Abstract
Abstract
Total hip arthroplasty (THA) in young patients has a high loosening rate, due in part to acetabular deformities that may compromise bone fixation and polyethylene wear. We therefore asked whether wear or osteolysis and loosening differ in patients under 40 years of age with alumina-on-alumina THA compared to those who are older. We prospectively followed 56 patients (63 hips) younger than 40 years (Group 1) and 247 patients (274 hips) older than 40 (Group 2) who had an alumina-on-alumina THA. The minimum followup was 4 years (mean, 5.6 years; range, 4–9 years). The two groups differed in various features: there were no patients with primary osteoarthritis in Group 1 and they had worse preoperative function and range of mobility, while weight, activity level, and implant size were greater in Group 2. The survival rate for cup loosening at 80 months postsurgery was 90.8% (95% confidence interval, 82.9–98.6%) for Group 1 and 96.5% (95% confidence interval, 94.2–98.7%) for Group 2. Cup loosening was less frequent with primary osteoarthritis than with severe developmental dysplasia of the hip. Although an alumina-on-alumina THA provided similar midterm survival and radiographic loosening in both age groups, the preoperative diagnosis seems more important than age for outcome. Continued followup will be required to determine if the alumina-on-alumina bearings in young patients result less risk of osteolysis and loosening.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Performing total hip arthroplasty (THA) in patients younger than 40 remains a concern. Both patients with secondary arthritis and those who are active are at risk due to their substantially higher revision rates of more than 90% at 20 years of followup in patients older than 40 years of age, and less than 70% in younger patients [14–16, 24, 28, 32, 37, 50]. Although other options, such as osteotomy and hip resurfacing, have been considered, THA is still the most frequent indication for a great number of cases and surgeons [15, 16, 32, 36, 37, 43]. However, poor long-term survival has been described with both cemented and uncemented cups in this population because of high wear in conventional polyethylene and loosening rates [25]. While both cemented and uncemented femoral stems usually provide better fixation [15, 16, 23, 34, 36, 37, 47, 50], different options are used to decrease wear and consequently osteolysis with alternate bearing surfaces including new highly cross-linked polyethylene, metal-on-metal, and alumina-on-alumina. Since the latter was introduced in the early 1970s by Boutin, minimal wear has been reported [26, 45, 46]. The same group reported unmeasurable alumina wear and lower rates of liner rupture in young patients if bone fixation was adequate [2–4, 42, 45, 46].
THA in patients younger than 40 years old also presents another challenge related to the preoperative diagnosis. Developmental dysplasia and posttraumatic and inflammatory conditions may produce insufficient bone stock and/or anatomic abnormalities requiring complex acetabular reconstruction that increases surgical difficulty and the risk of revision [12, 16, 17, 33, 39, 41, 46].
We asked (1) whether modern alumina-on-alumina bearing surfaces matched with metal-backed sockets would have comparable revision rates in patients younger and older than 40 years old; (2) whether preoperative conditions other than primary osteoarthritis, such as arthritis secondary to developmental diseases, (Perthes-Legg-Calvé disease and slipped capital femoral epiphysis), to moderate or severe developmental dysplasia of the hip, or to inflammatory conditions would be more important than age and would influence the clinical and radiographic outcome and rates of cup loosening in our patients. Finally, we report the incidence of osteolysis, radiolucent lines and femoral head penetration into the alumina liner as well as all the complications, liner fractures and noises, related to this alternate bearing surface.
Material and Methods
We prospectively followed 309 patients with 343 primary uncemented Cerafit®-Multicone hydroxyapatite (HAP) prostheses (Ceraver Osteal, Roissy, France) implanted between January 1999 and December 2003 in four institutions (Hospital La Paz de Madrid, Hospital General Yagüe de Burgos, Hospital Cabueñes de Gijon, and Hospital Provincial de Castellon). All procedures were performed by four high-volume (more than 50 cases per year) hip arthroplasty surgeons (EGC [author], and ABP, AMM and EM). In most patients, the choice of the alumina-on-alumina coupling was based mainly on age (less than 70 years old), but in a few active patients between 70 and 72 years old (10 patients) we implanted the same system. Of the 343 hips, we excluded six: one hip with a deep infection 27 months postoperatively in a 70-year-old woman (0.29%); one hip with a cup revision after a posttraumatic acetabular fracture at 27 months postsurgery in a 40-year-old man; one hip with a femoral stem revision after a periprosthetic fracture at 11 months after surgery in a 53-year-old man; and three patients (three hips) who died from nonimplant-related causes: lymphoma at 36 months postoperatively in a 59-year-old man; kidney cancer at 42 months postoperatively in a 63-year-old man; and rectal cancer at 35 months postoperatively in a 60-year-old man. The THA was functioning well in all three cases. The remaining 337 hips formed the basis of the followup study. Fifty-six patients (63 hips) were less than 40 years old (Group 1) and 247 patients (274 hips) were older than 40 years (Group 2). The mean age was 30.7 years (range, 14–40 years) in Group 1 and 57.9 years in Group 2 (range, 41–72 years). (We did not perform an a priori power analysis to assess number of patients required to discern differences in some key variables.) Patients were divided into five groups regardless of age: Group A included primary osteoarthritis, posttraumatic arthritis and avascular necrosis (233 hips); Group B included developmental arthritis (19 hips); Group C arthritis included secondary moderate developmental dysplasia according to Crowe et al. [6] (30 hips); Group D included arthritis secondary to severe developmental dysplasia [6] (13 hips); and Group E included inflammatory arthritis (42 hips). We recorded the gender, weight, and preoperative diagnosis. The minimum clinical and radiographic followup at the last evaluation was 4 years in nonrevised cases (mean, 5.6 years; range, 4–9 years). No patients were lost to followup.
