Abstract
In the 1980s, zirconia was introduced for THA with the expectation of lower polyethylene wear and better clinical results. However, several studies have reported poor survivorship of zirconia-polyethylene THA. We performed a systematic review and meta-analysis of zirconia-polyethylene THA to confirm or refute the theoretical advantages of this combination. Of 163 studies identified by a comprehensive search, seven met our selection criteria. These involved 769 hips of 586 patients with a mean age of 56.8 years and a minimum followup of 60 months (mean, 89.2 months; range, 60–155 months). The consolidated revision rate of zirconia-polyethylene THA at 89.2 months was higher than that of nonzirconia-polyethylene THA by 5% (risk difference, 0.05; 95% confidence interval, 0.02–0.08). Subgroup meta-analysis suggested THAs with zirconia heads from Ceraver had more revision surgery than nonzirconia heads (risk difference, 0.08; 95% confidence interval, 0.03–0.14), whereas zirconia heads from DePuy did not (risk difference, 0.02; 95% confidence interval, −0.01–0.06). The meta-analysis for annual linear polyethylene wear (which did not involve zirconia heads from Ceraver because of insufficient descriptions) showed no difference between zirconia and control groups. Collectively, THAs with high-quality zirconia heads appear to have prosthesis survivorship and polyethylene wear equivalent to those of THAs with traditional materials, but differing quality among zirconia heads could lead to poor survivorship of prostheses.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Ultrahigh-molecular-weight polyethylene (UHMWPE) acetabular components provide one of the most promising bearing surfaces in THA [18], and almost one million UHMWPE components are used worldwide annually [18]. Although better implant design and surgical techniques have improved the clinical results of THA, polyethylene wear debris remains a major cause of periprosthetic osteolysis and aseptic loosening, leading to revision surgery. UHMWPE acetabulum and metal heads such as stainless steel or cobalt-chromium (Co-Cr) are well-established combinations for the bearing surface used in THA.
Alumina ceramic heads were introduced in the early 1970s and had low polyethylene wear and revision rates [20]. However, they are brittle and can fracture, with an incidence ranging from 0.02% to 0.14% [3, 9, 13, 25]. Tougher and stronger zirconia ceramics were introduced clinically in the early 1980s. These produced lower polyethylene wear in vitro, equaling that of alumina ceramics [4, 17, 26]. Despite such promising data, one report suggests poor survivorship of zirconia-polyethylene THA, with 14% of aseptic loosening at a mean followup of 5.8 years [1], and another reported intensive phase transformation in the crystal structure of retrieved zirconia head ranging from 20% to 30% [10], which can lead to increased roughness of the zirconia head [26], increased polyethylene wear [3], and poor survivorship of the prosthesis [3].
We conducted a meta-analysis to (1) compare the survivorship of zirconia-polyethylene THA with that of nonzirconia-polyethylene THA, (2) ascertain interstudy heterogeneity, (3) determine whether manufacturers or fixation method influenced survivorship, and (4) determine whether there was any difference in mean annual polyethylene wear between zirconia and nonzirconia groups.
Materials and Methods
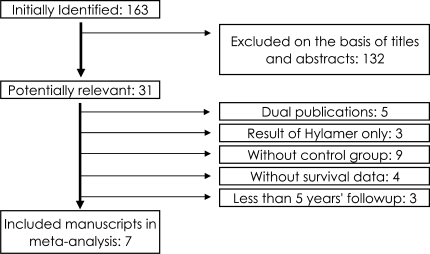
We searched for reports of clinical trials that compared THA using zirconia heads combined with UHMWPE with those using other head materials. The studies needed to have followups more than 5 years regardless of the size of the femoral head used or whether cemented or cementless arthroplasty was used. The study designs comprised randomized controlled trials (RCTs) and nonrandomized trials (non-RCTs), including cohort studies and historical cohort studies. We excluded uncontrolled case series (ie, Level IV) in the meta-analysis, because it is difficult to make definitive and reproducible criteria for nonzirconia studies that criteria covers the duration of followup, background of patients, fixation method and type of implants, and year of surgery. We initially searched PubMed (1966 to July 2007), EMBASE (1974 to July 2007), and the Cochrane Central Register of Controlled Trials (Issue 4, July 2007) with the Boolean operators “zirconia AND (head OR arthroplasty OR replacement)” without MEDLINE field tags. The Japan Centra Revuo Medicina also was searched for such articles written in Japanese (1983 to July 2007). Of 163 articles identified by our initial search, one article was in French and three in German, both with English abstracts; 132 were in English; and 27 were in Japanese with Japanese abstracts. We excluded irrelevant studies if the title and abstract indicated obviously irrelevant topics, such as dental materials, knee arthroplasty, and basic research including animal experiments, biomaterials, and simulators. We excluded dual publication, uncontrolled studies, studies without the number of revised cases, clinical studies that covered only enhanced UHMWPE (Hylamer™), which produced disastrous clinical results compared with UHMWPE [24], or if they only discussed ceramic-on-ceramic THA (Fig. 1).
