Abstract
Childhood lead exposure is associated with decreased cognitive abilities and executive functioning localized within the prefrontal cortex. Several studies have observed stronger associations between blood lead measurements obtained later in life than earlier measures, but there are no imaging studies investigating the developmental trajectory of blood lead levels taken during childhood on adult gray matter volume. In this study, we recruited 157 adults (20.8 ± 1.5 years of age) from the Cincinnati Lead Study to undergo high-resolution volumetric magnetic resonance imaging. Adjusted voxel-wise regression analyses were performed for associations between adult gray matter volume loss and yearly mean blood lead levels from one to six years of age in the entire cohort and by sex. We observed significant inverse associations between gray matter volume loss and annual mean blood lead levels from three to six years of age. The extent of prefrontal gray matter associated with yearly mean blood lead levels increased with advancing age of the subjects. The inverse associations between gray matter volume loss and yearly mean blood lead measurements were more pronounced in the frontal lobes of men than women. Analysis of women yielded significantly weaker associations between yearly mean blood lead levels and gray matter volume at all ages than either men or the combined cohort of men and women together. These results suggest that blood lead concentrations obtained during later childhood demonstrate greater loss in gray matter volume than childhood mean or maximum values. The relationship between childhood blood lead levels and gray matter volume loss was predominantly observed in the frontal lobes of males. This study demonstrates that maximum blood lead levels do not fully account for gray matter changes associated with childhood lead exposure, particularly in the frontal lobes of young men.
Keywords: Lead, brain, gray matter, magnetic resonance imaging, voxel based morphometry, development
1. Introduction
Lead is an environmental toxicant with documented effects on human cognition (Baghurst et al., 1992; Bellinger et al., 1992; Canfield et al., 2003; Dietrich et al., 1993a; Lanphear et al., 2005; Schnaas et al., 2000; Wasserman et al., 1997), behavior (Dietrich et al., 2001; Needleman et al., 1996; Stretesky and Lynch, 2004; Wright et al., 2008), and brain structure (Cecil et al., 2008; Stewart et al., 2006).
While mean and maximum childhood blood lead levels have long been regarded as the standard metrics of childhood lead exposure, several studies have noted stronger associations between neurobehavioral outcomes and blood lead levels measured in later childhood than either mean or maximum childhood blood lead levels (Bellinger et al., 1992; Chandramouli et al., 2009; Chen et al., 2007; Chen et al., 2005; Ris et al., 2004; Schnaas et al., 2000; Tong et al., 1996; Wasserman et al., 1997). In Cecil, et al. 2008, we demonstrated the adjusted association between mean childhood lead levels and adult gray matter volume loss using a voxel based morphometric analysis of volumetric magnetic resonance imaging (MRI) obtained from a longitudinal birth cohort. The purpose of this study was to investigate if later childhood blood levels were more strongly associated with neuroanatomical changes than mean or maximum childhood blood lead levels.
2. Methods
2.1 Participants
The Cincinnati Lead Study (CLS) is an urban, inner-city birth cohort with detailed prenatal and postnatal histories of low to moderate lead exposure and behavioral outcomes monitored over 30 years. The CLS enrolled pregnant women between 1979 and 1984 who lived in neighborhoods with historically high levels of childhood lead exposure. Women were excluded if they were known to be addicted to drugs, diabetic or had any known neurological or psychiatric disease. Infants were excluded if their birth weight was less than 1,500 g or if genetic or other serious medical issues were present at birth (Dietrich, Krafft 1987). This process netted newborns that were followed up quarterly through 5 y of age, semiannually from 5 to 6.5 y of age, again at age 10 y and between the ages of 15 and 17 y. A total of 157 CLS participants between the ages of 19 and 24 years provided informed consent and participated in this imaging study (Table 1). Some CLS participants in this imaging study did not have complete lead exposure histories; rather than try to impute the missing values these subjects were excluded from analysis of the years for which there was no lead exposure record. A summary of the cohort size and lead level by age of the participants is shown in Table 2.
Table 1.
Characteristics of the Children and of their Mothers in the Cincinnati Lead Study (N=157) with Comparison by Sex
| Characteristic | Cohort | Range | Men (N=83) | Women (N=74) | p value |
|---|---|---|---|---|---|
| Mean childhood blood lead concentration (μg/dL) | 13.3 ± 5.9 | (4.6 - 37.2) | 13.6 ± 6.3 | 13.1 ± 5.5 | 0.50 |
| Maximum blood lead concentration (μg/dL) | 23.1 ± 11.2 | (7.8 – 83.2) | 23.5 ± 11.0 | 22.7 ± 11.6 | 0.64 |
| Age at Max Pb level (months) | 23.4 ± 10.8 | (3 – 66) | 23.6 ± 11.5 | 23.2 ± 10.0 | 0.85 |
| Age at imaging (years) | 20.8 ± 0.9 | (19.7 – 24.3) | 20.8 ± 0.9 | 20.9 ± 0.9 | 0.50 |
| Gestational age (weeks) | 39.4 ± 1.7 | (35 – 43) | 39.5 ± 1.7 | 39.4 ± 1.7 | 0.38 |
| Birth weight (grams) | 3103 ± 468 | (1814 – 4260) | 3137 ± 508 | 3075 ± 412 | 0.63 |
| SES, 78 months | 18.1 ± 5.1 | (11 – 49) | 18.9 ± 5.7 | 17.6 + 4.3 | 0.80 |
| IQ-FSIQ at 7 years | 86.7 ± 11.9 | (50 – 116) | 85.2 ± 12.1 | 88.2 ± 11.6 | 0.89 |
| Educational Level (20 yrs) | 11.5 ± 1.4 | (8 – 16) | 11.3 ± 1.3 | 11.6 ± 1.6 | 0.78 |
| Maternal FSIQ | 75.3 ± 8.7 | (55 – 100) | 74.6 ± 8.1 | 76.0 ± 9.4 | 0.75 |
| Marijuana Usage | 76 positive (48%) | 46 positive (55%) | 30 positive (41%) | 0.95 | |
| Maternal Alcohol Usage | 24 yes (15%) | 11 yes (13%) | 13 yes (18%) | 0.65 | |
| Maternal Tobacco Usage | 71 yes (45%) | 35 yes (42%) | 36 yes (49%) | 0.68 | |
| Maternal Marijuana Usage | 18 yes (11%) | 9 yes (11%) | 9 yes (12%) | 0.43 | |
Abbreviations: SES-Hollingshead Socioeconomic Status, FSIQ-Full Scale Intelligence Quotient, p value represents difference between men and women using the student's t-test.
