Abstract
Purpose
The purpose of this review is to clarify the role of botulinum toxin serotype A (BTX-A) in the treatment of children with cerebral palsy (CP), with a special focus on the lower limb.
Background
The treatment of spasticity is central in the clinical management of children with CP. BTX-A blocks the release of acetylcholine at the motor end plate, causing a temporary muscular denervation and, in an indirect way, a reduced spasticity. Children with increased tone develop secondary problems over time, such as muscle contractures and bony deformities, which impair their function and which need orthopaedic surgery. However in these younger children, delaying surgery is crucial because the results of early surgical interventions are less predictable and have a higher risk of failure and relapse. As BTX-A treatment reduces tone in a selective way, it allows a better motor control and muscle balance across joints, resulting in an improved range of motion and potential to strengthen antagonist muscles, when started at a young age. The effects are even more obvious when the correct BTX-A application is combined with other conservative therapies, such as physiotherapy, orthotic management and casts. There is now clear evidence that the consequences of persistent increased muscle tone can be limited by applying an integrated multi-level BTX-A treatment approach. Nevertheless, important challenges such as patient selection, defining appropriate individual goals, timing, dosing and dilution, accuracy of injection technique and how to measure outcomes will be questioned. Therefore, “reflection is more important than injection” remains an actual statement.
Keywords: Cerebral palsy, Botulinum toxin A, Multi-level treatment, Lower limb
Introduction
Cerebral palsy (CP) has been described by Mercer Rang as “an insult of the developing brain that produces a disorder of movement and posture that is permanent but not unchanging” [1]. It is the most frequent cause of motor disability amongst children in Europe [2]. The prevalence in Europe has been rather stable over the last 30 years and ranges between 1.5 and 3.0 per 1,000 live births [3].
Children with CP may present with a variety of motor problems, changing with growth and development. Primary problems are directly related to the lesion in the central nervous system, influencing muscle tone, balance, strength and selectivity, whereas static muscle contractures and bony deformities (secondary problems) develop slowly over time in response to the primary problems. Furthermore, the child often develops adaptive mechanisms or ‘coping responses’ in gait to overcome the primary and secondary problems.
Of all primary problems, spasticity is the main cause of the development of secondary problems. A treatment programme should, therefore, be focused on the reduction or normalisation of tone to prevent the development of secondary problems and delaying or obviating the need for surgical intervention.
Spasticity can be addressed with oral medication, phenol, selective dorsal rhizotomy and intrathecal baclofen. In the past two decades, botulinum toxin serotype A (BTX-A) has been introduced as a selective treatment option for spasticity in children with CP. BTX-A, when injected into the muscles, will reduce muscle tone. It became clear that the use of BTX-A was a major advance in the treatment of CP and it is now widely accepted in the management of paediatric posture and movement disorders.
Figure 1 gives an overview of the different motor problems in children with CP and the action location of BTX-A.
Fig. 1.
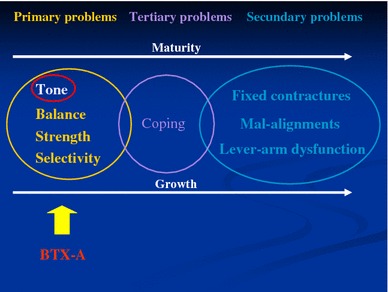
Motor problems experienced by children with cerebral palsy (CP) and the action location of botulinum toxin serotype A (BTX-A)
BTX-A is one of the seven different serotypes of botulinum toxin (A–G) produced by the anaerobic bacterium Clostridium botulinum [4]. The serotypes differ in neurotoxin complex size, activation level, intracellular site of action, acceptor/receptor sites, muscle-weakening efficacy, duration of action and target affinity [5]. BTX-A has been commercially available for clinical use for the longest time. There are currently four commercially available preparations of BTX-A: Botox® (Allergan), Dysport® (Ipsen), Xeomin® (Merz Pharmaceuticals GmbH; only available in Germany) and Hengli, a Chinese form [6, 7]. The only approved botulinum toxin type B (BTX-B) formulation is known as Myobloc® in the United States and as Neurobloc® outside of the United States. The clinical trials with BTX-B for CP are limited, mostly open-label pilot studies, and include patients who were secondary non-responders to BTX-A therapy. The regional and systemic anticholinergic adverse side effects of BTX-B limit its clinical use [8].
After BTX-A has been injected directly into the muscle, it is selectively taken up by endocytosis at the cholinergic nerve terminal, where it blocks the release of acetylcholine. This chemical denervation causes a temporary reduced muscular activity in the injected muscles. The process is reversible. Recovery occurs by terminal sprouting and definitive repair is established by the return of vesicle turnover to the original terminals. The return of synaptic function to the original neuromuscular junction associated with elimination of the sprouts requires approximately 91 days. The period of clinically useful relaxation is usually 12–16 weeks [7, 9, 10].
Although BTX-A has a high potential therapeutic value as a tone reducer, it should be noted that it also has to be considered as one of the strongest poisons of the world and is potentially lethal if not used in a safe way. Because the different commercial preparations have different formulations, molecular structures and purification methods, they are unlikely to be clinically equivalent. Individual dosages should be calculated independently for the preparations, guided by the dosing instructions specific to each product and based on previous response and clinical experience. Fixed dose-conversion factors are not applicable in the treatment of spasticity in children with CP [11]. While BTX-A produces a dose-dependent chemical denervation, systematic side effects or untoward responses occur as the total dose of BTX-A increases. Because several muscles are often injected simultaneously within one treatment session, multi-level treatments may involve a higher total dosage when compared to single-level treatments [12, 13]. Each dose should be expressed in units/kg/muscle. The total dose also has to be expressed in units/kg/body weight (U/kg/bw).
There has been enormous progress in the treatment of gait problems in children with CP. In the last 20 years, orthopaedic surgery in CP has evolved from staged surgery performed on an annual basis, where each deformity was corrected individually, to the current practice of a single-event multi-level procedure [1, 14, 15]. The previously used routine led to the so-called ‘birthday syndrome,’ where the child with CP spent a large part of his youth hospitalised or in intensive rehabilitation after surgery, instead of playing around with other children.
For purely orthopaedic interventions, Wenger and Rang [16] and Gage [14] convinced us that the overall result is better if all major muscles involved are lengthened and/or transferred, and bony deformities are corrected during the course of a single surgical procedure, so that all lower extremity joints are balanced simultaneously.
Three-dimensional gait analysis was crucial in proving that a better functional outcome was achieved with the single-event multi-level procedure [17–19]. The ability to objectively document kinematics, kinetics and electromyography (EMG) of the lower extremities, pelvis and trunk has resulted in a better understanding of the pathomechanics of gait and treatment outcomes [18].
A good understanding of the maturity of gait for normal children and for each individual child with CP is crucial in planning the treatment.
From the scientific literature, it can be concluded that many research groups are convinced that mature gait is achieved after the age of 6 years in normal children [20, 21]. Children with CP develop mature gait and/or other motor capacities at a more advanced age.
There is general agreement that surgical intervention to improve gait should be avoided until gait has matured, usually between the ages of 8 and 10 years. Before the age of 8 years, the gait of children with CP is often characterised by inconsistency, which complicates a clear recognition of all major problems in gait and motor function [14].
Moreover, delaying surgery is important because the results of early surgery are less predictable and have a higher risk of failure and relapse. Before the age of 8 years, the recurrence rate or need for a secondary procedure for equinus gait increases in children who have undergone heel cord procedures [14, 15, 22, 23].
Furthermore, it is also well described that the surgical manipulation of soft tissues affect the moment-generating capacity, indicating that repeated muscle and, certainly, tendon lengthening should be avoided to prevent weakness [24].
A delay of orthopaedic surgery should be complemented by a conservative treatment regimen that improves, if possible, the overall condition of the child and optimises motor function, thereby, reducing the development of secondary problems and the need for complex surgery.