There were no differences in gender distribution between groups (Table 1). There were 41 male patients (65.1%) and 22 female patients (34.9%) in Group 1 versus 163 male patients (59.5%) and 111 female patients (40.5%) in Group 2. Mean weight was greater in Group 2. There were no cases of primary osteoarthritis in Group 1, in which avascular necrosis of the femoral head, severe developmental dysplasia (as judged by the criteria of Crowe et al. [5]), and rheumatoid arthritis were more frequent. Preoperative function (p = 0.03) and range of mobility (p < 0.001) were worse in younger patients (Table 2).
Table 1.
Patient data (preoperative diagnosis)
| Diagnosis | Group 1 N = 63 |
Group 2 N = 274 |
|---|---|---|
| Primary osteoathrosis | 0 (0%) | 135 (49.3%) |
| Developmental arthritis (Legg-Calvé-Perthes, slipped capital femoral epiphysis) | 6 (9.5%) | 13 (4.7%) |
| Posttraumatic arthritis | 2 (3.2%) | 23 (8.4%) |
| Congenital dysplasia (Crowe Grades I–II) | 4 (6.3%) | 26 (9.5%) |
| Congenital dysplasia (Crowe Grades III–IV) | 8 (12.7%) | 5 (1.8%) |
| Inflammatory arthritis | 19 (30.2%) | 23 (8.4%) |
| Avascular necrosis | 23 (36.5%) | 48 (17.5%) |
| Septic arthritis | 1 (1.6%) | 0 (0%) |
| Acromegaly | 0 (0%) | 1 (0.4%) |
p < 0.001, linear-by-linear association.
Table 2.
Preoperative and postoperative clinical results according to the Merle D’Aubigné and Postel scale [39]
| Group 1 | Group 2 | p Values | |
|---|---|---|---|
| Preoperative Rating | |||
| Pain | 2.5 ± 0.8 | 2.7 ± 0.9 | 0.072 |
| Function | 2.8 ± 1.1 | 3.1 ± 1.0 | 0.038 |
| Range of mobility | 2.5 ± 0.9 | 2.8 ± 0.9 | 0.015 |
| Postoperative Rating | |||
| Pain | 5.9 ± 0.6 | 5.8 ± 0.5 | 0.309 |
| Function | 5.8 ± 0.7 | 5.8 ± 0.5 | 0.613 |
| Range of mobility | 5.6 ± 0.8 | 5.7 ± 0.6 | 0.944 |
Mean ± standard deviation.
We implanted a Cerafit®-Triradius press-fit cup (Ceraver), a fully HAP-coated TiAl6V4 rough shell with optional screws fitted with an Al2O3 liner, in every hip. The cup was paired to an uncemented Multicone stem (Ceraver), a fully HAP-coated tapered straight stem that has a rectangular geometry and is made of TiAl6V4. The liner was inserted using a Morse taper shape with an angle of 5º40′. Its purpose was to achieve better fixation and decrease the risk of liner dislocation. Characteristics of the alumina were 99.8% purity, 3.98 density, 2-μm grain size, and hipped in all cases. The HAP coating was applied by plasma spray, and according to the manufacturer’s specifications, the coating had a relative crystallinity of 37%, thickness of 80 ± 20 μm, bond shear strength of 15/25 MPa, and mean roughness of 3 μm. Regarding stem and shell surface finish, the arithmetic rugosity Ra beneath HAP was 3 to 4 μm.
All operations were performed with the same technique using a posterior approach. However, femoral head autograft taken from the femoral head was used in 13 hips (20.6%) in Group 1; autograft was used as segmental reinforcement in developmental dysplasia in three hips and as acetabular medial wall reinforcement using an impacted morselized autograft bone in 10 hips with acetabular protrusion. This last technique was used in eight hips (2.9%) with acetabular protrusion in Group 2. With a cup diameter of less than 50 mm, the femoral head diameter must be 28 mm. The minimum ceramic liner thickness was 4.2 mm for 46-mm (28-mm femoral head) and 50-mm (32-mm femoral head) shells and 6.2 mm for 48-mm (28-mm femoral head) and 52-mm (32-mm femoral head) shells (Table 3). We recorded the cup and stem sizes, the use of screws for the acetabular component, and femoral sizes for each patient group. Acetabular cup and femoral stem sizes were greater in Group 2. A small femoral head (46 or 48 mm) was used more frequently in Group 1.
Table 3.
Size of cups and number of hips in which screws for cup fixation were used
| Variable | Group 1 | Group 2 | p Values |
|---|---|---|---|
| Mean cup | 51.8 ± 3.2 | 53.1 ± 3.3 | 0.011 |
| Mean stem | 9.4 ± 1.7 | 10.1 ± 1.8 | 0.005 |
| Screws for cup fixation (hips) | |||
| No screws | 38 (60.3%) | 184 (67.2%) | p = 0.306 |
| Screws | 25 (39.7%) | 90 (32.8%) | Fisher’s exact test |
| Femoral head size (hips) | |||
| 28-mm (shell, 46 and 48-mm) | 13 (20.6%) | 24 (8.8%) | p = 0.012 |
| 32-mm (shell, more than 48 mm) | 50 (79.4%) | 250 (91.2%) | Fisher’s exact test |
Postoperatively, all patients received antibiotics and low-molecular-weight heparin subcutaneously to prevent thromboembolic incidents. At 2 days after surgery patients were walking with crutches for toe-touch partial weight bearing for 3 weeks. Thereafter they were allowed to weight bear as tolerated using two crutches for the next 6 weeks.
