4:222–223. [Google Scholar]
- Hof AL, Elzinga H, Grimmius W, Halbertsma JPK. Speed dependence of averaged EMG profiles in walking. Gait and Posture. 2002;16:78–86. doi: 10.1016/s0966-6362(01)00206-5. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Roth RS, Riley PO. The modeling of adult spastic paretic stiff-legged gait swing period based on actual kinematic data. Gait and Posture. 1998;7:117–124. doi: 10.1016/s0966-6362(97)00040-4. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Glenn MB. An illustration of clinical gait laboratory use to improve rehabilitation management. American Journal of Physical Medicine and Rehabilitation. 1994;73:421–427. doi: 10.1097/00002060-199411000-00007. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Gronley J, Perry J. Stiff-legged gait in spastic paresis: a study of quadriceps and hamstrings muscle activity. American Journal of Physical Medicine and Rehabilitation. 1991;70:294–300. [PubMed] [Google Scholar]
- Kirtley C, Whittle MW, Jefferson RJ. Influence of walking speed on gait parameters. Journal of Biomedical Engineering. 1985;7:282–288. doi: 10.1016/0141-5425(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Kuo AD. Energetics of actively powered locomotion using the simplest walking model. Journal of Biomechanics. 2002;124:113–120. doi: 10.1115/1.1427703. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. Journal of Biomechanics. 2006;39:2623–2630. doi: 10.1016/j.jbiomech.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Schwartz MH, Delp SL. Muscle contributions to support and progression over a range of walking speeds. Journal of Biomechanics. 2008;41:3243–3252. doi: 10.1016/j.jbiomech.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer T. Passive dynamic walking. International Journal of Robotics Research. 1990;9:62–82. [Google Scholar]
- Mochon S, McMahon TA. Ballistic walking: an improved model. Mathematical Biosciences. 1980;52:241–260. [Google Scholar]
- Murray MP, Mollinger LA, Gardner GM, Sepic SB. Kinematic and EMG patterns during slow, free, and fast walking. Journal of Orthopaedic Research. 1984;2:272–280. doi: 10.1002/jor.1100020309. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factorof gait speed in stroke subjects and the compensating role of hip flexors. Clinical Biomechanics. 1999;14:125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of Biomechanics. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait and Posture. 2008;28:135–143. doi: 10.1016/j.gaitpost.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark JR, Balmer SJ, Melis EH, Lemaire ED, Millar S. Electromyographic and kinematic nondisabled gait differences at extremely slow overground and treadmill walking speeds. Journal of Rehabilitation Research and Development. 2005;42:523–534. doi: 10.1682/jrrd.2004.05.0059. [DOI] [PubMed] [Google Scholar]
- Õunpuu S, Muik E, Davis RB, Gage JR, DeLuca PA. Rectus femoris surgery in children with cerebral palsy. Part I: The effect of rectus femoris transfer location on knee motion. Journal of Pediatric Orthopaedics. 1993a;13:325–330. doi: 10.1097/01241398-199305000-00010. [DOI] [PubMed] [Google Scholar]
- Perry J. Distal rectus femoris transfer. Developmental Medicine and Child Neurology. 1987;29:153–158. doi: 10.1111/j.1469-8749.1987.tb02130.x. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait Analysis: Normal and Pathological Function. SLACK Inc.; Thorofare, NJ: 1992. [Google Scholar]
- Piazza SJ, Delp SL. The influence of muscles on knee flexion during the swing phase of gait. Journal of Biomechanics. 1996;29:723–733. doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Reinbolt JA, Fox MD, Arnold AS, Õunpuu S, Delp SL. Importance of preswing rectus femoris activity in stiff-knee gait. Journal of Biomechanics. 2008;41:2362–2369. doi: 10.1016/j.jbiomech.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethlefsen S, Tolo VT, Reynolds RA, Kay R. Outcome of hamstring lengthening and distal rectus femoris transfer surgery. Journal of Pediatric Orthopaedics. 1999;8:75–79. [PubMed] [Google Scholar]