Fig. 1.
A flowchart illustrates how we selected the seven studies included in our meta-analysis according to our inclusion and exclusion criteria.
One reviewer (HY) extracted the age, gender, and diagnosis of patients; duration of followup; fixation method and type of implants; number of revised and unrevised cases; and the mean, standard deviation, and measurement method of annual linear polyethylene wear in groups receiving THA with either zirconia or nonzirconia heads. Another reviewer (HI) checked the accuracy of data and disagreements were resolved by discussion. Cases that were revised because of infection or fracture were removed. Revision surgeries attributable to polyethylene failure were not described in the included studies.
Seven studies (four non-RCTs and three RCTs) published in English met our inclusion criteria providing clinical results, including survivorship of zirconia-polyethylene and nonzirconia-polyethylene THA with more than 5 years’ followup. These seven studies involved 769 hips of 586 patients with a mean age of 56.8 years and a minimum followup of 60 months (mean, 89.2 months; range, 60–155 months). Of the THAs, 57.0% were in women, 53.3% were for osteoarthritis, 33.3% for aseptic necrosis of the femoral head, and 6.4% for rheumatoid arthritis [1, 11, 14–16, 21, 30]. Control materials of femoral heads consisted of stainless steel, Co-Cr, and alumina ceramics.
Two reviewers (HY, HI) independently used a checklist of 11 items or 40 items to evaluate the internal validity or the generalizability of included studies [22, 29]. Disagreements were resolved by discussion. The mean scores for the four non-RCTs and three RCTs were 4.5 (range, 4–5) and 6.3 (range, 6–7), respectively, for internal validity and 19 (range, 18–20) and 21.3 (range, 21–22), respectively, for generalizability.
Two of the four non-RCT studies used historical cohorts [1, 21], and one of these cited historical results of alumina head THA in the same institute published in another study [20] (Table 1). The other two non-RCT studies were cohort studies [11, 14]. Two studies were RCTs [15, 30]. In one trial, 50 patients received bilateral THAs with a zirconia head in one hip and a Co-Cr head in the other; 48 patients had a unilateral THA with a Co-Cr head [16]. Prosthesis survival data from the 50 patients with bilateral THAs were treated as a RCT, and the linear wear rate of polyethylene from all 98 patients was treated as a non-RCT. All seven studies provided the number of cases needing revision after zirconia and nonzirconia THA. Acetabular and femoral components were fixed by cement in five studies [1, 11, 14, 21, 30], whereas they were fixed by a cementless method in one study [16]. In one study, all the acetabular components were cementless, but the femoral components were cemented or cementless [15]. The zirconia heads came from four manufacturers: Ceraver (Roissy, France), Nippon Tokushu Tougyou (Nagoya, Japan), JMM (Kobe, Japan), and DePuy (Leeds, United Kingdom). Three studies were conducted in Europe and four in Asia. The studies from Europe consisted of two studies for Ceraver and one for DePuy, and the studies from Asia consisted of one for JMM, one for Toyo Tokushu Tougyou, and two for DePuy. All polyethylenes used in the included studies were traditional UHMWPE (Table 2). Although the details of UHMWPE in one study were unavailable [1], the material and sterilization of UHMWPE in the zirconia and nonzirconia groups in the remaining studies were the same. UHMWPE was provided by Ceraver, Mizuho (Tokyo, Japan), JMM, or DePuy. The annual linear polyethylene wear was measured directly on films in two studies, on digitized radiographs in one study, and with computer aid in four studies (Table 2). Two studies using zirconia from Ceraver did not provide the standard deviation of annual linear polyethylene wear [1, 11]. The annual linear polyethylene wear rates of the remaining five studies were combined [14–16, 21, 30].