We followed patients postdischarge at 6 weeks, at 3, 6, and 12 months, and annually thereafter. Surgeons from different institutions (EGC, ABP, AMM, and EM) clinically evaluated preoperative and postoperative pain, function, and range of mobility using the six-level scales described by Merle D’Aubigné and Postel [39]. Patients were also asked about the location of pain.
Standard anteroposterior radiographs of the pelvis and lateral radiographs of the hip were obtained preoperatively, immediately after the operation, and at each followup visit. The patient was positioned supine, with his/her feet together. The xray tube was positioned over the symphysis pubis 1 m from and perpendicular to the table with a symmetric obturator foramen and visible lesser trochanter and iliac crest. Measurements were made by a single author (EGR) not involved in the surgery. Cup position was assessed according to the acetabular abduction angle, the height of the center of the hip (as measured from the center of the femoral head to the interteardrop line), and the horizontal distance of the cup (measured from the center of the femoral head to the Köhler line [29]. The distribution of any radiolucent gaps on the initial postoperative radiograph and of radiolucent lines or osteolysis at the acetabular bone-prosthesis interface on the subsequent radiographs was recorded in the three zones described by DeLee and Charnley [8]. We judged radiographic bone ingrowth into an acetabular component by indirect inference based on (1) the absence of radiolucent lines around the cups of at least 2 mm and (2) cup tilting greater than 5° or superior migration greater than 3 mm [40]. Linear femoral head penetration was recorded according to the method of Kim et al. [33, 34] using scanned digitized radiographs and analyzed with a software package (AutoCAD 2000; AutoDesk Inc, Sausalito, CA) [18]. The 6-week postoperative radiograph was used as initial reference for subsequent measurements.
Femoral canal filling, measured as the ratio of the width of the stem to the width of the medullary canal, was determined at two levels: Level A (at the middle of the stem) and Level B (1 cm proximal to the tip). The distribution of any radiolucent lines or osteolysis as seen on the anteroposterior radiographs was recorded in the zones described by Gruen et al. [21]. Osteolysis was classified according to the criteria described by Goetz et al. [20]. Subsidence was defined as a decrease of at least 5 mm in the distance between the top of the stem and the greater trochanter when the initial postoperative radiographs were compared to those made at the followup evaluations. Femoral osteopenia due to stress shielding was graded according to the system described by Engh et al. [11]. Femoral component fixation was graded as radiographic ingrowth, fibrous stable, or unstable according to the criteria for porous prostheses described by Engh et al. [12]. Postoperative acetabular cup position and femoral canal filling were similar in both groups (Table 4). Forty-six hips had cup lucency on the initial postoperative radiograph in nonrevised cases.
Table 4.
Postoperative radiographic data
| Variable | Group 1 | Group 2 | p Values |
|---|---|---|---|
| Acetabular cup position | Mann-Whitney test | ||
| Acetabular abduction angle (degrees) | 46.5 ± 4.4 | 46.5 ± 5.6 | 0.834 |
| Horizontal position (mm) | 32.4 ± 7.3 | 33.5 ± 6.4 | 0.171 |
| Vertical position (mm) | 22.5 ± 6.8 | 21.6 ± 7.0 | 0.482 |
| Femoral canal filling (%) | Pearson’s chi square test | ||
| Level A | 83.1 ± 7.5 | 83.1 ± 6.7 | 0.848 |
| Level B | 89.8 ± 8.7 | 91.1 ± 8.6 | 0.363 |
| Level C | 85.4 ± 10.1 | 87.5 ± 8.4 | 0.194 |
Qualitative data (ie, gender, activity level, preoperative diagnosis, and implant size) are expressed as counts and percentages within groups and quantitative data by mean ± standard deviation or range. Qualitative data between the two groups were compared with use of the chi square test or Fisher’s exact test, and quantitative data (postoperative acetabular abduction angle, horizontal and vertical cup position) with the Mann-Whitney test. Kaplan-Meier survivorship analysis [30], with 95% confidence intervals, was used to estimate the cumulative probability of not having a revision of one or both prosthetic components and to estimate the cumulative probability of not having cup loosening in the whole series. Differences in survival were determined using the log-rank test. Cox regression analysis was used to detect possible survival differences between different diagnoses nonadjusted by age and adjusted by age.