Table 2.
Annual Characteristics of the Cincinnati Lead Study
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Mean Yrs 1-6 | |
|---|---|---|---|---|---|---|---|
| Cohort | |||||||
| Number | 157 | 157 | 156 | 156 | 153 | 149 | 157 |
| Mean Pb level | 10.6 ± 5.4 | 17.2 ± 8.5 | 16.3 ± 7.7 | 14 ± 6.8 | 11.8 ± 5.9 | 9.6 ± 5.2 | 13.3 ± 5.9 |
| Pb level range | 3.1 to 35 | 5.7 to 49.3 | 4.3 to 50.3 | 3.1 to 45.2 | 3.3 to 38.3 | 2.4 to 32.7 | 4.7 to 37.2 |
| Men | |||||||
| Number | 83 | 83 | 82 | 82 | 79 | 77 | 83 |
| Mean Pb level | 10.8 ± 5.8 | 17.7 ± 9.2 | 16.4 ± 8.1 | 14 ± 6.9 | 11.8 ± 6 | 10.1 ± 5.4 | 13.5 ± 6.3 |
| Pb level range | 3.1 to 35 | 5.4 to 47.8 | 4.3 to 43.8 | 3.1 to 38.2 | 3.3 to 31.8 | 2.4 to 24.3 | 4.7 to 34.8 |
| Women | |||||||
| Number | 74 | 74 | 74 | 74 | 74 | 72 | 74 |
| Mean Pb level | 10.3 ± 4.8 | 16.7 ± 7.8 | 16.2 ± 7.3 | 14 ± 6.8 | 11.8 ± 5.9 | 9.2 ± 5.0 | 13.1 ± 5.5 |
| Pb level range | 3.9 to 23.5 | 6.4 to 49.3 | 4.3 to 50.3 | 3.9 to 45.5 | 3.3 to 38.8 | 3.5 to 32.7 | 4.8 to 37.2 |
Note to Table 2: The yearly mean blood lead levels were not significantly different between men and women, men and the combined cohort, or women and the whole cohort (data not shown.)
2.2 Imaging Analysis
We acquired whole-brain, three-dimensional, high resolution volumetric 1.5 Tesla MR data (General Electric Medical Systems, Milwaukee, WI, Signa LX EXCITE scanner operating at software platforms of 11.0 and 12.0) using a T1-weighted, axial inversion recovery prepped, fast spoiled gradient echo (3D IR FSPGR) sequence (echo time (TE) of 5 msec, repetition time (TR) of 12 msec, inversion time (TI) 300 msec, field of view (FOV) = 24 cm × 19.2 cm, 1.5-mm thick contiguous slices in a 256 × 192 × 124 matrix for a resolution of 0.94 mm * 1 mm * 1.5 mm) to assess global and regional changes in brain tissue (gray matter, white matter, and cerebrospinal fluid [CSF]) volume for comparison with the yearly mean of childhood blood lead concentrations (measured in μg/dL) collected between 3 and 78 months of life using voxel-based morphometry (VBM) (Ashburner and Friston, 2000). VBM requires normalizing individual structural MRI scans to a study-specific template to allow voxel-by-voxel comparisons between individuals. This approach allows for statistical analyses throughout the brain without a priori designation of structures of interest or manual delineation of brain structures.
2.3 Blood Lead Concentrations
Blood lead samples were collected and analyzed for lead by anodic stripping voltametry as been previously described in extensive detail (Dietrich et al., 1993b, Dietrich et al., 1987, and Roda et al., 1987) at the Hematology and Environmental Chemistry Laboratory in the University of Cincinnati Department of Environmental Health. This laboratory was fully accredited to carry out this work and served as a reference laboratory for several blood lead assessment programs throughout the United States. Blood lead concentrations were measured in this cohort every 3 months from birth for the first 5 y of life and every 6 months from 5 to 6.5 y. To investigate the influence of lead exposure at different ages, yearly mean blood lead levels were calculated for all individuals in this study. The mean of all blood lead levels in the preceding year was used to calculate the yearly mean blood lead level. For example, the mean of blood lead levels recorded at birth through 12 months was designated Year 1 mean blood lead level. Similarly, the mean of blood lead levels from 15 months to 24 months gave the Year 2 mean blood lead level, and so on for years 3 through 5. In year 6, blood lead levels were collected semiannually, and the mean of the 66 and 72-month blood lead levels was used to calculate the year 6 mean blood lead level. Maximum values were also noted for each individual, and associations between individual maximum lead levels and adult gray and white matter volumes were also investigated.