When this conservative therapy only includes physiotherapy and the use of orthoses, the dynamic contractures often progress to fixed contractures and even skeletal deformations, causing severe biomechanical lever-arm dysfunction. However, when these therapies are complemented by a selective treatment for the spasticity, such as BTX-A injections, it is hoped that the consequences of the persistent muscle tone can be limited. The reduction in muscle tone allows a combined treatment and is intended to provide an opportunity to optimise the effects of casting and orthotic management, which enhance both motor ability and functional skills and potentially delay the need for surgery [19, 25].
Studies suggest that BTX-A treatment approach may lessen the complexity of future surgery and may help to delay surgery until the optimal timing is achieved, because repeated BTX-A injections can help to prevent the development of muscle contractures and bony deformities if started at an early age [11, 15, 19, 22, 26, 27].
BTX-A injections were first given therapeutically for strabismus in the early 1980s by Allan Scott in the USA [28]. In the following years, the therapeutic spectrum of BTX-A has been successively expanding. The treatment was adopted for other neurologic conditions, such as blepharospasm, cervical dystonia and hemifascial spasm. The use of BTX-A in spasticity was first tried in multiple sclerosis in 1990 [29]. In 1988, the first clinical trials using BTX-A for spasticity in patients with CP were started by Andrew Koman and co-workers. The preliminary results were reported in Koman et al. [30].
The original application of BTX-A in CP was limited to the treatment at one level (mainly to treat an equinus problem). However, a child with CP rarely presents with an isolated problem at one level. Based on the results of gait analysis and clinical examination, the necessity for multi-level treatment with BTX-A became apparent. Many of the common gait patterns in CP can only be adequately treated if several muscles are addressed simultaneously in one treatment session. This is why multi-level treatments with BTX-A are more appropriate.
A multitude of BTX-A studies has been published in the last decade. Most of them, however, focus on single, one-level BTX-A treatment in CP [30–35]. A few studies, though, highlight the need and overall better response of multi-level injections [12, 36–39]. Bakheit et al. [36] concluded, from a study of 1,594 treatments in children with muscle spasticity, that multi-level treatments with BTX-A resulted in a better overall response than single-level treatments. Galli et al. [37] and Mall et al. [38] also emphasised the need for multi-level treatment.
In summary, BTX-A can be seen as a valuable treatment option within the variety of tone reduction treatments, because it:
can reduce muscle tone
is safe at a young age
is reversible
is selective
allows combined treatment
is dose-dependent
Application of BTX-A injections: an integrated multi-level treatment
In order to influence all aspects of the child with CP, an ultimate treatment strategy (Fig. 2) has been set up, in which BTX-A is optimally combined with the common conservative treatment options (physiotherapy, orthotic management, casting and even oral medication). The aim of the combined treatment is to change and to improve the motor pattern of children with CP. This innovative approach has been used at the Pellenberg University Hospital since 1996.
Fig. 2.
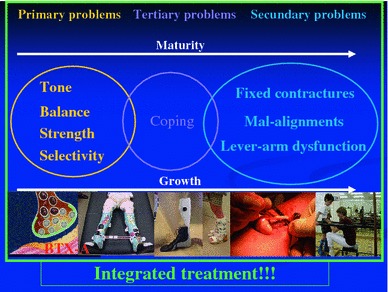
Integrated treatment in children with CP
The fundamentals of this integrated approach are proper muscle selection, an appropriate dosage of BTX-A, and an accurate injection technique. These three aspects are absolute prerequisites to assure a good outcome.
Once these fundamentals have been established, it is clear that other factors are crucial to the optimum ‘long-term’ outcome, namely, pre- and post-injection care, patient selection, timing, appropriate goal settings and an extended evaluation of the outcome. Only when all of these aspects are properly addressed is the success of BTX-A treatment guaranteed.
Within the integrated approach, the interdisciplinary team is of major importance. Because of the complexity of the motor disorder in children with CP and a variety of neurological deficits compounded by the effect of growth on the pathological process, the treatment of a child with CP should utilise a team approach with a variety of medical professionals.
Muscle selection
BTX-A injections should be fine-tuned for each patient individually following an extended standardised clinical examination and an evaluation of posture, gait and/or other motions.
The clinical examination focuses on spasticity, range of motion, strength and selective muscle control. However, even a well standardised physical examination cannot provide a complete description of the complex pathology of CP. Due to the dynamic nature of spastic CP, an accurate assessment of the child should include motion (spasticity is velocity-dependent). Desloovere et al. [40] studied the correlation between gait analysis data and clinical measurements, and evaluated the combined predictive value of static and dynamic clinical measurements on the gait data of children with CP. They found that gait analysis data cannot be sufficiently predicted by a combination of clinical measurements and concluded that both clinical examination and gait analysis data provide important information for delineating the problems of children with CP.
The observation of movement is thought to be a decisive factor in the ‘fine-tuning’ of BTX-A treatment, and, hence, gait analysis plays a crucial role in the identification of target muscles [41]. Objective gait/motion analysis allows the specific description of the pattern of motion at each joint and the identification of the muscles that cause the pathological pattern, according to which the treatment can be modified [14].
It should be noted that the motion analysis is limited to a standardised video recording (walking, crawling, sitting, rolling) in the different anatomical planes for children who are too young to maintain concentration or with limited anatomical height and for more involved children (Gross Motor Function Classification System [GMFCS] IV and V). It is known that children with GMFCS IV and V are more vulnerable to develop hip dysplasia and scoliosis/kyphosis, so X-rays are mandatory in their regular follow-up. Conclusions from these objective evaluations, related to individually defined goal settings, will be crucial for the final selection of the muscles that should be injected. A supplementary clinical evaluation under anaesthesia can provide additional information.
Appropriate dosage
As confidence with BTX-A has grown over the years, increasingly higher doses have been used. These dose increases have involved not only more units of BTX-A per injected muscle, but also the injection of multiple muscle groups in one session. As verified by the objective evaluations, many of the common pathological patterns in CP can only be adequately treated if several muscles are addressed simultaneously in one treatment session.
The optimal dosage per muscle depends on the muscle volume, the amount of spasticity and the degree of the muscle’s involvement in the pathological pattern. Less involved muscles need a lower dosage [12] compared with severely involved muscles, which dictate the pathological pattern of posture, gait or movement.
Using the multi-level approach to administer BTX-A, it was necessary to increase the total dosage. In the literature, total dosages ranging from 2 to 29 U/kg/bw can be found. Because most early studies included only equinus treatment, the most frequently referred dose range referred to was 4–8 U/kg/bw. When a multi-level treatment was used, the maximal doses in the literature ranged from 10 to 29 U/kg/bw. In one study [42, 43], a dose of up to 40 U/kg/bw was used within multi-level treatment. These authors concluded that BTX-A (Botox®) treatment at the higher dose is safe. In an overview of the studies that were performed, with the multi-level/multi-site technique, on monkeys, Aoki et al. [44] stated that there were no observable systematic effects at doses below 33 U/kg/bw. However, there was toxicity progressing to death at doses of 38–42 U/kg/bw.
Safe recommended total dosages recently reported in the literature for children with CP are [11]:
| Botox® | Dysport® | Neuroblock®/Myoblock® | |
|---|---|---|---|
| Range (U/kg bw) | 1–20 (25) | 1–20 (25) | Not established |
| Maximum total dose (U) | 400 (−600) | 500–1,000 | Not established |
| Range maximum dose/site (U) | 10–50 | 50–250 | Not established |
Increasing the dose of BTX-A brings an increased potential for adverse side effects. However, widespread use support the safety of high-dosage BTX-A treatments in children with CP, as the total dose is distributed over multiple muscles and over multiple injection sites per muscle [11, 12, 41, 45]. Significant unwanted adverse effects are rare [11, 36, 46, 47]. Described adverse events tend to be expected consequences of muscle relaxation, such as weakness or initial loss of function, which can occur as patients learn to readjust their postural control in response to altered muscle tone [45, 48]. Recovery and strengthening exercises and orthotics should control these problems. Temporary incontinence has been reported occasionally.