Results
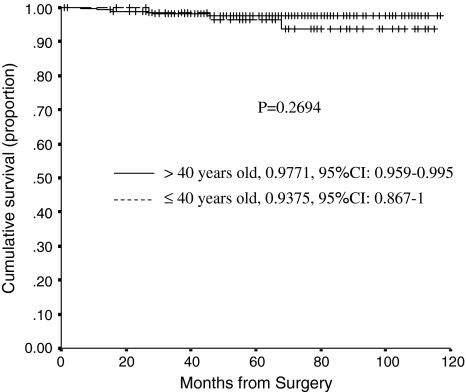
The cumulative probability of not having a revision at 80 months postsurgery was similar (p = 0.2694) for Group 1 (93.7%, 95% confidence interval, 86.7%–100%) and Group 2 (97.7%, 95% confidence interval, 95.9%–99.5%) (Fig. 1). At last followup, we had revised eight cups: six for loosening (two in Group 1 and four in Group 2), one for an acetabular periprosthetic fracture after a high-energy trauma, and one for an alumina liner fracture occurring from a fall 40 months after the operation. The cup with the liner fracture had a 50-mm outer-diameter cup (4.2-mm alumina liner) and a 35º acetabular abduction angle. Of the six cups revised for loosening, two had loosened because of poor press-fit (Table 5). We revised three stems, one for infection and two for aseptic loosening (one in a patient with acromegaly after implanting a very thin stem, which resulted in early subsidence, and one owing to a periprosthetic fracture from a fall 2 months after surgery).
Fig. 1.
A graph shows Kaplan-Meier survivorship curves comparing the cumulative probability of not having a cup revision for aseptic loosening in patients younger and older than 40 years. Cross lines represent censored hips. Ranges represent the 95% confidence intervals.
Table 5.
Data on loosened cups
| Case | Age | Group | Gender | Diagnosis | Acetabular anatomy | Cup size (mm) | Head diameter (mm) | Screws | Abduction angle (°) | Cup loosening (months) | Revision cup (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | 2 | Female | RA | A | 54 | 32 | yes | 55 | 28 | no |
| 2 | 67 | 2 | Female | OA | B | 50 | 32 | no | 55 | 48 | no |
| 3 | 64 | 2 | Female | Dysplasia | A | 46 | 28 | no | 45 | 25 | 46 |
| 4 | 39 | 1 | Female | Dysplasia | C | 48 | 28 | yes | 40 | 14 | no |
| 5 | 52 | 2 | Male | Legg-Calvè-Perthes | B | 48 | 28 | no | 55 | 1* | 6 |
| 6 | 45 | 2 | Female | RA | A | 50 | 32 | yes | 75 | 12* | 16 |
| 7 | 53 | 2 | Male | Avascular necrosis | B | 56 | 32 | no | 45 | 10 | no |
| 8 | 21 | 1 | Female | RA | A | 52 | 32 | yes | 52 | 22 | no |
| 9 | 46 | 2 | Female | Dysplasia | C | 52 | 32 | yes | 55 | 20 | 29 |
| 10 | 28 | 1 | Female | Legg-Calvè-Perthes | B | 56 | 32 | yes | 50 | 60 | 68 |
| 11 | 34 | 1 | Male | Dysplasia | B | 54 | 32 | no | 45 | 38 | 47 |
| 12 | 29 | 1 | Female | Dysplasia | C | 46 | 28 | no | 45 | 36 | no |
| 13 | 60 | 2 | Female | OA | B | 52 | 32 | no | 45 | 48 | no |
| 14 | 62 | 2 | Female | RA | A | 54 | 32 | yes | 42 | 36 | no |
RA = rheumatoid arthritis; * = poor pressfit.
The risk of cup loosening increased in patients with developmental arthritis (hazard ratio = 8.1), moderate developmental dysplasia (hazard ratio = 8.7), severe developmental dysplasia (hazard ratio = 16.2), and inflammatory arthritis (hazard ratio = 7.8). The increased odds based on diagnosis were similar for both age groups (Table 6).
Table 6.
Cox regression analysis (nonadjusted and adjusted by age) of time to cup loosening according to preoperative diagnosis
| Group | Nonadjusted by age | Adjusted by age | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% C.I. HR | p Values | Hazard ratio | 95% C.I. HR | p Values | |
| A | 1 | 1 | ||||
| B | 8.117 | 1.356–48.589 | 0.022 | 8.742 | 1.362–56.100 | 0.022 |
| C | 8.757 | 1.766–43.419 | 0.008 | 9.035 | 1.796–45.452 | 0.008 |
| D | 16.183 | 2.686–97.497 | 0.002 | 17.833 | 2.637–120.594 | 0.003 |
| E | 7.835 | 1.753–35.021 | 0.007 | 8.610 | 1.697–43.694 | 0.009 |
| Age | – | – | – | 1.006 | 0.966–1.048 | 0.774 |
Group A: Primary osteoarthrosis, posttraumatic arthritis, and avascular necrosis; Group B: Developmental arthritis; Group C: Moderate congenital dysplasia; Group D: Severe congenital dysplasia; Group E: Inflammatory arthritis. 95% C.I. HR: 95% confidence interval hazard ratio.
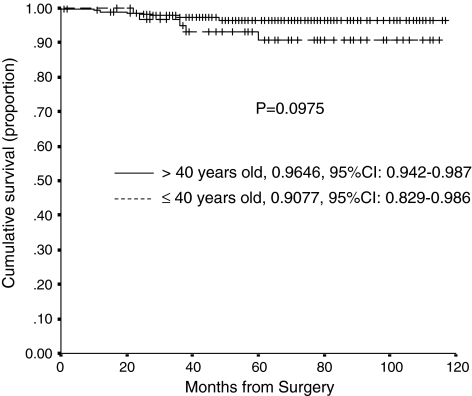
The cumulative probability of not having radiographic cup loosening as an end point at 80 months postsurgery was similar (p = 0.0974) for Group 1 (90.8%, 95% confidence interval, 82.9%–98.6%) and Group 2 (96.5%, 95% confidence interval, 94.2%–98.7%) (Fig. 2). At last followup there were eight radiographically loosened cups that had not been revised, five of which had radiolucency in two DeLee zones (two in Group 1 and three in Group 2, one of the latter also had cup migration), and three hips had circumferential lucency (one in Group 1 and two in Group 2) at 5 years postoperatively. Eleven of the 14 patients with radiographically loose hips (whether revised or not) were women. A larger abduction acetabular angle was more frequent (p = 0.068) in the loosened cup group; the mean angle was 52.8º ± 14.4° in this group, whereas the mean angle was 46.2º ± 4.6° in the nonloosened cup group.