2.4 VBM Approach
Separate multiple regression models were developed to investigate associations between each yearly mean blood lead level on changes in volume for white matter, gray matter, or cerebrospinal fluid. All initial exploratory analyses were 2-tailed for positive or negative associations, representing volume gain or loss, between yearly mean lead levels and volume in all tissue classes.
Because multiple environmental and developmental factors could influence adult brain volumes, we considered several potential covariates for inclusion in the final regression models. Covariate selection was performed using a modified version of our method reported previously (Cecil et al., 2008). In summary, a simple regression analysis between blood lead level and gray matter volume was used to identify regions where mean blood lead level was associated with gray matter volume change (P ≤ 0.001 unadjusted, 700 voxel minimum cluster size). After performing simple regressions, possible covariates were then added individually to the otherwise simple regression model. The change in the regression coefficient (beta 1) was calculated on voxel-byvoxel basis. Covariates were retained if the addition of the potential covariate caused a change of more than 10% in beta 1 in more than 20% of the voxels where a significant association was found in the simple regression between mean childhood blood lead and volume change. This process was repeated in a stepwise fashion until no further covariates met the inclusion criteria. This method is similar to that reported previously (Cecil et al., 2008), but uses a stepwise, rather than single-step, selection of covariates.
Covariates considered included participant age at time of imaging, current marijuana use (obtained from a urine drug screening collected at time of imaging), sex, birth weight, gestational age at birth, maternal IQ (Silverstein, 1985) maternal alcohol consumption during pregnancy, maternal marijuana use during pregnancy, maternal tobacco use during pregnancy, mean childhood Hollingshead socioeconomic status (SES) score (Cirino et al., 2002), current SES score, and HOME Inventory (using the mean Home Observation for Measurement of the Environment score measured in early childhood (Bradley and Caldwell, 1979). Two variables—age at time of imaging and birth weight—satisfied these criteria and were included in the final multiple regression models. No significant differences were observed between males and females in the distribution of any of the potential covariates, and separate analyses for each sex were not performed.
2.5 Ethical Oversight
