Abstract
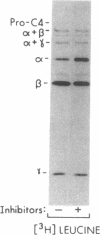
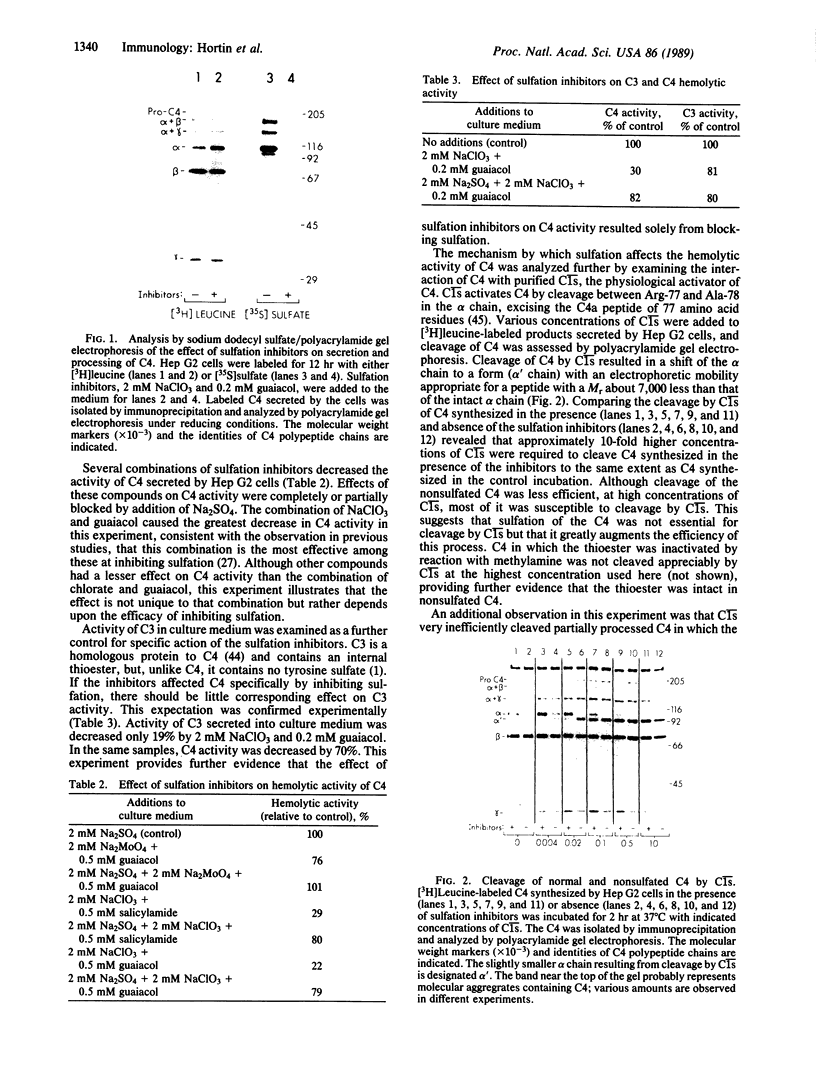
Sulfation of tyrosine residues recently has been recognized as a biosynthetic modification of many plasma proteins and other secretory proteins. Effects of this site-specific modification on protein function are not known, but the activity of several peptides such as cholecystokinin is greatly augmented by sulfation. Here, we examine the role of sulfation in the processing and activity of C4 (the fourth component of complement), one of the few proteins in which sites and stoichiometry of tyrosine sulfation have been characterized. Our results, with C4 as a paradigm, suggest that sulfation of tyrosine residues can have major effects on the activity of proteins participating in protein-protein interactions. Sulfation of C4 synthesized by Hep G2 cells was blocked by incubating the cells with NaClO3 and guaiacol. These sulfation inhibitors did not alter secretion or other steps in the processing of C4. However, hemolytic activity of C4 was decreased more than 50%. The inhibitors' effect on C4 activity was prevented by adding Na2SO4 to restore sulfation of C4. Activity of C3, a complement component homologous to C4 but lacking tyrosine sulfate residues, was minimally reduced (19%) by the inhibitors. Decreased hemolytic activity of nonsulfated C4 apparently resulted from impaired interaction with complement subcomponent C1s (EC 3.4.21.42), the protease that physiologically activates C4. Purified C1s was able to cleave nonsulfated C4, but approximately 10-fold higher concentrations of C1s were required for that cleavage than to yield equivalent cleavage of sulfated C4. Our results suggest that activation of C4, a central component in the classical pathway of complement activation, is influenced by the level of sulfation of the protein. Thus, sulfation of C4 provides a potential locus for physiological or pharmacological modulation of complement-mediated opsonization and inflammation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Bertaccini G., Erspamer V. Pharmacological data on phyllokinin (bradykinyl-isoleucyl-tyrosine o-sulphate) and bradykinyl-isoleucyl-tyrosine. Br J Pharmacol Chemother. 1966 Sep;27(3):479–485. doi: 10.1111/j.1476-5381.1966.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B. N., Abramovich D., Brand S. J., Petersen B., Rehfeld J. F. Complete sulfation of jejunal gastrin in the human fetus. Regul Pept. 1985 Apr;10(4):329–338. doi: 10.1016/0167-0115(85)90045-x. [DOI] [PubMed] [Google Scholar]
- Atkinson J. P., McGinnis K., Shreffler D. Development and characterization of a hemolytic assay for mouse C4. J Immunol Methods. 1980;33(4):351–368. doi: 10.1016/0022-1759(80)90005-8. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Chlorate--a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Bing D. H., Andrews J. M., Morris K. M., Cole E., Irish V. Purification of subcomponents Clq, Cl(-)r and Cl(-)s of the first component of complement from Cohn Fraction I by affinity chromatography. Prep Biochem. 1980;10(3):269–296. doi: 10.1080/10826068009412829. [DOI] [PubMed] [Google Scholar]
- Brand S. J., Andersen B. N., Rehfeld J. F. Complete tyrosine-O-sulphation of gastrin in neonatal rat pancreas. 1984 May 31-Jun 6Nature. 309(5967):456–458. doi: 10.1038/309456a0. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Atkinson J. P. Functional studies on the secreted form of human C4 (C4s), two incompletely processed two-subunit C4 molecules (beta - alpha + gamma and beta + alpha - gamma), and pro-C4. J Immunol. 1984 Apr;132(4):1967–1971. [PubMed] [Google Scholar]
