Abstract
Kaposiform hemangioendothelioma is a rare vascular tumor of childhood that is locally aggressive but has little metastatic potential and by itself is not known to be lethal. It most commonly presents as a superficial or deep soft tissue mass with associated cutaneous lesions. Kasabach-Merritt phenomenon, a condition characterized by profound thrombocytopenia and life-threatening hemorrhage, often is associated with kaposiform hemangioendothelioma. Six cases of kaposiform hemangioendothelioma have been reported in bone, two of which were located in extracraniofacial bones. We report a diagnostically challenging case of a 6-year-old girl with kaposiform hemangioendothelioma of the thoracolumbar spine without Kasabach-Merritt phenomenon or cutaneous lesions.
Introduction
Kaposiform hemangioendothelioma (KH) is a rare vascular tumor that occurs most often in infants and adolescents. It was first described by Zukerberg et al. [47] in 1993 as an entity distinct from juvenile hemangioma. Since then, there have been an increasing number of reports of kaposiform hemangioendothelioma in the skin and deep soft tissue [1–13, 15–19, 21–29, 32–47].
KH usually appears as an ill-defined cutaneous, red to purple indurated plaque at birth or in early childhood with an equal gender ratio [41]. KH most often occurs in the trunk, extremities, and retroperitoneum, although the lesions sometimes occur on the head and neck [22]. Although KH lesions generally are considered to be nonmetastasizing, they can be locally aggressive and are characterized by rapid growth and extension locally involving skin, soft tissues, and occasionally bone [31].
KH is classically associated with Kasabach-Merritt phenomenon (KMP), characterized by a consumptive coagulopathy and profound thrombocytopenia [41]. KH associated with KMP carries a worse prognosis because of bleeding or tumor invasion into vital organs. It is important to differentiate KH from other vascular entities, such as juvenile hemangioma or tufted angioma, because KH has no tendency to spontaneously involute and often is associated with KMP, a potentially life-threatening disorder [44].
We present a case of KH in a child presenting with chronic, atraumatic back pain without associated cutaneous changes or coagulopathy with multiple bony lesions in the thoracolumbar spine concerning for malignancy.