The institutional review boards of the Cincinnati Children's Hospital Medical Center and the University of Cincinnati approved the study protocol. A Certificate of Confidentiality for the study was obtained from the National Institutes of Health.
3. Results
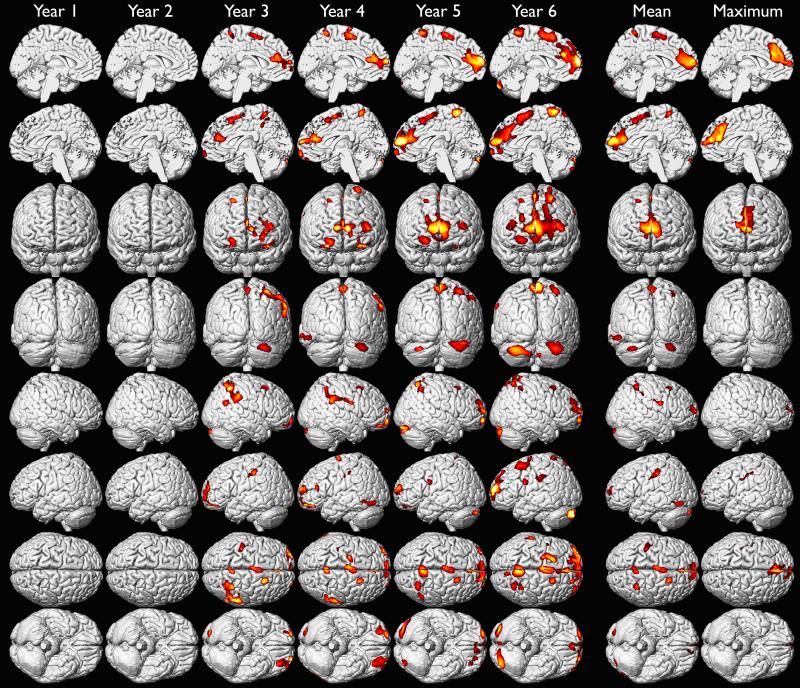
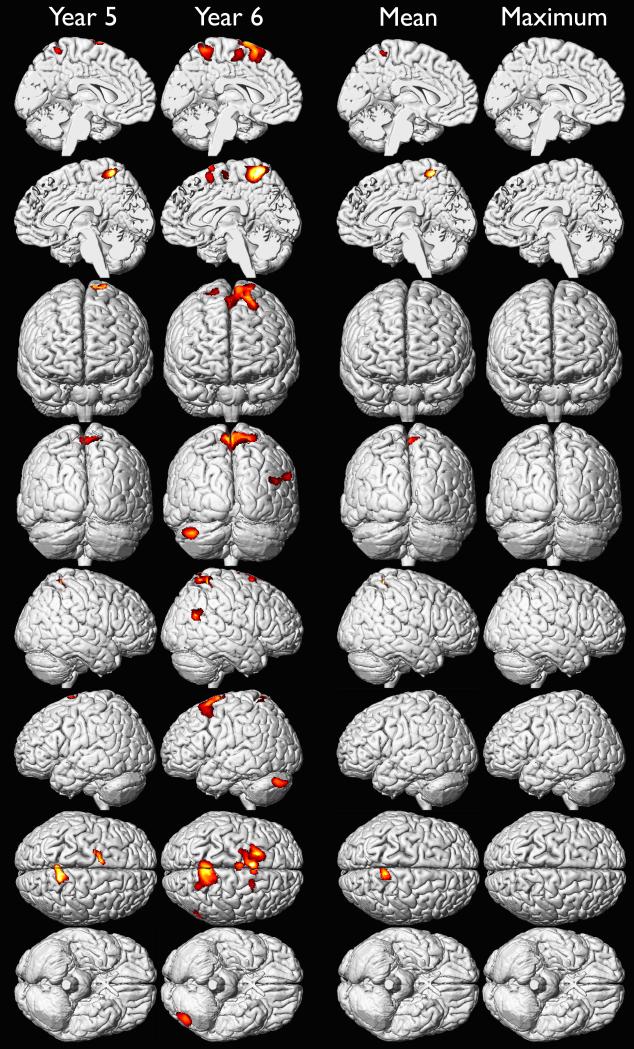
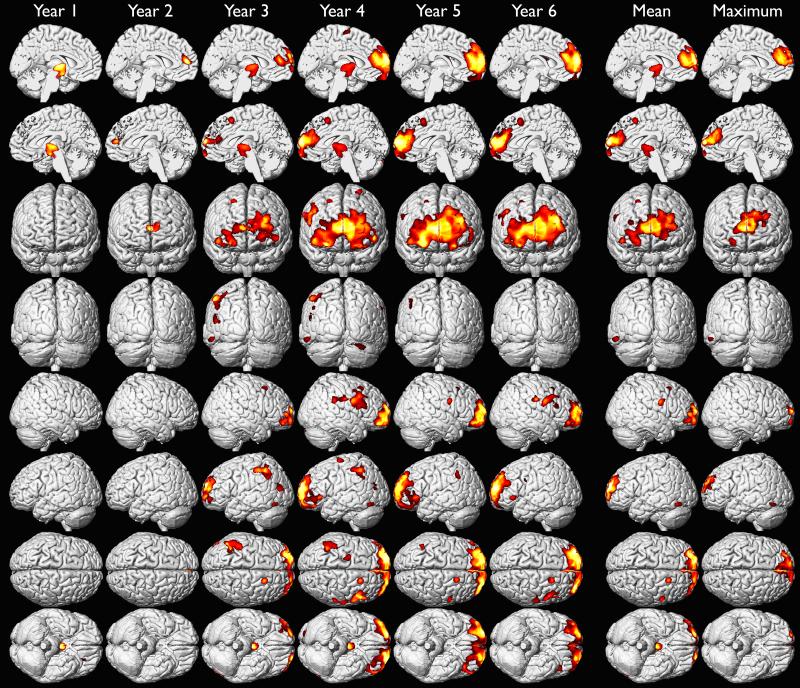
We investigated the effects of yearly mean blood lead from 1 to 6 years of age on adult gray matter volume in a voxel-wise analysis of high resolution volumetric MR images (Table 3). We found that later ages of blood lead assessment were more strongly associated with gray matter volume loss than earlier ages of assessment, and that males were more affected than females at all ages (Figures 1A-1C). These findings were most prominent in the frontal lobes. We found the largest regions of gray matter volume loss were in males associated with mean blood lead levels measured during the fifth and sixth year of life (Figure 1B). Although maximum blood lead measures were recorded at approximately 2 years of age, the strongest associations with adult gray matter volume were observed in association with blood lead levels measured at 5 and 6 years of age (Figures 1A-1C). These results suggest that blood lead measurements obtained early in childhood, mean, or maximum blood lead levels may not fully represent the extent of lead-associated gray matter changes observed in young adults.
Table 3.
Number of Voxels within Significant Gray Matter Clusters Associated with Mean Childhood Blood Lead
| Year | Population | All | Frontal | Temporal | Parietal | Cerebellar |
|---|---|---|---|---|---|---|
| 1 | Group | 855 | 0 | 0 | 855 | 0 |
| Men | 5334 | 842 | 0 | 0 | 0 | |
| Women | 0 | 0 | 0 | 0 | 0 | |
| 2 | Group | 0 | 0 | 0 | 0 | 0 |
| Men | 5202 | 2502 | 0 | 0 | 0 | |
| Women | 0 | 0 | 0 | 0 | 0 | |
| 3 | Group | 26304 | 14151 | 871 | 938 | 1944 |
| Men | 34279 | 22788 | 1246 | 4898 | 1246 | |
| Women | 0 | 0 | 0 | 0 | 0 | |
| 4 | Group | 34253 | 22913 | 0 | 5746 | 1557 |
| Men | 86815 | 74836 | 1026 | 5203 | 872 | |
| Women | 0 | 0 | 0 | 0 | 0 | |
| 5 | Group | 41116 | 29538 | 0 | 5949 | 5629 |
| Men | 91692 | 89867 | 0 | 812 | 0 | |
| Women | 2992 | 0 | 0 | 0 | 0 | |
| 6 | Group | 68028 | 44207 | 0 | 10978 | 12843 |
| Men | 84784 | 78956 | 0 | 1982 | 0 | |
| Women | 22770 | 0 | 1791 | 0 | 2101 |
Figure 1A.
Gray matter volume loss associated with adjusted yearly mean, childhood mean and maximum blood lead levels in the whole CLS cohort. Hotter colors indicate greater strength of association between blood lead level (annual, mean and maximum of childhood) and gray matter volume loss upon adjustment for age at imaging and birth weight. Significance thresholds were set at uncorrected p < 0.001 and 700 voxel minimum contiguous cluster size.
Figure 1C.