Willis et al. [49] suggest that doses of BTX-A between 15 and 25 U/kg can be administered to the lower limbs of children with CP regardless of aetiology, clinical phenotype, severity, functional ability or medication use, with an adverse event rate that is comparable with lower doses. However, caution has been expressed regarding children with severe spastic quadriplegia who have dysphagia, for whom the total dosage should be limited (<18 U/kg/bw). Adair and Graham [50] found that the incidence of adverse events increased sequentially from GMFCS I to GMFCS V.
In children who are overweight, we advise to adjust their total body weight to the reference of their typical pairs’ height/weight ratio.
The multi-sites theory (with a safe distance between injection sites) is of major importance. This theory is based on the principle that a muscle is like a sponge, which can absorb a certain amount of fluid. If we exceed that volume, the muscle will leak and the toxin will enter the general blood circulation, provoking adverse side effects. Therefore, the total dose per muscle always has to be divided between more sites, with an absolute maximum of 25–50 U/site, and, due to different dilutions, to an absolute maximum of 1 ml/site (in our hands, never more than 25 U/site and/or 1 syringe of 1 ml/site) and an inter-site distance of a minimum of 4–5 cm [51, 52].
Appropriate ranges for Botox® per muscle group of the lower limbs are indicated Table 1.
Table 1.
Indications for appropriate ranges for Botox® per muscle group of the lower limbs
| Muscle group | Dosage (units/kg/body weight) |
|---|---|
| Gastrocnemius | 3–6 |
| Soleus | 1–3 |
| Tibialis posterior | 1–2 |
| Medial hamstrings (semitendinosus, semimembranosus, gracilis) | 3–5 |
| Lateral hamstrings | 1–2 |
| Hip adductors | 1–3 |
| Rectus femoris | 1–2 |
| Iliopsoas | 1–4 |
| Dosage per injection site | Max of 50 units Botox®/site |
Each 100-U vial of Botox® is usually reconstituted with 1–2 ml of saline. Two controlled studies found no differences in therapeutic effect between high- and low-volume preparations [53, 54]. However, recently study results, suggest an improved effect for higher dilutions. A possible explanation for the increased effects of higher dilution compared to lower dilution could be that a larger volume/dilution enables a greater spread of the toxin to neuromuscular junctions and, therefore, improved paralysis and reduction in muscle tone [55, 56]. Gracies et al. [56] also showed the superior efficacy of BTX-A in a spastic muscle when injected using an end plate targeting technique.
Guidelines indicate that the frequency of injecting should not be more than one session of injections every three months. By applying the integrated approach in which BTX-A injections are combined with casting, orthotic management and intensive physical therapy, the duration of the BTX-A effect is increased. The averaged duration of effect according to this approach was found to be more than one year [57]. This is an important finding because the higher the frequency of treatments, the higher the risk of antibody formation [58]. Brashear et al. [59] investigated the long-term dose consistency of BTX-A and the intervals between treatments over a period of two years in cervical dystonia patients. Their outcomes indicated that doses and intervals between BTX-A treatments were consistent throughout two years of observation, thereby, indirectly indicating the non-development of antibody formation. Recent data confirm these findings in children with CP and found that total dosages and treatment intervals remained stable within subsequent BTX-A treatments [57]. More research is needed to expand the results of this study.
Accurate injection technique
BTX-A injections can be administered under local anaesthesia, conscious sedation or general anaesthesia [27]. If multiple levels are involved, it is recommended to administer BTX-A under general anaesthesia. This also permits an additional clinical evaluation while the patient is under anaesthesia.
Correct needle placement (usually 26 gauge × 23 mm and 22 gauge × 30 mm) for the different selected muscle groups is defined by using palpation with the muscle under stretching and by manual testing. By applying a passive motion to the joint, the needle will be moving with the muscle and the correct needle placement can be confirmed. This is particularly interesting for separating bi- and mono-articular muscle groups.
However we are convinced that ultrasonography with or without EMG guidance or electrical stimulation is the most appropriate technique to localise and identify the muscles to be injected, especially for the smaller muscle groups [11].
Once the fundamentals of the multi-level BTX-A treatment are properly addressed, several other crucial factors are important in achieving successful outcomes. The first factor is the optimum pre- and post-injection care (which is a combination of casting, physical therapy and orthotic management). To set up a long-term treatment plan, appropriate patient selection with ideal timing and individually defined goal-settings is crucial to obtain successful results of BTX-A treatment. Finally, including perfectly timed BTX-A treatments, detailed measurement of the outcome is also crucial.
Aftercare
Alhusaini et al. [60] found no changes in the passive tissue characteristic of the calf muscles following BTX-A, despite a reduction in spasticity. They concluded that additional treatment approaches are required to supplement the effects of BTX-A injections when managing children with calf muscle spasticity in CP. Desloovere [61] confirmed that aftercare issues, such as casting, orthotic management and physical therapy, significantly influenced a successful outcome after BTX-A injection in children with CP.
Casting and day and night orthoses are used in conjunction with physical therapy to prolong improved muscle length and facilitate the carry-over of improved motor control following BTX-A injections. The combination of these different conservative treatments is crucial within the integrated approach and should be seen as a continuum in which the treatments are strongly linked to each other, mainly by the use of physical therapy.
Casting
For some time, the general indication for toxin injection was “the presence of a dynamic contracture, interfering with function, in the absence of a fixed myostatic contracture” [62]. However, in many cases, there are components of both dynamic muscle shortening and early contractures. Various combinations of injections with periods of casting may then be appropriate, and may extend the window of a strict BTX-A treatment. There is evidence that BTX-A alone is as effective as casting alone in the management of dynamic equinus, having a similar magnitude of response but a longer duration [27, 63, 64]. From the work of Molenaers et al. [65] and Desloovere et al. [66], the additional benefits of the combined treatment have become clear. Children that were treated with BTX-A injections combined with casting showed an improved second-ankle rocker and longer-lasting effects as compared to children that were treated without casting. From a prospective study, Desloovere et al. [41] concluded that more benefits, mainly in the proximal joints, were seen for the children who were casted after injections as compared to the children who were casted before injections.
Most of the studies have examined the effects of casting on spastic equinus. However, casting can also be applied for other muscles at other levels. Because of the inconvenience of casting proximal muscles and proximal joints, these casts should be removable and be worn for only a part of the day. It should be noted that, although several studies and long-term clinical practice indicated the advantages of combining BTX-A injections with casting, Blackmore et al. [67] concluded in their review that there is still no strong and consistent evidence that combining casting and BTX-A is superior to using either intervention alone, or that either casting or BTX-A is superior to the other immediately after treatment.
Nevertheless, our own data, objective and even dynamic, prove, on several different occasions, that combining BTX-A with casting is far more effective in short- and long-term outcomes than casting or BTX-A as a standalone procedure. But we have to admit that we always combine casting and BTX-A with the use of orthoses as part of the aftercare and treatment protocol (which is what we strongly recommend).
Physical therapy
As previously mentioned, the overall aim is to make functional progress or at least to preserve a status quo in the medium- to long-term. In physical therapy treatment of children with CP, varying approaches and techniques are used, ranging from very conservative and conventional techniques like tonification, manual stretching, massage etc., to more complex motor learning-based theories like neurodevelopmental treatment, Vojta, Petö and several others.
A number of studies emphasise the importance of physical therapy combined with BTX-A treatment [39, 68–75]. Because of the shortage of knowledge on therapy contents and the different outcome measures used in these studies, no consensus can be reached concerning the content of the physiotherapy programme after treatment with BTX-A. Desloovere et al. [26] showed that an individually defined specific physical therapy programme after BTX-A results in an improved effect of the combined treatment (physical therapy and BTX-A) as compared to traditional therapy post-BTX-A.
At the University Hospital of Pellenberg, Belgium, special treatment plans have been developed for children that were planned for BTX-A treatment, based on general principles in motor training.
First, the physical therapist should ensure the child’s optimum preparation before the BTX-A treatment, including the definition of goal-setting, starting new specific motor training (training new postures and specific movements), warning the child of a possible initial loss of functionality shortly after the injections and, as a consequence, the need to start a more intensive physical therapy programme.