Table 1.
Summary of analyzed studies
| Study | Control materials | Head diameter (mm) | Fixation | Design | Number of zirconia | Number of control | Manufacturer of zirconia |
|---|---|---|---|---|---|---|---|
| Allain et al. [1] (1999) | Alumina | 28, 32 | Cement | HC | 78 | 117 | Ceraver |
| Hernigou and Bahrami [11] (2003) | Alumina, stainless steel | 28, 32 | Cement | Cohort | 40 | 96 | Ceraver |
| Inoue et al. [14] (2006) | Co-Cr | 22 | Cement | Cohort | 13 | 13 | Nippon Tokushu Tougyou |
| Liang et al. [21] (2007) | Alumina | 22 | Cement | HC | 58 | 46 | JMM |
| Kim [15] (2005) | Co-Cr | 28 | Cement, cementless | RCT | 52 | 52 | DePuy |
| von Schewelov et al. [30] (2005) | Stainless steel | 22 | Cement | RCT | 28 | 28 | DePuy |
| Kim et al. [16] (2003) | Co-Cr | 22 | Cementless | * | 50 | 98 | DePuy |
* Survival data from 50 patients with bilateral THAs were treated as a RCT and the linear wear rate from 98 patients including bilateral and unilateral THAs was treated as a non-RCT; HC = studies with historical cohort; RCT = randomized controlled trial.
Table 2.
Details of polyethylene
| Study | Material | γ irradiation | Manufacturer | Measurement of wear |
|---|---|---|---|---|
| Allain et al. [1] (1999) | UHMWPE | No information | Ceraver | Film |
| Hernigou and Bahrami [11] (2003) | UHMWPE GUR415 | γ in air | Ceraver | Digitized radiograph |
| Inoue et al. [14] (2006) | UHMWPE with MW > 5500 kDa* | No γ irradiation | Mizuho | Computer aid |
| Liang et al. [21] (2007) | UHMWPE GUR402 | 2.5 Mrad γ in air | JMM | Computer aid |
| Kim [15] (2005) | UHMWPE ram-extruded GUR1050 | 2.4–4 Mrad γ in vacuum | DePuy | Computer aid |
| von Schewelov et al. [30] (2005) | UHMWPE GUR415 | γ in air | DePuy | Film |
| Kim et al. [16] (2003) | UHMWPE ram-extruded GUR1050* | 2.4–4 Mrad γ in air* | DePuy | Computer aid |
* Information obtained directly from authors; UHMWPE = ultrahigh-molecular-weight polyethylene; MW = molecular weight.
For the analysis of the risk of revision surgery, we used the fixed-effects model weighted by the Mantel-Haenszel method following a test of heterogeneity [23]. The quantity I2 was used for assessing heterogeneity between trials in meta-analysis. A value of I2 greater than 50% indicates substantial heterogeneity [12]. If the hypothesis of heterogeneity was accepted, we used a random-effects model, the DerSimonian-Laird method [5]. In five of seven studies for this meta-analysis, there were no revised cases in the control group [14–16, 21, 30]. No events in an outcome were considered a zero cell in the 2 × 2 table. Zero cells create problems in calculation of odds or risk ratio caused by the division by zero. Although this problem is commonly dealt with by adding 0.5 to each cell of the 2 × 2 table, this addition causes inaccuracy of odds and risk ratios if there is a high frequency of zero cells [7]. Therefore, we estimated the risk difference of revision surgery with 95% confidence intervals (CIs) between zirconia and control groups instead of odds or risk ratio. The risk difference of revision surgery indicates the difference of the frequency of revision surgery during followup between groups. Furthermore, we analyzed subgroups consisting of the manufacturers of zirconia or the fixation methods of implants. To determine the existence of heterogeneous studies, an informal graphic exploration was performed using a L’Abbe plot, and sensitivity analysis was performed [19, 28]. The combined mean difference of annual linear polyethylene wear between zirconia-polyethylene and nonzirconia-polyethylene THAs was calculated according to the empirical Bayes method [27]. A random-effects model was used if I2 was greater than 50%. If not, a fixed-effects model was used.