Case Report
A 6-year, 3-month-old girl presented with a 6-month history of discrete pain localized to the right of midline at the thoracolumbar junction. She denied any inciting trauma but stated the pain was worsening with time. She reported difficulty with all activities including swimming. Her recent medical history revealed the occurrence of atypical pneumonia 4 months before presentation treated with azithromycin and a urinary tract infection 2 months before presentation treated with cotrimoxazole. She denied any numbness, tingling, or weakness in her lower extremities. She denied any bowel or bladder changes.
Physical examination revealed a healthy-appearing child with localized tenderness to palpation over the right paraspinous musculature at the thoracolumbar junction. No masses were palpable and there were no overlying skin changes, erythema, discoloration, or other deformities. Neurovascular examination of her lower extremities was normal.
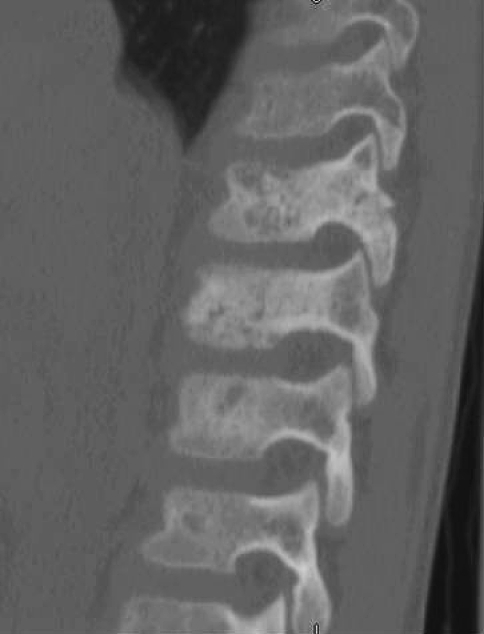
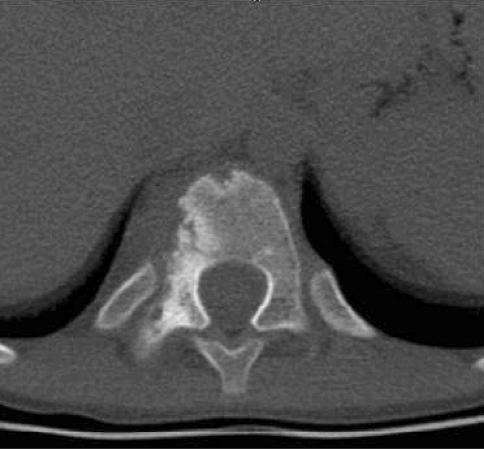
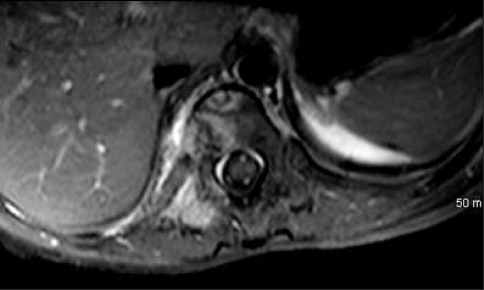
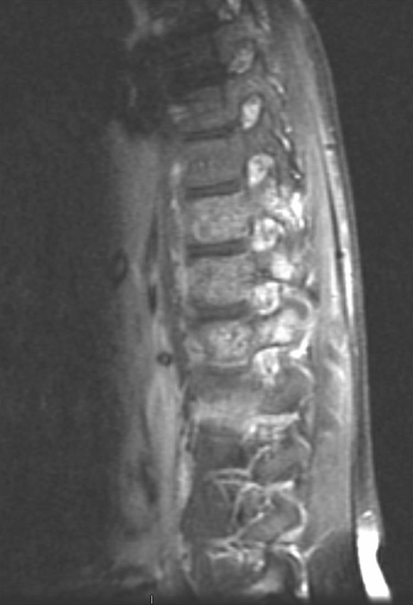
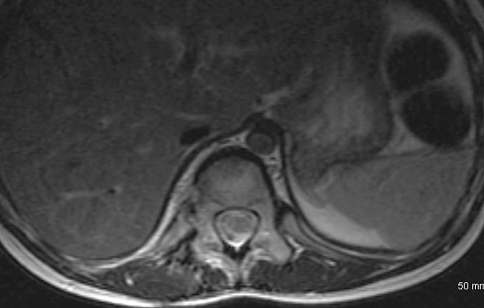
Initial radiographic studies included plain radiographs, CT, MRI of the thoracic and lumbar spine, and bone scan. Plain radiographs revealed sclerotic lesions involving the right pedicles and vertebral bodies of T11 and T12 (Figs. 1, 2). CT scans showed mixed sclerotic and lytic lesions involving the right side of the vertebral bodies of T11–L2, and sclerotic lesions in the right pedicles, transverse processes, and rib heads of T11 and T12 (Figs. 3, 4). MRI revealed hypointense signal abnormality on T1-weighted images in T11–L2, indicating sclerosis with marked enhancement after infusion of gadolinium. There was an associated abnormal soft tissue mass paralleling the right lateral aspect of the vertebral bodies from T11–L2, and the intervertebral foramina of the right side at T10–L2. There was no cord compression or epidural mass (Figs. 5, 6). Bone scan revealed mild increased radiotracer uptake at the pedicles of T11 and T12.
Fig. 1.
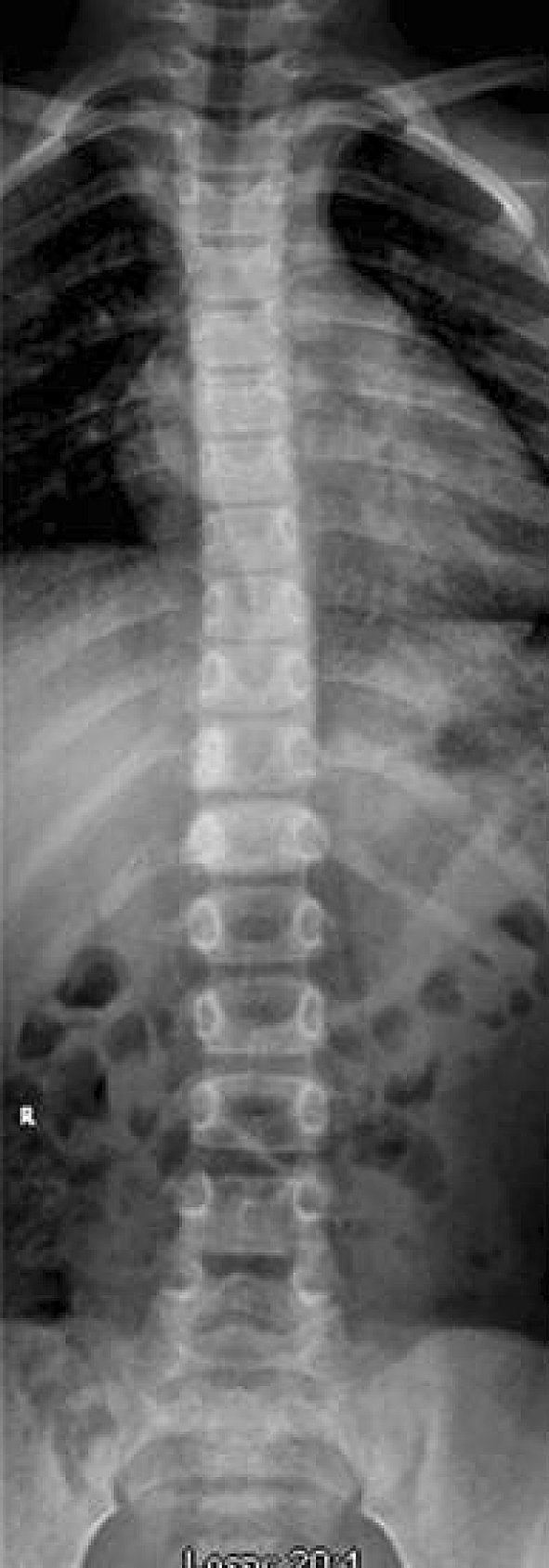
An anteroposterior radiograph of the patient’s thoracolumbar spine shows sclerotic changes of the right pedicles of T11 and T12.
Fig. 2.