Fig. 2.
A graph shows Kaplan-Meier survivorship curves comparing the cumulative probability of not having an aseptic cup loosening in patients younger and older than 40 years. Cross lines represent censored hips. Ranges represent the 95% confidence intervals.
One patient had intermittent pain in the thigh during the first year after surgery. At the last followup evaluation, there was one hip with Level 4 pain, 27 hips with Level 5 pain, and 298 hips with Level 6 pain among the nonrevised hips. Although mean preoperative ranges of function and range of mobility were worse in Group 1 than in Group 2, mean postoperative ranges were similar in both groups We did not observe differences between the four institutions with the number of available hips (Table 3). No patient spontaneously reported any hip noise and none reported hip noises when specifically questioned. There was no osteolysis around any component. There were no instances of Grade 2 or higher proximal femoral osteopenia or cortical widening in any case. With the available technology we could not detect radiographic penetration of the femoral head into the alumina liner after the 6-week postoperative reference radiograph in any hip.
Discussion
Wear is the most important factor limiting long-term results in THA [4, 19, 23, 25, 32–34]. Alternative bearing surfaces, such as the new highly cross-linked polyethylene, metal-on-metal and ceramic-on-ceramic, are being specifically used for young patients [4, 7, 42, 43]. This population presents several problematic conditions other than wear, such as developmental dysplasia of the hip and inflammatory disease and, since these preoperative diagnoses are different from those in older people, bone fixation in the former might be limited by bone deficiencies [13–16, 27, 31, 35, 42, 44, 49]. We asked whether modern alumina-on-alumina bearing surfaces matched with metal-backed sockets would have comparable revision rates in patients younger and older than 40 years old. We then asked whether preoperative diagnoses would be more important than age and would influence the survival and cup loosening. Finally, we report the incidence of osteolysis, radiolucent lines and femoral head penetration into the alumina liner as well as all the complications, liner fractures and noises, related to this alternate bearing surface.
There were several limitations, including the relatively small available study cohort, especially in Group 1. In some diagnoses, this resulted in very wide confidence intervals for some groups. Secondly, each group has subjects with different diagnoses so the short followup and the small number of patients limited our ability to generate conclusions, especially in relation to different diagnoses and cup loosening. Our conclusions should therefore be considered preliminary.
Our data suggest that patients younger than 40 years old have different diagnoses than older patients. Despite these differences, a Cerafit® alumina-on-alumina THA provides similar midterm survival in patients younger and older than 40 years old. Bizot et al. [2] reported less than 95% survival at 7 years in patients younger than 40 years of age using different sockets and suggested that an improvement in acetabular fixation was needed to increase survival rates. The same group reported 98.4% survival rate for aseptic loosening in patients younger than 55 years using a press-fit metal-backed cup [3]. Nizard et al. [42] reported 13% revision for aseptic loosening in a series of patients younger than 30 years using the same prosthesis. Poor survivorship was observed in THA performed after secondary arthritis related to a slipped capital epiphysis or trauma. The main reason for failure in the Nizard et al. [42] study was aseptic loosening of the acetabular component. We also observed a relatively high failure rate for cup loosening using this acetabular component. The socket has a HA coated rough surface but is not tridimensional, the manufacturer has recently improved bone fixation by adjusting surface and alumina liner width. In the new shells (implanted since 2004) the titanium alloy shell is 3 mm instead of 5 mm thick, and this has made it possible to increase the alumina insert thickness by 2 mm, consequently improving the mechanical resistance. Moreover, the outer macrostructure of the new shells also provides a larger contact area for osteointegration thus further enhancing secondary stability.
The main reason for failure in our series using the press-fit metal-backed shell was aseptic loosening of the acetabular component; this observation confirms that of other series [3, 15, 42]. Earlier studies of a young population noted the higher risk for THA revision, especially cup revision (Table 7). An association between early loosening of the acetabular cup and deficient acetabular bone structure has been reported previously [17, 19]. Fixation of a HAP cup remains difficult in dysplasia even when the surgeon uses screws and a small cup; and this is true whatever the age of the patient. High rates of loosening have been reported in patients who had developmental dysplasia [5, 27, 41, 44, 48] and in patients who had rheumatoid arthritis; loosening was ascribed to poor bone quality and the rheumatoid disease [13–15, 17, 31, 35, 44, 49]. The original poor bone quality in these patients can result in insufficient cup fixation. These observations indicate that, when necessary, the acetabulum must be reconstructed with grafts or metallic devices, when necessary, before a cup can be implanted to ensure sufficient fixation.
Table 7.