Studies with significant differences or those with generally expected results tend to be submitted and accepted, leading to publication bias in meta-analyses. The funnel plot, Begg’s test, and Egger’s test were used to evaluate the potential for publication bias (ie, for better or worse survivorship) associated with the survivorship of THA [2, 8]. A p value of publication bias less than 0.10 was considered significant. The funnel plot was not symmetric and both tests revealed publication bias (Begg’s test, p = 0.004; Egger’s test, p = 0.000), indicating there might be unpublished studies with insignificant results or unexpected results.
We used the Stata® software package (Version 9.2; StataCorp LP, College Station, TX) for all analyses.
Results
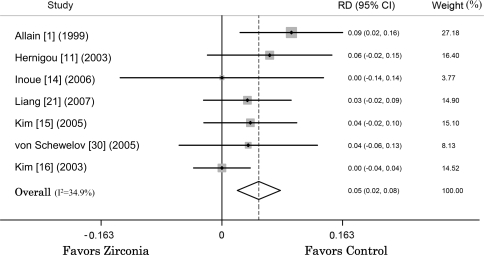
Zirconia-polyethylene THAs required more revision surgery at a mean followup of 89.2 months (range, 60–155 months) than nonzirconia-polyethylene THAs (Fig. 2). The estimated pooled risk difference of revision surgery from seven studies in the fixed-effects model was 0.05 (95% CI, 0.02–0.08; p = 0.001; homogeneity I2 = 34.9%). The pooled risk difference estimated from the three RCT studies suggests similar risk of revision at a mean followup of 113.2 months with zirconia heads and nonzirconia heads (risk difference, 0.02; 95% CI, −0.01–0.06; p = 0.221; homogeneity I2 = 0.0%).
Fig. 2.
When seven studies were pooled in a fixed-effects model, the risk difference (RD) of revision surgery between zirconia-polyethylene and nonzirconia-polyethylene THAs was 0.05 (95% CI, 0.02–0.08).
We identified the study by Allain et al. [1] as the source of heterogeneity. That study used so-called first-generation zirconia ceramics, which are believed to provide inferior clinical results [3]. The combined revision rate of zirconia-polyethylene THA at a mean followup of 108.1 months estimated from the remaining six studies also was greater than that of nonzirconia-polyethylene THA by 3.4% (risk difference, 0.034; 95% CI, 0.003–0.06; p = 0.03) and interstudy heterogeneity of the remaining six studies was similar (homogeneity, p = 0.03).
THAs with zirconia heads made by Ceraver led to more revision surgery than control materials (risk difference, 0.08; 95% CI, 0.03–0.14; p = 0.02; homogeneity I2 = 0.0%), whereas THA with zirconia heads from DePuy did not (risk difference, 0.02; 95% CI, −0.01–0.06; p = 0.212; homogeneity I2 = 0.0%). These results suggest there are differences in clinical results between manufacturers of zirconia probably because of the quality of the zirconia heads.
To assess the influence of fixation methods of implants on the survivorship of THA with zirconia, we excluded each study observing only cementless THAs [16] or observing cemented and cementless THAs [15]. Cemented THAs with zirconia heads had more revision surgery than cemented THAs with control materials (risk difference, 0.06; 95% CI, 0.02–0.10; p = 0.001; homogeneity I2 = 2.8%). This result implies fixation methods of implants have little influence on the difference of survivorship between zirconia and nonzirconia groups.
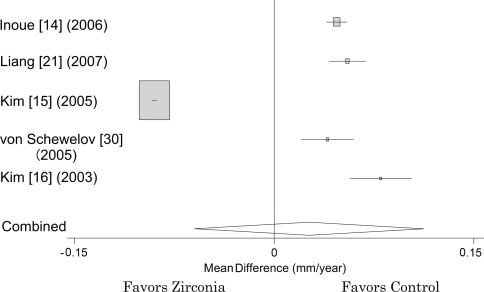
Five studies providing the means and standard deviations of annual linear polyethylene wear did not include studies using zirconia of Ceraver (Table 2). There was no difference in the pooled annual polyethylene wear between zirconia head groups and control groups from these five studies. The pooled difference of annual polyethylene wear in the random-effects model was 0.026 mm per year (95% CI, −0.060–0.113; p = 0.552; homogeneity I2 = 99%) (Fig. 3).
