Abstract
A new device and automated measurement technology known as OroSTIFF is described to characterize non-participatory perioral stiffness in healthy adults for eventual application to patients with orofacial movement disorders associated with neuromotor disease, traumatic injury, or congenital clefts of the upper lip. Previous studies of perioral biomechanics required head stabilization for extended periods of time during measurement which precluded sampling patients with involuntary body/head movements (dyskinesias), or pediatric subjects. The OroSTIFF device is face-referenced and avoids the complications associated with head-restraint. Supporting data of non-participatory perioral tissue stiffness using OroSTIFF are included from 10 male and 10 female healthy subjects. The OroSTIFF device incorporates a pneumatic glass air cylinder actuator instrumented for pressure, and an integrated subminiature displacement sensor to encode lip aperture. Perioral electromyograms were simultaneously sampled to confirm passive muscle state for the superior and inferior divisions of the orbicularis oris muscles. Perioral stiffness, derived as a quotient from resultant force (ΔF) and interangle span (ΔX), was modeled with multilevel regression techniques. Real-time calculation of the perioral stiffness function demonstrated a significant quadratic relation between imposed interangle stretch and resultant force. This stiffness growth function also differed significantly between males and females. This study demonstrates the OroSTIFF ‘proof-of-concept’ for cost-effective non-invasive stimulus generation and derivation of perioral stiffness in a group of healthy unrestrained adults, and a case study to illustrate the dose-dependent effects of Levodopa on perioral stiffness in an individual with advanced Parkinson’s disease who exhibited marked dyskinesia and rigidity.
Keywords: upper lip, lower lip, biomechanics, force, displacement, elastic recoil
1. Introduction
Limb and orofacial stiffness are modulated by central descending inputs to lower motor neurons, reflex gain, muscle and connective tissue properties, postural orientation to gravitational loads, and geometry of muscle attachments (Barlow & Müller, 1991; Oluwatosin & Oluwatosin, 1998; Shiller et al., 2002). Quantifying stiffness typically involves imposing a specific displacement (ΔX) on a muscle-tissue system (e.g., whole body, limb, jaw) and measuring the resultant force (ΔF). The ratio of the resultant force to displacement yields a stiffness quotient (ΔF/ΔX). Muscle rigidity, regarded a clinical correlate of stiffness, is used to evaluate neurologic status, pharmacological efficacy (Caligiuri & Galasko, 1992), and neurosurgical intervention (Prochazka et al., 1997; Barlow et al., 1998; Sepehri et al., 2007).
Although biomechanical studies of limb rigidity have provided valuable insight into the neural regulation of limb movement disorders (Webster, 1964; Rushworth, 1964; Houk, 1979), similar application to the perioral system has been tenuous due to inadequate methods of transduction. Perioral anatomy is complex with its constituent muscles arranged in four layers according to their origins (Frellinger et al., 1987). The upper lip is associated with at least six muscles, including the orbicularis oris superior, depressor septi, levator labii superioris, levator labii superioris alaeque nasi, zygomaticus major, and levator anguli oris muscles. The lower lip is associated with four, including the orbicularis oris inferior, depressor labii inferioris, depressor anguli oris, and mentalis muscles (Zemlin, 1998). Several muscles converge just lateral to the buccal angle and interlace to form a dense, three-dimensionally mobile, fibromuscular mass known as the modiolus (Al-Hoqail & Meguid, 2009). Nearly all muscles converging toward the modiolus have dermal terminations. The modiolus supports decussation between fibers of the orbicularis oris and labial tractors terminating in the modiolus, and is involved with all forces acting on the oral angle (Pellisier et al., 2000; Zufferey, 2002). Several types of deep and superficial fascia also converge on the modiolus (Mitz & Peyronie, 1976) and function to organize and stabilize muscles, nerves, and vessels (Dzubow, 1986).
Eric Müller (Müller et al., 1985), a physicist and pioneer for speech biomechanics, developed the first mass-spring based three-dimensional space-frame model of the perioral system to simulate and test hypotheses concerning articulatory performance dynamics. He suggested that assessment of perioral stiffness would provide a potentially useful set of biomarkers to clarify the effects of progressive neuromotor disease, traumatic brain injury, pharmacologic treatment, neurosurgical, or maxillofacial surgical intervention on facial animation and speech motor control.
Previous studies have primarily focused on active rigidity during voluntary movement (Caligiuri & Galasko, 1992; Webster, 1966). However, some neurological disorders manifest elevated (e.g., Parkinson’s disease - PD) or decreased muscle activity (e.g., cerebellar disease) at rest. This nonparticipatory ‘resting’ state is known as passive rigidity. In patients with idiopathic PD, labial stiffness was positively correlated with perioral muscle activity and inversely related to the magnitude of lip movements during speech (Hunker et al., 1982). Another report challenged this finding and noted no clear statistical relation between lip stiffness and lip kinematics but observed that PD speakers became more hypokinetic as a function of speech rate (Caliguiri, 1989). The precise effects of rigidity or increased stiffness on lip displacement amplitude, velocity, and speaking rate remain poorly understood in this neurologic disorder.
Active and passive force were measured in the perioral system of healthy young adults (Barlow & Müller, 1991) in order to determine the relation between muscle length (interangle span) and in vivo resultant force in the perioral region. Active force during maximum voluntary contraction (MVC) of the orbicularis oris muscles increased quadratically with increases in the span (aperture) between the corners of the mouth, and were significantly greater for male than female subjects, whereas passive tissue forces were nearly equal as a function of span. In healthy adults, the lower lip is also stiffer than its upper lip counterpart, and may be due in part to its antigravity role in postural control.
Stiffness as a regulated variable has been hypothesized to play a significant role in movement, associated with equilibrium position and end-point accuracy (Shadmehr, 1993; Feldman & Levin, 2009), force recruitment, and velocity scaling among articulators during speech production (Gracco, 1994; Löfqvist & Gracco, 1997; Shaiman & Gracco, 2002). Separate cortical control systems have been hypothesized for movement and stiffness regulation related to reciprocal activation and coactivation of antagonist muscle groups (Humphrey & Reed, 1983). Precise regulation of lip stiffness is essential for accurate production of fricative sounds such as [f] and [v] (Ito et al., 2004) and the duration of lip closure during consonant production (Löfqvist, 2005). Altered stiffness regulation between articulators is another feature of orofacial motor control which could impact facial movements. For example, jaw perturbation experiments revealed that stiffness is up-regulated between the upper lip and jaw in order to maintain the constriction area between lips during the production of a fricative consonant (Gomi et al., 2002). Increased jaw stiffness is associated with a decrease in the variability of speech kinematics (Shiller et al., 2002). Stiffness regulation appears to play a central role for speech motor learning and adaptation (Nasir & Ostry, 2006; Tremblay et al., 2008).