- Schwartz MH, Rozumalski A, Trost JP. The effect of walking speed on the gait of typically developing children. Journal of Biomechanics. 2008;41:1639–1650. doi: 10.1016/j.jbiomech.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Shiavi R, Bugle HJ, Limbird T. Electromyographic gait assessment, Part 1: Adult EMG profiles and walking speed. Journal of Rehabilitation Research and Development. 1987;24:13–23. [PubMed] [Google Scholar]
- Stansfield BW, Hillman SJ, Hazlewood ME, Lawson AA, Mann AM, Loudon IR, Robb JE. Normalized speed, not age, characterizes ground reaction force patterns in 5- to 12-year-old children walking at self-selected speeds. Journal of Pediatric Orthopedics. 2001a;21:395–402. [PubMed] [Google Scholar]
- Stansfield BW, Hillman SJ, Hazlewood ME, Lawson AA, Mann AM, Loudon IR, Robb JE. Sagittal joint kinematics, moments, and powers are predominantly characterized by speed of progression, not age, in normal children. Journal of Pediatric Orthopedics. 2001b;21:403–411. [PubMed] [Google Scholar]
- Stansfield BW, Hillman SJ, Hazlewood ME, Robb JE. Regression analysis of gait parameters with speed in normal children walking at self-selected speeds. Gait and Posture. 2006;23:288–294. doi: 10.1016/j.gaitpost.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Sutherland DH, Santi M, Abel MF. Treatment of stiff-knee gait in cerebral palsy: a comparison by gait analysis of distal rectus femoris transfer versus proximal rectus release. Journal of Pediatric Orthopaedics. 1990;10:433–441. [PubMed] [Google Scholar]
- Thelen DG, Anderson FC, Delp SL. Generating dynamic simulations of movement using computed muscle control. Journal of Biomechanics. 2003;36:321–328. doi: 10.1016/s0021-9290(02)00432-3. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Anderson FC. Using computed muscle control to generate forward dynamic simulations of human walking from experimental data. Journal of Biomechanics. 2006;39:1015–1107. doi: 10.1016/j.jbiomech.2005.02.010. [DOI] [PubMed] [Google Scholar]
- van der Linden M, Kerr A, Hazlewood ME, Hillman SJ, Robb JE. Kinematic and kinetic gait characteristics of normal children walking at a range of clinically relevant speeds. Journal of Pediatric Orthopedics. 2002;22:800–806. [PubMed] [Google Scholar]
- Waters RL, Garland DE, Perry J, Habig T, Slabaugh P. Stiff-legged gait in hemiplegia: surgical correction. Journal of Bone & Joint Surgery. 1979;61-A:927–933. [PubMed] [Google Scholar]
- Yamaguchi GT, Zajac FE. Restoring unassisted natural gait to paraplegics via functional neuromuscular stimulation: a computer simulation study. IEEE Transactions in Biomedical Engineering. 1990;37:886–902. doi: 10.1109/10.58599. [DOI] [PubMed] [Google Scholar]
- Yngve DA, Scarborough N, Goode B, Haynes R. Rectus and hamstring surgery in cerebral palsy: a gait analysis study of results by functional ambulation level. Journal of Pediatric Orthopaedics. 2002;22:672–676. [PubMed] [Google Scholar]
- Zajac FE, Gordon ME. Determining muscle's force and action in multi-articular movement. Exercise and Sport Sciences Reviews. 1989;17:187–230. [PubMed] [Google Scholar]
- Zmitrewicz RJ, Neptune RR, Kotaro S. Mechanical energetic contributions from individual muscles and elastic prosthetic feet during symmetric unilateral transtibial amputee walking: a theoretical study. Journal of Biomechanics. 2007;40:1824–1831. doi: 10.1016/j.jbiomech.2006.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. Gravity accelerates the preswing knee into flexion during double support.
Movie 2. Contralateral muscles contribute an extension accelerations to the preswing knee during double support.