Series in young patients
| Study | Number of hips | Mean age (range) | Type of implant | Mean followup, years (range) | Survival rate, revision (time) | Survival rate, aseptic loosening (time) | Risk factors |
|---|---|---|---|---|---|---|---|
| Berger et al. [1] | 79 | 37 (20–49) | HG I | 8.8 (6.5–0.5) | 98.8% (10 years) | Osteolysis | |
| Delaunay et al. [7] | 83 | 40.7(23–49) | Alloclassic-Metasul | 7.3 (2–10.4) | 100% at 10 years | ||
| Devitt et al. [9] | 132 | 42.2 (16–49) | Charnley | 18.1 (16–252) | 75% (20 years) | ||
| Emery et al. [10] | 57 | 41 (17–49) | Stanmore | 13 (0.4–21) | 68% (15 years) | ||
| Garcia-Cimbrelo et al. [16] | 67 | 32.4 (18–39) | Cemented Charnley | 21.7 | 69.9% (20 years) | 60% cup (20 years) 74% stem (20 years) | Acetabular bone deficiency Polyethylene wear |
| Ha et al. [22] | 78 | 37 (19–49) | Bicontact Al-on-Al | 5.5 (5–6) | 100% (5 years) | ||
| Halley and Wroblewski [24] | 49 | 26 (17–30) | Charnley | 9.5 (5–15.5) | 14.3% socket failure | Acetabular bone | |
| Hartofilakidis et al. [27] | 93 | 50 (25– 69) | 46 Charnley | 16.6 (12–18) | 67.4% | Polyethylene wear uncemented | |
| Kearns et al. [32] | 299 | 47 (22–70) | 47 uncemented | 13.4 (10–16) | 72.3% | ||
| 41.1 (18–50) | Uncemented | 8.4 (5–19) | 46.8% (15 years) | Dysplasia | |||
| Lewthwaite et al. [36] | 130 | 42 | Exeter Universal Hip | 12.5 (10–17) | 92.6% (12.5 years) | Acetabular loosening | |
| McAuley et al. [38] | 561 | 40 (16–50) | AML | 6.9 (0–19) | 60.4% (15 years) | ||
| Nizard et al. [42] | 132 | 23.4 (13–30) | Alumina-on-alumina (different cups) | 6.9 (1–26.5) | 82.1% (10 years) 72.4% (15 years) |
74.5% cup (15 years) 88.1% stem (15 years) |
Posttraumatic arthritis SCFE |
| Spangehl et al. [48] | 44 | 39 (12– 67) | Hemispheric Uncemented porous-coated Bulk femoral head autograft | 7.5 (5–12) | 91% | 97.8% | Polyethylene wear |
| Williams et al. [49] | 57 | 16.4 (13–24) | Cemented Charnley | 4.6 (20 months to 9 years) | 96.5% | 73.4% 56.5% (more than 5 years followup) | Pelvic growth (younger patient) |
| Wroblewski et al. [50] | 1092 | 41 (12–51) | Charnley | 17.5 | 55.3% (27 years) |
Although the followup of this series is too short to allow definite conclusions, the Cerafit® alumina-on-alumina THA provided similar midterm survival in the older and younger groups. Cup loosening was the main concern in both groups. Failure of a HAP-coated cup is more frequently associated with dysplasia than with the patient’s age, even when screws and a small cup are used. The preoperative diagnosis seems more important than age for outcome. Continued followup will be required to determine if the alumina-on-alumina bearings in young patients result in less osteolysis and loosening. As we have no power analysis, we consider this a preliminary study to generate hypotheses.
Acknowledgments
We thank Agustin Blanco-Pozo (Hospital General Yagüe, Burgos, Spain), Antonio Murcia-Mazón (Hospital de Cabueñes, Gijon, Spain), and Eduardo Martí (Hospital Provincial de Castellón, Spain) for providing the clinical data and radiographs of their patients in this multicenter study.
Footnotes
This work was performed at Hospital la Paz, Madrid, Spain, Hospital General Yagüe, Burgos, Spain, Hospital Cabueñes, Gijon, Spain, and Hospital Provincial Castellon, Spain.
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Berger RA, Jacobs JJ, Quigley LR, Rosenberg AG, Galante JP. Primary cementless acetabular reconstruction in patients younger than 50 years old. 7- to 11-year results. Clin Orthop Relat Res. 1997;344:216–226. doi: 10.1097/00003086-199711000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Bizot P, Banallec L, Sedel L, Nizard R. Alumina-on-alumina total hip prosthesis in patients 40 years or younger. Clin Orthop Relat Res. 2000;379:68–76. doi: 10.1097/00003086-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bizot P, Hannouche D, Nizard R, Witvoet J, Sedel L. Hybrid alumina total hip arthroplasty using a press-fit metal-backed socket in patients younger than 55 years: a six- to 11-year evaluation. J Bone Joint Surg Br. 2004;86:190–194. doi: 10.1302/0301-620X.86B2.14026. [DOI] [PubMed] [Google Scholar]
- 4.Bizot P, Nizard R, Hamadouche M, Hannouche D, Sedel L. Prevention of wear and osteolysis: alumina-on-alumina bearing. Clin Orthop Relat Res. 2000;393:85–93. doi: 10.1097/00003086-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Chougle A, Hemmady MV, Hodgkinson JP. Severity of hip dysplasia and loosening of the socket in cemented total hip replacement: a long-term follow-up. J Bone Joint Surg Br. 2005;87:16–20. [PubMed] [Google Scholar]
- 6.Crowe JF, Mani VJ, Ranawat CS. Total hip replacement in congenital dislocaion and dysplasia of the hip. J Bone Joint Surg Am. 1979;61:15–23. [PubMed] [Google Scholar]