Gray matter volume loss associated with adjusted yearly mean, childhood mean and maximum blood lead levels in the females of CLS cohort. Hotter colors indicate greater strength of association between blood lead level (Year 5, Year 6, mean and maximum of childhood) and gray matter volume loss in females upon adjustment for age at time of imaging and birth weight. Significance thresholds were set at uncorrected p < 0.001 and 700 voxel minimum contiguous cluster size. No significant findings for years 1-4.
Figure 1B.
Gray matter volume loss associated with adjusted yearly mean, childhood mean and maximum blood lead levels in the males of CLS cohort. Hotter colors indicate greater strength of association between blood lead level (annual, mean and maximum of childhood) and gray matter volume loss in males upon adjustment for age at time of imaging and birth weight. Significance thresholds were set at uncorrected p < 0.001 and 700 voxel minimum contiguous cluster size.
4. Discussion
While most developed countries have reduced the major sources of lead exposure in the general population, particularly from the combustion of gasoline (petrol), lead remains a threat to healthy development in children. Lead exposure in US children results primarily from the ingestion of leaded paint residues in dust and soil, although other sources such as lead in imported toys, art materials, candies, and folk medicines can still present a risk. Blood lead levels during childhood usually correspond to the intensity of normal hand-to-mouth and ambulatory behavior, and peak at approximately 2 years of age (Ris et al., 2004). Blood lead concentrations decline in older children as they grow out of their mouthing behaviors and absorption is diminished, but there is still ongoing exposure that reflects ingestion, environmental exposure, and resorption from mineral deposits in the body throughout life.
4.1 Maximum blood lead levels
Until only recently, it was widely believed that that neurocognitive and behavioral changes in children were latent effects of peak lead exposure occurring years earlier (Bellinger et al., 1992; Pocock et al., 1994; Schwartz, 1994). Several recent studies utilizing serial blood lead measurements have observed that maximum blood lead levels were not strong predictors of all lead-associated findings (Chen et al., 2005; Tong et al., 1996; Tong et al., 1998), and that blood lead levels recorded later in childhood yielded stronger associations between cognitive (Baghurst et al., 1992; Bellinger et al., 1992; Chen et al., 2007; Chen et al., 2005; Factor-Litvak et al., 1999; Lanphear et al., 2005; Schnaas et al., 2000; Tong et al., 1996; Tong et al., 1998; Wasserman et al., 1997) and behavioral (Burns et al., 1999; Ris et al., 2004) outcomes. Comparing the effects of earlier versus later blood lead concentrations on IQ, Hornung, Lanphear, et al. (Hornung et al., 2009) found that the greatest global cognitive deficits were observed among Cincinnati and Rochester children whose blood lead concentrations continued to rise from 2 to 6 years of age. Furthermore, adult criminal arrest rates in the Cincinnati cohort were a 3.35 times higher in subjects whose 6-year blood lead level was 50% higher than their 2-year blood lead (Hornung et al., 2009).
Our findings are consistent with the cognitive and behavioral studies by demonstrating a stronger and more widespread association for blood lead levels in years five and six of life when compared with the maximal or early childhood blood lead levels (Figures 1A & 1B). However, it is noteworthy that the maximum blood lead level did demonstrate a relationship with volume loss in the frontal lobes.
4.2 Sex Differences in lead exposure outcomes
We have previously found that associations between mean childhood blood lead level and gray matter volume loss were much more widespread and significant in males than females despite comparable mean childhood blood lead levels (Cecil et al., 2008). Our previous study was the first to observe sex differences in a radiologic outcome of childhood lead exposure. Several studies, including many performed in this cohort, have observed stronger associations between lead levels in males than females by diverse neurocognitive (Bellinger et al., 1990; Cecil et al., 2008; Dietrich et al., 1987; Froehlich et al., 2007; Pocock et al., 1987; Ris et al., 2004) and behavioral (Wright et al., 2008) outcomes, though these findings are not universal (Baghurst et al., 1992; Rabinowitz et al., 1991; Tong et al., 1996). The consistency of findings of greater lead-associated neurocognitive and behavioral findings in males, and our prior work showing greater extent and significance of lead-associated gray matter volume loss in males, suggests an underlying physiologic difference in how the brains of men and women respond to childhood lead exposure.
4.3 Sex differences and developmental trajectories
Men and women have brains of different sizes, and different trajectories of gray matter maturation. White matter volume increases in a closely linear fashion from birth to early adulthood (Giedd et al., 1999; Lenroot et al., 2007), reflecting consistently increasing myelination with age (Gulani et al., 2001; Hildebrand and Waxman, 1984; Partridge et al., 2004; Suzuki et al., 2003), while total gray matter volume increases in a non-linear and region-specific manner (Giedd, 2004; Giedd et al., 1999; Lenroot and Giedd, 2006; Lenroot et al., 2007; Shaw et al., 2008). Total gray matter volume peaks at approximately 11 years of age in boys and 9 years of age in girls (Lenroot et al., 2007). Frontal and parietal gray matter volumes peak at approximately 12 years of age in boys and 10 years of age in girls (Giedd et al., 1999; Lenroot et al., 2007). Peaks in gray matter volume are thought to correspond to maximum neuronal number and synaptic density, and the subsequent decline to arise from normal pruning of synapses and neurons (Huttenlocher, 1984; Low and Cheng, 2006). While the rate of volume change is comparable between boys and girls in most regions (Giedd et al., 1999; Lenroot et al., 2007), normal gray matter volume loss occurs at a faster rate in the frontal lobes of boys than girls (De Bellis et al., 2001; Giedd et al., 1999), though this finding has not been consistently replicated (Giedd, 2004; Lenroot et al., 2007).
