Abstract
Leptin, a 16-kDa cytokine produced mainly by the adipose tissue, is known to increase energy expenditure while at the same time lowering food intake by acting directly on the hypothalamus. ObRb, the leptin receptor mostly involved in intracellular signaling, is expressed in a wide range of tissues, thus allowing leptin to affect a much broader diversity of biological processes. High concentrations of leptin are encountered in patients with hyperleptinemia, a condition which very often accompanies obesity and which is a direct result of leptin resistance. In the present study, moderate and high concentrations of leptin (16 and 160 ng/ml) were mostly utilized in order to investigate the role of this cytokine in oxidative stress levels in human monocytes. Leptin was found to increase oxidative species production as measured with 2′,7′-dichlorodihydrofluorescein diacetate (general marker of oxidative species, but not O−.2) and dihydroethidium (marker of O−.2). Surprisingly, it also augmented superoxide dismutase activity. Inhibition of the Na+–H+ exchanger isoform 1 (NHE1) also inhibited leptin-induced superoxide anion production but at the same time amplified leptin-induced production of other oxidative species. Signaling proteins such as phosphoinositide 3 kinase and conventional isoforms of protein kinase C (α-, βi-, βii-), as well as NADPH oxidase, also participated in leptin signaling. Finally, leptin was found to increase glutathionylation levels of NHE1-bound heat shock protein 70 kDa (Hsp70) but not Hsp70 binding to NHE1.
Keywords: Atherosclerosis, Leptin, Monocytes, NHE1, Oxidative stress, Signaling
Introduction
Leptin, the product of the ob gene, is a 16-kDa cytokine produced mainly, but not exclusively, by the adipose tissue (Sweeney 2002). Soon after its identification in 1994 and as its role in body energy regulation became increasingly apparent, it was heralded as the “cure for obesity” (Zhang et al. 1994; Auwerx and Staels 1998). Hyperleptinemia very often accompanies obesity and can thus be viewed as the body’s attempt to reduce food intake while at the same time increasing energy expenditure (Considine et al. 1996). Following extensive studies, it is clear today that human obesity is too complex a condition to be combated with mere leptin level manipulation. However, it is also clear that leptin is not confined to just regulating body weight and energy expenditure but has a pleiotropic role, affecting such diverse processes as the reproductive system rhythm and the immune response (Ahima and Osei 2004).
Leptin mediates its action mainly through its receptor, Ob gene receptor b (ObRb). At least six leptin receptor isoforms have been characterized so far (ObRa–f) with ObRb being the only one with cytoplasmic domains capable of participating in signal transduction (Sweeney 2002). Binding of leptin to ObRb initiates the Janus kinases/signal transducers and activators of transcription pathway as is common with type II cytokine receptors, thus allowing the signal to reach a number of downstream protein targets. ObRb is expressed in smooth muscle cells and endothelial cells, as well as in most (if not all) types of blood cells, including the subject cells of our present study, monocytes (Peelman et al. 2004).
Monocytes are cells of the immune system that are the precursors of macrophages, cells capable of phagocytosing entities they encounter that are either foreign to the organism such as bacteria and other large enough particles or originate within the organism but need to be cleared, such as apoptotic cells (Gregory and Devitt 2004). As a consequence, phagocytosis is crucial to survival as it neutralizes potential threats to the life of the organism. However, phagocytosis of non-degradable materials leads to their cytoplasmic accumulation, which in time can seriously disrupt normal macrophage function. Oxidized low-density lipoproteins (oxLDLs) are such materials. Their intracellular accumulation can lead macrophages towards becoming foam cells, fundamental components of atheromatous plaques (Glass and Witztum 2001).
Monocytes, along with neutrophils, are cells capable of executing the respiratory burst, the rapid release of reactive oxygen species (ROS) aimed at eliminating bacteria or other infectious threats (Demaurex et al. 1996; Sanchez-Pozo et al. 2003). Low-density lipoproteins (LDLs) in the vicinity of the burst can be oxidized towards oxLDLs (Cathcart 2004). High concentrations of oxidative species cause damage to proteins and can pose a serious threat for cells. As a result, cells have anti-oxidant mechanisms such as binding of the protein heat shock protein 70 kDa (Hsp70) to sensitive proteins and high concentrations of the anti-oxidant tripeptide glutathione (Xu and Giffard 1997; Suzuki et al. 2005). However, apart from their crude oxidizing role, reactive oxygen species, as well as reactive nitrogen species (RNS), are increasingly being recognized as also having a role in intracellular signaling (Gutierrez et al. 2006).