- 7.Delaunay CP, Bonnomet F, Clavert P, Laffargue P, Migaud H. THA using metal-on-metal articulation in active patients younger than 50 years. Clin Orthop Relat Res. 2008;466:340–346. doi: 10.1007/s11999-007-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 9.Devitt A, O’Sullivan T, Quinlan W. 16- to 25-year follow-up study of cemented arthroplasty of the hip in patients aged 50 years or younger. J Arthroplasty. 1997;12:479–489. doi: 10.1016/S0883-5403(97)90169-8. [DOI] [PubMed] [Google Scholar]
- 10.Emery DF, Clarke HJ, Grover ML. Stanmore total hip replacement in younger patients: review of a group of patients under 50 years of age at operation. J Bone Joint Surg Br. 1997;79:240–246. doi: 10.1302/0301-620X.79B2.7165. [DOI] [PubMed] [Google Scholar]
- 11.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement: the factors governing bone ingrowth, stress shielding and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 12.Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants: the femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed] [Google Scholar]
- 13.Eskelinen A, Paavolainen P, Helenius I, Pulkkinen P, Remes V. Total hip arthroplasty for rheumatoid arthritis in young patients. Acta Orthop. 2006;77:853–865. doi: 10.1080/17453670610045704. [DOI] [PubMed] [Google Scholar]
- 14.Eskelinen A, Remes V, Helenius I, Pulkkinen P, Nevalainen J, Paavolainen P. Total hip arthroplasty for primary osteoarthritis in young patients in the Finnish Arthroplasty Register. 4,611 primary replacements followed for 0–22 years. Acta Orthop. 2005;76:28–41. doi: 10.1080/00016470510030292. [DOI] [PubMed] [Google Scholar]
- 15.Eskelinen A, Remes V, Helenius I, Pulkkinen P, Nevalainen J, Paavolainen P. Uncemented total hip arthroplasty for primary osteoarthritis in young patients: a mid- to long-term follow-up study from the Finnish Arthroplasty Register. Acta Orthop. 2006;77:57–70. doi: 10.1080/17453670610045704. [DOI] [PubMed] [Google Scholar]
- 16.García-Cimbrelo E, Cruz-Pardos A, Cordero J, Sanchez-Sotelo J. Low friction arthroplasty in patients younger than 40 years old: 20- to 25-year results. J Arthroplasty. 2000;15:825–832. doi: 10.1054/arth.2000.8097. [DOI] [PubMed] [Google Scholar]
- 17.García-Cimbrelo E, Díaz-Martin A, Madero R, Munuera L. Loosening of the cup after low-friction arthroplasty in patients with acetabular protrusion: the importance of the position of the cup. J Bone Joint Surg Br. 2000;82:108–115. doi: 10.1302/0301-620X.82B1.9796. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Cimbrelo E, Garcia-Rey E, Murcia-Mazon A, Blanco-Pozo A, Marti E. Alumina-on-alumina in THA: a multicenter prospective study. Clin Orthop Relat Res. 2008;466:309–316. doi: 10.1007/s11999-007-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Cimbrelo E, Munuera L. Early and late loosening of the acetabular cup after low-friction arthroplasty. J Bone Joint Surg Am. 1992;74:1119–1129. [PubMed] [Google Scholar]
- 20.Goetz DD, Smith JJ, Harris WH. The prevalence of femoral osteolysis associated with components inserted with or without cement in total hip replacements. J Bone Joint Surg Am. 1994;76:1121–1128. doi: 10.2106/00004623-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 22.Ha YC, Koo KH, Jeong ST, Joon Yoo J, Kim YM, Joong Kim H. Cementless alumina-on-alumina total hip arthroplasty in patients younger than 50 years: a 5-year minimum follow-up study. J Arthroplasty. 2007;22:184–188. doi: 10.1016/j.arth.2006.02.169. [DOI] [PubMed] [Google Scholar]
- 23.Hallan G, Lie SA, Havelin LI. High wear rates and extensive osteolysis in 3 types of uncemented total hip arthroplasty: a review of the PCA, the Harris-Galante and the Profile/Tri-Lock Plus arthroplasties with a minimum of 12 years median follow-up in 96 hips. Acta Orthop. 2006;77:575–584. doi: 10.1080/17453670610012638. [DOI] [PubMed] [Google Scholar]
- 24.Halley DK, Wroblewski BM. Long-term results of low friction arthroplasty in patients 30 years of age or younger. Clin Orthop Relat Res. 1986;211:43–50. [PubMed] [Google Scholar]
- 25.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty: a minimum 18.5 year follow-up study. J Bone Joint Surg Am. 2002;84:69–77. [PubMed] [Google Scholar]
- 26.Harris WH. Results of uncemented cup: a critical appraisal at 15 years. Clin Orthop Relat Res. 2003;417:121–125. doi: 10.1097/01.blo.0000096824.67494.aa. [DOI] [PubMed] [Google Scholar]
- 27.Hartofilakidis G, Georgiades G, Babis GC, Yiannakopoulos CK. Evaluation of two surgical techniques for acetabular reconstruction in total hip replacement for congenital hip disease: results after a minimum ten-year follow-up. J Bone Joint Surg Br. 2008;90:724–730. doi: 10.1302/0301-620X.90B6.20490. [DOI] [PubMed] [Google Scholar]