The different trajectories of gray matter volume change in males and females suggest different windows of neuronal vulnerability during development. Lead has been shown to be neurotoxic to cultures of developing neurons (Basha et al., 2003; Chetty et al., 2001; Reddy and Zawia, 2000), but whether the toxicity of lead varies along the course of neuronal development is unknown. If lead-associated gray matter volume loss results from lead acting during a window of developmental neuronal vulnerability, we would expect to see roughly parallel, chronologically offset patterns of gray matter volume loss in males and females, corresponding roughly to the parallel trajectories of developmental gray matter volume change. Instead, we observed widespread regions of lead-associated gray matter volume loss in males at several time points and an almost complete lack of findings in females. These results suggest males are intrinsically vulnerable, or females are intrinsically protected, from lead-associated gray matter volume loss. Female sex has been shown to be protective in epidemiologic studies of stroke, schizophrenia, and Parkinson's disease (Amantea et al., 2005). If the observed differences in male and female lead-associated gray matter volume loss are due to different physiology, estrogens may explain why the brains of men and women respond differently to similar lead exposures.
4.4 Sex differences and estradiol
Males and females have dramatically different circulating levels of sex hormones throughout life; prepubertal estradiol is approximately 8-fold higher in girls than boys (Cutler, 1997) and 25-fold higher during puberty(Ducharme et al., 1976). Estradiol enhances cell proliferation (Tanapat et al., 1999) and neuronal density (Gould et al., 1990; Hao et al., 2006; Morrison and Hof, 2007; Pozzo-Miller et al., 1999; Tang et al., 2004) and synaptic density (Woolley et al., 1996). Multiple studies have shown that estradiol protects neurons from oxidative stress (Green and Simpkins, 2000; Kolsch et al., 2001; Schmidt et al., 2002; Teepker et al., 2003; Vedder et al., 1999; Wang et al., 2001). The mechanisms of neuroprotective actions of estradiol include activation of the mitogen activated kinase pathway, altered expression of anti-apoptotic bcl-2 genes, maintentance of calcium homeostasis via the NMDA channel, and direct antioxidative action. (Green and Simpkins, 2000; Kolsch et al., 2001; Rao and Kolsch, 2003; Schmidt et al., 2002; Teepker et al., 2003; Vedder et al., 1999; Wang et al., 2001).
In a neuronal culture model, lead-exposed neurons pretreated with estradiol showed reduced expression of the antioxidant glutathione, reduced expression of the pro-apoptotic protein caspase-3, and decreases in the number of apoptotic neurons (Chetty et al., 2007). The neuroprotective properties of estrogens (Amantea et al., 2005; Rao and Kolsch, 2003), particularly estradiol (Chetty et al., 2007), provide a potential etiology for the observed neuroanatomical differences between males and females exposed to comparable levels of lead during childhood.
5. Study Limitations
A single blood lead measurement reflects both recent exposure and the ongoing resorption of lead from deep physiological depots such as bone. Individual serial blood lead measurements show that individual variability decreases with age (Mushak, 1998), a finding replicated in this cohort, resulting in a stabilization of rank order of lead exposure over time(Ris et al., 2004). This stabilization of rank order should result in stronger associations between lead levels and lead-associated outcomes with age, making it difficult to determine the extent to which studies such as this one and others (Chen et al., 2007; Chen et al., 2005; Lanphear et al., 2005; McMichael et al., 1988; Song et al., 2003) reflect the decreased variability of blood lead over time.
Our study benefits from a large sample size and detailed histories of lead exposures and perinatal, environmental and sociohereditary influences on development. This study is limited by relatively high mean blood lead concentrations at enrollment and limited generalizability due to the primarily African-American, urban, and impoverished demographics of this cohort (Chen et al., 2007; Ris et al., 2004; Wright et al., 2008). This study utilized a cross-sectional sample of volumetric magnetic resonance images obtained during adulthood as outcome measures. While this outcome measure allows for separation of concurrent from earlier developmental effects of lead exposure, a longitudinal volumetric MRI study of lead exposure during childhood and adolescence would be helpful in delineating effects of lead exposure at different ages on developing brain structures. At the time this study was designed, such an experiment was not technically feasible. While these results reflect altered neuroanatomy associated with lead exposure, the functional consequences of gray matter volume loss, or gain, are often difficult to interpret (Giedd, 2004; Gogtay et al., 2004; Lenroot and Giedd, 2006; Shaw et al., 2008). This problem is compounded when attempting to explain differences in cognitive outcome measures, such as IQ, which utilize widespread, non-distinct brain regions (Haier et al., 2004). More studies are necessary to fully understand the connections between neurocognitive and behavioral outcomes and the neuroanatomic findings described herein.
