Abstract
The nucleus paragigantocellularis (PGi) has been proposed to play a role in opiate dependence/withdrawal. In the present study, we examined the discharge activity of PGi neurons before and after the development of morphine tolerance/dependence in rats. A multi-wire electrode was chronically implanted in the PGi, which allowed us to monitor the effects of both acute and chronic morphine treatments on the activity of PGi neurons recorded from the same site. We found that acute morphine excited, inhibited or had no effect on 36%, 35% or 29% of PGi neurons (N=556), respectively. After 3 days of continuous morphine infusion, which led to morphine tolerance/dependence, the firing rates of both excitatory and inhibitory PGi neurons returned to pre-morphine treatment levels, indicating that the PGi neurons developed tolerance to both excitatory and inhibitory effects of morphine. Naltrexone-precipitated withdrawal from chronic morphine treatment also induced heterogeneous responses in the PGi. On a site-by-site basis, most of the sites that showed excitatory response to acute morphine exhibited inhibitory response during withdrawal, and all the sites that showed inhibitory response to acute morphine exhibited excitatory response during withdrawal. Correlation analysis further quantitatively showed that PGi neurons’ responses to acute morphine and that during withdrawal were inversely correlated with a correlation coefficient of 0.73, suggesting that adaptations in the PGi during the development of morphine dependence share common neural mechanisms with the acute effect of morphine. These results provide new insights into the role of the PGi in the development of morphine tolerance/dependence.
Keywords: PGi, morphine tolerance/dependence, electrophysiology, multi-electrode recording, opiate, rat
1. Introduction
Opiate drugs, such as morphine, are widely used in clinical management of pain. However, chronic use of opiate drugs leads to the development of tolerance and dependence that limit their therapeutic usefulness and contributes to a serious social and health problem. Extensive research has been conducted to identify the neural structures and neural mechanisms underlying this undesired process. Several lines of evidence suggest that the nucleus paragigantocellularis (PGi), which is located in the rostral ventral medulla, is involved in the opiate physical dependence and withdrawal symptoms. First, the PGi provides the major excitatory amino acid input to the noradrenergic locus coeruleus (LC) (Aston-Jones et al., 1986; Ennis and Aston-Jones, 1988; Ennis et al., 1992), which is a well-known structure involved in opiate dependence and withdrawal (for review see Nestler, 1992). During morphine withdrawal, there is an increase in gluatamate release in the LC (Aghajanian et al., 1994; Zhang et al., 1994), which is believed to contribute to the withdrawal-induced hyperactivity of LC neurons. Lesions of the PGi or administration of glutamate antagonists attenuated the withdrawal-induced hyperactivity of LC neurons (Rasmussen and Aghajanian, 1989; Akaoka and Aston-Jones, 1991; Rasmussen et al., 1996). Second, chronic morphine induces c-Fos expression in the PGi (Beckmann et al., 1995; Stornetta et al., 1993; Johnson et al., 2002). Third, in conscious, opioid-naïve rats, local electrical stimulation of the PGi yields a series of behaviors that are similar to those seen during opioid antagonist-precipitated withdrawal (Liu et al., 1999). Although these studies established the role of PGi in the morphine dependence, the underlying mechanisms remain unknown. The goal of the present study was to elucidate the mechanisms by examining the PGi discharge activity during the development of morphine tolerance and dependence.
Several studies suggest that the responses of PGi neurons to morphine are heterogeneous (Satoh et al., 1979; Azami et al., 1981; Baraban et al., 1995; Haghparast et al., 1998; Saiepour et al., 2001). Since the earlier neurophysiological studies employed acute recording model, the responses of PGi neurons to acute and chronic morphine treatment had to be studied in different animals (Haghparast et al., 1998; Saiepour et al., 2001). Thus, these studies were unable to identify the adaptive changes of each type of PGi neurons during the development of opiate tolerance/dependence. In the present study, a multi-wire electrode was chronically implanted on each animal so that we were able to monitor the discharge activities of the PGi neurons recorded by the same single wire before and after the induction of morphine tolerance/dependence. The chronically implanted rat model provided us the capacity that is essential to study the heterogeneous PGi neurons’ activity during the development of morphine tolerance/dependence. Furthermore, unlike previous studies that employed single electrodes that can only record one neuron from each animal, the multi-wire electrode recording method allows us to sample a large population of PGi neurons and also allows us study the temporal relationship among the discharge activities of PGi neurons. The model provided new insights into the role of PGi in morphine tolerance/dependence.
2. Material and methods
2.1. Animals and surgeries
All procedures were approved by the Institutional Animal Care and Use Committee at University of Mississippi Medical Center. Adult male Sprague-Dawley rats (300–400 grams) were used in this study. Aseptic techniques were employed during all surgical procedures. Each rat was anesthetized by sodium pentobarbital (50 mg/kg, i.p.) and fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The skull was exposed, and the head was oriented to place two skull landmarks, bregma and lamda, at the same horizontal level. Small holes were drilled in the skull at coordinates overlying the PGi (12.0 mm posterior to bregma, 1.5 mm lateral to midline) and lateral cerebral ventricle (0.5 mm posterior to bregma, 1.3 mm lateral to midline). The coordinates were estimated from the rat brain atlas of Paxinos and Watson (1998). An eight-wire electrode bundle (NB LABS, Dennison, TX) was then advanced into the PGi (8.8 mm depth). A 23-gauge guide cannula (Plastic One, Inc., Roanoke VA, USA) was implanted into the lateral ventricle (4.5 mm below the dura) for intracerebroventricular (i.c.v.) drug injection. The presence of cerebrospinal fluid in the guide cannula was examined as verification of proper placement. A stylet was placed into the guide cannula to ensure that the cannula would remain patent during the post-surgical recovery period. The microwire bundle and guide cannula were secured in place with 4 stainless steel screws trepanned through the skull and adhered with dental acrylic (Lang Dental MFG Co., Wheeling, IL). In some rats, a PE-50 catheter was implanted into femoral vein for intravenous (i.v.) drug injection. Housed individually, animals were allowed at least a week to recover from the surgeries.