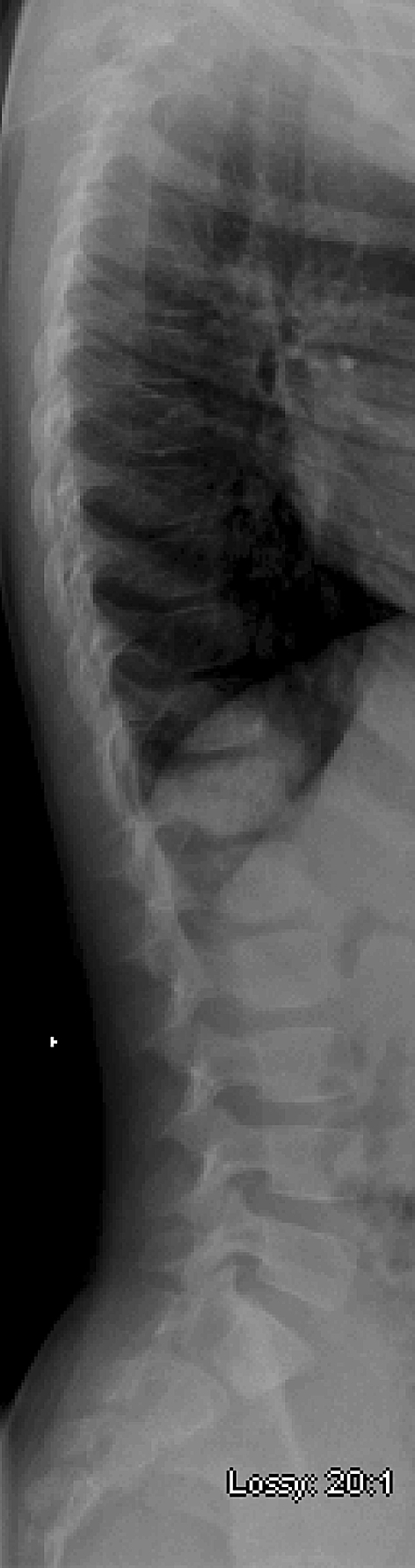
A lateral radiograph of the patient’s thoracolumbar spine shows sclerotic changes at T11 and T12 vertebral bodies.
Fig. 3.
A CT scan in the sagittal plane of the patient’s thoracolumbar spine from T9 to L2 shows mixed lytic and sclerotic changes of T11–L2 vertebral bodies and posterior elements.
Fig. 4.
A CT scan in the axial plane of T11 shows mixed lytic and sclerotic bone lesions in the right vertebral body and right pedicle.
Fig. 5.
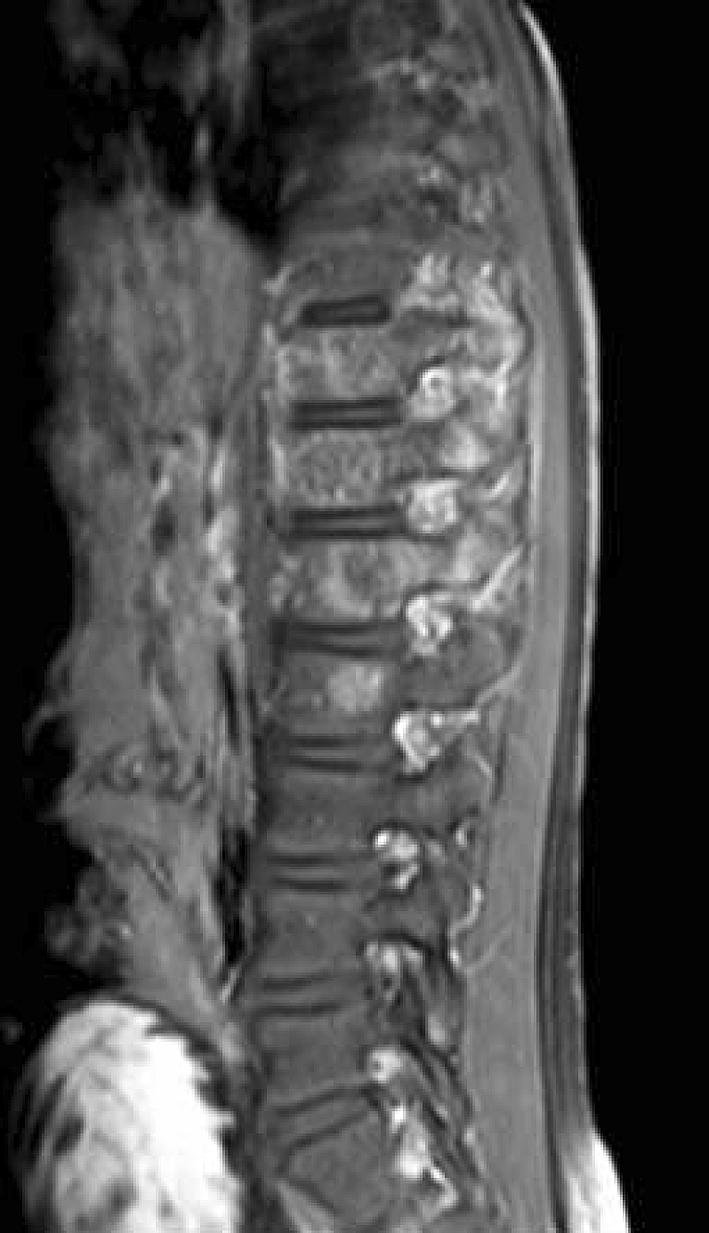
A T1-weighted postcontrast sagittal MR image of the patient’s thoracolumbar spine shows signal changes in bone and soft tissue of T11–L2.
Fig. 6.
A T1-weighted postcontrast axial MR image of the patient’s thoracolumbar spine at T11 shows right-sided soft tissue and bone signal changes.
