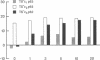Abstract
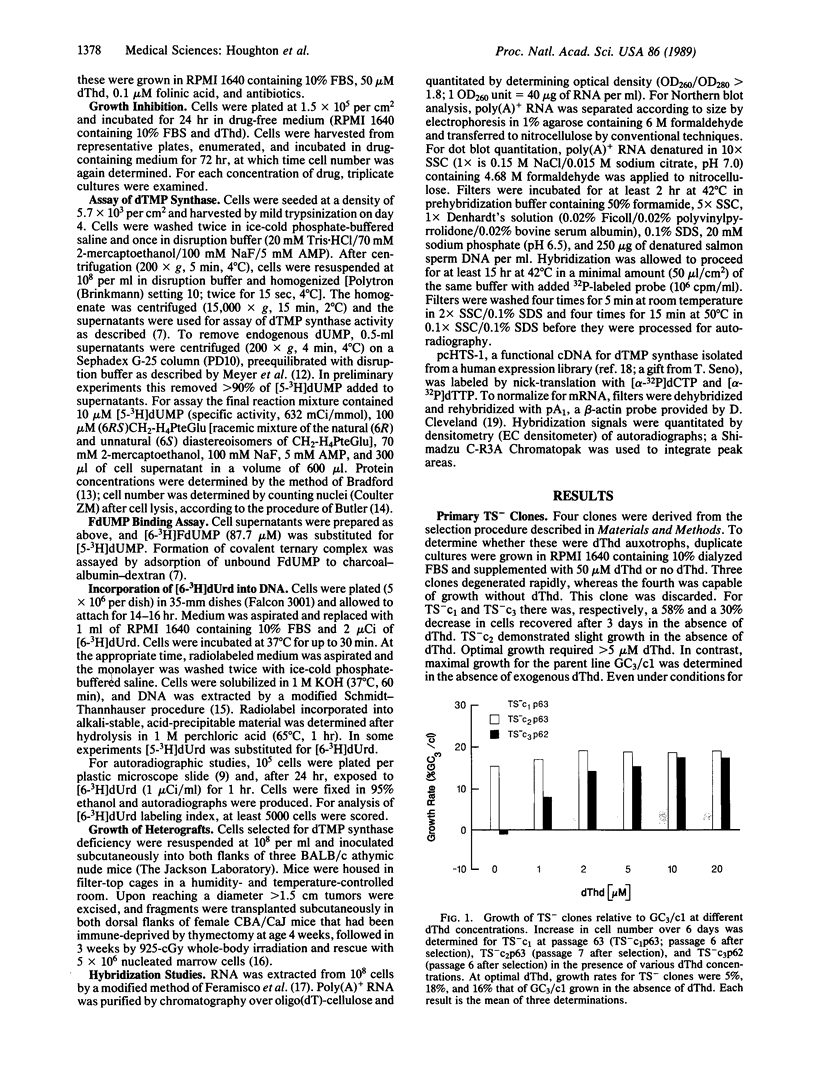
GC3/c1 human colon adenocarcinoma cells were treated with the mutagen ethyl methanesulfonate, and three clones deficient in thymidylate synthase (5,10-methylenetetrahydrofolate:dUMP C-methyltransferase, EC 2.1.1. 45) activity were selected and characterized. Growth in medium deficient in thymidine caused cell death in two clones (TS- c1 and TS- c3), whereas one clone (TS- c2) showed limited growth. Growth correlated with thymidine synthase activity and 5-fluoro-2'-deoxyuridine 5'-monophosphate-binding capacity and with incorporation of 2'-deoxy[6-3H]uridine into DNA. In the presence of optimal thymidine, growth rates were only 5-18% that of the parental clone (GC3/c1), which grew equally well in thymidine-deficient or -replete medium. Analysis of poly(A)+ RNA showed normal levels of a 1.6-kilobase transcript in TS- c1 and TS- c2 but decreased levels (approximately 6% control) in TS- c3. Clone TS- c3 was 32-, 750-, and greater than 100,000-fold more resistant than the parental clone to 5-fluorouracil, 5-fluoro-2'-deoxyuridine, and methotrexate, respectively. When inoculated into athymic nude mice, each TS- clone produced tumors, demonstrating continued ability to proliferate in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akazawa S., Kumai R., Yoshida K., Ayusawa D., Shimizu K., Seno T. The cytotoxicity of 5-fluorouracil is due to its incorporation into RNA not its inhibition of thymidylate synthase as evidenced by the use of a mouse cell mutant deficient in thymidylate synthase. Jpn J Cancer Res. 1986 Jul;77(7):620–624. [PubMed] [Google Scholar]
- Allegra C. J., Chabner B. A., Drake J. C., Lutz R., Rodbard D., Jolivet J. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. J Biol Chem. 1985 Aug 15;260(17):9720–9726. [PubMed] [Google Scholar]
- Allegra C. J., Drake J. C., Jolivet J., Chabner B. A. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4881–4885. doi: 10.1073/pnas.82.15.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Koyama H., Iwata K., Seno T. Single-step selection of mouse FM3A cell mutants defective in thymidylate synthetase. Somatic Cell Genet. 1980 Mar;6(2):261–270. doi: 10.1007/BF01538800. [DOI] [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Kaneda S., Takeishi K., Seno T. Cell-cycle-directed regulation of thymidylate synthase messenger RNA in human diploid fibroblasts stimulated to proliferate. J Mol Biol. 1986 Aug 20;190(4):559–567. doi: 10.1016/0022-2836(86)90241-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butler W. B. Preparing nuclei from cells in monolayer cultures suitable for counting and for following synchronized cells through the cell cycle. Anal Biochem. 1984 Aug 15;141(1):70–73. doi: 10.1016/0003-2697(84)90426-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Danenberg P. V. Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochim Biophys Acta. 1977 Dec 23;473(2):73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Fernandes D. J., Bertino J. R., Hynes J. B. Biochemical and antitumor effects of 5,8-dideazaisopteroylglutamate, a unique quinazoline inhibitor of thymidylate synthase. Cancer Res. 1983 Mar;43(3):1117–1123. [PubMed] [Google Scholar]