2.2. Induction of morphine tolerance and dependence
Morphine tolerance/dependence was induced by continuous i.c.v. infusion with morphine sulfate (26 nmol/µl/hr, Sigma-Aldrich Inc., St. Louis, MO) for 3 days through an osmotic minipump (Alzet 2001, Alza, Palo Alto, CA). Both the infusion period and dose paradigm have been well documented by previous studies to produce robust physical dependence and tolerance (Horan and Ho, 1991; Feng et al., 1994; Zhu and Ho, 1998). The control group received equal volume of saline vehicle infusion (1 µl/hr). Before induction into the pump, the solutions were passed through 0.2 mm sterile Acrodisk filters (Gelman Science, Ann Arbor, MI). The minipumps were primed overnight at 35 °C in sterile saline so that the nominal flow rate (1 µl/hr) was attained. Under halothane anesthesia, osmotic minipumps were implanted subcutaneously between the scapulae. A 4-cm piece of Tygon tubing (0.38 mm inner diameter, Cole-Palmer, Chicago, IL) was used to connect the outlet of the minipump to an internal cannula that was placed into the i.c.v. guide cannula.
2.3. Electrophysiological recordings
PGi neuronal activities were recorded under halothane anesthesia (1.25%, mixed with oxygen) as described before (Zhu and Zhou, 2001). Body temperature was maintained at 37°C with a heating pad. Online isolation and discrimination of PGi neuronal activity was accomplished using a commercial multi-channel neuronal acquisition processor (MNAP system, Plexon Inc., Dallas, TX, USA) that allows one to monitor groups (up to 4 neurons per wire) of neurons simultaneously. Identifying different neurons on a single wire was accomplished by real-time discrimination of individual waveforms using template analysis procedures provided by the MNAP system. To ensure that neurons recorded by different wires were distinct, we compared the shape of their waveforms, firing rates, and patterns (e.g. interspike interval histograms) before further analysis.
2.4. Histology
To check the location of electrodes, at the end of recording, a current (20 µA, 10 s) was passed through a selected wire to create lesions. Three days after the lesion, rats were sacrificed with overdose of pentobarbital, and their brains were removed and fixed in phosphate-buffered paraformaldehyde. Brains were then frozen in liquid nitrogen and 45-µm coronal sections were cut in a cryostat and mounted on glass slides. The sections were stained with cresyl violet and examined under light microscope. The locations of gliosis were plotted onto drawings of the sections using a microprojector.
2.5. Experimental procedures
One group of rats was used for determination of the effects of a single dose of morphine on PGi neuronal activity only. The rats received a single dose of morphine through either i.c.v. (26 nmol, 5µl) or i.v. (2.5 mg/kg) route. The dosages have been shown to produce significant analgesia effects in previous studies (Feng et al., 1994; Dickenson et al., 1979). To test the involvement of opioid receptors, opioid antagonist naltrexone (50 nmol, i.c.v. or 5 mg/kg, i.v.) or saline vehicle was applied about 20 min after morphine injection. The spontaneous activities of PGi neurons were recorded under halothane anesthesia before and after the drug injections.
Another set of rats received both acute and chronic morphine treatment. Before the induction of morphine tolerance/dependence, the rats were challenged with a single dose of morphine (26 nmol, 5µl, i.c.v.). Then, the rats received continuous morphine infusion for 3 days to induce tolerance and physical dependence. The control group received equal volume of saline infusion. On day 4, after termination of the infusions, the morphine-dependent rats were challenged with opioid antagonist naltrexone (50 nmol, i.c.v.) to precipitate withdrawal. The responses of PGi neurons to acute and chronic morphine treatment were recorded under halothane anesthesia.
2.6. Data analysis
Mean firing rates, auto-correlograms and cross-correlograms were analyzed using Neuroexplorer (Nex Technologies, Lexington, MA, USA) and Matlab (Mathworks, Natick, MA, USA) software. The degree of oscillation was quantified by an oscillatory index, which was computed as the ratio of the amplitude of the first satellite peak to the offset of the auto-correlogram (König, 1994). The strength of synchrony was quantified by a synchrony index, which was computed as the ratio of the amplitude of the central peak to the offset of the cross-correlogram (König, 1994). A change in activity was defined as an increase or decrease in firing rate by mean baseline activity ± two SEM, respectively. The effects of morphine on the mean firing rates, oscillatory indexes, and synchrony indexes were analyzed by t-test, paired t-test or one-way repeated-measures ANOVA. Data are presented as mean ± S.E.M.
3. Results
3.1. PGi neurons’ responses to acute morphine treatment
The effects of acute morphine treatment on PGi neurons’ spontaneous firing were examined in 556 neurons from 23 rats. Of the 23 rats, 20 rats received i.c.v. morphine injection (26 nmol, 5µl) and 3 rats received i.v. morphine injection (2.5 mg/kg). The PGi neurons exhibited heterogeneous responses to acute morphine treatment. Fig. 1 shows typical excitatory responses (A and C) and inhibitory responses (B and D) of PGi neurons to acute morphine treatment.
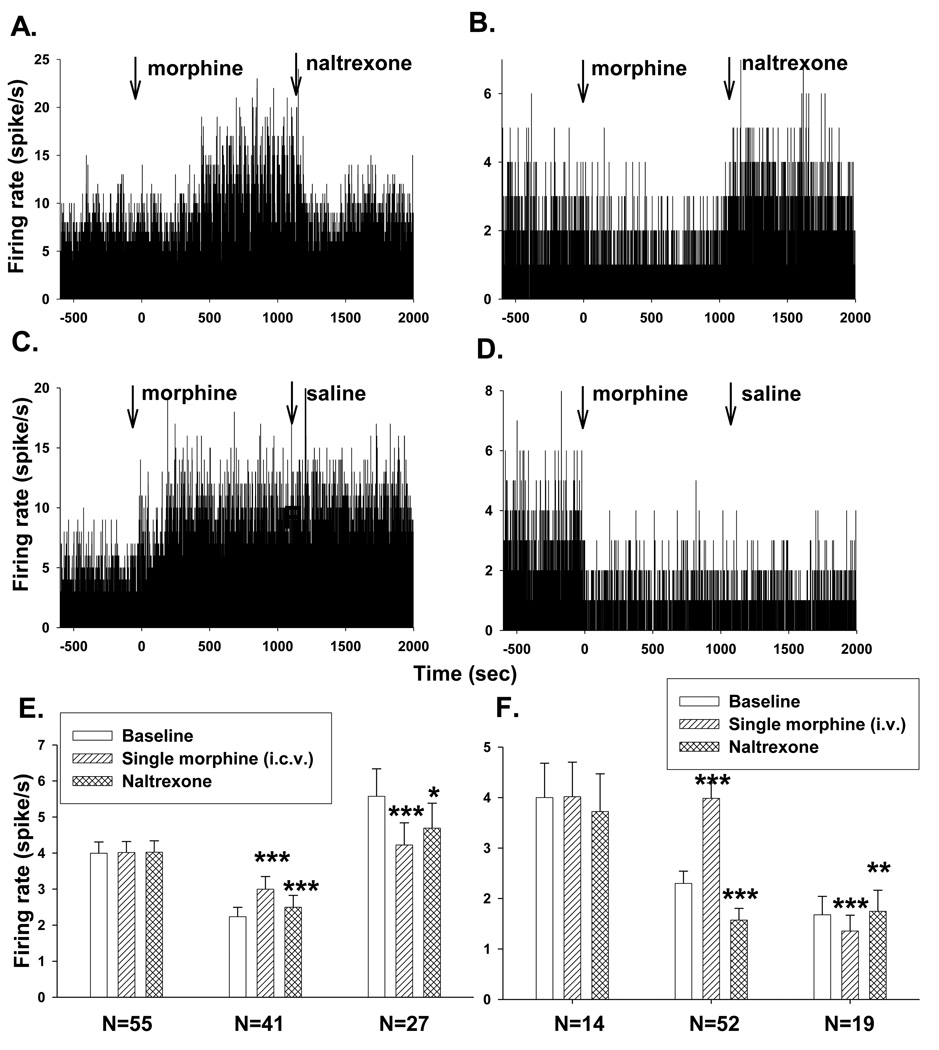
Fig.1.