Laboratory workup included complete blood count with differential, comprehensive metabolic panel (including liver enzymes, calcium, phosphorus, and alkaline phosphatase), C-reactive protein, erythrocyte sedimentation rate, Brucella titer, Lyme titer, and sputum analysis for acid fast bacilli. All results were within normal range except for mild anemia with a hemaglobin of 10.8 g/dL.
The compilation of clinical, radiographic, and laboratory features yielded a broad differential including benign and malignant entities, including infection (specifically mycobacterial or fungal), eosinophilic granuloma, hemangiomatosis, lymphangiomatosis, desmoplastic fibromatosis, lymphoma, leukemia, Ewing’s sarcoma, and metastatic neuroblastoma. The patient underwent fluoroscopically guided biopsy of the T12 pedicle lesion; the biopsy was histologically nondiagnostic. Gram stain for anaerobes and aerobes, acid-fast bacilli and fungal stain, and cultures of the biopsy specimen, were all negative. Further imaging with combined 18F-fluorodeoxyglucose positron emission tomography and CT scan revealed mild activity at T10, T11, L1, and L2 that was similar to the background osseous activity. There was a paucity of radiotracer activity at the T12 vertebral body.
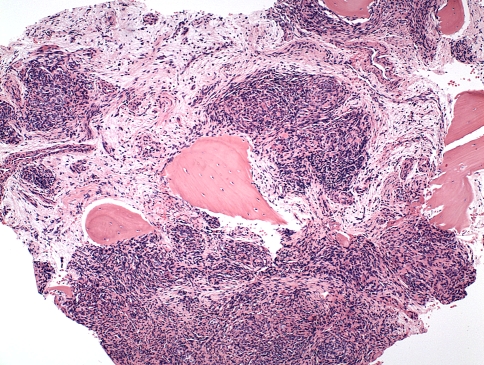
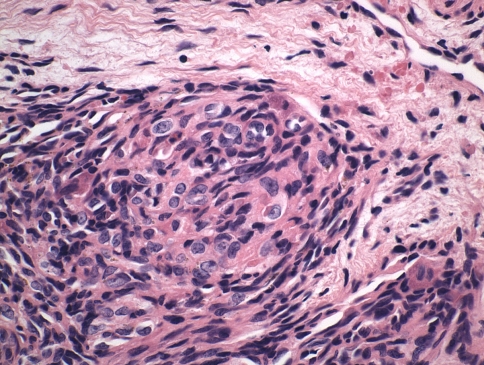
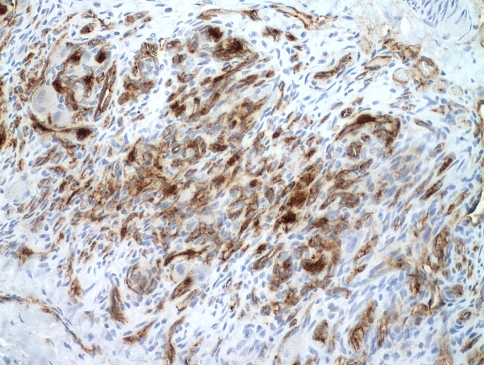
Because of the lack of formal diagnosis and increasing back pain, the patient underwent open biopsy of the right rib head, transverse process, and vertebral bodies of T11 and T12. Histologic sections consisted of a lobulated proliferation of thin-walled blood vessels with a prominent spindled pericytic component, involving the trabecular bone, and the adjacent ligamentous soft tissue (Fig. 7). The spindled zones merged with glomeruloid-like nests of rounded to oblong epithelioid cells (Fig. 8). There were admixed osteoclastic giant cells. Immunohistochemistry showed spindle cells with focal immunoreactivity for α-smooth muscle actin, suggesting a pericytic component. The lobulated vascular proliferation exhibited immunoreactivity for CD31, an immunomarker specific for vascular endothelium (Fig. 9). Immunohistochemistry for pancytokeratin AE1/AE3 and epithelial membrane antigen was negative.
Fig. 7.
A photomicrograph shows KH composed of cellular nests of spindled and epithelioid cells with adjacent islands of trabecular bone (Stain, hematoxylin and eosin; original magnification, ×100).
Fig. 8.
A high-power view shows glomeruloid-like areas characterized by small, thin-walled vascular channels merging with rounded, epithelioid cells (Stain, hematoxylin and eosin; original magnification, ×600).
Fig. 9.
Immunohistochemistry for CD31 highlights small, slit-like vascular spaces of KH (original magnification, ×600).
The initial interpretation of the pathology was that of infantile myofibromatosis. Interdisciplinary review of the clinical, pathologic, and radiographic findings at a specialized Vascular Anomaly Center rendered a diagnosis of KH of bone without KMP features.
Because of the morbidity involved in resection of multiple thoracolumbar spinal levels, treatment with the antiangiogenic agent, thalidomide, and celecoxib was initiated. Eight months after initiation of treatment, the patient’s radiographs and MRI showed regression of the disease (Figs. 10, 11).
Fig. 10.
A T1-weighted postcontrast sagittal MR image of the patient’s thoracolumbar spine shows regression of the vertebral changes 8 months after initiation of antiangiogenic therapy.
Fig. 11.
A T1-weighted postcontrast axial MR image of the patient’s thoracolumbar spine at T11 shows regression of the soft tissue mass and bone changes 8 months after initiation of antiangiogenic therapy.