Recently, perioral tissue stiffness has been measured using a wall-mounted linear actuator operating under position feedback to sequentially impose step increases in interangle lip span in healthy female (Seibel & Barlow, 2007) and male adults (Chu et al., 2009) sampled under a passive (no-contraction) condition. The methods used however required head stabilization in a cephalostat for several minutes during the presentation of lip displacements. This approach precludes study in clinical populations, including pediatric patients (i.e., orofacial cleft), or in cases where dyskinesia is present (e.g., progressive neuromotor disease, traumatic brain injury).
To solve this limitation, a new face-referenced interangle device known as OroSTIFF was developed in our bioengineering laboratory to examine the feasibility of real-time perioral stiffness measurements in unrestrained participants in health and disease. It was hypothesized that passive ‘non-participatory’ perioral stiffness would vary quadratically as a function of interangle span displacement. Sex was also regarded as a potentially significant independent variable because of known differences in lip dynamics (Barlow & Rath, 1985; Barlow & Müller, 1991). The OroSTIFF device application and related test results are expected to provide clinicians a new method for evaluating the efficacy of pharmacological and surgical intervention in the perioral system of patients with neuromotor disease (e.g., Parkinson’s disease), craniofacial anomalies (e.g., cleft lip), lip/face carcinoma, or traumatic injury to the face and/or brain centers involved in facial animation (e.g., bomb blast, missile wounds, vehicular).
2. Materials and methods
2.1 Subjects
Twenty healthy adults (10 males, 10 females), 19 to 31 years of age with no prior history of neurological and craniofacial disorder, and/or speech impairment participated in this study (Table 1). Informed consent, approved by the University of Kansas Human Subjects Internal Review Board, was obtained from all subjects.
Table 1.
Physical characteristics of participants.
| Male | Female | |
|---|---|---|
| Number of Subjects | 10 | 10 |
| Age (yrs) | 24.59 (SD=3.07) | 23.18 (SD=2.76) |
| Weight (kg)* | 67.78 (SD=7.35) | 57.52 (SD=8.44) |
| Height (cm)* | 174.22 (SD=11.12) | 164.74 (SD=4.22) |
| Head circumference (cm) | 57.05 (SD=1.66) | 55.69 (SD=2.07) |
| Nasion-to-inion (cm) | 39.22 (SD=2.23) | 37.54 (SD=1.75) |
| Lip Resting Span (mm) | 47.32 (SD=2.82) | 46.46 (SD=2.16) |
Significant difference at p<.05
2.2 Protocol
Subjects were seated in a comfortable chair and instructed to remain speechless and motionless, and relax facial muscles during the 2 minute sampling (Figure 1). To ensure subjects’ nonparticipation during the imposed perioral stretch, Ag/AgCl 4-mm diameter bipolar electrodes (2-cm interelectrode distance) were placed over the orbicularis oris superior (OOSm) and orbicularis oris inferior (OOIm) muscles. Biopotentials were conditioned with Grass P511 bioamplifiers (30Hz-1kHz bandpass, Gain=20K). A 1-cm interincisal bite block was molded (KERR Xtrude-XP™) for each subject to stabilize the mandible during stiffness sampling.
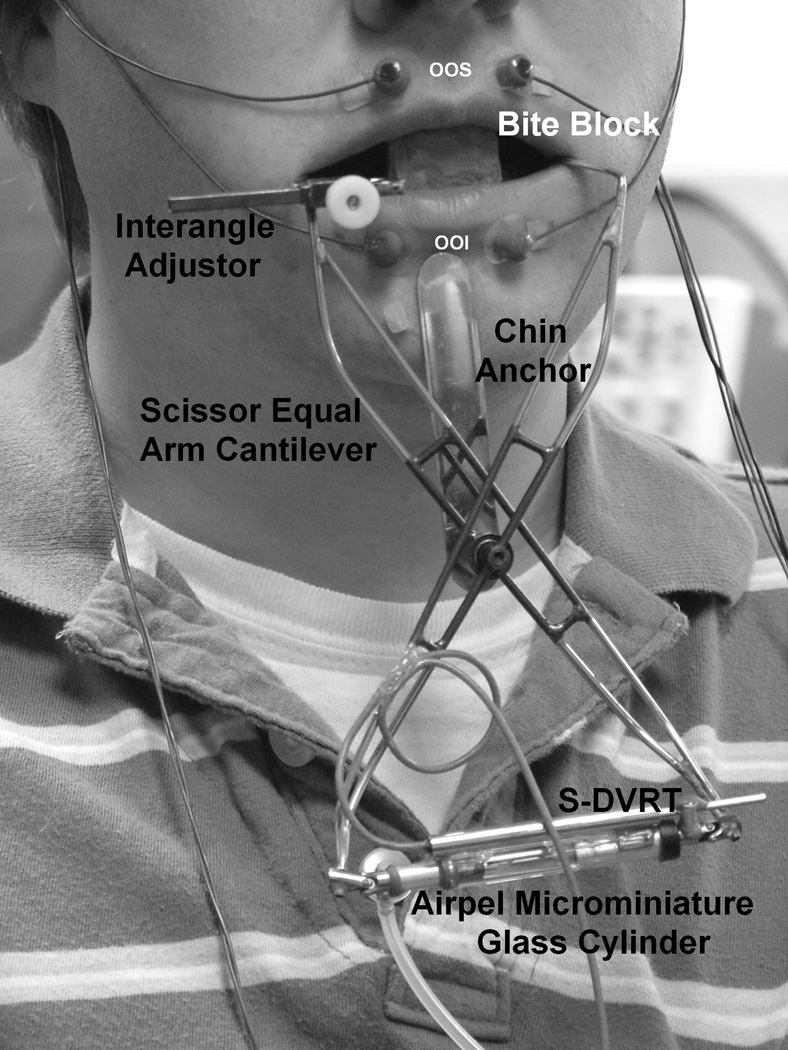
Figure 1.
Instrumentation and configuration to assess the force-displacement relation (stiffness) for the perioral tissues. The equal-arm scissor cantilever and stainless steel lip saddles (hooks) positioned for imposing increases in interangle span and automated stiffness sampling. A lip span adjustor accommodates to individual differences in lip aperture and used to set initial position (L0 + 15 mm). The stainless steel 0.020” shimstock chin anchor articulated on a needle bearing and was positioned over the subject’s mental symphysis for vertical stabilization. InVivo Metrics 4 mm Ag/AgCl surface electrodes placed over orbicularis oris superior (OOS) and orbicularis oris inferior (OOI) muscle recording sites.