Effects of a single dose of morphine on PGi neuronal activities. (A–D) Histograms of excitatory and inhibitory effects of a single dose of morphine on PGi neurons. Morphine was injected i.c.v. at time 0. Naltrexone or saline vehicle was injected 20 min after morphine injection. (E and F) Summary of the reversal effects of naltrexone on acute morphine-induced inhibition and excitation of PGi neurons (E. i.c.v. injection, F. i.v. injection). Bars represent mean firing rate of PGi neurons 10 min before morphine injection (baseline), 10–20 min after morphine injection and 0–10 min after naltraxone injection, respectively. Error bars represent S.E.M. ***P< 0.001, **P<0.005, *P<0.05 (One-way repeated measures analysis of variance and Boferonii test).
Of a total of 471 PGi neurons recorded from the 20 rats that received i.c.v. injection of a single dose of morphine, 149 (32%) neurons exhibited an increase in firing rate (baseline: 1.74±0.23 spike/s; 10 min after morphine administration: 2.56±0.31 spike/s, paired t-test, P<0.000), 175 (37%) neurons exhibited a decrease in firing rate (baseline: 2.76±0.34 spike/s; after morphine administration: 1.93±0.76 spike/s, P<0.000, paired t-test) and 147 neurons (31%) exhibited no change in firing rate (baseline: 4.15±0.60 spike/s; after morphine administration: 4.09±0.48 spike/s, P>0.8, paired t-test). There was no significant difference between the baseline firing rates of the excitatory neurons and the inhibitory neurons (P>0.05, t-test). However, the baseline firing rate of the neurons that showed no significant response to morphine was significantly higher than those exhibited responses to morphine (P=0.01, t-test).
A single dose of morphine injected through i.v. route produced similar effects. Of a total of 85 PGi neurons recorded from 3 rats, 52 (61%) neurons exhibited an increase in firing rate (baseline: 2.30±0.24 spike/s; after morphine administration: 4.00±0.30 spike/s, P<0.000, paired t-test), 19 (22%) neurons exhibited a decrease in firing rate (baseline: 1.68±0.37 spike/s; after morphine administration: 1.35±0.32 spike/s, P<0.000, paired t-test), and 14 (17%) neurons exhibited no response in firing rate (baseline: 4.0±0.68 spike/s; after morphine administration: 4.02±0.68 spike/s, P>0.3). These results indicated that the morphine-induced heterogeneous responses in the PGi were not due to an unexpected effect of morphine that was injected by the i.c.v. route.
Opioid antagonist naltrexone was applied to 6 rats that received i.c.v. morphine injection and the 3 rats that received i.v. morphine injection. Naltrexone was injected about 20 min after morphine injection. The morphine-induced excitation or inhibition was reversed by administration of naltrexone (Fig. 1A and 1B) but not saline vehicle (Fig. 1C and 1D). The reversal effects of naltrexone are summarized in Fig. 1E (i.c.v. injection) and 1F (i.v. injection). One-way repeated measures analysis of variance and Boferonii test showed significant differences in mean firing rate before and after naltrexone injection.