- Chan A. C., Atkinson J. P. Oligosaccharide structure of human C4. J Immunol. 1985 Mar;134(3):1790–1798. [PubMed] [Google Scholar]
- Colten H. R. Biosynthesis of the MHC-linked complement proteins (C2, C4 and factor B) by mononuclear phagocytes. Mol Immunol. 1982 Oct;19(10):1279–1285. doi: 10.1016/0161-5890(82)90294-2. [DOI] [PubMed] [Google Scholar]
- Dunckley H., Gatenby P. A., Hawkins B., Naito S., Serjeantson S. W. Deficiency of C4A is a genetic determinant of systemic lupus erythematosus in three ethnic groups. J Immunogenet. 1987 Aug-Oct;14(4-5):209–218. doi: 10.1111/j.1744-313x.1987.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Farries T. C., Finch J. T., Lachmann P. J., Harrison R. A. Resolution and analysis of 'native' and 'activated' properdin. Biochem J. 1987 Apr 15;243(2):507–517. doi: 10.1042/bj2430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielder A. H., Walport M. J., Batchelor J. R., Rynes R. I., Black C. M., Dodi I. A., Hughes G. R. Family study of the major histocompatibility complex in patients with systemic lupus erythematosus: importance of null alleles of C4A and C4B in determining disease susceptibility. Br Med J (Clin Res Ed) 1983 Feb 5;286(6363):425–428. doi: 10.1136/bmj.286.6363.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978 Oct 1;148(4):1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY H., HARDY P. M., JONES D. S., KENNER G. W., SHEPPARD R. C. THE ANTRAL HORMONE GASTRIN. STRUCTURE OF GASTRIN. Nature. 1964 Dec 5;204:931–933. doi: 10.1038/204931a0. [DOI] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J. P., Howard J. B. Effect of methylamine on the structure and function of the fourth component of human complement, C4. J Biol Chem. 1980 Nov 10;255(21):10025–10028. [PubMed] [Google Scholar]
- Green E. D., Boime I., Baenziger J. U. Differential processing of Asn-linked oligosaccharides on pituitary glycoprotein hormones: implications for biologic function. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):81–100. doi: 10.1007/BF00230637. [DOI] [PubMed] [Google Scholar]
- Hortin G. L., Graham J. P. Sulfation of tyrosine residues does not influence secretion of alpha 2-antiplasmin or C4. Biochem Biophys Res Commun. 1988 Feb 29;151(1):417–421. doi: 10.1016/0006-291x(88)90609-2. [DOI] [PubMed] [Google Scholar]
- Hortin G. L., Schilling M., Graham J. P. Inhibitors of the sulfation of proteins, glycoproteins, and proteoglycans. Biochem Biophys Res Commun. 1988 Jan 15;150(1):342–348. doi: 10.1016/0006-291x(88)90526-8. [DOI] [PubMed] [Google Scholar]
- Hortin G., Chan A. C., Fok K. F., Strauss A. W., Atkinson J. P. Sequence analysis of the COOH terminus of the alpha-chain of the fourth component of human complement. Identification of the site of its extracellular cleavage. J Biol Chem. 1986 Jul 5;261(19):9065–9069. [PubMed] [Google Scholar]
- Hortin G., Fok K. F., Toren P. C., Strauss A. W. Sulfation of a tyrosine residue in the plasmin-binding domain of alpha 2-antiplasmin. J Biol Chem. 1987 Mar 5;262(7):3082–3085. [PubMed] [Google Scholar]
- Hortin G., Folz R., Gordon J. I., Strauss A. W. Characterization of sites of tyrosine sulfation in proteins and criteria for predicting their occurrence. Biochem Biophys Res Commun. 1986 Nov 26;141(1):326–333. doi: 10.1016/s0006-291x(86)80372-2. [DOI] [PubMed] [Google Scholar]