Discussion
KH is a rare, locally aggressive endothelial-derived spindle cell neoplasm that occurs nearly exclusively during childhood [1]. Although it can be locally aggressive, KH has little metastatic potential [47], although death has resulted from severe coagulopathy associated with KMP [18, 39, 47]. KH has features common to capillary hemangioma and Kaposi sarcoma with kaposiform features, including formation of slit-like lumens by bland, spindled cells [44]. The designation of hemangioendothelioma implies the uncertainty regarding the biologic behavior of the tumor, which is situated in the intermediate category between infantile hemangioma and angiosarcoma [39, 41, 47]. KMP describes a syndrome of profound thrombocytopenia and coagulopathy that may be associated with KH [41]. KH generally occurs in the soft tissues, often with KMP, and only rarely has been reported to invade bone [16, 22, 31, 32, 34, 35]. Of the cases of KH invasion into bone, only two have been reported in extracraniofacial sites [31, 35].
Our case presented a difficult diagnostic challenge, given the confusing clinical, imaging, and histologic findings. The patient did not present with any of the currently described characteristic physical examination findings of KH, such as palpable mass or blue-red skin lesions, nor did she have abnormal laboratory values suggestive of thrombocytopenia related to KMP. Extensive mixed lytic and sclerotic bony involvement was evident at multiple thoracolumbar spine levels on plain radiographs and CT with minimal soft tissue involvement on MRI. The radiographic findings raised an extensive and varied differential that included osteomyelitis (specifically fungal or mycobacterial), Langerhans cell histiocytosis, hemangiomatosis, lymphangiomatosis, skeletal angiomatosis, desmoplastic fibromatosis, metastatic neuroblastoma, lymphoma, leukemia, and Ewing’s sarcoma. Initial fluoroscopically guided percutaneous core biopsy at the pedicle of T11, an area with radiographic evidence of extensive involvement, resulted in a pathologic assessment that was inconclusive, but the possibility of a vascular lesion was raised in the histologic differential. Open biopsy then was performed with sampling of the transverse process, pedicles, vertebral bodies, and adjacent soft tissue mass from two levels. After a multidisciplinary review by pathologists and clinicians with expertise in sarcomas and vascular malformations, the diagnosis of KH was rendered.
It is challenging to make a diagnosis of KH in a patient who does not have cutaneous skin changes, laboratory changes (specifically thrombocytopenia), and bony changes associated with minimal soft tissue mass. We identified six patients with KH with bone involvement reported in the literature[16, 22, 31, 32, 34, 35] (PubMed search using keyword “kaposiform hemangioendothelioma”) (Table 1). All of these patients presented with accompanying cutaneous changes. Gruman et al. [22] described a patient with mixed lytic and sclerotic bony lesions in the mandible and maxilla and an associated indurated, ecchymotic mass and facial swelling. Although our patient lacked the associated skin changes and swelling, these radiographic changes mimic closely those found in her thoracolumbar spine. Other patients with reported bony changes had a large, soft tissue mass associated with the bone lesion [16, 22, 31, 32, 34, 35]. In the absence of adjuvant therapy, the bone lesions that were surgically unresectable continued to grow and invade adjacent structures [22, 31, 32].
Table 1.
Reported cases of kaposiform hemangioendothelioma with bony lesions
| Study | Age (years) | Gender | Location | Cutaneous lesion | KMP | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Lai et al. [31] | 2 | Male | Radius/ulna/forearm | Yes | Yes | Amputation, XRT | ANED |
| Lyons et al. [34] | 2 | Male | Temporal bone/neck | Yes | No | Excision | ANED |
| DeFatta et al. [16] | 3 | Male | Hard palate/soft palate/mucosa/submucosa | Yes | NA | Excision | ANED |
| Lalaji et al. [32] | 1 | Female | Temporal bone/lateral mass C1/occipital bone/ear | Yes | Yes | Oral prednisone | Unknown |
| Gruman et al. [22] | 2 | Female | Mandible/maxilla/cheek | Yes | No | Interferon α-2b/prednisone | AWD |
| Mac-Moune Lai et al. [35] | 5 | Female | Humerus | Yes | NA | Excision | AWD |
KMP = Kasabach-Merritt phenomenon; NA = not available; XRT = radiation therapy; ANED = alive with no evidence of disease; AWD = alive with disease.
Treatment options for KH are diverse and pose a distinct challenge. Because of the rarity of KH and lack of reported long-term followup, most regimens have been based on other clinically aggressive vascular malformations. Further complicating treatment is the fact that KH occurs as a distinct entity and associated with KMP. Numerous reports recommend primary wide local excision followed by supportive therapy for associated symptoms [13, 32, 33, 40, 43, 47]. Several nonsurgical treatments, such as radiation [24], embolization [16, 41], interferon [15, 22, 29, 34], and the chemotherapeutic regimen of actinomycin D, cyclophosphamide, and vincristine [22, 26] have proved successful in managing KH. In cases with KH associated with KMP, no reports in the literature recommended either observation alone or steroids alone. The best results were obtained with interferon alone or combined with excision or steroids, multiagent chemotherapy, or excision alone or combined with radiation or steroids and vincristine (Table 2). In cases with isolated KH, excision alone or combined with radiation therapy or other neoadjuvant therapy was the only treatment that eradicated the disease (Table 3). San Miguel et al. [40] published a thorough review of 158 patients with KH in which 28 of 29 patients treated with surgical excision and some type of initial nonoperative therapy had eradication of their disease. In the six cases reporting primary bony involvement, as is present in this case, four cases involved surgical excision, whereas prednisone and interferon were used in the other two cases [16, 22, 31, 32, 34, 35] (Table 1).