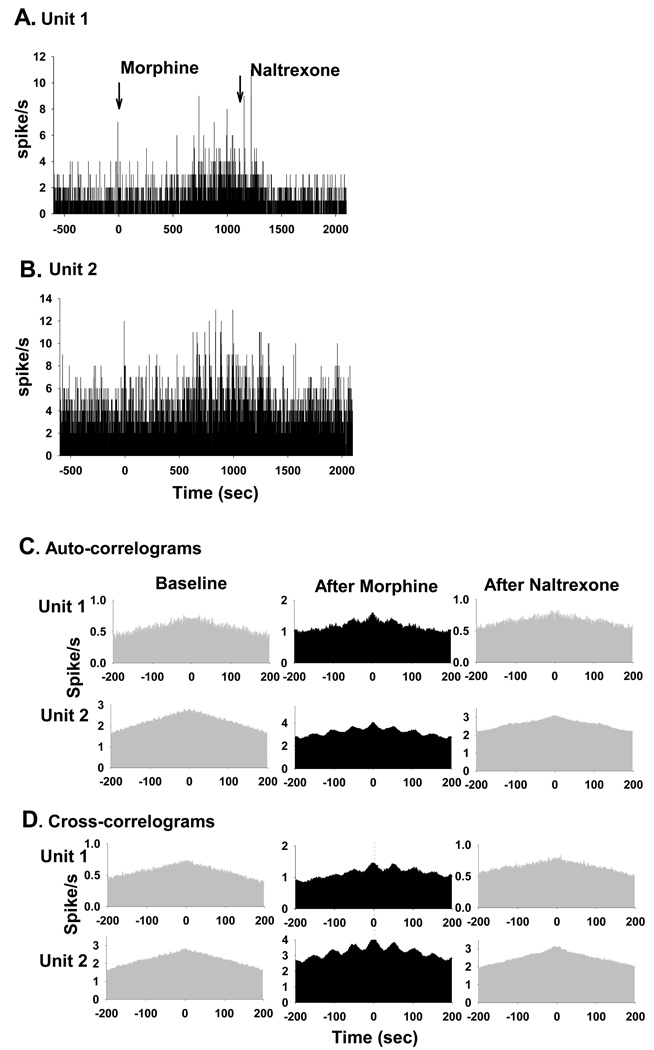
Among the total of 556 PGi neurons, 13 neurons (2%) from 3 rats exhibited synchronized oscillatory burst activities after acute morphine treatment. All but one (12/13) exhibited an increase in mean firing rate after morphine injection. Fig. 2shows the auto-correlograms and cross-correlograms of two simultaneously recorded PGi neurons. The regularity of the burst activity of each neuron was assessed by auto-correlograms, which show distinctive satellite peaks after morphine injection. The degree of synchrony between the pair of oscillatory PGi neurons was assessed by cross-correlograms, which show a distinctive central peak indicating synchronous activity after morphine injection (Fig. 2B). Auto-correlation analysis revealed that 7.82±1.36% of the neuronal activity was deemed oscillatory after morphine injection, compared to 0% of the activity before morphine (P <0.003, One-way repeated measures analysis of variance and Boferonii test, n=13 neurons). The duration of the oscillatory cycle was about 50–60 sec, which is similar to that observed in the LC (Zhu and Zhou, 2001). Cross-correlation analysis revealed that all of the 13 neurons that exhibited oscillatory discharges were synchronized with at least one other neuron. Ten to 20 min after morphine injection, 17.63±2.06 % of the PGi neuronal activity was synchronous, compared to 7.36±0.96% before morphine injection (P<0.001, One-way repeated measures analysis of variance and Boferonii test, n=15 pairs). The morphine-induced synchronous burst activities were reversed by i.c.v (50 nmol) or i.v. (5 mg/kg) injection of opioid antagonist naltrexone (Fig. 2A and 2B). The oscillatory indexes were 0% after naltrexone (P<0.05, One-way repeated measures analysis of variance and Boferonii test, n=13). The synchronous indexes were 4.95±0.17 % after administration of naltrexone (P<0.005, One-way repeated measures analysis of variance and Boferonii test, n=15 pairs).
Fig. 2.
Effects of morphine on temporal relationship among PGi neurons’ activities. (A) and (B) Histograms of spontaneous firing rates of 2 simultaneously recorded PGi neurons (Unit 1 and Unit 2) before and after morphine and naltrexone injections. Morphine was injected at time 0 (26 nmol, i.c.v.). Naltrexone was injected 20 min after morphine administration. (C) Auto-correlograms of the two PGi neurons before and after morphine and naltrexone injections. Note the large satellite peaks after morphine and lack of satellite peaks after naltrexone. (D) Cross-correlograms of the two neurons before and after morphine and naltrexone injections. Note the large central peaks after morphine and lack of central peaks after naltrexone.
3.2. Correlation between PGi neurons’ responses to acute morphine treatment and that to chronic morphine treatments
The effects of both acute and chronic morphine treatments on PGi neurons’ activity were studied in 6 rats. Before the induction of morphine tolerance/dependence, the responses of 147 PGi neurons to acute morphine treatment were recorded from 42 wires (i.e., sites). After chronic morphine treatment, the responses of 154 PGi neurons from the same sites were recorded before and after naltrexone injection (withdrawal). For each wire, up to 4 neurons were isolated. Since the neurons isolated by a single wire usually exhibited the same type of response, their responses were averaged to represent the response of the site (Table 1).
Table1.
Effects of acute and chronic morphine treatment on the spontaneous firing rates of PGi neurons
| Pre-infusion | After infusion(Tolerance /Dependence) | |||
|---|---|---|---|---|
| Baseline | Acute morphine | Baseline | Withdrawal | |
| Excitation site(18) | 1.86±0.29 | 2.87±0.57a | 1.98±0.17 | 1.42±0.20c |
| Inhibition site(11) | 1.97±0.33 | 1.41±0.23b | 2.48±0.49 | 2.98±0.58b |
| Non-response site(14) | 5.45±1.17 | 5.40±1.16 | 3.51±0.51 | 3.19±0.49 |
Data are presented as mean ± S.E.D (spike/s).
The responses of PGi neurons to acute and chronic morphine treatment were recorded from 42 wires from 6 rats.
‘Excitatory site’, i.e., the recording sites (wire) that showed excitatory response to acute morphine injection.
‘Inhibitory site’, i.e., the recording sites (wire) that showed inhibitory response to acute morphine injection.
‘Non-response site’, i.e., the recording sites (wire) that did not show no significant response to acute morphine injection.
Numbers in parentheses refer to the number of wires.
P< 0.005,
P≤0.002,
P<0.0005, compared to baseline values (paired t-test).
First, we examined whether PGi neurons developed tolerance to morphine after continuous morphine infusion. Since the PGi neurons exhibited heterogeneous responses to acute morphine treatment, we performed separate analysis for the sites that exhibited excitatory response to acute morphine (excitatory sites) and the sites that exhibited inhibitory response (inhibitory sites). We found that there were no significant differences in neurons’ baseline firing rates before and after chronic morphine treatment for both the excitatory sites (1.86±0.29 spike/s before vs. 1.98±0.17 spike/s after, t-test, P=0.72, n=18) and the inhibitory sites (1.97±0.33 spike/s before vs. 2.48±0.49 spike/s after, t-test, P=0.40, n=11), indicating that PGi neurons developed tolerance to morphine after continuous morphine infusion.
Second, we examined the effects of naltrexone-precipitated withdrawal on PGi neurons’ activity. We found that withdrawal from morphine also induced heterogeneous responses in the PGi. Of the total of 42 sites, naltrexone injection induced a decrease in firing rate in 16 sites, an increase in firing rate in 12 sites and no significant changes in 14 sites.