- Hortin G., Sims H., Strauss A. W. Identification of the site of sulfation of the fourth component of human complement. J Biol Chem. 1986 Feb 5;261(4):1786–1793. [PubMed] [Google Scholar]
- Hortin G., Strauss A. W. Effects of acidotropic compounds on the secretory pathway: inhibition of secretion and processing of the third and fourth components of complement. Biochem Biophys Res Commun. 1986 Apr 29;136(2):603–609. doi: 10.1016/0006-291x(86)90483-3. [DOI] [PubMed] [Google Scholar]
- Hortin G., Tollefsen D. M., Strauss A. W. Identification of two sites of sulfation of human heparin cofactor II. J Biol Chem. 1986 Dec 5;261(34):15827–15830. [PubMed] [Google Scholar]
- Howard P. F., Hochberg M. C., Bias W. B., Arnett F. C., Jr, McLean R. H. Relationship between C4 null genes, HLA-D region antigens, and genetic susceptibility to systemic lupus erythematosus in Caucasian and black Americans. Am J Med. 1986 Aug;81(2):187–193. doi: 10.1016/0002-9343(86)90250-0. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Tyrosine sulfation and the secretory pathway. Annu Rev Physiol. 1988;50:363–376. doi: 10.1146/annurev.ph.50.030188.002051. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Young J. R. Covalent binding properties of the C4A and C4B isotypes of the fourth component of human complement on several C1-bearing cell surfaces. J Immunol. 1986 Apr 1;136(7):2542–2550. [PubMed] [Google Scholar]
- Isenman D. E., Young J. R. The molecular basis for the difference in immune hemolysis activity of the Chido and Rodgers isotypes of human complement component C4. J Immunol. 1984 Jun;132(6):3019–3027. [PubMed] [Google Scholar]
- Jackson C. G., Ochs H. D., Wedgwood R. J. Immune response of a patient with deficiency of the fourth component of complement and systemic lupus erythematosus. N Engl J Med. 1979 May 17;300(20):1124–1129. doi: 10.1056/NEJM197905173002002. [DOI] [PubMed] [Google Scholar]
- Janatova J., Tack B. F. Fourth component of human complement: studies of an amine-sensitive site comprised of a thiol component. Biochemistry. 1981 Apr 28;20(9):2394–2402. doi: 10.1021/bi00512a005. [DOI] [PubMed] [Google Scholar]
- Jukkola A., Risteli J., Niemelä O., Risteli L. Incorporation of sulphate into type III procollagen by cultured human fibroblasts. Identification of tyrosine O-sulphate. Eur J Biochem. 1986 Jan 2;154(1):219–224. doi: 10.1111/j.1432-1033.1986.tb09382.x. [DOI] [PubMed] [Google Scholar]
- Karp D. R. Post-translational modification of the fourth component of complement. Sulfation of the alpha-chain. J Biol Chem. 1983 Nov 10;258(21):12745–12748. [PubMed] [Google Scholar]
- Karp D. R., Shreffler D. C., Atkinson J. P. Characterization of the Mr difference between secreted murine fourth component of complement and the major plasma form: evidence for carboxyl-terminal cleavage of the alpha chain. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6666–6670. doi: 10.1073/pnas.79.21.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Dorner A. J. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988 May 5;263(13):6352–6362. [PubMed] [Google Scholar]