Post-BTX-A injection physical therapy starts at the time of the casting period and should focus on: (1) analytical therapy by electrostimulation and/or proprioceptive training of, for instance, the tibialis anterior and gluteus maximus muscles, specific muscle training in open and closed loops, fast motion exercises and the training of specific muscle activities in parts of the active range of motion, such as full hip and knee extension, that are unknown and not used by the child and (2) functional therapy by active gait rehabilitation and the use of newly recovered muscle activity in daily life. The long-term physical therapy should then focus on preserving gained muscle length by stretching and the use of orthoses, casts and positioning, continuing strengthening and proprioceptive training of the antagonists and/or agonists and (3) automation of new motor development (Lokomat, treadmill). Treadmill walking provides increased opportunity to repetitively train the whole gait cycle and facilitate an improved gait pattern [76].
For the functional children, training the sense of new movements and the full joint amplitude during active motion is especially important. An analytical approach may be used to assist in establishing the balance between agonists, antagonists and synergistic muscles [77]. Converting muscle function into functional activities is also important because it allows more automatic performance of new motions (for instance, by treadmill training), ensuring the carry-over effect. Employing a dynamic approach and building variability into the functional activities will make the learning process more interesting for the child, provide more functional possibilities and help to prevent the child from relapsing to the same posture and movement as before.
In the more involved child, postural problems caused by muscle contractions and skeletal/joint deformations are of major concern. Through the use of tone-controlling injections and rehabilitation, physical therapy stimulates a more symmetrical active posture, with major focus on active trunk control. In addition, these children are stimulated by sitting, standing and other modalities of treatment, such as a walking belt (robotic training), standing table, sitting aid, pressure splint and/or body jacket. This will enable them to be more active and gain better motor control, which will improve muscle length and stiffness.
Orthotic management
After BTX-A injections, the use of night splinting and day orthoses appears to be a critical factor in influencing the long-term effects of BTX-A treatment (the effects on both preserving muscle length and providing stability to distal joints are thought to be important). This allows selective training of more proximal muscle groups (‘target training’). For some children, day splinting contributes to proprioceptive training, and for children who lack selective control of certain muscle groups, the day splints are crucial for normalising gait (for instance, for correcting a drop foot) [12]. Orthoses supply the appropriate biomechanical alignment to allow practice and ensure functional carry-over outside periods of targeted motor training conducted by the physical therapist. Bilateral leaf springs are common post-injection day orthoses. For spastic adductors (with hips at risk), the combination of BTX-A and the use of variable hip abduction orthoses may be indicated [27, 78]. At night (or in the evening), fixed ankle–foot orthoses (AFOs) with knee extension braces and an abduction–external rotation rod are often applied [79].
Patient selection, timing and goal settings
BTX-A treatments in children with CP are usually referred when there is a lack in motor control progress, development of muscle contractures, intolerance of day and night splinting, and/or decrease in functionality. Treatments are appropriate for a variety of diagnoses (predominantly spastic type of hemiplegia, diplegia, triplegia and quadriplegia) and GMFCS functional levels I–V. Treatment goals for BTX-A have been expanded in recent times. Because treatment indications have been extended and because children with CP reflect a very heterogeneous group with regard to motor impairment and ability, treatment goals need to be well defined and tailored to the individual needs of the patient. More involved children, as well as more functional children, can benefit from BTX-A treatment, as long as the goal settings are adapted to the specific problems. The study of Fagard et al. [80] showed that BTX-A treatment was successful in more functional as well as in less functional children with CP. They also found differences in goal setting and the success rate of these goals between both groups. Goals around the hip were more frequent in less functional children, but the success rate was higher in the more functional children. The second-ankle rocker also showed a higher success rate in the functional group. Further research is needed to evaluate if specific physiotherapy exercises with special attention to these goals lead to a higher success rate in less functional children.
It should be noted that BTX-A is not only used to treat spasticity in children with CP, but it is also an effective therapy in children and adolescents with an acquired brain injury to improve leg and arm function, comfort and well-being [81].
Treatment goals for different indications may be focused on improving function (gait) and, thereby, influencing the pathological process for the functional child (GMFCS level I–III) and improving balance, control of sitting, positioning and facilitating hygienic care and bracing for non-ambulatory patients (GMFCS level IV–V). More specific goals are listed below [27, 46, 68, 82–84]:
Facilitation of orthotic management
Continuation of conservative management until maturity of gait is achieved
Evaluation of short-term functional gain, providing crucial information for the future treatment plan
Simulation of orthopaedic or neurosurgery facilitating training in order to achieve a better condition before going into surgery
Assisting in the prevention of hip subluxation by controlling spasticity in hip adductors and flexors, with a hip abduction brace
Decreasing spasms for patients with highly fluctuating tone in the upper and lower limbs
Allowing improved positioning and control of posture
Treatment of pain caused by spasms (spastic-athetoid)
Treatment of back pain due to hyperlordosis, where tone reduction of the psoas muscle can help
Relief of pain post-operatively
Use of BTX-A as an adjunctive treatment for regional or generalised spasticity
Our data revealed some differences in treatment with BTX-A between children with hemiplegia and children with diplegia:
Children with hemiplegia mostly have spasticity in the soleus muscle. Therefore, the soleus is often injected with BTX-A in these children. In children with diplegia, however, the soleus muscle is usually rather weak and not very spastic. So, treatment of the soleus is not indicated in patients with diplegia
The adductor muscles are more often treated in children with hemiplegia and quadriplegia
Children with diplegia receive more repeated BTX-A injections compared to children with hemiplegia
Between 1996 and 2006, 906 children were treated with BTX-A in the University Hospital of Pellenberg. More than half of them were classified as diplegic CP (Fig. 3). When we evaluated 116 children who received at least two repeated injections, most of them (79%; 92/116) were children with diplegia (Fig. 4).
Fig. 3.
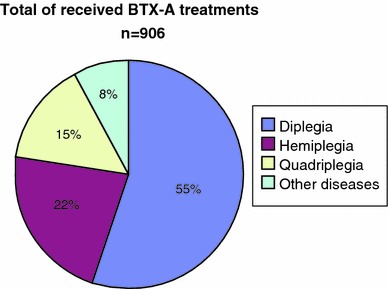
Number of BTX-A treatments between 1996 and 2006 at University Hospital of Pellenberg
Fig. 4.
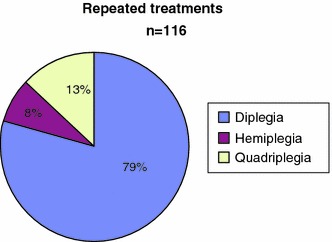
Repeated treatments in function of diagnosis
Spasticity will usually develop quickly within the first years of age. From the onset of spasticity, the motor development will be influenced and the contractures will start to develop.
Ideally, therefore, BTX-A treatment should start at a young age when gait patterns and motor function are still flexible, allowing gross motor function learning during the time window of tone reduction. The optimal timing is often reported to be between 2 and 6 years of age [27, 62, 85]. Older children usually benefit from a more targeted treatment approach.
BTX-A is contraindicated in the presence of infection at the proposed injection site(s), individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation, and in patients with myasthenia gravis, Eaton–Lambert syndrome, amyotrophic lateral sclerosis or other significant diseases that might interfere with neuromuscular function.
In 2008, a multi-centre study (UZ Pellenberg, UZ Gent, UZ Antwerp, UCL Brussels and ULB-VUB Brussels) for the Belgium government was set up to evaluate the effect of integrated multi-level BTX-A treatment both in young and older children with CP (287 children). Therefore, the study group was divided into two age groups (<9 years and ≥9 years). Although the mean GAS scores slightly decreased with increasing age, they remained above the expected outcome of 50 in both age groups and the difference in mean GAS scores between the two age groups was not significant. This implicates that BTX-A is effective in younger as well as in older children.
Desloovere [61] delineated crucial factors within the BTX-A treatment strategy which may predict a positive outcome. The results indicated that age, diagnosis, muscle selection, and frequency of physical therapy and orthotic management after injection can be considered as crucial factors influencing the effect of BTX-A.
Evaluation of outcome
In the post-BTX-A treatment evaluation, we are interested in the individual’s treatment result and in evaluating the treatment hypothesis. In addition, also the correlation between outcome results and the subjective experience of the patient is important.