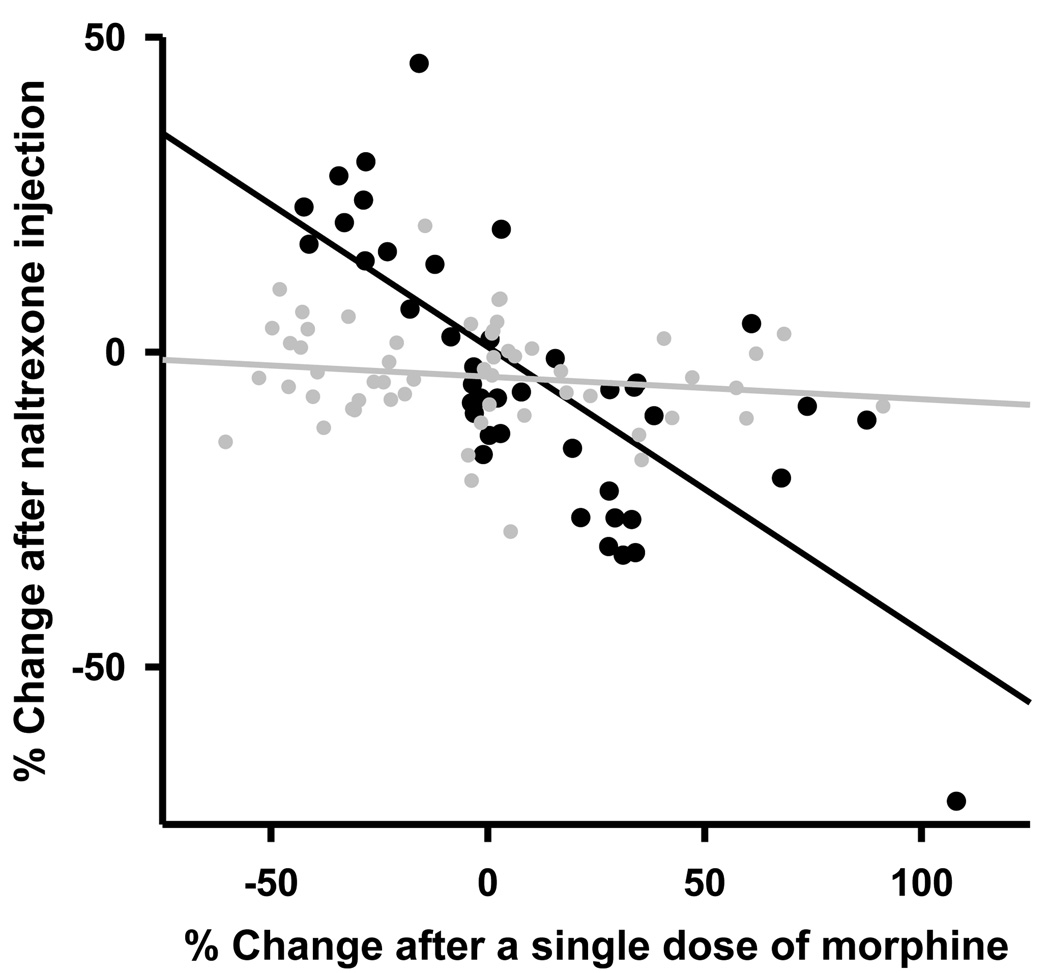
Third, we compared the PGi neurons’ responses to acute morphine treatment and that during the naltrexone-precipitated withdrawal. Among the 18 sites that exhibited excitatory response to acute morphine, 12 of them (66.7%) exhibited a decrease in firing rate during the naltrexone-precipitated withdrawal (1.94±0.21 spike/s before naltrexone vs. 1.42±0.20 spike/s after naltrexone, P<0.0003, n=12 paired t-test). Among the 11 sites that exhibited inhibitory response to acute morphine treatment, all of them showed an increase in firing rate during the withdrawal (2.48±0.49 vs. 2.98±0.58 spike/s, P=0.002, n=11, paired t-test). For the 13 sites that exhibited no change in firing rate to acute morphine treatment, 9 of them (69.2%) also exhibited no change in firing rate during withdrawal (3.72±0.67 vs. 3.50±0.60 spike/s, P>0.05, n=9, paired t-test). Regression analysis revealed quantitatively that the responses of PGi neurons to acute morphine treatment were inversely correlated to that to naltrexone-precipitated withdrawal (R=0.73, R2=0.53, P<0.0001, Fig. 3, black symbols). A slope of −0.45 indicates that chronic morphine treatment induces an adaptive change in the PGi neurons that compensates about 45% of their responses to acute morphine treatment.
Fig. 3.
Correlation between the responses of PGi neurons to a single dose of morphine and that during the naltrexone-precipitated withdrawal from chronic morphine infusion (black circle, and black line, R=0.73, R2=0.53, P<0.0001). In the control groups, there was no significant correlation between the response of PGi neurons to a single dose of morphine and that to naltrexone after chronic saline infusion (R = 0.17, R2 = 0.03, P>0.2, grey circles and grey line).
In the control group (n=8), saline vehicle was infused through osmotic mini pump for 3 days. Of a total of 51 sites (wires) in the PGi, acute morphine treatment induced a decrease in firing rate in 24 recording sites (47%), an increase in firing rate in 13 sites (25.5%) and no change in firing rate in 14 sites (27.5%). After chronic saline infusion, naltrexone injection induced an increase in firing rate in 1 site (2%), a decrease in firing rate in 8 sites (15.6%) and no change in firing rate in 42 (82.4%) sites. Regression analysis showed that there was no significant correlation between the responses of PGi neurons to acute morphine treatment and that to naltrexone injection after chronic saline infusion (R=0.17, R2=0.03, P>0.2, Fig. 3, grey symbols).