- Keogh S. J., Harding D. R., Hardman M. J. The complement component C1s catalysed hydrolysis of peptide 4-nitroanilide substrates. Biochim Biophys Acta. 1987 May 27;913(1):39–44. doi: 10.1016/0167-4838(87)90229-9. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Dodds A. W., Porter R. R. A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. EMBO J. 1984 Aug;3(8):1819–1823. doi: 10.1002/j.1460-2075.1984.tb02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Yu S., Sy J., Redman C. M., Lipmann F. Tyrosine sulfation of proteins from the human hepatoma cell line HepG2. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7160–7164. doi: 10.1073/pnas.82.21.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauff G., Alper C. A., Awdeh Z., Batchelor J. R., Bertrams J., Bruun-Petersen G., Dawkins R. L., Démant P., Edwards J., Grosse-Wilde H. Statement on the nomenclature of human C4 allotypes. Immunobiology. 1983 Mar;164(2):184–191. doi: 10.1016/s0171-2985(83)80009-6. [DOI] [PubMed] [Google Scholar]
- Miura N., Prentice H. L., Schneider P. M., Perlmutter D. H. Synthesis and regulation of the two human complement C4 genes in stable transfected mouse fibroblasts. J Biol Chem. 1987 May 25;262(15):7298–7305. [PubMed] [Google Scholar]
- Morris K. M., Aden D. P., Knowles B. B., Colten H. R. Complement biosynthesis by the human hepatoma-derived cell line HepG2. J Clin Invest. 1982 Oct;70(4):906–913. doi: 10.1172/JCI110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhaev V. V., Siksnis V. A., Melik-Nubarov N. S., Galkantaite N. Z., Denis G. J., Butkus E. P., Zaslavsky BYu, Mestechkina N. M., Martinek K. Protein stabilization via hydrophilization. Covalent modification of trypsin and alpha-chymotrypsin. Eur J Biochem. 1988 Apr 5;173(1):147–154. doi: 10.1111/j.1432-1033.1988.tb13978.x. [DOI] [PubMed] [Google Scholar]
- Nachman R. J., Holman G. M., Cook B. J., Haddon W. F., Ling N. Leucosulfakinin-II, a blocked sulfated insect neuropeptide with homology to cholecystokinin and gastrin. Biochem Biophys Res Commun. 1986 Oct 15;140(1):357–364. doi: 10.1016/0006-291x(86)91098-3. [DOI] [PubMed] [Google Scholar]
- Porter R. R. Complement polymorphism, the major histocompatibility complex and associated diseases: a speculation. Mol Biol Med. 1983 Jul;1(1):161–168. [PubMed] [Google Scholar]
- Press E. M., Gagnon J. Human complement component C4. Structural studies on the fragments derived from C4b by cleavage with C3b inactivator. Biochem J. 1981 Nov 1;199(2):351–357. doi: 10.1042/bj1990351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Roos M. H., Kornfeld S., Shreffler D. C. Characterization of the oligosaccharide units of the fourth component of complement (Ss protein) synthesized by murine macrophages. J Immunol. 1980 Jun;124(6):2860–2863. [PubMed] [Google Scholar]
- Schreiber R. D., Müller-Eberhard H. J. Fourth component of human complement: description of a three polypeptide chain structure. J Exp Med. 1974 Nov 1;140(5):1324–1335. doi: 10.1084/jem.140.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E., Gill E. W., Sim R. B. Drugs that induce systemic lupus erythematosus inhibit complement component C4. Lancet. 1984 Aug 25;2(8400):422–424. doi: 10.1016/s0140-6736(84)92905-2. [DOI] [PubMed] [Google Scholar]
- Wetsel R. A., Ogata R. T., Tack B. F. Primary structure of the fifth component of murine complement. Biochemistry. 1987 Feb 10;26(3):737–743. doi: 10.1021/bi00377a013. [DOI] [PubMed] [Google Scholar]
- Yu C. Y., Campbell R. D., Porter R. R. A structural model for the location of the Rodgers and the Chido antigenic determinants and their correlation with the human complement component C4A/C4B isotypes. Immunogenetics. 1988;27(6):399–405. doi: 10.1007/BF00364425. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Fey G. H. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci U S A. 1985 Feb;82(3):708–712. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]