In a recent study, we reported that leptin, at a concentration representative of high hyperleptinemic levels (160 ng/ml), amplified certain human monocyte atherosclerosis-related properties, such as monocyte adhesion to and migration through laminin 1 (a constituent element of the extracellular matrix), as well as oxLDL phagocytosis (Konstantinidis et al. 2009). It was observed that the Na+–H+ exchanger isoform 1 (NHE1) was implicated in the signaling pathways which regulated all the aforementioned atherosclerosis-related properties. This ubiquitously expressed isoform has a major housekeeping role through the regulation of intracellular pH (pHi) and cell volume via the exchange of extracellular Na+ with H+ in a 1:1 ratio (Putney et al. 2002). Additionally, through its cytoplasmic C-terminal domains, NHE1 is known to interact with the cytoskeleton as well as a variety of cytoplasmic proteins, through which it accepts signals initiated by molecules such as glucose, hormones, and others which affect its activation (Bourikas et al. 2003; Koliakos et al. 2004). Through these interactions, NHE1 is both itself regulated and also able to regulate cellular functions such as cell motility and cell survival (Malo and Fliegel 2006). A recent study concluded that Hsp70 had a crucial role in maintaining Na+–K+–ATPase cytoskeleton anchorage in rat renal epithelial cells during recovery after stress caused by a low-protein diet (Ruete et al. 2008). NHE1 is also anchored to the cytoskeleton and it is possible that this interaction is also protected by Hsp70 during stress (Koliakos et al. 2008).
The aim of the present study was to shed light on the effect of leptin on the quality and quantity of oxidant species production, as well as on intracellular anti-oxidant mechanisms in human monocytes. In addition, we attempted to study the position and role of NHE1 during this leptin effect.
Materials and methods
Materials
Recombinant human leptin was from R&D systems (Minneapolis, MN, USA). Ficoll-Paque Plus (1.077 g/ml), Percoll (1.130 g/ml), and the enhanced chemiluminescence (ECL) plus kit were from Amersham Biosciences (Sweden—now GE Healthcare, UK). 27′.7′-Dichlorodihydrofluorescein diacetate (H2DCFDA), dihydroethidium (DHE), anti-human CD14 R-phycoerythrin-conjugated antibody, 7-aminoactinomycin D (7-AAD), diphenyleneiodonium chloride (DPI), Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), nigericin, iodoacetic Na, methazolamide, 4,4’-diisothiocyanatostilbene-2,2’-disulfonic acid (DIDS), diethylenetriamine-penta-acetic acid (DTPA), superoxide dismutase (SOD) standards, and xanthine oxidase were from Sigma (St. Louis, MO, USA). Bis-(carboxyethyl)-5(6)-carboxy-fluorescein acetoxymethyl ester (BCECF-AM) and hypoxanthine were from AppliChem (Darmstadt, Hesse, Germany). Cariporide was from Sanofi-Aventis (Paris, France). Iscove’s Modified Dulbecco’s Medium (IMDM), fetal calf serum (FCS), penicillin/streptomycin, and l-glutamine were from Biochrom (Berlin, Germany). Cytochalasin D, wortmannin, and 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-1) were from Fluka (Switzerland). GF109203X and Gö6976 were from Alexis (Lausen, Switzerland). Protein A/G PLUS-Agarose, control immunoglobulin G (IgG), and primary (anti-NHE1, anti-Hsp70, anti-glutathione (GSH)) and horse radish peroxidase (HRP)-conjugated secondary (anti-rabbit, anti-goat, anti-mouse) antibodies were from Santa Cruz Biotechnology (CA, USA). The biotinylated protein ladder (markers) and anti-biotin secondary antibody were from Cell Signaling Technology (MA, USA). All other reagents were of analytical grades and were obtained from commercial sources.
Leptin and inhibitors
The three leptin concentrations used (1.6, 16, 160 ng/ml) for the dose–response tests represent normal, high normal or moderate hyperleptinemic, and high hyperleptinemic levels, respectively. The latter two can be observed in patients suffering from hyperleptinemia, a direct result of severe obesity and its associated leptin resistance (Zarkesh-Esfahani et al. 2001; Dong et al. 2006).