The thin wall tubular stainless steel face-referenced OroSTIFF device (mass = 40.7gm) was coupled bilaterally to the oral angles via lip saddles and supported on the mental symphysis with a double-adhesive tape collar for vertical stabilization (see chin anchor on Figure 1). The device incorporates an Airpel® custom microminiature pneumatic glass-cylinder actuator instrumented for pressure (Honeywell #26PCCFAG, +/− 15 psi) and an integrated custom subminiature displacement sensor (differential variable reluctance transformer [DVRT], MicroStrain® , Inc) to encode lip aperture. The pneumatic actuator was manually pressurized with a 10-cc syringe which in turn imposed an interangle stretch of approximately 20 mm. A 30-gauge blunt tip cannula, vented to atmosphere, was coupled in parallel with the OroSTIFF pneumatic system. This cannula provided a fixed resistive load, essentially a controlled air leak, upon which the perioral recoil force would act to allow the equal-arm scissor cantilevers to return to their initial lip aperture resting position (L0 + 15 mm). A block diagram of the instrumentation is shown in Figure 2.
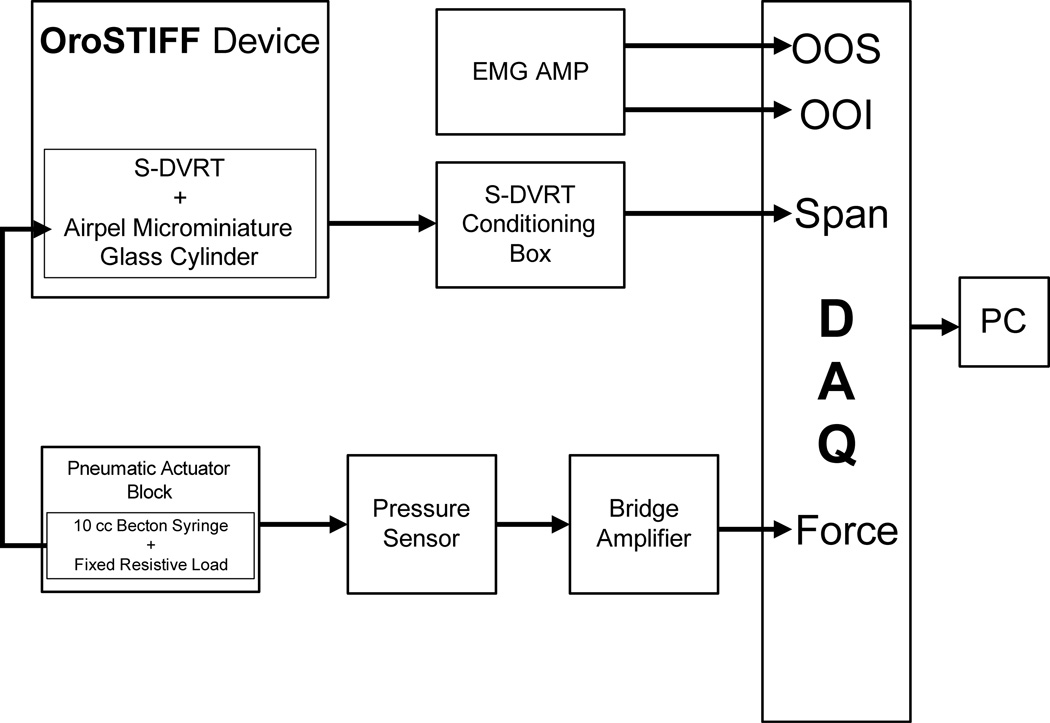
Figure 2.
A schematic block diagram of the OroSTIFF system. The device is equipped with a subminiature differential variable reluctance transducer to measure displacement of the lips, and a pressure transducer to derive the reactive force associated with perioral tissue recoil. A fixed pneumatic resistive load, in the form of 30 gauge blunt tip cannula, permits the moveable cantilevers of the OroSTIFF device to return to its initial position [L0 + 15 mm] following each imposed stretch trial.
Interangle oral aperture at rest provides an estimate of resting muscle length (L0) and was measured with a digital caliper for each subject. The OroSTIFF interangle span was initialized to [L0 + 15 mm] for all subjects. A series of 5 interangle stretch trials were completed while sampling force, displacement, and electromyograms (EMG) from bipolar electrodes placed on the upper lip (OOSm) and lower lip (OOIm) in real-time with custom software (OroSTIFF v.3.0.5, LabVIEW™ 8.0). Individual interangle stretch trials were completed within 10 seconds, and the perioral stiffness protocol was completed within 2 minutes for each participant.
2.3 Data acquisition and analysis
2.3.1 Identifying nonlinear segment of force-displacement curve
Air pressure within the microminiature pneumatic glass-cylinder actuator and the displacement signal from the DVRT were digitized at 2 ksamples/sec at 16-bits resolution. These waveforms were down-sampled to yield 100 pressure and position samples which were digitally low-pass filtered (flp = 30 Hz, 2-pole Butterworth), and subsequently averaged in 10 bins of 10, yielding an effective sample rate of 200 Hz for real-time calculation and display of force, displacement, and derived stiffness.
Stiffness coefficients (N/mm) were automatically calculated in real time during the phase of elastic recoil for each of 5 trials as the low-mass interangle yokes of the OroSTIFF device returned to the participant’s interangle rest position. The stiffness coefficient was calculated as the change in force over a 1 mm change in interangle span and sequentially evaluated at 1 mm intervals. Real-time display of stiffness coefficient versus span began when 3 conditions were met simultaneously: span > 0.5 mm, force decreasing, and a positive slope for a 10-point linear fit of force versus span. Graphic display continues until span < 0.5 mm (see Figure 3, points D to E). The absolute number of stiffness points along the recoil trajectory depends on the maximum interangle span achieved. To determine stiffness for a specific span a 100-point running cubic spline was evaluated at 0.5 mm above and below the desired span (i.e., force was evaluated at 19.5 and 18.5 mm to calculate stiffness for a nominal span of 19 mm). The cubic spline allows force to be determined at regular displacement intervals.