Table 2.
Treatment options for kaposiform hemangioendothelioma in the presence of Kasabach-Merritt phenomenon
| Regimen | Outcome (number of cases) | Reference | ||
|---|---|---|---|---|
| ANED | AWD | DOD | ||
| Interferon | 8 | 4 | 3 | [12, 15, 25, 34, 35, 40] |
| Vincristine/cyclophosphamide/actinomycin D/methotrexate | 1 | [26] | ||
| Excision | 2 | [34, 35] | ||
| Excision/radiation | 1 | [34] | ||
| Excision/interferon | 1 | 2 | [35, 41] | |
| Excision/steroids | 1 | [16] | ||
| Excision/steroids/vincristine | 1 | [16] | ||
| Steroids/chemotherapy | 2 | [16, 34] | ||
| Steroids/embolization | 1 | [16] | ||
| Steroids/interferon | 2 | 2 | [16, 29, 34] | |
ANED = alive with no evidence of disease; AWD = alive with disease; DOD = died of disease.
Table 3.
Treatment options for kaposiform hemangioendothelioma in the absence of Kasabach-Merritt phenomenon
| Regimen | Outcome (number of cases) | Reference | ||
|---|---|---|---|---|
| ANED | AWD | DOD | ||
| Interferon | 2 | [16, 34] | ||
| Steroids | 3 | [1, 22, 34] | ||
| Interferon/steroids | 1 | [22] | ||
| Vincristine/steroids | 3 | [22] | ||
| Observation | 6 | [22, 34] | ||
| Excision | 15 | [3, 10, 16, 34, 35, 43] | ||
| Excision/radiation | 1 | [24] | ||
| Excision/neoadjuvant therapy of some type | 28 | [40] | ||
ANED = alive with no evidence of disease; AWD = alive with disease; DOD = died of disease.
Our patient had multilevel spine involvement that was surgically unresectable lesions without associated KMP. She was treated with oral thalidomide and celecoxib at the recommendation from consultation with a center specializing in vascular anomalies. This recommendation was based on successful previous treatment of two cases of unresectable KH of bone with thalidomide and celecoxib. D’Amato et al. [14] first described the mechanism of oral thalidomide as an inhibitor of angiogenesis. Since that time, oral thalidomide has been shown to reduce growth of carcinomas in animals and multiple myeloma in humans through its antiangiogenic effect [20]. In a recent Phase II study for treatment of newly diagnosed glioblastoma, combined therapy of thalidomide and celecoxib, another antiangiogenic agent, reportedly produced good results [30]. Eight months after initiation of treatment with oral thalidomide and celecoxib, our patient had radiographic evidence of regression of her disease.
Acknowledgments
We thank the Vascular Anomaly Center at Children’s Hospital Boston for radiographic and pathologic review and their recommendations for treatment options.
Footnotes
Each author certifies that she has no commerical associations (eg. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Each author certifies that his or her institution has approved or waived approval for the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Beaubien ER, Ball NJ, Storwick GS. Kaposiform hemangioendothelioma: a locally aggressive vascular tumor. J Am Acad Dermatol. 1998;38:799–802. doi: 10.1016/S0190-9622(98)70461-X. [DOI] [PubMed] [Google Scholar]
- 2.Bienaime A, Rojat-Habib MC, Hesse S, Pelissier JF, Bonerandi JJ. [Giant vascular tumour in an adult: tufted angioma or kaposiform hemangioendothelioma] [in French. Ann Dermatol Venereol. 2006;133:553–556. doi: 10.1016/S0151-9638(06)70961-9. [DOI] [PubMed] [Google Scholar]
- 3.Birchler MT, Schmid S, Holzmann D, Stallmach T, Gysin C. Kaposiform hemangioendothelioma arising in the ethmoid sinus of an 8-year-old girl with severe epistaxis. Head Neck. 2006;28:761–764. doi: 10.1002/hed.20414. [DOI] [PubMed] [Google Scholar]
- 4.Blei F, Karp N, Rofsky N, Rosen R, Greco MA. Successful multimodal therapy for kaposiform hemangioendothelioma complicated by Kasabach-Merritt phenomenon: case report and review of the literature. Pediatr Hematol Oncol. 1998;15:295–305. doi: 10.3109/08880019809014013. [DOI] [PubMed] [Google Scholar]
- 5.Bodemer C, Fraitag S, Amoric JC, Benaceur S, Brunelle F, Prost Y. [Spindle-cell hemangioendothelioma with monomelic and multifocal form in a child] [in French. Ann Dermatol Venereol. 1997;124:857–860. [PubMed] [Google Scholar]
- 6.Bolde SA, Shete SS, Dantkale SS, Deshpande NM, Zawar MP. Kasabach-Merritt syndrome: a case report. Indian J Pathol Microbiol. 2005;48:27–29. [PubMed] [Google Scholar]