3.3. Distribution of morphine sensitive PGi neurons
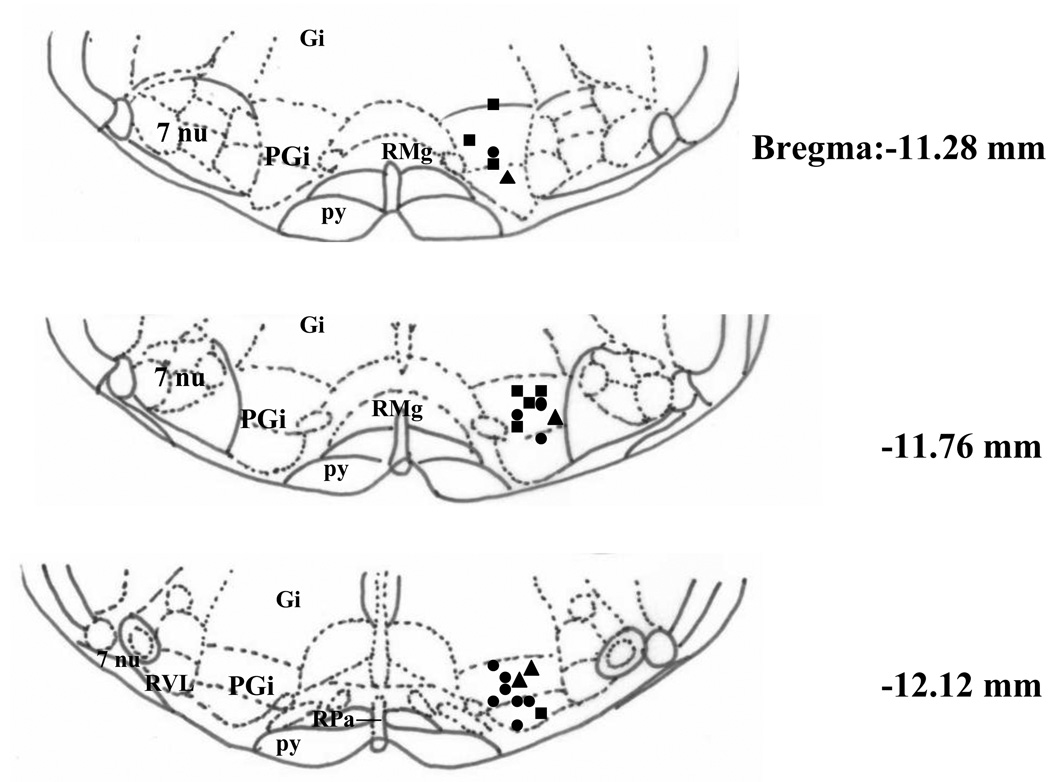
At the end of recording session, a small positive current (20 µA, 10 sec) was passed through a selected microwire to mark its location. The wire was selected based on the neurons’ responses to a single dose of morphine. Fig. 4 shows the distribution of 23 recording sites from a total of 23 rats (11 excitatory, 8 inhibitory and 4 no effect). Each type of recording site seems to distribute evenly through out the entire PGi.
Fig. 4.
Distribution of the recording sites in the PGi. The recording sites that exhibited inhibition, excitation or no change to a single dose of morphine are indicated by squares, circles and triangles, respectively. Figures are redrawn after Paxios and Watson (1998) and present as cross-sections of the medulla. Abbreviations include PGi (nucleus paragigantocellularis), Gi (gigantocellular reticular nucleus), RMg (raphe magnus nucleus), 7nu (facial nucleus), py (pyramidal tract), RPa (raphe pallidus nucleus) and RVL (rostroventrolateral reticular nucleus).
4. Discussion
In this study, we employed a chronically implanted rat model and for the first time examined the effects of acute and chronic morphine treatments on the same group of PGi neurons. First, by studying a large population of PGi neurons, we confirmed that acute morphine treatment induced heterogeneous responses in the PGi neurons. Both of the excitatory and inhibitory effects involved opioid receptors. Second, on a site-by-site basis, PGi neurons developed tolerance to morphine after continuous morphine infusion. Third, naltrexone-precipitated withdrawal also induced heterogeneous responses in the PGi. On a site-by-site basis, the responses of PGi neurons during withdrawal were inversely correlated to that to acute morphine. Whereas the sites with acute excitatory responses exhibited inhibitory responses during the withdrawal, the sites with acute inhibitory responses exhibited excitatory responses during the withdrawal. This specific correlation between acute and chronic effects of morphine suggests that adaptations in the PGi during the development of morphine dependence share common neural mechanisms with the acute effect of morphine.
4.1. Acute morphine effect on the PGi neurons
Earlier studies employed single electrode that not only limited their sample size, but also might bias their samples to certain types of PGi neurons. Inconsistent findings were reported: excitation only (Satoh et al., 1979), inhibition only (Haghparast et al., 1998) or both (Saiepour et al., 2001; Azami et al. 1981). Given the neurochemical heterogeneity and broad anatomical distribution of PGi neurons, it is expected that PGi neurons exhibit heterogeneous responses to acute morphine treatment. In order to characterize PGi neurons’ responses to acute morphine, a large population of PGi neurons needs to be sampled throughout the nucleus. This objective was very difficult to be achieved in the previous studies because only one neuron can be studied from each animal with a single electrode. In the present study, we employed the multi-wire recording technique and were able to study the responses of a large number of PGi neurons from each animal. A total of 556 PGi neurons were recorded. We confirmed the hypothesis that acute morphine treatment induces heterogeneous responses in the PGi neurons. In majority of animals, all the three types of responses were observed. Histology study revealed that each type of neurons was evenly distributed throughout the PGi.
Our previous studies (Zhu and Zhou, 2001, 2005) showed that morphine did not simply decrease the firing rate of neurons in the noradrenergic LC as previously believed, but that it induced synchronous oscillatory burst activities in the LC. The morphine-induced long-lasting synchronous oscillatory activity in the LC may be a neuronal signal that could induce synaptic plasticity leading to opioid addiction (Zhu and Zhou 2003). In the present study, only a tiny group of excitatory PGi neurons (~2%) exhibited synchronized burst activity. Thus, the PGi inputs to the LC may not provide the neural signals that directly drive the oscillatory activity in the LC. Nevertheless, the PGi inputs to the LC may contribute to the generation of the morphine-induced oscillation and synchrony in the LC by providing a sustained excitatory input. Consistent with this hypothesis, our previous study (Zhu and Zhou, 2005) showed that the morphine-induced oscillation and synchrony were reversed by injection of excitatory amino acid receptor antagonists.
Morphine may modulate PGi neuronal activity by both pre-synaptic and post-synaptic mechanisms. Studies of presympathetic neurons of the rat rostral ventrolateral medulla have demonstrated that μ-opioid receptors were expressed in both the neurons and their synaptic inputs (Aicher et al., 2001). In vitro recordings from neonatal rat brains demonstrated that opioid agonists produce inhibitory effects on both the presympathetic neurons (post-synaptic inhibition) and their GABAergic and glutamatergic inputs (pre-synaptic inhibition) (Hayar and Guyenet, 1998). It is also possible that systemic administration of morphine affects PGi neuronal activity by acting on opioid-sensitive neurons that project to PGi. Future studies are needed to elucidate the specific mechanisms that produce the heterogeneous responses to acute morphine treatment in the PGi.