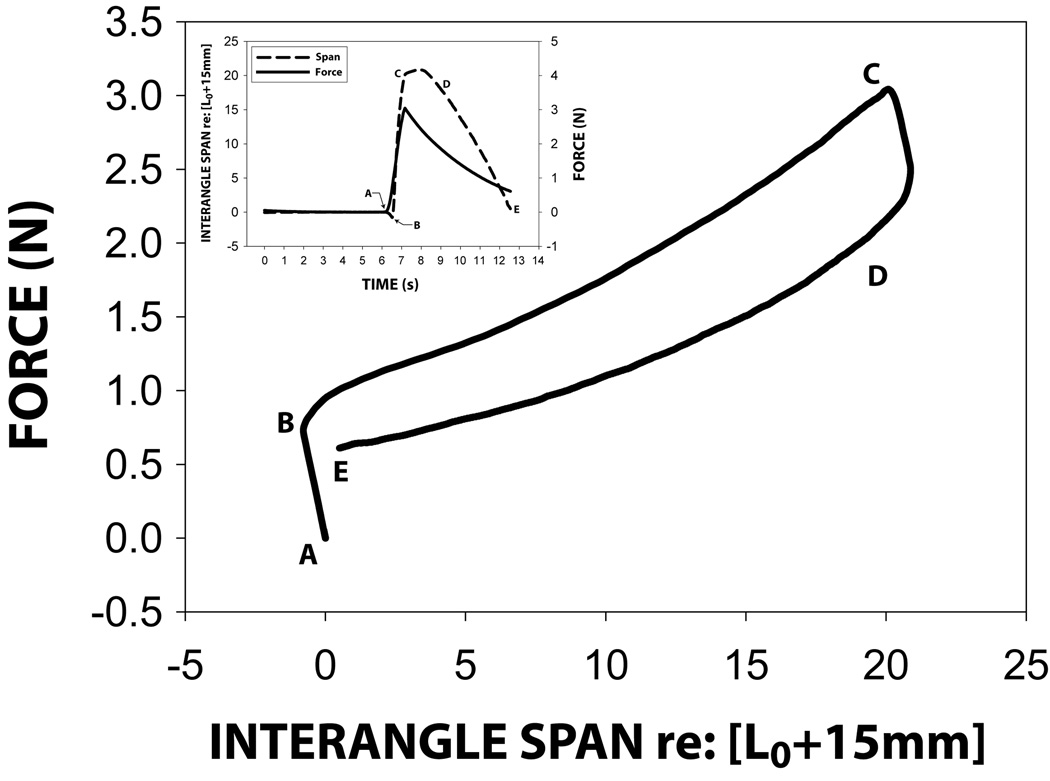
Figure 3.
Typical force-span hysteresis curve sampled from a normal adult subject with graphic insert showing span-time (black line) and force-time (dashed line) plots. Point A: preload condition of the OroSTIFF device on a subject's face; B: onset of interangle stretch phase ; C: peak interangle stretch; the recoil phase (D–E) during which stiffness is calculated (ΔForce/ΔSpan). Regions from A–E and just after C reflect bending (elasticity) of the OroSTIFF device without change in S-DVRT position output.
2.3.2 Determine muscle activity pattern during non-passive stretch
The root-mean-square (RMS) of the upper lip (OOSm) and lower lip (OOIm) EMG signals were computed during the phase of elastic recoil for each of 5 trials with an increment of 10 samples at 2000Hz (averaging time of 5 ms) to quantitatively verify non-participation of perioral muscles.
2.3.3 Calibration of the OroSTIFF device
The DVRT factory calibration data as well as digital caliper measurements were used to determine the ratio of DVRT position-to-interangle span. Force was calibrated with a load cell placed between the stainless steel interangle lip saddles of the OroSTIFF device. Device stiffness was determined by clamping the stainless steel interangle lip saddles and measuring position and force while modulating pressure with the 10-cc Becton syringe. The device functionality was verified using a precision linear spring.
After OroSTIFF program initialization, voltage offsets were determined with pressure vented to atmosphere, position set to zero, and EMG disconnected. All four signals were scaled linearly using these offsets and previously determined calibration slopes to yield force (N), displacement (mm), and EMG (uV). The DVRT position signal was converted to interangle span by multiplying by a constant to account for differences in the scissor-equal arm cantilever lengths on opposite sides of the central pivot needle bearing, and by correcting for device stiffness. Measured force is divided by this effective device stiffness and subtracted from position to yield interangle span. The negative slope seen in figure 3 between points A and B, and C and D represents the effective device stiffness.
2.3.4 Data analysis of force vs. displacement (ΔF/ΔX)
Given the hierarchically nested design of the data, in which the perioral stiffness was measured through a series of 5 interangle stretch trials (level-1) for each participant (level-2), multilevel regression analysis was conducted using SAS Version 9.1 (SAS Institute, 2004). First, an unconditional means model (i.e., null model) was fit in order to determine the random variance components. Then, level-1 and level-2 predictors and cross-level interaction terms were introduced into the null model with their significant random effects. The level-1 predictors represented the linear and quadratic regression slopes of the interangle span on the perioral stiffness. The cross-level interaction terms represented the sex differences in the linear and quadratic regression slopes.
3. Results
3.1 The regression result of force vs. displacement (ΔF/ΔX)
The fitted null model is given by the expression Yij = γ00 + uoj + rij , where uoj ~ N(0, τ00) and rij ~ N(0, σ2) for trial i and participant j. This model expresses the stiffness scores as the sum of an overall mean (γ00), a series of random deviations from that mean (uoj), and a random error (rij) associated with the ith trial in the jth participant. The null model also showed that the estimated mean stiffness score across all trials and all participants was .073 N/mm (SE = .002, t [19] = 40.90, p<.01). The estimated variances of level-1 and level-2 residual errors were .00180 (SE = .00006, z = 30.28.002, p<.01) and .00005 (SE = .00002, z = 2.17, p<.05), respectively. The intra-class correlation (ICC) indicated that most of the variability (97.6%) in the stiffness scores occurred within participants. These estimates suggested that the stiffness scores do differ within participants and there is less but significant variation between participants.