- 28.Havelin LI, Engesaeter L, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty register. 11 years and 73, 000 arthroplasties. Acta Orthop Scand. 2000;71:337–353. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- 29.Johnston RC, Fitzgerald RH, Harris WH, Poss R, Müller ME, Sledge CB. Clinical and radiographic evaluation of total hip arthroplasty: a standard system of terminology for reporting results. J Bone Joint Surg Am. 1990;72:161–168. [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 31.Katsimihas M, Taylor AH, Lee MB, Sarangi PP, Learmonth ID. Cementless acetabular replacement in patients with rheumatoid arthritis: a 6- to 14-year prospective study. J Arthroplasty. 2003;18:16–22. [DOI] [PubMed]
- 32.Kearns SR, Jamal B, Rorabeck CH, Bourne RB. Factors affecting survival of uncemented total hip arthroplasty in patients 50 years or younger. Clin Orthop Relat Res. 2006;453:103–109. doi: 10.1097/01.blo.0000238868.22852.dd. [DOI] [PubMed] [Google Scholar]
- 33.Kim YH, Kim JS, Cho SH. A comparison of polyethylene wear in hips with cobalt-chrome or zirconia heads: a prospective, randomised study. J Bone Joint Surg Br. 2001;83:742–750. doi: 10.1302/0301-620X.83B5.10941. [DOI] [PubMed] [Google Scholar]
- 34.Kim YH, Kook HK, Kim JS. Total hip replacement with a cementless acetabular component and a cemented femoral component in patients younger than fifty years of age. J Bone Joint Surg Am. 2002;84:770–774. doi: 10.2106/00004623-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Learmonth ID, Heywood AW, Kaye J, Dall D. Radiological loosening after cemented hip replacements for juvenile chronic arthritis. J Bone Joint Surg Br. 1989;71:209–212. doi: 10.1302/0301-620X.71B2.2925736. [DOI] [PubMed] [Google Scholar]
- 36.Lewthwaite SC, Squires B, Gie GA, Timperley AJ, Ling RS. The Exeter™ Universal Hip in patients 50 years or younger at 10–17 years’ follow. Clin Orthop Relat Res. 2008;466:324–331. doi: 10.1007/s11999-007-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malchau H, Herberts P, Eisler T, Gallerick G, Söderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 38.McAuley JP, Szuszczewicz ES, Young A, Engh CA., Sr Total hip arthroplasty in patients 50 years and younger. Clin Orthop Relat Res. 2004;418:119–125. doi: 10.1097/00003086-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Merle D’Aubigné R, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed] [Google Scholar]
- 40.Moore MS, McAuley JP, Young AM, Engh CA., Sr Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;444:176–183. doi: 10.1097/01.blo.0000201149.14078.50. [DOI] [PubMed] [Google Scholar]
- 41.Mulroy RD, Jr, Harris WH. Failure of acetabular autogenous grafts in total hip arthroplasty: increasing incidence: a follow-up note. J Bone Joint Surg Am. 1990;72:1536–1540. [PubMed] [Google Scholar]
- 42.Nizard R, Pourreyron D, Raould A, Hannouche D, Sedel L. Alumina-on-alumina hip arthroplasty in patients younger than 30 years old. Clin Orthop Relat Res. 2008;466:317–323. doi: 10.1007/s11999-007-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parvizi J, Campfield A, Clohisy JC, Rothman RH, Mont MA. Management of arthritis of the hip in the young adult. J Bone Joint Surg Br. 2006;88:1279–1285. doi: 10.1302/0301-620X.88B10.17859. [DOI] [PubMed] [Google Scholar]
- 44.Schreurs BW, Busch VJ, Welten ML, Verdonschot N, Slooff TJ, Gardeniers JW. Acetabular reconstruction with impaction bone-grafting and a cemented cup in patients younger than fifty years old. J Bone Joint Surg Am. 2004;86:2385–2392. doi: 10.2106/00004623-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Sedel L, Kerboull L, Christel P. Alumina on alumina hip replacement results and survivorship in young patients. J Bone Joint Surg Br. 1990;72:658–663. doi: 10.1302/0301-620X.72B4.2380223. [DOI] [PubMed] [Google Scholar]
- 46.Sedel L, Nizard RS, Kerboull L, Witwoet J. Alumina-alumina hip replacement in patients younger than 50 years old. Clin Orthop Relat Res. 1994;298:175–183. [PubMed] [Google Scholar]
- 47.Singh S, Trikha SP. Hydroxyapatite ceramic coated femoral stems in young patients: a prospective ten-year study. J Bone Joint Surg Br. 2004;86:1118–1123. doi: 10.1302/0301-620X.86B8.14928. [DOI] [PubMed] [Google Scholar]
- 48.Spangehl MJ, Berry DJ, Trousdale RT, Cabanela ME. Uncemented acetabular components with bulk femoral head autograft for acetabular reconstruction in developmental dysplasia of the hip: results at five to twelve years. J Bone Joint Surg Am. 2001;83:1484–1489. doi: 10.2106/00004623-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Williams WW, McCullough CJ. Results of cemented total hip replacement in juvenile chronic arthritis: a radiological review. J Bone Joint Surg Br. 1993;75:872–874. doi: 10.1302/0301-620X.75B6.8245074. [DOI] [PubMed] [Google Scholar]
- 50.Wroblewski BM, Siney PD, Fleming PA. Charnley low-frictional torque arthroplasty in patients under the age of 51 years: follow-up to 33 years. J Bone Joint Surg Br. 2002;84:540–543. doi: 10.1302/0301-620X.84B4.10293. [DOI] [PubMed] [Google Scholar]