4.2. Chronic morphine action in the PGi
It has been generally accepted that chronic administration of morphine induces adaptive changes in the central nervous system that counter the effects of acute morphine treatment and attempt to restore a normal equilibrium. The adaptations lead to tolerance, a reduction of sensitivity or loss of responsiveness to opioid, and/or dependence, a state in which opioid drug is required to maintain normal physiological function and to avoid a withdrawal syndrome. The tolerance and physical dependence following chronic exposure to opioid have been extensively investigated in the noradrenergic LC (for review see Nestler et al., 1994, Nestler and Aghajanian, 1997; Zhu et al., 1998), a brain region that was thought to be relatively homogeneous. However, it is very difficult to study tolerance and dependence in a heterogeneous brain region such as PGi because it requires comparison of the acute and chronic effects on the same group of neurons. In the present study, the chronically implanted rat model allowed us to study the effects of both acute and chronic morphine treatments on the activity of PGi neurons recorded from the same site.
Tolerance to the inhibitory effect of morphine has been found in the noradrenergic LC neurons after chronic morphine treatment (Aghajanian, 1978; Christie et al., 1987). The firing rate of LC neurons was initially suppressed by morphine, but activity returned towards normal rates by 48–72 hours of continuous morphine treatment. Several studies proposed that PGi neurons also became tolerant to morphine (Haghparast et al., 1998; Saiepour et al., 2001) based on two observations: 1) the averaged baseline firing rate of PGi neurons in chronically morphine-treated rats was not significant different from that in opioid naïve rats; 2) PGi neurons in the morphine-dependent rats did not respond to a single morphine challenge. However, these methods can not determine whether PGi neurons became tolerant because the effects of acute and chronic morphine treatments on PGi neurons were studied on different groups of rats that did not allow the comparison of the acute and chronic effects on the same group of neurons. In the present study, the changes in PGi neuronal activity were examined in the same rats on a site-by-site basis. After continuous morphine infusion, despite of the presence of high level of morphine, the spontaneous firing rates of either the excitatory or the inhibitory PGi neurons recovered toward the pre-morphine level, indicating that PGi neurons developed tolerance to both excitatory and inhibitory effects of morphine treatment.
At behavioral level, the opiate-withdrawal symptoms are generally opposite to the physiological responses observed after acute opioid administration. In the present study, on a site-by-site basis, we demonstrated that during the naltrexone-precipitated withdrawal, PGi neurons exhibited discharge changes that were opposite to that observed after acute treatment, i.e., most of the ‘excitatory sites’ exhibited inhibitory responses and most of the ‘inhibitory sites’ exhibited excitatory responses. Most of the non-response sites did not respond to naltrexone. Since after 3 days of continuous morphine infusion, the PGi neurons developed tolerance to acute effects of morphine, the naltrexone-induced changes in PGi activity could not be due to a simple reversal of the effects of morphine that was present in the brain. Our data not only revealed that the withdrawal-induced changes in PGi neuronal activity were qualitatively opposite to the acute morphine actions, but also that the withdrawal-induced changes were quantitatively correlated to that caused by acute morphine treatment. To the best of our knowledge, this is the first time that a quantitative correlation between acute and chronic effects of morphine treatment has been demonstrated in the same group of neurons in vivo. The correlation suggested that the adaptation that was expressed during withdrawal from continuous morphine exposure was proportional to the initial action of morphine, i.e., a PGi neuron’s rebound during withdrawal can be predicted by its response to acute morphine. The cellular events underlying the withdrawal-induced changes in PGi neuronal activity remain to be elucidated.
The withdrawal-induced adaptive changes in PGi neuronal activity may play an important role in the development and expression of dependence/withdrawal. Since endogenous opioid in the PGi neurons have been implicated in modulation of autonomic activity and pain (Dampney et al., 1982; Punnen et al., 1984; Sun et al., 1988; Ciriello et al., 1986; Akaike et al., 1978; Azami et al., 1982; Satoh et al., 1979; Van Bockstaele and Aston-Jones, 1995), the changes in PGi activity during withdrawal may account for certain physical and autonomic signs of withdrawal syndrome as well as withdrawal-induced hyperalgesia. This is supported by behavioral studies that demonstrated that direct stimulations of PGi in free moving rats induced behavioral signs that were similar to those seen during opiate withdrawal (Liu et al., 1999; Rockhold et al., 2000). The withdrawal-induced changes in PGi may also have significant influence on its target sites. The PGi provides the major excitatory amino acid input to the noradrenergic LC (Aston-Jones et al., 1986; Ennis and Aston-Jones, 1988; Ennis et al., 1992), which is strongly activated during opioid withdrawal in vivo (for review see Nestler, 1992). Future studies will further examine these issues with the chronically implanted rat model.