The final model included two level-1 predictors that represent the linear and quadratic regression slopes of the interangle span on the perioral stiffness. Two cross-level interaction terms were also included to test whether the regression slopes differ between males and females. This model can be written by
| (1) |
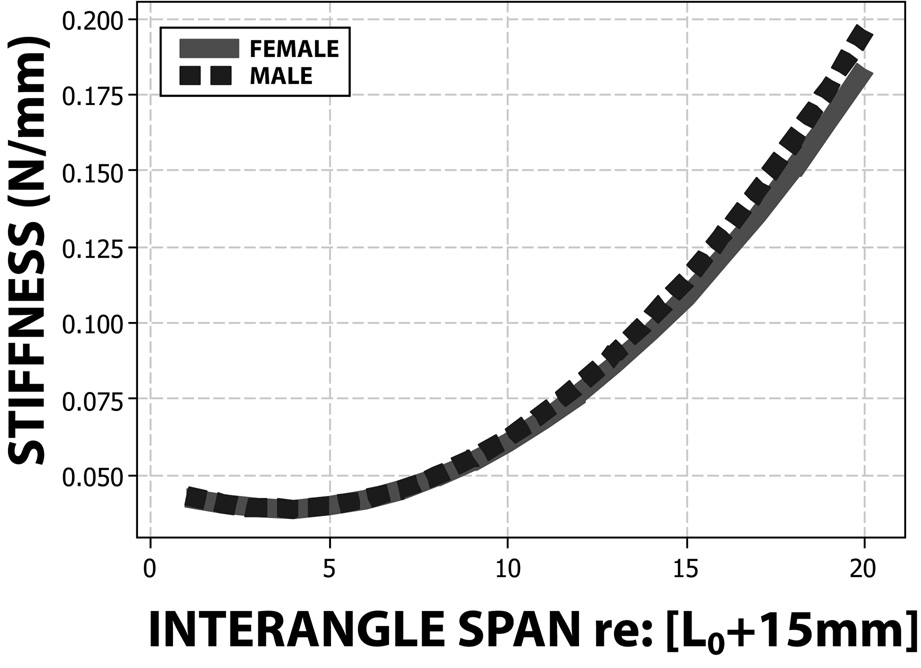
where and rij ~ N(0, σ2) for trial i and participant j. The random effect (u2j) for the was not included because its estimation made Newton-Raphson algorithm for optimizing residual likelihood function (REML) to yield a non-positive definite G matrix. The parameter estimates from the fitted final model are shown in Table 2. It was shown that the perioral stiffness increased as a quadratic function of the interangle span, γ̂10 = .00054, t (1811) = 37.28, p<.01. More importantly, this quadratic slope significantly differed between males and females, γ̂21 = .00005, t (1811) = 2.31, p<.05 (see Figure 4). Although the linear function of the interangle span was also significant, there was no sex difference in this linear slope.
Table 2.
Multilevel regression parameter estimates of between- and within- level components.
| Fixed Effect | Estimate | SE | t | p | |
|---|---|---|---|---|---|
| Intercept | .04583 | .00408 | 11.23 | < .01 | |
| Sex | .00099 | .00579 | .17 | .86 | |
| Span | −.00396 | .00107 | −3.70 | < .01 | |
| Span2 | .00054 | .00002 | 37.28 | < .01 | |
| Sex × Span | −.00046 | .00152 | −.30 | .76 | |
| Sex × Span2 | .00005 | .00002 | 2.31 | < .05 | |
| Random Effect | Estimate | SE | z | p | |
| σ2 | .00019 | .00000 | 30.09 | < .01 | |
| τ00 | .00015 | .00005 | 2.82 | < .01 | |
| τ11 | .00001 | .00000 | 2.97 | < .01 | |
| τ01 | −.00004 | .00001 | −2.81 | < .01 | |
| ICC | .431 | ||||
|
|
.893 | ||||
|
|
- | ||||
| −2lnL | −10360.1 | ||||
| AIC | −10352.1 | ||||
Figure 4.
The residual ICC showed that 43.1% of total residual variance occurred between participants. The squared multiple correlation indicated that approximately 89.3% of level-1 residual variance (σ̂2) was accounted for by this model. Since the estimated level-2 residual variance (τ̂00) increased with the random slope, the squared multiple correlation for this level could not be obtained. The likelihood-ratio (LR) test suggested that both the random intercept (LR χ2 = 232.10, p<.01) and the random linear slope (LR χ2 = 1439.00, p<.01) were tenable.
3.2 The muscle activity pattern during non-passive stretch
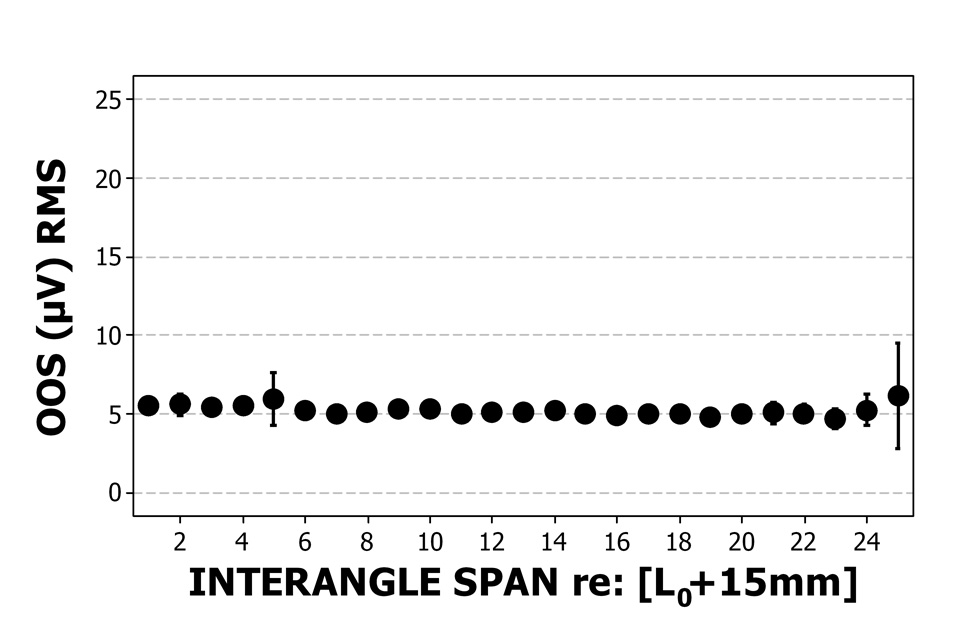
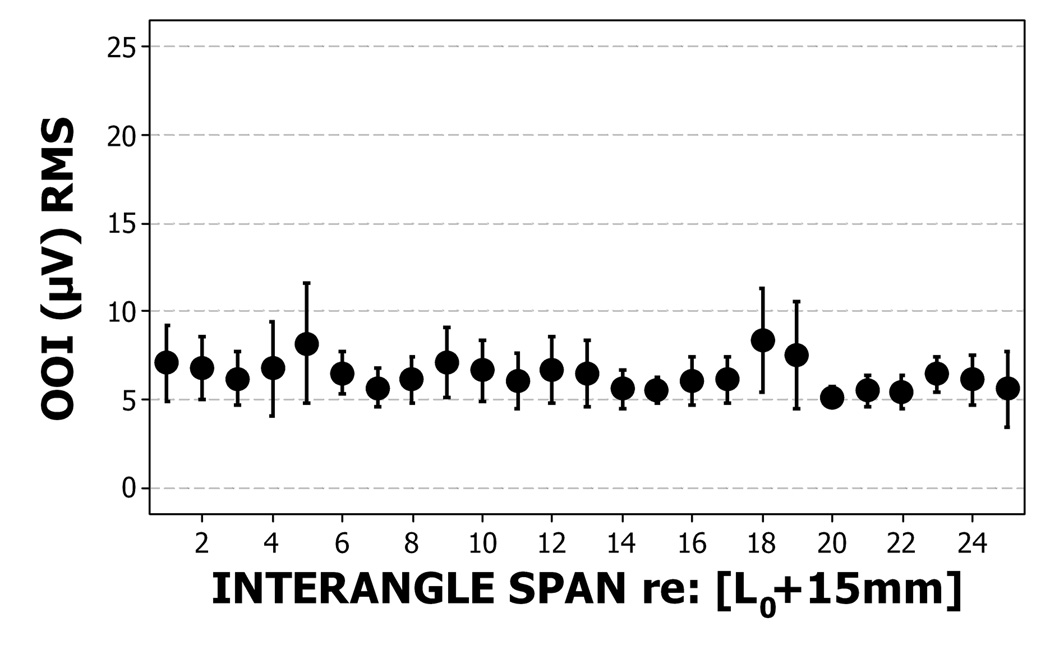
Perioral muscle activity remained remarkably constant as interangle span was increased confirming the non-participatory nature of the experimental task. The distribution of EMG RMS values for the OOS and OOI muscle recording sites pooled among subjects is shown in Figure 5a and 5b. A one-way ANOVA for EMG RMS versus interangle span indicated non-significance for both the upper lip (F=0.80, p=.737, R-sq (adj) =.00%), and lower lip (F=0.54, p=.967, R-sq (adj) =.00%) and confirmed the non-participatory nature of the stiffness sampling protocol.