Fig. 3.
When five studies providing the means and standard deviations of polyethylene wear rate were pooled in a random-effects model, the difference in annual linear polyethylene wear between zirconia-polyethylene and nonzirconia-polyethylene THAs was −0.023 mm per year (95% CI, −0.120–0.074).
Discussion
Zirconia was introduced for THA in the 1980s with the expectation of lower polyethylene wear and better clinical results. However, several studies have reported poor survivorship of zirconia-polyethylene THAs [1, 10]. In this study, we conducted a systematic review and a meta-analysis to assess the survivorship and polyethylene wear of zirconia-polyethylene THA compared with nonzirconia-polyethylene THA and the influence of zirconia manufacturers or implant fixation method on the survivorship.
Some study limitations of this meta-analysis should be considered. First, cross-linked polyethylene was available from 1998 and polyethylene for THA had almost shifted from traditional UHMWPE to cross-linked UHWMWPE because of low polyethylene wear [6]. For our meta-analysis, only clinical results of THA with traditional UHMWPE were available. Although full translation of this meta-analysis to THA with cross-linked UHMWPE is difficult, it is reasonable to anticipate head materials causing high wear of traditional UHMWPE and poor survivorship of THA also can cause high wear of cross-linked UHMWPE and poor clinical results compared with those of other materials. Therefore, we need to clarify the clinical outcomes of THA with a zirconia head to determine whether future use of zirconia heads is justified. Second, bias in material or sterilization methods of traditional UHMWPE or geographic area where studies were conducted might exist. Several kinds of materials or sterilization methods of traditional UHMWPE were used in the reported studies. Two studies using zirconia of Ceraver were conducted in France, whereas of the three studies of DePuy, one was conducted in Europe and two in Asia. However, the main difference between zirconia and nonzirconia groups in each study was head materials. Therefore, we considered it justified to consolidate the difference of risk of revision or linear polyethylene wear in the zirconia and nonzirconia groups among studies. Third, our analysis indicated the possibility of publication bias in the clinical results of zirconia-polyethylene THA. Thus, there might have been studies that remained unpublished for various reasons, which could not be included in our meta-analysis. If one considers a possible publication bias of this analysis toward worse clinical results for zirconia-polyethylene THA, unpublished studies, in theory, might have indicated better clinical outcomes. However, such studies would have been published actively, because zirconia was expected to provide clinical results as good as those seen in vitro. Another possibility is that revision rates of zirconia-polyethylene THAs were disastrous and surgeons simply stopped using a device without studying the differences. However, disastrous clinical results, even if in an unexpected direction, tend to be submitted and accepted, as in the cases of early zirconia heads [1, 10] or Hylamer™ [24]. Therefore, unpublished studies that would have shifted the meta-analysis toward better clinical results for zirconia are unlikely. Meta-analyses can use only peer-reviewed published data. The International Committee of Medical Journal Editors proposed a system of clinical trial registration in 2004, and many journals support this system. In the future, more RCTs examining the clinical results of zirconia heads are expected to be registered and published regardless of their results, and further meta-analyses involving such trials are expected.
Although the most likely cause of the poor survival of THAs with zirconia heads is polyethylene wear, we found no difference between zirconia and control groups. This might be attributed to the following factors. First, only five of seven studies provided necessary data for the consolidation of annual linear polyethylene wear [14–16, 21, 30]. Second, the data for zirconia from Ceraver, which considerably increased revision surgery of THA, was not involved in the meta-analysis for polyethylene wear [1, 11]. Hernigou and Bahrami [11] reported mean annual polyethylene wear rates against zirconia from Ceraver and stainless steel of 0.4 mm per year and 0.13 mm per year, respectively. Allain et al. [1] reported a mean annual linear polyethylene wear rate against zirconia from Ceraver in the revised cases of 0.5 mm per year, whereas total mean polyethylene wear rates against zirconia and alumina were 0.09 mm per year and 0.1 mm per year, respectively. Their results indicate heterogeneous quality among zirconia heads. Third, the polyethylene wear rate against zirconia reported by Kim [15] in 2005 was low with a narrow CI. This might cancel the other four studies, which favor the control group. Collectively, THAs with high-quality zirconia heads may have prosthesis survivorship and polyethylene wear equivalent to those of THAs with traditional materials, but heterogeneous quality among zirconia heads leads to poor survivorship of the prosthesis because of polyethylene wear.