The individual’s treatment result provides new and interesting information that may be important in the further development or fine-tuning of that child’s overall treatment strategy. By carefully evaluating the treatment outcome, we learn how the child develops new motor abilities in therapy, gait or movements, and normal daily life. In particular, the functional use of the antagonist and restoration of the agonist/antagonist balance is important in this respect. An objective evaluation after BTX-A injections can also help to highlight other problems, especially the contributions of weakness, poor balance and inadequate trunk stability. Moreover, post-injection gait analysis data are useful to distinguish between primary gait deviations and coping mechanisms. Differences between the two can be quite subtle; however, by definition, coping mechanisms will disappear spontaneously once the primary gait problems are resolved [14]. Finally, BTX-A treatment has a role as a pre-surgical evaluation method for children in whom the benefits of surgery (orthopaedic or neurosurgery) are difficult to predict, or for whom the fine-tuning of the operation requires more fundamental information about underlying motor problems [84, 86].
The post-BTX-A treatment evaluation can also be used to evaluate the present treatment hypothesis. BTX-A has a variety of short-term successful outcome parameters, such as a reduction of muscle tone [63, 85], an increased range of joint motion [32, 63, 85, 87], an improved gait pattern [32, 87], an increased muscle length [88] and improved function through the Gross Motor Function Measure [39]. Different types of assessment tools were used in the performed studies until now, which may explain the variety of treatment outcome parameters. Variability in outcome may also be related to crucial factors like dose, antibody formation, aftercare and age [45, 85].
There are only a limited number of studies on the long-term outcome. Desloovere et al. [25] demonstrated that BTX-A treatment, in combination with common conservative treatment options, delays and reduces the frequency of surgical procedures and result in a gait pattern that is less defined by secondary problems (e.g. bony deformities) at 5–10 years of age, minimising the need for complex surgery at a later age and enhancing quality of life. This is in agreement with the results of Molenaers et al. [19], which showed that botulinum toxin type A treatment can delay and reduce the need for surgery in the follow-up of children with cerebral palsy, provided that the treatment is started while gait patterns are still flexible.
Our patients’ subjective experience shows an overall satisfaction rate of 69.2%. Approximately 60% believe that the effect of treatment lasts for 6–12 months and 36% are convinced that the effect lasts for longer than 1 year.
Long-term use of BTX-A
Because of the temporary effect of BTX-A, for the majority of the patients repeated injections are needed. As mentioned before, dosages and treatment intervals of approximately one year remain stable within subsequent BTX-A treatments [57]. By evaluating the effect of two to four repeated BTX-A treatments in children with CP using the Goal Attainment Scale (GAS), we found that the GAS score decreased between the first and last BTX-A session, however the overall mean GAS T-score remained significantly higher than the expected mean of 50, indicating a successful outcome [89]. Further research is needed to expand these findings.
We also evaluated the ongoing treatment after four and five BTX-A treatments in 106 children with CP [57]. Fifty percent of the patients in the study group continued with BTX-A treatments after the four investigated BTX-A treatments. Another 19.8% of the patients received their last treatment one year or less before the end of data collection (and may or may not continue BTX-A treatment). Follow-up data after five treatment sessions disclosed 39.6% of patients who continued treatment and 22.6% who received their last injection of BTX-A 1 year or less before the end of data collection (and may or may not continue BTX-A treatment).
Financial cost
A discussion of the financial cost of BTX-A is complex, because the costs of one BTX-A vial varies between countries. However, a financial cost comparison between BTX-A injections and soft tissue surgery (lengthening of muscles) was made for children with diplegia (in both treatment of psoas, hamstrings and gastrocnemius bilateral), treated at the University Hospital of Pellenberg (Table 2). The total cost takes into account the cost for the technical act (operation), anaesthesia, the cost of the BTX-A product and hospital stay. On average, the cost of the BTX-A product is 770 Euros for a patient with diplegia between four and six years of age. Hospital stay was the primary cost driver for the BTX-A treatment sessions, as well as for soft tissue surgery. The hospital stay of 4–7 days after soft tissue surgery is much longer than the 1-day hospital stay (1-day clinic) after BTX-A treatment. Moreover for soft tissue surgery, there is still the cost for the rehabilitation period of about 4–6 weeks in rehabilitation hospital (where they can attend school), which was not included in the total cost.
Table 2.
Financial cost comparison between BTX-A treatment and soft tissue surgery in children with diplegia (in both treatment of psoas, hamstrings and gastrocnemius bilateral), treated at the University Hospital of Pellenberg, Belgium
| BTX-A treatment (Euros) | Soft tissue surgery (Euros) | |
|---|---|---|
| Operation | 60 | 750 |
| Anaesthesia | 50 | 300 |
| Hospital stay | 166.11 (1 day) | 2,500–3,500 (4–7 days) |
| BTX-A product | 770 | – |
| Total cost | 1,046.11 | >3,550–4,550 |
Conclusion
From different studies and two decades of clinical experience it can be concluded that BTX-A treatment, applied according to an integrated approach and started at a young age, will improve the overall condition of children with CP.
Children who received BTX-A demonstrate several advantages such as less loss of muscle strength, less financial costs, better objective gait data and less absences in school, compared to patients who already underwent a surgical intervention at a young age. Moreover, soft tissue surgery also has a high recurrence rate and a higher risk of lengthening muscles or tendons which were in fact dynamically not too short at all (objective gait data).
BTX-A treatment and surgical intervention can be viewed as complementary rather than mutually exclusive, and may be used concurrently or sequentially to increase the benefit, for instance, lever-arm deformities can be corrected simultaneous with BTX-A treatment for spasticity, and BTX-A treatment can be used as an outcome predictor for surgical interventions, such as in selective dorsal rhizotomy or intrathecal baclofen treatment.
BTX-A can also be used at an older age to control spasticity during the pubertal growth spurt, even when children already previously underwent a surgical intervention.
It should be noted that applying the BTX-A treatment in a careless manner, we may spoil the chances of maximal improvement of the child, and repeated injections may then be less effective, even without having antibody formation. Long-term repeated treatments are assured to be successful only if all conditions are fulfilled (integrated approach, multi-level multi-site injections when needed, appropriate muscle selection, secure injection technique). However, long-term BTX-A treatment cannot always prevent the development of secondary deformities such as lever-arm dysfunctions (due to underlying weakness, lack of good selective motor control etc.). These secondary problems can then be successfully addressed by orthopaedic surgical corrections with good long-standing outcomes.
We should be aware that BTX-A is still the most potent poison available, potentially lethal, and, therefore, the application rules should be followed strictly. In this respect, for a quadriplegic patient (GMFCS IV–V) with swallowing or respiration problems, the total doasage should never exceed 16–18U/kg/BW.
If a multi-level high-dose treatment is applied, the dose should always be divided over several sites per muscle with special attention to the inter-site distance (5 cm) and with the maximum dose per site (25 U and, if diluted by more than 4–5 ml, maximum 1 syringe/site).
Following the above precautions, adverse effects will be very rare or even absent.
Further instructions can be found in the consensus paper by Heinen et al. [11].
However, not all of our questions are yet solved. Different challenges in the future still require new studies such as:
How to organise the optimum treatment plan in one patient with CP for the different BTX-A treatment indications (such as hyperhidrosis, hyperactive bladder, migraine, drooling, spasticity, postoperative pain)?
What is the most appropriate dilution (1, 2, 3, 4… ml/vial)?
How to decrease the dosage by injecting at the motor end plate, while assuring a good efficacy? Will we be able to identify the motor end plates in a clinical setting?
Acknowledgments
Conflict of interest statement
For some of the referred studies, the authors received an unrestricted educational grant from Allergan N.V., Belgium.