Figure 5a & 5b.
The distribution of mean and standard error of EMG RMS values (µV) for upper lip (5a) and lower lip (5b) recording sites for all participants during the ‘face-relaxed’ non-participatory conditions. Eighty-five observations at each interangle span except at 25 mm, where there were only 3 observations.
4. Discussion
This present study demonstrated a non-invasive and rapid (< 2 mins) method for real-time data acquisition and analysis of perioral tissue stiffness without head restraint. The derived regression function for non-participatory perioral stiffness using OroSTIFF is consistent with previous reports in healthy participants using costly servo-controlled linear actuators that required head restraint (Seibel & Barlow, 2007). The results indicated that intersubject variability in stiffness growth functions increased as the interangle span increased (Figure 4). This is likely due to individual differences in perioral anatomy. Male subjects yielded significantly higher stiffness coefficients than female subjects. A similar trend was noted in stiffness measures sampled at lip midline (Ho et al., 1982). This may be due in part to undocumented differences in perioral anatomy, including the size and orientation of muscles, connective tissue, and vessels.
Sample Clinical Application
The clinical utility of the OroSTIFF application was put to the test in a participant with a significant movement disorder, a 68-year old male with a 12-year history of idiopathic Parkinson’s disease (PD). He exhibited moderate-severe dyskinesia of head, trunk, and extremities under the prescribed dosage of anti-PD medications and this is designated as the ‘ON’ state. He exhibited reduced dyskinesia, but increased dystonia and rigidity in the ‘OFF’ condition (unmedicated condition). This individual consented to refrain from taking his anti-PD medications from the previous evening for a period of 12-hours, and subsequently arrived at the laboratory at 9AM in the ‘OFF’ condition. An incisal bite block was molded, and the OroSTIFF protocol was completed in less than 2 minutes. The participant was given his prescribed dose of anti-PD medications with a glass of water. After 50 minutes, the participant was in the ‘ON’ state, and the OroSTIFF protocol was repeated once again to assess the effects of L-Dopa on perioral stiffness functions.
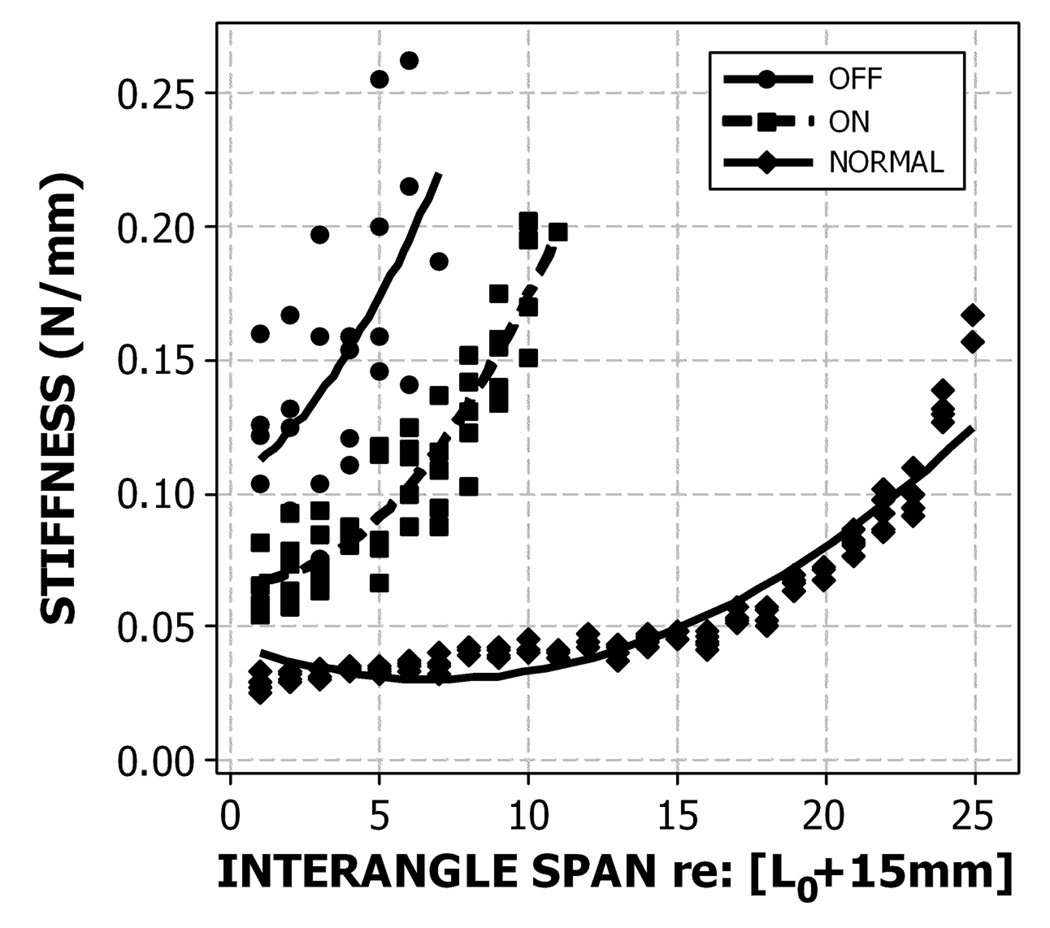
Several striking features are apparent from the PD stiffness plots given in Figure 6. First, in the ‘OFF’ state, perioral stiffness is approximately 7 times greater than normal, and because of this high level of tonic muscle activity, the imposed displacements generated by the OroSTIFF device were limited to approximately 10 mm beyond resting span (L0+15 mm). Administration of the anti-PD medications had a significant effect on reducing the slope and offset of the quadratic stiffness function, however perioral stiffness remained significantly elevated over normal levels. A video clip is included with this research report to demonstrate the OroSTIFF sampling protocol for this PD participant. Future studies will focus on the relation between perioral stiffness and orofacial hypokinesia during speech.