- 7.Brasanac D, Janic D, Boricic I, Jovanovic N, Dokmanovic L. Retroperitoneal kaposiform hemangioendothelioma with tufted angioma-like features in an infant with Kasabach-Merritt syndrome. Pathol Int. 2003;53:627–631. doi: 10.1046/j.1440-1827.2003.01518.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang JM, Kwon BJ, Han MH, Kang HS, Chang KH. Kaposiform hemangioendothelioma arising from the internal auditory canal. AJNR Am J Neuroradiol. 2006;27:931–933. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen RL, Chang PY, Hsu YH, Chang YH, Peng HC. Recurrent life-threatening hemothorax in an infant with pleurocutaneous kaposiform hemangio-endothelioma. J Pediatr Hematol Oncol. 2006;28:630–632. doi: 10.1097/01.mph.0000212957.81468.66. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YS, Kessler H, Rees TD, Philofsky D, Pontikas A. Gingival swelling in a 13-year-old girl with multiple recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:85–91. doi: 10.1016/j.tripleo.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 11.Cho SH, Na KS. Haemangioendothelioma on the conjunctiva of the upper eyelid. Clin Experiment Ophthalmol. 2006;34:794–796. doi: 10.1111/j.1442-9071.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 12.Chung MT, Chen CH, Chiu CH, Yang CP, Hsueh C, Jaing TH. Successful nonoperative therapy for Kaposiform hemangioendothelioma involving the neck: report of 1 case. Otolaryngol Head Neck Surg. 2003;129:605–607. doi: 10.1016/S0194-5998(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JG, Edwards SL, Holmes JD. Kaposiform haemangioendothelioma: case report and review of the literature. Br J Plast Surg. 2002;55:163–165. doi: 10.1054/bjps.2001.3769. [DOI] [PubMed] [Google Scholar]
- 14.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deb G, Jenkner A, Sio L, Boldrini R, Bosman C, Standoli N, Donfrancesco A. Spindle cell (Kaposiform) hemangioendothelioma with Kasabach-Merritt syndrome in an infant: successful treatment with alpha-2A interferon. Med Pediatr Oncol. 1997;28:358–361. doi: 10.1002/(SICI)1096-911X(199705)28:5<358::AID-MPO6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.DeFatta RJ, Verret DJ, Adelson RT, Gomez A, Myers LL. Kaposiform hemangioendothelioma: case report and literature review. Laryngoscope. 2005;115:1789–1792. doi: 10.1097/01.mlg.0000176539.94515.75. [DOI] [PubMed] [Google Scholar]
- 17.Deraedt K, Vander Poorten V, Geet C, Renard M, Wever I, Sciot R. Multifocal kaposiform haemangioendothelioma. Virchows Arch. 2006;448:843–846. doi: 10.1007/s00428-006-0177-6. [DOI] [PubMed] [Google Scholar]
- 18.Ekfors TO, Kujari H, Herva R. Kaposi-like infantile hemangioendothelioma. Am J Surg Pathol. 1993;17:314–317. doi: 10.1097/00000478-199303000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Enjolras O, Mulliken JB, Wassef M, Frieden IJ, Rieu PN, Burrows PE, Salhi A, Leaute-Labreze C, Kozakewich HP. Residual lesions after Kasabach-Merritt phenomenon in 41 patients. J Am Acad Dermatol. 2000;42:225–235. doi: 10.1016/S0190-9622(00)90130-0. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J. Angiogenesis-dependent diseases. Semin Oncol. 2001;28:536–542. doi: 10.1016/S0093-7754(01)90021-1. [DOI] [PubMed] [Google Scholar]
- 21.Gianotti R, Gelmetti C, Alessi E. Congenital cutaneous multifocal kaposiform hemangioendothelioma. Am J Dermatopathol. 1999;21:557–561. doi: 10.1097/00000372-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Gruman A, Liang MG, Mulliken JB, Fishman SJ, Burrows PE, Kozakewich HP, Blei F, Frieden IJ. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616–622. doi: 10.1016/j.jaad.2004.10.880. [DOI] [PubMed] [Google Scholar]
- 23.Haisley-Royster C, Enjolras O, Frieden IJ, Garzon M, Lee M, Oranje A, Laat PC, Madern GC, Gonzalez F, Frangoul H, Le Moine P, Prose NS, Adams DM. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459–462. doi: 10.1097/00043426-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Hardisson D, Prim MP, Diego JI, Patron M, Escribano A, Rabanal I. Kaposiform hemangioendothelioma of the external auditory canal in an adult. Head Neck. 2002;24:614–617. doi: 10.1002/hed.10074. [DOI] [PubMed] [Google Scholar]
- 25.Harper L, Michel JL, Enjolras O, Raynaud-Mounet N, Riviere JP, Heigele T, Napoli-Cocci S. Successful management of a retroperitoneal kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon using alpha-interferon. Eur J Pediatr Surg. 2006;16:369–372. doi: 10.1055/s-2006-924615. [DOI] [PubMed] [Google Scholar]
- 26.Hauer J, Graubner U, Konstantopoulos N, Schmidt S, Pfluger T, Schmid I. Effective treatment of kaposiform hemangioendotheliomas associated with Kasabach-Merritt phenomenon using four-drug regimen. Pediatr Blood Cancer. 2007;49:852–854. doi: 10.1002/pbc.20750. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao CC, Chen CC, Ko SF, Huang CC, Chuang JH. A case of axillary kaposiform hemangioendothelioma resembles a soft tissue sarcoma. J Pediatr Hematol Oncol. 2005;27:596–598. doi: 10.1097/01.mph.0000184310.60701.c6. [DOI] [PubMed] [Google Scholar]