- Hori T., Ayusawa D., Shimizu K., Koyama H., Seno T. Chromosome breakage induced by thymidylate stress in thymidylate synthase-negative mutants of mouse FM3A cells. Cancer Res. 1984 Feb;44(2):703–709. [PubMed] [Google Scholar]
- Houghton J. A., Maroda S. J., Jr, Phillips J. O., Houghton P. J. Biochemical determinants of responsiveness to 5-fluorouracil and its derivatives in xenografts of human colorectal adenocarcinomas in mice. Cancer Res. 1981 Jan;41(1):144–149. [PubMed] [Google Scholar]
- Houghton J. A., Taylor D. M. Growth characteristics of human colorectal tumours during serial passage in immune-deprived mice. Br J Cancer. 1978 Feb;37(2):213–223. doi: 10.1038/bjc.1978.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J. A., Weiss K. D., Williams L. G., Torrance P. M., Houghton P. J. Relationship between 5-fluoro-2'-deoxyuridylate, 2'-deoxyuridylate, and thymidylate synthase activity subsequent to 5-fluorouracil administration, in xenografts of human colon adenocarcinomas. Biochem Pharmacol. 1986 Apr 15;35(8):1351–1358. doi: 10.1016/0006-2952(86)90281-9. [DOI] [PubMed] [Google Scholar]
- Houghton P. J., Houghton J. A., Germain G., Torrance P. M. Development and characterization of a human colon adenocarcinoma xenograft deficient in thymidine salvage. Cancer Res. 1987 Apr 15;47(8):2117–2122. [PubMed] [Google Scholar]
- Hughes W. L., Christine M., Stollar D. A radioimmunoassay for measurement of serum thymidine. Anal Biochem. 1973 Oct;55(2):468–478. doi: 10.1016/0003-2697(73)90137-1. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Calvert A. H., Jackman A. L., Brown S. J., Jones M., Harrap K. R. A potent antitumour quinazoline inhibitor of thymidylate synthetase: synthesis, biological properties and therapeutic results in mice. Eur J Cancer. 1981 Jan;17(1):11–19. doi: 10.1016/0014-2964(81)90206-1. [DOI] [PubMed] [Google Scholar]
- Kaneda S., Takeishi K., Ayusawa D., Shimizu K., Seno T., Altman S. Role in translation of a triple tandemly repeated sequence in the 5'-untranslated region of human thymidylate synthase mRNA. Nucleic Acids Res. 1987 Feb 11;15(3):1259–1270. doi: 10.1093/nar/15.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li I. C., Chu E. H. Mutants of Chinese hamster cells deficient in thymidylate synthetase. J Cell Physiol. 1984 Aug;120(2):109–116. doi: 10.1002/jcp.1041200202. [DOI] [PubMed] [Google Scholar]
- Meyer W. H., Houghton J. A., Lutz P. J., Houghton P. J. Hypoxanthine:guanine phosphoribosyltransferase activity in xenografts of human osteosarcoma. Cancer Res. 1986 Oct;46(10):4896–4899. [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974 Jan 29;13(3):471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- Semon J. H., Grindey G. B. Potentiation of the antitumor activity of methotrexate by concurrent infusion of thymidine. Cancer Res. 1978 Sep;38(9):2905–2911. [PubMed] [Google Scholar]
- Yoshioka A., Tanaka S., Hiraoka O., Koyama Y., Hirota Y., Ayusawa D., Seno T., Garrett C., Wataya Y. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J Biol Chem. 1987 Jun 15;262(17):8235–8241. [PubMed] [Google Scholar]