Figure 6.
Perioral stiffness data and quadratic functions for a 68-year old male with Parkinson’s disease in the ON (with anti-PD meds) and OFF (without meds) conditions plotted relative to an age- and sex-matched healthy control.
The current instantiation of the OroSTIFF device is limited to sampling relatively low levels of interangle lip force associated with non-participatory (passive) conditions in health and disease. High stiffness, short-throw titanium cantilevers are under development to reduce the size and mass of the OroSTIFF device, and permit measurement of active force production up to 25 N among pediatric and adult populations. The current instrument design also precludes unilateral assessment of stiffness in the perioral region. This feature would be useful for patients who have sustained unilateral injury to the lower face, undergoing lip revision surgery due to uni- or bilateral congenital cleft of the upper lip, manifest laterality differences in perioral stiffness due to a central brain lesion or progressive neuromotor disease (e.g., Parkinson’s disease, cerebrovascular stroke), or peripheral cranial mononeuropathy (Bell’s palsy). One possible solution for unilateral measurement is to mechanically reference lip stiffness measures sampled from either the right or left oral angle to a midline transducer yoke positioned in a custom dental impression mold within the occlusal plane. Such a configuration has been used successfully to sample maximum voluntary contraction (tetany) and controlled force dynamics in a midline vertical lip compression task in young adults with moderate-to-severe congenital forms of upper motor neuron syndrome (Barlow & Abbs, 1986).
Supplementary Material
Acknowledgements
This study was supported by the Sutherland Foundation, NIH R01 DC003311 (Barlow), NIH R01 DE13814 (Trotman), and NIH P30 DC005803.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors confirm that there are no conflicts of interest associated with this publication and there has been no financial support for this work that could have influenced its outcome.
Contributor Information
Shin-Ying Chu, Department of Speech-Language-Hearing: Sciences and Disorders, Communication Neuroscience Laboratories, University of Kansas, 1000 Sunnyside Avenue, Rm 3001, Lawrence, Kansas 66045-7555, shinying@ku.edu, TL 785-864-1196, FX 785-331-4345
Steven M. Barlow, Professor, SPLH, Neuroscience, Human Biology, and Bioengineering, Director, Communication Neuroscience Laboratories, University of Kansas, 1000 Sunnyside Avenue, Rm 3001, Lawrence, Kansas 66045-7555, smbarlow@ku.edu, TL 785-749-1004, FX 785-312-5344
Douglas Kieweg, Project Coordinator, Digital and Electrical Engineering Core, The Center for Biobehavioral Neurosciences in Communication Disorders, University of Kansas, 1000 Sunnyside Avenue, Lawrence, KS 66045, dkieweg@ku.edu, TL: 785-864-4539, FX: 785-864-4571
Jaehoon Lee, Center for Research Methods and Data Analysis, Schiefelbusch Institute for Life Span Studies, University of Kansas, 1301 Sunnyside Ave, Lawrence, KS 66045, jaehoon@ku.edu
References
- Al-Hoqail RA, Meguid EMA. An anatomical and analytical study of the modiolus: enlightening its relevance to plastic surgery. Aesthetic Plastic Surgery. 2009;33:147–152. doi: 10.1007/s00266-008-9187-x. [DOI] [PubMed] [Google Scholar]
- Barlow SM. The effects of speech-orofacial motor performance by means of motion sense. Journal of Communication Disorders. 1998;31:511–534. doi: 10.1016/s0021-9924(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Barlow SM. Mechanical frequency detection thresholds in the human face. Experimental Neurology. 1987;96:253–261. doi: 10.1016/0014-4886(87)90044-6. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Abbs JH. Fine force and position control of select orofacial structures in the Upper Motor Neuron Syndrome. Experimental Neurology. 1986;94:699–713. doi: 10.1016/0014-4886(86)90248-7. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Iacono RP, Paseman LA, Biswas A, D’Antonio LD. The effects of experimental posteroventral pallidotomy on force and speech aerodynamics in Parkinson’s disease. In: Cannito MP, Yorkston KM, Beukelman DR, editors. Speech Motor Control. Baltimore: Paul H. Brookes Publishing Company; 1998. pp. 117–156. [Google Scholar]
- Barlow SM, Müller EM. The relation between interangle span and in vivo resultant force in the perioral musculature. Journal of Speech, Language, and Hearing Research. 1991;34:252–259. doi: 10.1044/jshr.3402.252. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Rath EM. Maximum voluntary closing forces in the upper and lower lips of humans. Journal of Speech, Language, and Hearing Research. 1985;28:373–376. doi: 10.1044/jshr.2803.373. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP. The influence of speaking rate on articulatory hypokinesia in Parkinsonian dysarthria. Brain and Language. 1989;36:493–502. doi: 10.1016/0093-934x(89)90080-1. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Galasko DR. Quantifying drug-induced changes in Parkinsonian rigidity using an instrumental measure of activated stiffness. Clinical Neuropharmacology. 1992;15:1–12. doi: 10.1097/00002826-199202000-00001. [DOI] [PubMed] [Google Scholar]
- Chu SY, Barlow SM, Lee J. Nonparticipatory Stiffness in the Male Perioral Complex. Journal of Speech, Language, and Hearing Research. 2009;52:1353–1359. doi: 10.1044/1092-4388(2009/08-0101). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzubow LM. The fasciae of the face: an anatomic and histologic analysis. Journal of the American Academy of Dermatology. 1986;14:502–507. doi: 10.1016/s0190-9622(86)70065-0. [DOI] [PubMed] [Google Scholar]
- Felman AG, Levin MF. The equilibrium-point hypothesis- past, present, and future. In: Sternad D, editor. Progress in Motor Control. New York, NY: Springer Science; 2009. pp. 699–726. [DOI] [PubMed] [Google Scholar]