References
- 1.Rang M (1993) In: Wenger DR, Rang M (eds) Cerebral palsy in the art and practice of children’s orthopaedics. Raven Press, New York
- 2.Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287–294. doi: 10.1111/j.1651-2227.2005.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 3.McManus V, Guillem P, Surman G, Cans C. SCPE work, standardization and definition—an overview of the activities of SCPE: a collaboration of European CP registers. Zhongguo Dang Dai Er Ke Za Zhi. 2006;8(4):261–265. [PubMed] [Google Scholar]
- 4.Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol. 2001;8:21–29. doi: 10.1046/j.1468-1331.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 5.Aoki KR. Immunologic and other properties of therapeutic botulinum toxin serotypes. In: Brin MF, Hallett M, Jankovic J, editors. Scientific and therapeutic aspects of botulinum toxin. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 103–113. [Google Scholar]
- 6.Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki KR, Ranoux D, Wissel J. Using translational medicine to understand clinical differences between botulinum toxin formulations. Eur J Neurol. 2006;13:10–19. doi: 10.1111/j.1468-1331.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 8.Lukban MB, Rosales RL, Dressler D. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm. 2009;116(3):319–331. doi: 10.1007/s00702-008-0175-8. [DOI] [PubMed] [Google Scholar]
- 9.Aoki KR. Pharmacology and immunology of botulinum toxin type A. Clin Dermatol. 2003;21:476–480. doi: 10.1016/j.clindermatol.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 10.de Paiva A, Meunier FA, Molgó J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci USA. 1999;96(6):3200–3205. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinen F, Desloovere K, Schroeder AS, Berweck S, Borggraefe I, van Campenhout A, Andersen GL, Aydin R, Becher JG, Bernert G, Caballero IM, Carr L, Valayer EC, Desiato MT, Fairhurst C, Filipetti P, Hassink RI, Hustedt U, Jozwiak M, Kocer SI, Kolanowski E, Krägeloh-Mann I, Kutlay S, Mäenpää H, Mall V, McArthur P, Morel E, Papavassiliou A, Pascual-Pascual I, Pedersen SA, Plasschaert FS, van der Ploeg I, Remy-Neris O, Renders A, Di Rosa G, Steinlin M, Tedroff K, Valls JV, Viehweger E, Molenaers G (2010) The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur J Paediatr Neurol 1:45–66 [DOI] [PubMed]
- 12.Molenaers G, Desloovere K, Eyssen M, Decat J, Jonkers I, De Cock P. Botulinum toxin type A treatment of cerebral palsy: an integrated approach. Eur J Neurol. 1999;6:S51–S57. doi: 10.1111/j.1468-1331.1999.tb00035.x. [DOI] [Google Scholar]
- 13.Kinnett D. Botulinum toxin A injections in children: technique and dosing issues. Am J Phys Med Rehabil. 2004;83:S59–S64. doi: 10.1097/01.PHM.0000141131.66648.E9. [DOI] [PubMed] [Google Scholar]
- 14.Gage JR. Gait analysis in cerebral palsy. London: Mac Keith Press; 1991. pp. 101–131. [Google Scholar]
- 15.Fabry G, Liu XC, Molenaers G. Gait pattern in patients with spastic diplegic cerebral palsy who underwent staged operations. J Pediatr Orthop B. 1999;8:33–38. [PubMed] [Google Scholar]
- 16.Wenger DR, Rang M. The art and practice of children’s orthopaedics. New York: Raven Press; 1993. [Google Scholar]
- 17.Gage JR, DeLuca PA, Renshaw TS. Gait analysis: principles and applications. J Bone Joint Surg Am. 1995;77:1607–1623. [Google Scholar]
- 18.DeLuca PA, Davis RB, 3rd, Ounpuu S, Rose S, Sirkin R. Alterations in surgical decision making in patients with cerebral palsy based on three-dimensional gait analysis. J Pediatr Orthop. 1997;17:608–614. doi: 10.1097/01241398-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Molenaers G, Desloovere K, Fabry G, De Cock P. The effects of quantitative gait assessment and botulinum toxin A on musculoskeletal surgery in children with cerebral palsy. J Bone Joint Surg Am. 2006;88:161–170. doi: 10.2106/JBJS.C.01497. [DOI] [PubMed] [Google Scholar]
- 20.Gómez Pellico L, Rodríguez Torres R, Dankloff Mora C. Changes in walking pattern between five and six years of age. Dev Med Child Neurol. 1995;37:800–806. doi: 10.1111/j.1469-8749.1995.tb12063.x. [DOI] [PubMed] [Google Scholar]
- 21.Desloovere K, Molenaers G, Eyssen M. The three dimensional kinematics, kinetics and EMG pattern of the walking gait cycle of children aged between three and six years. Gait Posture. 1998;8:76. [Google Scholar]
- 22.Zurcher AW, Molenaers G, Fabry G. Treatment of equinus in young children with hemiplegic cerebral palsy: recurrence after Achilles tendon lengthening and kinematic and kinetic evaluation of treatment with botulinum toxin. Gait Posture. 1999;10:90. doi: 10.1016/S0966-6362(99)90462-9. [DOI] [Google Scholar]
- 23.Borton DC, Walker K, Pirpiris M, Nattrass GR, Graham HK. Isolated calf lengthening in cerebral palsy. Outcome analysis of risk factors. J Bone Joint Surg Br. 2001;83:364–370. doi: 10.1302/0301-620X.83B3.10827. [DOI] [PubMed] [Google Scholar]
- 24.Delp SL, Arnold AS, Piazza SJ. Graphics-based modeling and analysis of gait abnormalities. Biomed Mater Eng. 1998;8:227–240. [PubMed] [Google Scholar]
- 25.Desloovere K, Molenaers G, De Cat J, Pauwels P, Van Campenhout A, Ortibus E, Fabry G, De Cock P. Motor function following multilevel botulinum toxin type A treatment in children with cerebral palsy. Dev Med Child Neurol. 2007;49:56–61. doi: 10.1017/S001216220700014X.x. [DOI] [PubMed] [Google Scholar]
- 26.Desloovere K, De Cat J, Schörkhuber V, Van den Broeck C, Persyn A, Huenaerts C, Callewaert B, Molenaers G. The effect of individually defined physiotherapy program based on gait analysis after BTX-A treatment in children with CP. Gait Posture. 2007;26:S26–S27. [Google Scholar]
- 27.Graham HK, Aoki KR, Autti-Rämö I, Boyd RN, Delgado MR, Gaebler-Spira DJ, Gormley ME, Guyer BM, Heinen F, Holton AF, Matthews D, Molenaers G, Motta F, García Ruiz PJ, Wissel J. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture. 2000;11:67–79. doi: 10.1016/S0966-6362(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 28.Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol Vis Sci. 1973;12:924–927. [PubMed] [Google Scholar]
- 29.Snow BJ, Tsui JK, Bhatt MH, Varelas M, Hashimoto SA, Calne DB. Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol. 1990;28(4):512–515. doi: 10.1002/ana.410280407. [DOI] [PubMed] [Google Scholar]
- 30.Koman LA, Mooney JF, 3rd, Smith B, Goodman A, Mulvaney T. Management of cerebral palsy with botulinum-A toxin: preliminary investigation. J Pediatr Orthop. 1993;13:489–495. doi: 10.1097/01241398-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Koman LA, Mooney JF, 3rd, Smith BP, Goodman A, Mulvaney T. Management of spasticity in cerebral palsy with botulinum-A toxin: report of a preliminary, randomized, double-blind trial. J Pediatr Orthop. 1994;14:229–303. doi: 10.1097/01241398-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Koman LA, Mooney JF, 3rd, Smith BP, Walker F, Leon JM. Botulinum toxin type A neuromuscular blockade in the treatment of lower extremity spasticity in cerebral palsy: a randomized, double-blind, placebo-controlled trial. J Pediatr Orthop. 2000;20:108–115. [PubMed] [Google Scholar]