Abbreviations
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- VBM
voxel based morphometry
- GM
gray matter
- WM
white matter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52:119–32. doi: 10.1016/j.phrs.2005.03.002. Aug. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. Jun. [DOI] [PubMed] [Google Scholar]
- Baghurst PA, McMichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, Tong SL. Environmental exposure to lead and children's intelligence at the age of seven years. The Port Pirie Cohort Study. N Engl J Med. 1992;327:1279–84. doi: 10.1056/NEJM199210293271805. Oct 29. [DOI] [PubMed] [Google Scholar]
- Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH. Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. Int J Dev Neurosci. 2003;21:1–12. doi: 10.1016/s0736-5748(02)00137-5. Feb. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Sloman J. Antecedents and correlates of improved cognitive performance in children exposed in utero to low levels of lead. Environ Health Perspect. 1990;89:5–11. doi: 10.1289/ehp.90895. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Stiles KM, Needleman HL. Low-level lead exposure, intelligence and academic achievement: a long-term follow-up study. Pediatrics. 1992;90:855–61. Dec. [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM. Home observation for measurement of the environment: a revision of the preschool scale. Am J Ment Defic. 1979;84:235–44. Nov. [PubMed] [Google Scholar]
- Burns JM, Baghurst PA, Sawyer MG, McMichael AJ, Tong SL. Lifetime low-level exposure to environmental lead and children's emotional and behavioral development at ages 11-13 years. The Port Pirie Cohort Study. Am J Epidemiol. 1999;149:740–9. doi: 10.1093/oxfordjournals.aje.a009883. Apr 15. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr., Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Jarvis K, Lanphear BP. Decreased Brain Volume in Adults with Childhood Lead Exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli K, Steer CD, Ellis M, Emond AM. Effects of early childhood lead exposure on academic performance and behaviour of school age children. Arch Dis Child. 2009;94:844–8. doi: 10.1136/adc.2008.149955. Nov. [DOI] [PubMed] [Google Scholar]
- Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ. IQ and blood lead from 2 to 7 years of age: are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect. 2005;113:597–601. doi: 10.1289/ehp.7625. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Cai B, Dietrich KN, Radcliffe J, Rogan WJ. Lead exposure, IQ, and behavior in urban 5- to 7-year-olds: does lead affect behavior only by lowering IQ? Pediatrics. 2007;119:e650–8. doi: 10.1542/peds.2006-1973. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty CS, Reddy GR, Murthy KS, Johnson J, Sajwan K, Desaiah D. Perinatal lead exposure alters the expression of neuronal nitric oxide synthase in rat brain. Int J Toxicol. 2001;20:113–20. doi: 10.1080/109158101317097692. May-Jun. [DOI] [PubMed] [Google Scholar]
- Chetty CS, Vemuri MC, Reddy GR, Suresh C. Protective effect of 17-beta-estradiol in human neurocellular models of lead exposure. Neurotoxicology. 2007;28:396–401. doi: 10.1016/j.neuro.2006.03.012. Mar. [DOI] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. Jun. [DOI] [PubMed] [Google Scholar]
- Cutler GB., Jr The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61:141–4. Apr. [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–7. doi: 10.1093/cercor/11.6.552. Jun. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Krafft KM, Bornschein RL, Hammond PB, Berger O, Succop PA, Bier M. Low-level fetal lead exposure effect on neurobehavioral development in early infancy. Pediatrics. 1987;80:721–30. Nov. [PubMed] [Google Scholar]
- Dietrich KN, Berger OG, Succop PA, Hammond PB, Bornschein RL. The developmental consequences of low to moderate prenatal and postnatal lead exposure: intellectual attainment in the Cincinnati Lead Study Cohort following school entry. Neurotoxicol Teratol. 1993a;15:37–44. doi: 10.1016/0892-0362(93)90043-n. Jan-Feb. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Berger OG, Succop PA. Lead Exposure and the Motor Developmental Status of Urban Six-Year-Old Children in the Cincinnati Prospective Study. Pediatrics. 1993b;91:301–7. Feb. [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–8. doi: 10.1016/s0892-0362(01)00184-2. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- Ducharme JR, Forest MG, De Peretti E, Sempe M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42:468–76. doi: 10.1210/jcem-42-3-468. Mar. [DOI] [PubMed] [Google Scholar]
- Factor-Litvak P, Wasserman G, Kline JK, Graziano J. The Yugoslavia Prospective Study of environmental lead exposure. Environ Health Perspect. 1999;107:9–15. doi: 10.1289/ehp.991079. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Dietrich KN, Cory-Slechta DA, Wang N, Kahn RS. Interactive effects of a DRD4 polymorphism, lead, and sex on executive functions in children. Biol Psychiatry. 2007;62:243–9. doi: 10.1016/j.biopsych.2006.09.039. Aug 1. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. Oct. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. Jun. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. others. May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–91. doi: 10.1523/JNEUROSCI.10-04-01286.1990. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–58. doi: 10.1016/s0736-5748(00)00017-4. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–5. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. Feb. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–33. doi: 10.1016/j.neuroimage.2004.04.025. Sep. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. others. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C, Waxman SG. Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the development of nodes of Ranvier and axoglial relations. J Comp Neurol. 1984;224:25–37. doi: 10.1002/cne.902240103. Mar 20. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Lanphear BP, Dietrich KN. Age of Greatest Susceptiblity to Childhood Lead Exposure: A New Statistical Approach. Environ Health Perspect. 2009 doi: 10.1289/ehp.0800426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–96. Mar. [PubMed] [Google Scholar]