Acknowledgement
Supported by Center for Psychiatric Neuroscience Small Grant Programs and NIDA R03 (016440). We thank Xiaomei Zhu, Mathew Burford, and Yutong Liu for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Tolerance of locus coeruleus neurons to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Kogan JH, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Schreihofer AM, Kraus JA, Sharma S, Milner TA, Guyenet PG. μ-opioid receptors are present in functionally identified sympathoexcitatory neurons in the rat rostral ventrolateral medulla. J. Comp. Neurol. 2001;433:34–47. doi: 10.1002/cne.1123. [DOI] [PubMed] [Google Scholar]
- Akaike A, Shibata T, Satoh H, Tagaki H. Analgesia induced by microinjection of morphine into, and electrical stimulation of, the nucleus reticularis paragigantocellularis of rat medulla oblongata. Neuropharmacology. 1978;17:775–778. doi: 10.1016/0028-3908(78)90093-x. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J. Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a board afferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Azami J, Wright DM, Roberts MH. Effects of morphine and naloxone on the responses to noxious stimulation of neurones in the nucleus reticularis paragigantocellularis. Neuropharmacology. 1981;20:869–876. doi: 10.1016/0028-3908(81)90080-0. [DOI] [PubMed] [Google Scholar]
- Azami J, Llewelyn MB, Roberts MH. The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain. 1982;12:229–246. doi: 10.1016/0304-3959(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Stornetta RL, Guyenet PG. Effects of morphine and morphine withdrawal on adrenergic neurons of the rat rostral ventrolateral medulla. Brain Res. 1995;676:245–257. doi: 10.1016/0006-8993(95)00097-a. [DOI] [PubMed] [Google Scholar]
- Beckmann AM, Matsumoto I, Wilce PA. Immediate early gene expression during morphine withdrawal. Neuropharmacology. 1995;34:1183–1189. doi: 10.1016/0028-3908(95)00089-o. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol. Pharmacol. 1987;32:633–638. [PubMed] [Google Scholar]
- Ciriello J, Caverson MM, Polosa C. Function of the ventrolateral medulla in the control of the circulation. Brain Res. 1986;396:359–391. doi: 10.1016/0165-0173(86)90005-6. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Goodchild AK, Robertson LG, Montgomery W. Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res. 1982;249:223–235. doi: 10.1016/0006-8993(82)90056-7. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Oliveras JL, Besson JM. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res. 1979;170:95–111. doi: 10.1016/0006-8993(79)90943-0. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J. Neurosci. 1988;8:3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G, Shiekhattar R. Activation of locus coeruleus neurons by nucleus paragigantocellularis or noxious sensory stimulation is mediated by intracoerulear excitatory amino acid neurotransmission. Brain Res. 1992;598:185–195. doi: 10.1016/0006-8993(92)90182-9. [DOI] [PubMed] [Google Scholar]
- Feng YZ, Tseng YT, Jaw SP, Hoskins B, Ho IK. Tolerance development to butorphanol: comparison with morphine. Pharmacol. Biochem. Behav. 1994;49:649–655. doi: 10.1016/0091-3057(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Haghparast A, Semnanian S, Fathollahi Y. Morphine tolerance and dependence in the nucleus paragigantocellularis: Single unit recording study in vivo. Brain Res. 1998;814:71–77. doi: 10.1016/s0006-8993(98)01029-4. [DOI] [PubMed] [Google Scholar]
- Hayar A, Guyenet PG. Pre- and postsynaptic inhibitory actions of methionine-enkephalin on identified bulbospinal neurons of the rat RVL. J. Neurophysiol. 1998;80:2003–2014. doi: 10.1152/jn.1998.80.4.2003. [DOI] [PubMed] [Google Scholar]
- Horan PJ, Ho IK. The physical dependence liability of butorphanol; a comparative study with morphine. Eur. J. Pharmacol. 1991;203:387–391. doi: 10.1016/0014-2999(91)90895-w. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Peoples J, Stornetta RL, Van Bockstaele EJ. Opioid circuits originating from the nucleus paragigantocellularis and their potential role in opiate withdrawal. Brain Res. 2002;955:72–84. doi: 10.1016/s0006-8993(02)03367-x. [DOI] [PubMed] [Google Scholar]
- König P. A method for the quantification of synchrony and oscillatory properties of neuronal activity. J. Neurosci. Meth. 1994;54:31–37. doi: 10.1016/0165-0270(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Liu N, Rockhold RW, Ho IK. Electrical stimulation of nucleus paragigantocellularis induces opioid withdrawal-like behaviors in the rat. Pharmacol. Biochem. Behav. 1999;62:263–271. doi: 10.1016/s0091-3057(98)00164-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J. Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Altreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res. Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Punnen S, Willette R, Krieger AJ, Sapru HN. Cardiovascular response to injections of enkephalin in the pressor area of the ventrolateral medulla. Neuropharmacology. 1984;23:939–946. doi: 10.1016/0028-3908(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: Attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 1989;505:346–350. doi: 10.1016/0006-8993(89)91466-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Kendrick WT, Kogan JH, Aghajanian GK. A selective AMPA antagonist, LY293558, suppresses morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropsychopharmacology. 1996;15:497–505. doi: 10.1016/S0893-133X(96)00094-2. [DOI] [PubMed] [Google Scholar]
- Rockhold RW, Liu NS, Coleman D, Commiskey S, Shook J, Ho IK. The nucleus paragigantocellularis and opioid withdrawal-like behavior. J. Biomed. Sci. 2000;7:270–276. doi: 10.1007/BF02255476. [DOI] [PubMed] [Google Scholar]
- Saiepour MH, Semnanian S, Fathollahi Y. Occurrence of morphine tolerance and dependence in the nucleus paragigantocellularis neurons. Eur. J. Pharmacol. 2001;411:85–92. doi: 10.1016/s0014-2999(00)00862-1. [DOI] [PubMed] [Google Scholar]
- Satoh M, Akaike A, Takagi H. Excitation by morphine and enkephalin of single neurons of nucleus reticularis paragigantocellularis in the rat: a probable mechanism of analgesic action of opioids. Brain Res. 1979;169:406–410. doi: 10.1016/0006-8993(79)91043-6. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Norton FE, Guyenet PG. Autonomic areas of rat brain exhibit increased Fos-like immunoreactivity during opiate withdrawal in rats. Brain Res. 1993;624:19–28. doi: 10.1016/0006-8993(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Sun M, Hackett JT, Guyenet PG. Sympathoexcitatory neurons of rostral ventrolateral medulla exhibit pacemaker properties in the presence of a glutamate-receptor antagonist. Brain Res. 1988;438:23–40. doi: 10.1016/0006-8993(88)91320-0. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G. Integration in the ventral medulla and coordination of sympathetic, pain and arousal functions. Clin. Exper. Hypertension. 1995;17:153–165. doi: 10.3109/10641969509087062. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 2001;81:299–330. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Zhang T, Feng YZ, Rockhold RW, Ho IK. Naloxone-precipitated morphine withdrawal increases pontine glutamate levels in the rat. Life Sci. 1994;55:25–31. doi: 10.1016/0024-3205(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Zhu H, Ho IK. NMDA-R1 antisense oligonucleotide attenuates withdrawal signs from morphine. Eur. J. Pharmacol. 1998;352:151–156. doi: 10.1016/s0014-2999(98)00367-7. [DOI] [PubMed] [Google Scholar]
- Zhu H, Rockhold RW, Ho IK. The role of glutamate in physical dependence on opioids. Jpn. J. Pharmacol. 1998;76:1–14. doi: 10.1254/jjp.76.1. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhou W. Morphine induces synchronous oscillatory discharges in the rat locus coeruleus. J. Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-21-j0003.2001. RC179 (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhou W. Morphine-induced potentiation in the dentate gyrus of the hippocampus involves norepinephrine. Eur. J. Pharmacol. 2003;467:141–144. doi: 10.1016/s0014-2999(03)01601-7. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhou W. Excitatory amino acid receptors are involved in the morphine-induced synchronous oscillatory discharges in the locus coeruleus. Eur. J. Pharmacol. 2005;528:73–78. doi: 10.1016/j.ejphar.2005.10.048. [DOI] [PubMed] [Google Scholar]