- Frellinger G, Gruber H, Happak W, Pechmann U. Surgical anatomy of the mimic muscle system and the facial nerve: importance for reconstructive and aesthetic surgery. Plastic Reconstructive Surgery. 1987;80:686–690. doi: 10.1097/00006534-198711000-00005. [DOI] [PubMed] [Google Scholar]
- Gomi H, Honda M, Ito T, Murano EZ. Compensatory articulation during bilabial fricative production by regulating muscle stiffness. Journal of Phonetics. 2002;30:261–279. [Google Scholar]
- Gracco VL. Some organizational characteristics of speech movement control. Journal of Speech, Language, and Hearing Research. 1994;37:4–27. doi: 10.1044/jshr.3701.04. [DOI] [PubMed] [Google Scholar]
- Ho TP, Azar K, Weinstein S, Bowley WW. Physical properties of human lips: experimental and theoretical analysis. Journal of Biomechanics. 1982;15:859–866. doi: 10.1016/0021-9290(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Houk JC. Regulation of stiffness by skeletomotor reflexes. Annual Review Physiology. 1979;41:99–114. doi: 10.1146/annurev.ph.41.030179.000531. [DOI] [PubMed] [Google Scholar]
- Humphrey DR, Reed DJ. Separate cortical systems for control of joint movement and joint stiffness: reciprocal activation and coactivation of antagonist muscles. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York, NY: Raven Press; 1983. pp. 347–372. [PubMed] [Google Scholar]
- Hunker CJ, Abbs JH, Barlow SM. The relationship between parkinsonian rigidity and hypokinesia in the orofacial system: a quantitative analysis. Neurology. 1982;32:749–754. doi: 10.1212/wnl.32.7.749. [DOI] [PubMed] [Google Scholar]
- Ito T, Gomi H, Honda M. Dynamical simulation of speech cooperative articulation by muscle linkages. Biological Cybernetics. 2004;91:275–282. doi: 10.1007/s00422-004-0510-6. [DOI] [PubMed] [Google Scholar]
- Löfqvist A. Lip kinematics in long and short stop and fricative consonants. Journal of the Acoustical Society of America. 2005;117:858–878. doi: 10.1121/1.1840531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfqvist A, Gracco VL. Lip and jaw kinematics in bilabial stop consonant production. Journal of Speech, Language, and Hearing Research. 1997;40:877–893. doi: 10.1044/jslhr.4004.877. [DOI] [PubMed] [Google Scholar]
- Mitz V, Peyronie M. The superficial musculo-aponeurotic system (SMAS) in the parotid and cheek area. Plastic Reconstructive Surgery. 1976;58:80–88. doi: 10.1097/00006534-197607000-00013. [DOI] [PubMed] [Google Scholar]
- Müller EM, Milenkovic PH, MacLeod GE. Perioral tissue mechanics during speech production. In: Eisenfeld J, DeLisi C, editors. Mathematics and Computers in Biomedical Application. Amsterdam: Elsevier; 1985. pp. 363–371. [Google Scholar]
- Nasir SM, Ostry DJ. Somatosensory precision in speech production. Current Biology. 2006;16:1918–1923. doi: 10.1016/j.cub.2006.07.069. [DOI] [PubMed] [Google Scholar]
- Oluwatosin OM, Oluwatosin OA. Orofacial indices: a study in 240 Nigerian children. African Journal of Medicine and Medical Sciences. 1998;27:39–42. [PubMed] [Google Scholar]
- Pellisier P, Pistre V, Bustamante K, Baudet J. The modiolus: comparative anatomy, embryological and physiological review. Annales de chirurgie plastique et esthetique. 2000;41:41–47. [PubMed] [Google Scholar]
- Prochazka A, Bennett DJ, Stephens MJ, Patrick SK, Sears-Duru R, Roberts T, Jhamandas JH. Measurement of rigidity in Parkinson’s disease. Movement Disorders. 1997;12:24–32. doi: 10.1002/mds.870120106. [DOI] [PubMed] [Google Scholar]
- Rushworth G. Some aspects of the pathophysiology of spasticity and rigidity. Clinical Pharmacology Therapy. 1964;5:828–836. [Google Scholar]
- SAS Institute. SAS 9.1.3 Language Reference: Concepts. North Carolina: Author; 2004. [Google Scholar]
- Seibel LM, Barlow SM. Automatic measurement of nonparticipatory stiffness in the perioral complex. Journal of Speech, Language, and Hearing Research. 2007;50:1272–1279. doi: 10.1044/1092-4388(2007/089). [DOI] [PubMed] [Google Scholar]
- Sepehri B, Esteki A, Ebrahimi-Takamjani E, Shahidi G-A, Khamseh F, Moinodin M. Quantification of rigidity in Parkinson’s disease. Annals of Biomedical Engineering. 2007;35:2196–2203. doi: 10.1007/s10439-007-9379-6. [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Control of equilibrium position and stiffness through postural modules. Journal of Motor Behavior. 1993;25:228–241. doi: 10.1080/00222895.1993.9942052. [DOI] [PubMed] [Google Scholar]
- Shaiman S, Gracco VL. Task-specific sensorimotor interactions in speech production. Experimental Brain Research. 2002;146:411–418. doi: 10.1007/s00221-002-1195-5. [DOI] [PubMed] [Google Scholar]
- Shiller DM, Laboissiere R, Ostry DJ. Relationship between jaw stiffness and kinematic variability in speech. Journal of Neurophysiology. 2002;88:2329–2340. doi: 10.1152/jn.00286.2002. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Houle G, Ostry DJ. Specificity of speech motor learning. Journal of Neuroscience. 2008;28:2426–2434. doi: 10.1523/JNEUROSCI.4196-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DD. The dynamic quantification of spasticity with automated integrals of passive motion resistance. Clincial Pharmacology and Therapeutics. 1964;5:299–309. [Google Scholar]
- Webster DD. Rigidity in extrapyramidal disease. Journal of Neurosurgery. 1966;24 supl II:299–309. [Google Scholar]
- Zemlin WR. Articulation. In: Dragin SD, editor. Speech and Hearing Science: Anatomy and Physiology. 4th ed. Needham Heights, Massachusetts: Allyn & Bacon; 1998. pp. 197–225. [Google Scholar]
- Zufferey JA. Importance of the modiolus in plastic surgery. Plastic Reconstructive Surgery. 2002;110:331–334. doi: 10.1097/00006534-200207000-00058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.