- Kolsch H, Ludwig M, Lutjohann D, Rao ML. Neurotoxicity of 24-hydroxycholesterol, an important cholesterol elimination product of the brain, may be prevented by vitamin E and estradiol-17beta. J Neural Transm. 2001;108:475–88. doi: 10.1007/s007020170068. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9. doi: 10.1289/ehp.7688. others. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–73. doi: 10.1016/j.neuroimage.2007.03.053. others. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Cheng HJ. Axon pruning: an essential step underlying the developmental plasticity of neuronal connections. Philos Trans R Soc Lond B Biol Sci. 2006;361:1531–44. doi: 10.1098/rstb.2006.1883. Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Baghurst PA, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ. Port Pirie Cohort Study: environmental exposure to lead and children's abilities at the age of four years. N Engl J Med. 1988;319:468–75. doi: 10.1056/NEJM198808253190803. Aug 25. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging cerebral cortex. Int Rev Neurobiol. 2007;81:41–57. doi: 10.1016/S0074-7742(06)81004-4. [DOI] [PubMed] [Google Scholar]
- Mushak P. Uses and limits of empirical data in measuring and modeling human lead exposure. Environ Health Perspect. 1998;106(Suppl 6):1467–84. doi: 10.1289/ehp.98106s61467. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Jama. 1996;275:363–9. Feb 7. [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–14. doi: 10.1016/j.neuroimage.2004.02.038. others. Jul. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Ashby D, Smith MA. Lead exposure and children's intellectual performance. Int J Epidemiol. 1987;16:57–67. doi: 10.1093/ije/16.1.57. Mar. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Smith M, Baghurst P. Environmental lead and children's intelligence: a systematic review of the epidemiological evidence. Bmj. 1994;309:1189–97. doi: 10.1136/bmj.309.6963.1189. Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J Neurophysiol. 1999;81:1404–11. doi: 10.1152/jn.1999.81.3.1404. Mar. [DOI] [PubMed] [Google Scholar]
- Rabinowitz MB, Wang JD, Soong WT. Dentine lead and child intelligence in Taiwan. Arch Environ Health. 1991;46:351–60. doi: 10.1080/00039896.1991.9934402. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- Rao ML, Kolsch H. Effects of estrogen on brain development and neuroprotection--implications for negative symptoms in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):83–96. doi: 10.1016/s0306-4530(02)00126-9. Apr. [DOI] [PubMed] [Google Scholar]
- Reddy GR, Zawia NH. Lead exposure alters Egr-1 DNA-binding in the neonatal rat brain. Int J Dev Neurosci. 2000;18:791–5. doi: 10.1016/s0736-5748(00)00048-4. Dec. [DOI] [PubMed] [Google Scholar]
- Ris MD, Dietrich KN, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and neuropsychological outcome in adolescence. J Int Neuropsychol Soc. 2004;10:261–70. doi: 10.1017/S1355617704102154. Mar. [DOI] [PubMed] [Google Scholar]
- Roda SM, Greenland RD, Bornschein RL, Hammond PB. Anodic stripping voltammetery procedure modified for improved accuracy of blood lead analysis. Clin. Chem. 1988;34:563–567. Mar. [PubMed] [Google Scholar]
- Schmidt AJ, Krieg JC, Vedder H. Differential effects of glucocorticoids and gonadal steroids on glutathione levels in neuronal and glial cell systems. J Neurosci Res. 2002;67:544–50. doi: 10.1002/jnr.10146. Feb 15. [DOI] [PubMed] [Google Scholar]
- Schnaas L, Rothenberg SJ, Perroni E, Martinez S, Hernandez C, Hernandez RM. Temporal pattern in the effect of postnatal blood lead level on intellectual development of young children. Neurotoxicol Teratol. 2000;22:805–10. doi: 10.1016/s0892-0362(00)00101-x. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Low-level lead exposure and children's IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65:42–55. doi: 10.1006/enrs.1994.1020. Apr. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. others. Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AB. Two- and four-subtest short forms of the WAIS-R: a closer look at validity and reliability. J Clin Psychol. 1985;41:95–7. doi: 10.1002/1097-4679(198501)41:1<95::aid-jclp2270410116>3.0.co;2-v. Jan. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, Todd AC, Shi W, Bassett S, Youssem D. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66:1476–84. doi: 10.1212/01.wnl.0000216138.69777.15. May 23. [DOI] [PubMed] [Google Scholar]
- Stretesky PB, Lynch MJ. The relationship between lead and crime. J Health Soc Behav. 2004;45:214–29. doi: 10.1177/002214650404500207. Jun. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Matsuzawa H, Kwee IL, Nakada T. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR Biomed. 2003;16:257–60. doi: 10.1002/nbm.848. Aug. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–23. doi: 10.1093/cercor/bhg121. others. Feb. [DOI] [PubMed] [Google Scholar]
- Teepker M, Anthes N, Krieg JC, Vedder H. 2-OH-estradiol, an endogenous hormone with neuroprotective functions. J Psychiatr Res. 2003;37:517–23. doi: 10.1016/s0022-3956(03)00068-2. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- Tong S, Baghurst P, McMichael A, Sawyer M, Mudge J. Lifetime exposure to environmental lead and children's intelligence at 11-13 years: the Port Pirie cohort study. Bmj. 1996;312:1569–75. doi: 10.1136/bmj.312.7046.1569. Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Baghurst PA, Sawyer MG, Burns J, McMichael AJ. Declining blood lead levels and changes in cognitive function during childhood: the Port Pirie Cohort Study. Jama. 1998;280:1915–9. doi: 10.1001/jama.280.22.1915. Dec 9. [DOI] [PubMed] [Google Scholar]
- Vedder H, Anthes N, Stumm G, Wurz C, Behl C, Krieg JC. Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J Neurochem. 1999;72:2531–8. doi: 10.1046/j.1471-4159.1999.0722531.x. Jun. [DOI] [PubMed] [Google Scholar]
- Wang J, Green PS, Simpkins JW. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem. 2001;77:804–11. doi: 10.1046/j.1471-4159.2001.00271.x. May. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Lolacono NJ, Factor-Litvak P, Kline JK, Popovac D, Morina N, Musabegovic A, Vrenezi N. Capuni-Paracka S and others.Lead exposure and intelligence in 7-year-old children: the Yugoslavia Prospective Study. Environ Health Perspect. 1997;105:956–62. doi: 10.1289/ehp.97105956. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–17. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. Sep 9. [DOI] [PubMed] [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]