- 33.Boyd RN, Pliatsios V, Starr R, Wolfe R, Graham HK. Biomechanical transformation of the gastroc-soleus muscle with botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2000;42:32–41. doi: 10.1017/S0012162200000074. [DOI] [PubMed] [Google Scholar]
- 34.Cosgrove AP, Graham HK. Botulinum toxin A prevents the development of contractures in the hereditary spastic mouse. Dev Med Child Neurol. 1994;36:379–385. doi: 10.1111/j.1469-8749.1994.tb11863.x. [DOI] [PubMed] [Google Scholar]
- 35.Sławek J, Klimont L. Functional improvement in cerebral palsy patients treated with botulinum toxin A injections—preliminary results. Eur J Neurol. 2003;10:313–317. doi: 10.1046/j.1468-1331.2003.00582.x. [DOI] [PubMed] [Google Scholar]
- 36.Bakheit AMO, Severa S, Cosgrove A, Morton R, Roussounis SH, Doderlein L, Lin JP. Safety profile and efficacy of botulinum toxin A (Dysport) in children with muscle spasticity. Dev Med Child Neurol. 2001;43(4):234–238. doi: 10.1017/S0012162201000445. [DOI] [PubMed] [Google Scholar]
- 37.Galli M, Crivellini M, Santambrogio GC, Fazzi E, Motta F. Short-term effects of ‘botulinum toxin A’ as treatment for children with cerebral palsy: kinematic and kinetic aspects at the ankle joint. Funct Neurol. 2001;16:317–323. [PubMed] [Google Scholar]
- 38.Mall V, Berweck S, Kirschner J, Herrmann J, Schelle A, Linder M, Michaelis U, Stein S, Korinthenberg R, Heinen F. Die Therapie Spastischer Bewegungsstörungen im Kindesalter mit Botulinumtoxin A. Klin Neurophysiol. 2001;2001:218–224. doi: 10.1055/s-2001-18957. [DOI] [Google Scholar]
- 39.Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, Becher JG. The combined effect of lower-limb multilevel botulinum toxin type A and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Arch Phys Med Rehabil. 2006;87:1551–1558. doi: 10.1016/j.apmr.2006.08.342. [DOI] [PubMed] [Google Scholar]
- 40.Desloovere K, Molenaers G, Feys H, Huenaerts C, Callewaert B, Van de Walle P. Do dynamic and static clinical measurements correlate with gait analysis parameters in children with cerebral palsy? Gait Posture. 2006;24:302–313. doi: 10.1016/j.gaitpost.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Desloovere K, Molenaers G, Jonkers I, De Cat J, De Borre L, Nijs J, Eyssen M, Pauwels P, De Cock P. A randomized study of combined botulinum toxin type A and casting in the ambulant child with cerebral palsy using objective outcome measures. Eur J Neurol. 2001;8:75–87. doi: 10.1046/j.1468-1331.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 42.Awaad Y, Tayem H, Elgamal A, Coyne MF. Treatment of childhood myoclonus with botulinum toxin type A. J Child Neurol. 1999;14:781–786. doi: 10.1177/088307389901401203. [DOI] [PubMed] [Google Scholar]
- 43.Awaad Y, Tayem H, Munoz S, Thomas R, Soliman S, Michon A, Minarik S. High dose of botulinum toxin type-A (BTX/A): safety and efficacy in patients with cerebral palsy. J Pediatr Neurol. 2000;2:91–96. [Google Scholar]
- 44.Aoki KR, Ismail M, Tang-Lui D, Brar B, Wheeler LA. Botulinum toxin type A: from toxin to therapeutic agent. Eur J Neurol. 1997;4:S1–S3. doi: 10.1111/j.1468-1331.1997.tb00292.x. [DOI] [Google Scholar]
- 45.Goldstein EM. Safety of high-dose botulinum toxin type A therapy for the treatment of pediatric spasticity. J Child Neurol. 2006;21:189–192. doi: 10.2310/7010.2006.00041. [DOI] [PubMed] [Google Scholar]
- 46.Flett PJ. Rehabilitation of spasticity and related problems in childhood cerebral palsy. J Paediatr Child Health. 2003;39:6–14. doi: 10.1046/j.1440-1754.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 47.Naumann M, Jankovic J. Safety of botulinum toxin type A: a systematic review and meta-analysis. Curr Med Res Opin. 2004;20:981–990. doi: 10.1185/030079904125003962. [DOI] [PubMed] [Google Scholar]
- 48.Koman LA, Brashear A, Rosenfeld S, Chambers H, Russman B, Rang M, Root L, Ferrari E, García de Yebenes Prous J, Smith BP, Turkel C, Walcott JM, Molloy PT. Botulinum toxin type A neuromuscular blockade in the treatment of equinus foot deformity in cerebral palsy: a multicenter, open-label clinical trial. Pediatrics. 2001;108:1062–1071. doi: 10.1542/peds.108.5.1062. [DOI] [PubMed] [Google Scholar]
- 49.Willis AW, Crowner B, Brunstrom JE, Kissel A, Racette BA. High dose botulinum toxin A for the treatment of lower extremity hypertonicity in children with cerebral palsy. Dev Med Child Neurol. 2007;49:818–822. doi: 10.1111/j.1469-8749.2007.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adair B, Graham K. Systemic adverse events following injection of botulinum toxin A (BoNT-A) in children with cerebral palsy. Dev Med Child Neurol. 2009;51(2):54–55. doi: 10.1111/j.1469-8749.2009.03583.x. [DOI] [PubMed] [Google Scholar]
- 51.Sanders I, Shaari C, Amirali LAY (1999) The glycogen depletion assay and the measurement of botulinum toxin injections. Abstract from International Conference on Basic and Therapeutic Aspects of Botulinum and Tetanus Toxins, Orlando, Florida, November 16–18 1999, p 33
- 52.Chell J, Hunter JB (2001) Urinary incontinence following botulinum toxin A injection in cerebral palsy. Abstract of the 20th EPOS Meeting, Montpellier, France, April 4–7 2001
- 53.Francisco GE, Boake C, Vaughn A. Botulinum toxin in upper limb spasticity after acquired brain injury: a randomized trial comparing dilution techniques. Am J Phys Med Rehabil. 2002;81:355–363. doi: 10.1097/00002060-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Lee LR, Chuang YC, Yang BJ, Hsu MJ, Liu YH. Botulinum toxin for lower limb spasticity in children with cerebral palsy: a single-blinded trial comparing dilution techniques. Am J Phys Med Rehabil. 2004;83:766–773. doi: 10.1097/01.PHM.0000137314.38806.95. [DOI] [PubMed] [Google Scholar]
- 55.Kawamura A, Campbell K, Lam-Damji S, Fehlings D. A randomized controlled trial comparing botulinum toxin A dosage in the upper extremity of children with spasticity. Dev Med Child Neurol. 2007;49:331–337. doi: 10.1111/j.1469-8749.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 56.Gracies JM, Lugassy M, Weisz DJ, Vecchio M, Flanagan S, Simpson DM. Botulinum toxin dilution and endplate targeting in spasticity: a double-blind controlled study. Arch Phys Med Rehabil. 2009;90:9–16. doi: 10.1016/j.apmr.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 57.Molenaers G, Schörkhuber V, Fagard K, Van Campenhout A, De Cat J, Pauwels P, Ortibus E, De Cock P, Desloovere K. Long-term use of botulinum toxin type A in children with cerebral palsy: treatment consistency. Eur J Paediatr Neurol. 2009;13(5):421–429. doi: 10.1016/j.ejpn.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Herrmann J, Geth K, Mall V, Bigalke H, Schulte Mönting J, Linder M, Kirschner J, Berweck S, Korinthenberg R, Heinen F, Fietzek UM. Clinical impact of antibody formation to botulinum toxin A in children. Ann Neurol. 2004;55:732–735. doi: 10.1002/ana.20098. [DOI] [PubMed] [Google Scholar]
- 59.Brashear A, Hogan P, Wooten-Watts M, Marchetti A, Magar R, Martin J. Longitudinal assessment of the dose consistency of botulinum toxin type A (Botox®) for cervical dystonia. Adv Ther. 2005;22:49–55. doi: 10.1007/BF02850184. [DOI] [PubMed] [Google Scholar]
- 60.Alhusaini AA, Crosbie J, Shepherd R, Dean C. Do botulinum toxin injections (BTX) alter the passive mechanical properties of the calf muscles in children with cerebral palsy (CP)? Dev Med Child Neurol. 2009;51(2):54. [Google Scholar]