Transformation from the tetragonal to a monoclinic phase of yttrium-stabilized tetragonal zirconia polycrystals leads to volumetric expansion of approximately 3% to 4% [26], increased roughness of the zirconia head [26], and increased polyethylene wear [3]. Although zirconia manufacturers predicted there would be less than 2% monoclinic sites on zirconia femoral heads during the first 10 years in patients, one retrieval study suggests transformation of as much as 80% by 9 years [3]. That the area of monoclinic transformation corresponds to the contact region against polyethylene indicates tribologic conditions also trigger phase transformation. Hydrothermal conditions such as autoclaving for sterilization also are involved in phase transformation. The manufacturing process, manufacturers, and year of manufacture can be crucial for stability of the tetragonal phase in zirconia. The first generation of zirconia from some manufacturers has undergone substantial transformation of its crystal structure to a monoclinic phase and these implants have inferior survivorship [1, 10]. Our subgroup meta-analysis supports the importance of the manufacturing process and manufacturers. Studies using zirconia from DePuy showed no difference in risk difference between the zirconia and control heads. This implies zirconia heads from specific manufacturers could give clinical results at least equivalent to those of traditional head materials.
Should we now stop using zirconia heads? We believe urgent cessation of zirconia-polyethylene THA would not be justified, because the zirconia supplied by some manufacturers seems to have clinical results equivalent to those of traditional materials. Obviously, alumina ceramics had clinically lower polyethylene wear than conventional metal heads and better ceramics with toughness and low polyethylene wear desired. Yttrium-stabilized tetragonal zirconia polycrystals with 0.25% (w/w) Al2O3 (Al-doped) has been available since 2002. Al-doped zirconia ceramics are approximately fivefold more resistant to phase transformation by hydrothermal conditions than non-Al-doped zirconia and are expected to have lower polyethylene wear and provide better clinical results in THA [3, 21]. Additional clinical confirmation of trials for zirconia ceramics, including such improved materials in combination with cross-linked UHMWPE, by meta-analyses of published and registered studies is required.
Acknowledgments
We thank Keiichi Kawanabe for discussion and Koji Asaumi and Young-Hoo Kim for providing information about polyethylene.
Footnotes
One or more of the authors (HY, HI, TN, TN) have received funding from Health and Labour Sciences Research Grants.
References
- 1.Allain J, Le Mouel S, Goutallier D, Voisin MC. Poor eight-year survival of cemented zirconia-polyethylene total hip replacements. J Bone Joint Surg Br. 1999;81:835–842. doi: 10.1302/0301-620X.81B5.9454. [DOI] [PubMed] [Google Scholar]
- 2.Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 3.Brown S, Green D, Pezzotti G, Donaldson T, Clarke I. Possible triggers for phase transformation in zirconia hip balls. J Biomed Mater Res B Appl Biomater. 2008;85:444–452. doi: 10.1002/jbm.b.30964. [DOI] [PubMed] [Google Scholar]
- 4.Cales B. Zirconia as a sliding material: histologic, laboratory, and clinical data. Clin Orthop Relat Res. 2000;379:94–112. doi: 10.1097/00003086-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 5.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 6.Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: two randomized studies using radiostereometric analysis. Acta Orthop. 2007;78:746–754. doi: 10.1080/17453670710014518. [DOI] [PubMed] [Google Scholar]
- 7.Egger M, Altman DG, Smith GD. Systematic Reviews in Health Care: Meta-Analysis in Context. 2. London, UK: BMJ Books; 2001. [Google Scholar]
- 8.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritsch E, Gleitz M. Ceramic femoral head fractures in total hip arthroplasty. Clin Orthop Relat Res. 1996;328:129–136. doi: 10.1097/00003086-199607000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi K, Sugano N, Nishii T, Miki H, Oka K, Yoshikawa H. Phase transformation of a zirconia ceramic head after total hip arthroplasty. J Bone Joint Surg Br. 2001;83:996–1000. doi: 10.1302/0301-620X.83B7.12122. [DOI] [PubMed] [Google Scholar]