- 28.Hu B, Lachman R, Phillips J, Peng SK, Sieger L. Kasabach-Merritt syndrome-associated kaposiform hemangioendothelioma successfully treated with cyclophosphamide, vincristine, and actinomycin D. J Pediatr Hematol Oncol. 1998;20:567–569. doi: 10.1097/00043426-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Iwami D, Shimaoka S, Mochizuki I, Sakuma T. Kaposiform hemangioendothelioma of the mediastinum in a 7-month-old boy: a case report. J Pediatr Surg. 2006;41:1486–1488. doi: 10.1016/j.jpedsurg.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Kesari S, Schiff D, Henson JW, Muzikansky A, Gigas DC, Doherty L, Batchelor TT, Longtine JA, Ligon KL, Weaver S, Laforme A, Ramakrishna N, Black PM, Drappatz J, Ciampa A, Folkman J, Kieran M, Wen PY. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. Neuro Oncol. 2008;10:300–308. doi: 10.1215/15228517-2008-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai FM, Allen PW, Yuen PM, Leung PC. Locally metastasizing vascular tumor: spindle cell, epithelioid, or unclassified hemangioendothelioma? Am J Clin Pathol. 1991;96:660–663. doi: 10.1093/ajcp/96.5.660. [DOI] [PubMed] [Google Scholar]
- 32.Lalaji TA, Haller JO, Burgess RJ. A case of head and neck kaposiform hemangioendothelioma simulating a malignancy on imaging. Pediatr Radiol. 2001;31:876–878. doi: 10.1007/s002470100009. [DOI] [PubMed] [Google Scholar]
- 33.Lee E, Billings SD, Roumpf S, Mousdicas N. Abdominal plaque in a 10-day-old boy. Arch Dermatol. 2006;142:641–646. doi: 10.1001/archderm.142.5.641-b. [DOI] [PubMed] [Google Scholar]
- 34.Lyons LL, North PE, Mac-Moune Lai F, Stoler MH, Folpe AL, Weiss SW. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559–568. doi: 10.1097/00000478-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Mac-Moune Lai F, To KF, Choi PC, Leung PC, Kumta SM, Yuen PP, Lam WY, Cheung AN, Allen PW. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087–1092. doi: 10.1038/modpathol.3880441. [DOI] [PubMed] [Google Scholar]
- 36.Martinez AE, Robinson MJ, Alexis JB. Kaposiform hemangioendothelioma associated with nonimmune fetal hydrops. Arch Pathol Lab Med. 2004;128:678–681. doi: 10.5858/2004-128-678-KHAWNF. [DOI] [PubMed] [Google Scholar]
- 37.Mendez R, Capdevila A, Tellado MG, Somoza I, Liras J, Pais E, Vela D. Kaposiform hemangioendothelioma associated with Milroy’s disease (primary hereditary lymphedema) J Pediatr Surg. 2003;38:E9–E12. doi: 10.1016/S0022-3468(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 38.Mentzel T, Mazzoleni G, Dei Tos AP, Fletcher CD. Kaposiform hemangioendothelioma in adults: clinicopathologic and immunohistochemical analysis of three cases. Am J Clin Pathol. 1997;108:450–455. doi: 10.1093/ajcp/108.4.450. [DOI] [PubMed] [Google Scholar]
- 39.Niedt GW, Greco MA, Wieczorek R, Blanc WA, Knowles DM. Hemangioma with Kaposi’s sarcoma-like features: report of two cases. Pediatr Pathol. 1989;9:567–575. doi: 10.3109/15513818909026915. [DOI] [PubMed] [Google Scholar]
- 40.San Miguel FL, Spurbeck W, Budding C, Horton J. Kaposiform hemangioendothelioma: a rare cause of spontaneous hemothorax in infancy. Review of the literature. J Pediatr Surg. 2008;43:e37–e41. doi: 10.1016/j.jpedsurg.2007.08.068. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach-Merritt phenomenon) is associated with Kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg. 1997;100:1377–1386. doi: 10.1097/00006534-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Senturk N, Yyldiz L, Aydin F, Eroglu L, Canturk T, Turanli AY. Kaposiform hemangioendothelioma in an adult with an unusual presentation. J Eur Acad Dermatol Venereol. 2006;20:630–632. doi: 10.1111/j.1468-3083.2006.01524.x. [DOI] [PubMed] [Google Scholar]
- 43.Vetter-Kauczok CS, Strobel P, Brocker EB, Becker JC. Kaposiform hemangioendothelioma with distant lymphangiomatosis without an association to Kasabach-Merritt-Syndrome in a female adult! Vasc Health Risk Manag. 2008;4:263–266. doi: 10.2147/vhrm.2008.04.01.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss SW, Goldblum JR, eds. Hemangioendothelioma: vascular tumors of intermediate malignancy. Enzinger and Weiss’s Soft Tissue Tumors. 5th Ed. St Louis, MO: Mosby Elsevier; 2008, pp. 688–693.
- 45.Wilken JJ, Meier FA, Kornstein MJ. Kaposiform hemangioendothelioma of the thymus. Arch Pathol Lab Med. 2000;124:1542–1544. doi: 10.5858/2000-124-1542-KHOTT. [DOI] [PubMed] [Google Scholar]
- 46.Zamecnik M, Mikleova Z, Michal M. Kaposiform hemangioendothelioma in adult: report of a case with amianthoid-like fibrosis and angiectases. Cesk Patol. 2000;36:163–167. [PubMed] [Google Scholar]
- 47.Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood: an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321–328. doi: 10.1097/00000478-199304000-00001. [DOI] [PubMed] [Google Scholar]