- 61.Desloovere K. Efficacy of botulinum toxin A treatment in children with cerebral palsy is defined by crucial factors within the treatment strategy. Gait Posture. 2008;28:S1–S2. doi: 10.1016/S0966-6362(08)70002-X. [DOI] [Google Scholar]
- 62.Boyd R, Graham HK. Botulinum toxin A in the management of children with cerebral palsy: indications and outcome. Eur J Neurol. 1997;4:S15–S22. doi: 10.1111/j.1468-1331.1997.tb00295.x. [DOI] [Google Scholar]
- 63.Corry IS, Cosgrove AP, Duffy CM, McNeill S, Taylor TC, Graham HK. Botulinum toxin A compared with stretching casts in the treatment of spastic equinus: a randomised prospective trial. J Pediatr Orthop. 1998;18:304–311. [PubMed] [Google Scholar]
- 64.Flett PJ, Stern LM, Waddy H, Connell TM, Seeger JD, Gibson SK. Botulinum toxin A versus fixed cast stretching for dynamic calf tightness in cerebral palsy. J Paediatr Child Health. 1999;35:71–77. doi: 10.1046/j.1440-1754.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- 65.Molenaers G, Eyssen M, Desloovere K, Jonkers I, de Cock P. The effect of multilevel botulinum toxin type A treatment combined with short leg casting and orthotic management on the gait of CP children. Gait Posture. 1999;10:74. doi: 10.1016/S0966-6362(99)90432-0. [DOI] [Google Scholar]
- 66.Desloovere K, Molenaers G, Jonkers I, Van Deun S, Nijs J. The effect of combined botulinum toxin injections and serial casting on gait disorders in cerebral palsy. Gait Posture. 2000;12:57. [Google Scholar]
- 67.Blackmore AM, Boettcher-Hunt E, Jordan M, Chan MD. A systematic review of the effects of casting on equinus in children with cerebral palsy: an evidence report of the AACPDM. Dev Med Child Neurol. 2007;49:781–790. doi: 10.1111/j.1469-8749.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 68.Boyd RN, Morris ME, Graham HK. Management of upper limb dysfunction in children with cerebral palsy: a systematic review. Eur J Neurol. 2001;8:150–166. doi: 10.1046/j.1468-1331.2001.00048.x. [DOI] [PubMed] [Google Scholar]
- 69.Damiano DL, Quinlivan J, Owen BF, Shaffrey M, Abel MF. Spasticity versus strength in cerebral palsy: relationships among involuntary resistance, voluntary torque, and motor function. Eur J Neurol. 2001;8:40–49. doi: 10.1046/j.1468-1331.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 70.Leach J. Children undergoing treatment with botulinum toxin: the role of the physical therapist. Muscle Nerve Suppl. 1997;6:S194–S207. doi: 10.1002/(SICI)1097-4598(1997)6+<194::AID-MUS14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 71.Love SC, Valentine JP, Blair EM, Price CJ, Cole JH, Chauvel PJ. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia: a randomized controlled trial. Eur J Neurol. 2001;8:50–58. doi: 10.1046/j.1468-1331.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 72.Ong HT, Chong HN, Yap SSP. Comprehensive management of spasticity in cerebral palsy: role of physical therapy and other adjunctive treatments. Singapore Paediatr J. 2001;43:133–136. [Google Scholar]
- 73.Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, Becher JG. Effect of multilevel botulinum toxin A and comprehensive rehabilitation on gait in cerebral palsy. Pediatr Neurol. 2007;36(1):30–39. doi: 10.1016/j.pediatrneurol.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Smedal T, Gjelsvik B, Lygren H, Borgmann R, Waje-Andreassen U, Grønning M. Botulinum toxin A—effect on spasticity. Tidsskr Nor Laegeforen. 2001;121:3277–3280. [PubMed] [Google Scholar]
- 75.Speth LAWM, Leffers P, Janssen-Potten YJM, Vles JSH. Botulinum toxin A and upper limb functional skills in hemiparetic cerebral palsy: a randomized trial in children receiving intensive therapy. Dev Med Child Neurol. 2005;47:468–473. doi: 10.1017/S0012162205000903. [DOI] [PubMed] [Google Scholar]
- 76.Willoughby KL, Dodd KJ, Shields N. A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disabil Rehabil. 2009;31(24):1971–1979. doi: 10.3109/09638280902874204. [DOI] [PubMed] [Google Scholar]
- 77.Hoare BJ, Imms C. Upper-limb injections of botulinum toxin-A in children with cerebral palsy: a critical review of the literature and clinical implications for occupational therapists. Am J Occup Ther. 2004;58:389–397. doi: 10.5014/ajot.58.4.389. [DOI] [PubMed] [Google Scholar]
- 78.Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G, Thomason P, Wolfe R, Reddihough D. Does botulinum toxin A combined with bracing prevent hip displacement in children with cerebral palsy and “hips at risk”? A randomized, controlled trial. J Bone Joint Surg Am. 2008;90:23–33. doi: 10.2106/JBJS.F.01416. [DOI] [PubMed] [Google Scholar]
- 79.Huenaerts C, Desloovere K, Molenaers G, Nijs J, Callewaert B. The effects of ankle-foot-orthoses on the gait of children with cerebral palsy after treatment with botulinum toxin A: effects on temporal-spatial parameters and kinematics and kinetics of the proximal joints. Gait Posture. 2004;20:S63. [Google Scholar]
- 80.Fagard K, Desloovere K, Molenaers G (2009) The influence of the functional level of children with CP on the success rate for BTX-A treatment, defined by the goal attainment scale. Gait Posture
- 81.van Rhijn J, Molenaers G, Ceulemans B. Botulinum toxin type A in the treatment of children and adolescents with an acquired brain injury. Brain Inj. 2005;19:331–335. doi: 10.1080/02699050400013675. [DOI] [PubMed] [Google Scholar]
- 82.Chambers HG. Treatment of functional limitations at the knee in ambulatory children with cerebral palsy. Eur J Neurol. 2001;8:59–74. doi: 10.1046/j.1468-1331.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- 83.O’Brien CF. Treatment of spasticity with botulinum toxin. Clin J Pain. 2002;18:S182–S190. doi: 10.1097/00002508-200211001-00011. [DOI] [PubMed] [Google Scholar]
- 84.Rutz E, Hofmann E, Brunner R. Preoperative botulinum toxin to avoid poor surgical results of muscle lengthening in patients with cerebral palsy. Gait Posture. 2008;28:S2. doi: 10.1016/S0966-6362(08)70003-1. [DOI] [Google Scholar]
- 85.Wissel J, Heinen F, Schenkel A, Doll B, Ebersbach G, Müller J, Poewe W. Botulinum toxin A in the management of spastic gait disorders in children and young adults with cerebral palsy: a randomized, double-blind study of “high-dose” versus “low-dose” treatment. Neuropediatrics. 1999;30:120–124. doi: 10.1055/s-2007-973475. [DOI] [PubMed] [Google Scholar]
- 86.Molenaers G, Desloovere K, Van Campenhout A, Pauwels P, De Cat J, Nijs J, Feys H, De Cock P. Can multilevel BTX-A treatment predict the effect of SDR on gait in children with spastic diplegia? Gait Posture. 2005;22:S1–S2. [Google Scholar]
- 87.Sutherland DH, Kaufman KR, Wyatt MP, Chambers HG, Mubarak SJ. Double-blind study of botulinum A toxin injections into the gastrocnemius muscle in patients with cerebral palsy. Gait Posture. 1999;10:1–9. doi: 10.1016/S0966-6362(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 88.Eames NW, Baker R, Hill N, Graham K, Taylor T, Cosgrove A. The effect of botulinum toxin A on gastrocnemius length: magnitude and duration of response. Dev Med Child Neurol. 1999;41:226–232. doi: 10.1017/S0012162299000493. [DOI] [PubMed] [Google Scholar]
- 89.Molenaers G (2008) Treatment efficacy of long-term use of botulinum toxin type A (BTXA) in children with cerebral palsy. Gait Posture 28:S1