- 11.Hernigou P, Bahrami T. Zirconia and alumina ceramics in comparison with stainless-steel heads: polyethylene wear after a minimum ten-year follow-up. J Bone Joint Surg Br. 2003;85:504–509. doi: 10.1302/0301-620X.85B4.13397. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higuchi F, Shiba N, Inoue A, Wakebe I. Fracture of an alumina ceramic head in total hip arthroplasty. J Arthroplasty. 1995;10:851–854. doi: 10.1016/S0883-5403(05)80086-5. [DOI] [PubMed] [Google Scholar]
- 14.Inoue A, Asaumi K, Endo H, Fujiwara K, Mitani S, Ozaki T. Assessment of head wear more than ten years after total hip arthroplasty: 22-mm zirconia vs. metal heads. Acta Med Okayama. 2006;60:311–318. doi: 10.18926/AMO/30721. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH. Comparison of polyethylene wear associated with cobalt-chromium and zirconia heads after total hip replacement: a prospective, randomized study. J Bone Joint Surg Am. 2005;87:1769–1776. doi: 10.2106/JBJS.D.02572. [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Oh SH, Kim JS, Koo KH. Contemporary total hip arthroplasty with and without cement in patients with osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:675–681. doi: 10.1302/0301-620X.85B2.13289. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P, Oka M, Ikeuchi K, Shimizu K, Yamamuro T, Okumura H, Kotoura Y. Low wear rate of UHMWPE against zirconia ceramic (Y-PSZ) in comparison to alumina ceramic and SUS 316L alloy. J Biomed Mater Res. 1991;25:813–828. doi: 10.1002/jbm.820250703. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz S, Muratoglu O, Evans M, Edidin A. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials. 1999;20:1659–1688. doi: 10.1016/S0142-9612(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 19.L’Abbé K, Detsky A, O’Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–233. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- 20.Le Mouel S, Allain J, Goutallier D. 10-year actuarial analysis of a cohort of 156 total hip prostheses of a cemented polished aluminum/polyethylene alloy] [in French. Rev Chir Orthop Reparatrice Appar Mot. 1998;84:338–345. [PubMed] [Google Scholar]
- 21.Liang B, Kawanabe K, Ise K, Iida H, Nakamura T. Polyethylene wear against alumina and zirconia heads in cemented total hip arthroplasty. J Arthroplasty. 2007;22:251–257. doi: 10.1016/j.arth.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Malmivaara A, Koes BW, Bouter LM, Tulder MW. Applicability and clinical relevance of results in randomized controlled trials: the Cochrane review on exercise therapy for low back pain as an example. Spine. 2006;31:1405–1409. doi: 10.1097/01.brs.0000219868.30427.66. [DOI] [PubMed] [Google Scholar]
- 23.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 24.Norton M, Yarlagadda R, Anderson G. Catastrophic failure of the Elite Plus total hip replacement, with a Hylamer acetabulum and zirconia ceramic femoral head. J Bone Joint Surg Br. 2002;84:631–635. doi: 10.1302/0301-620X.84B5.12679. [DOI] [PubMed] [Google Scholar]
- 25.Peiró A, Pardo J, Navarrete R, Rodriguez-Alonso L, Martos F. Fracture of the ceramic head in total hip arthroplasty: report of two cases. J Arthroplasty. 1991;6:371–374. doi: 10.1016/S0883-5403(06)80190-7. [DOI] [PubMed] [Google Scholar]
- 26.Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1–25. doi: 10.1016/S0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 27.Robbins H. An Empirical Bayes Approach to Statistics, Proceedings of the Third Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, CA: University of California Press; 1956. [Google Scholar]
- 28.Sharp S, Thompson S, Altman D. The relation between treatment benefit and underlying risk in meta-analysis. BMJ. 1996;313:735–738. doi: 10.1136/bmj.313.7059.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine. 1997;22:2323–2330. doi: 10.1097/00007632-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 30.Schewelov T, Sanzen L, Onsten I, Carlsson A, Besjakov J. Total hip replacement with a zirconium oxide ceramic femoral head: a randomised roentgen stereophotogrammetric study. J Bone Joint Surg Br. 2005;87:1631–1635. doi: 10.1302/0301-620X.87B12.16873. [DOI] [PubMed] [Google Scholar]