Cariporide (20 nM) inhibits NHE1. Wortmannin (50 nM) inhibits phosphoinositide 3 kinase (PI3K). GF109203X (10 μΜ) inhibits all protein kinase C (PKC) isoforms. Gö6976 (500 nM) inhibits alpha- and beta-PKC isoforms (PKCα, PKCβΙ, and PKCβΙΙ). DPI (10 μΜ) inhibits NADPH oxidase and nitric oxide synthase. l-NAME (100 μΜ) inhibits solely nitric oxide synthase.
Monocyte isolation
Monocytes were isolated from heparinized whole blood drawn from consenting individuals with normal body mass index, as described previously (Seager-Danciger et al. 2004). In brief, the drawn blood was diluted with phosphate-buffered saline (PBS) 1× (1 mM ethylenediaminetetraacetic acid (EDTA), pH 7.2) and underlayered using an 18-gauge spinal needle with Ficoll-Paque Plus (1.077 g/ml) in 50-ml falcon tubes. After centrifugation (400×g/20 min/RT/no brake), the peripheral blood mononuclear cell (PBMC) layer was collected and put in new clean 50-ml falcon tubes. There followed three washes with PBS 1× (1 mM EDTA, pH 7.2). The PBMCs were then diluted with complete IMDM and overlayered on 46% Percoll in 50-ml falcon tubes. After centrifugation (550×g/30 min/RT/no brake), the monocyte layer was collected, diluted with PBS 1× (1 mM EDTA, pH 7.2), and washed twice with PBS 1× before use in experiments. Monocyte purity in the end samples was measured on a BeckmanCoulter EPICS XL-MCL flow cytometer using anti-CD14-PE antibody and was found to be >85%. Flow cytometry viability measurements using 7-AAD showed <10% staining and thus >90% viability.
Intracellular pH determination
Changes in pHi were measured through modification of a previously described method (Incerpi et al. 1994). In brief, monocytes suspended in buffer containing (mM) 30 Tris–HCl, 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 5 glucose (pH 7.3) were loaded with 2 μg per 106 cells BCECF-AM (30 min/37 °C) in the dark. Once inside cells, BCECF-AM is readily cleaved towards the fluorescent form BCECF by intracellular esterases. Cells were then washed three times with the same buffer in order to remove the remaining fluorescent probe. When appropriate, cariporide was added and incubation took place (15 min/37 °C) in the dark. Along with the inhibitors, 1 mM iodoacetic Na (glycolysis inhibitor), 0.125 mM DIDS (HCO−3–Cl− exchanger inhibitor), and 0.4 mM methazolamide (carbonic anhydrase inhibitor) were added to all samples (including the control) in order to avoid any interference from other pHi-regulating systems. Fluorescence was measured immediately after incubation under continuous magnetic stirring at 20 °C in a 3-ml quartz cuvette in a Shimadzu fluorescence thermostatic spectrophotometer. Data were obtained as the ratio of the pH-sensitive excitation wavelength (495 nm) to the pH-insensitive excitation signal wavelength (440 nm) with the emission wavelength set at 530 nm. Routinely, at each experiment, calibration of fluorescence versus pH was conducted through the use of the polyether ionophore nigericin (13 μM) which couples K+ and H+ gradients across the plasma membrane, as previously described (Thomas et al. 1979). The calibration curves produced were roughly linear in the range of pH 6.5–7.5 (R2 = 0.81–1).
General oxidative species production measurement
General oxidative species production was assessed through the use of the fluorescent probe H2DCFDA. Monocytes were incubated with 5 μg/ml H2DCFDA for 20 min/37 °C in the dark in PBS 1× and then washed three times with the same buffer. Cells were then incubated with the chosen inhibitors (15 min/37 °C) in 1 ml PBS 1×, followed where appropriate by incubation with leptin (30 min/37 °C) in the same volume. At the same time, a control sample was kept under the same conditions but was treated neither with inhibitors nor leptin. After two washes with PBS 1×, monocytes were transferred as 200-μl samples to wells in a 96-well black plate and fluorescence was measured in an LS-55 Perkin-Elmer fluorescence spectrometer with the excitation and emission wavelengths set at 498 and 522 nm, respectively.
Superoxide (O−.2) production measurement
Monocytes were loaded with 25 μΜ DHE in the dark (20 min/37 °C) in complete IMDM (10% FCS) and were then washed twice with PBS 1×. Cells were then incubated with the chosen inhibitors (15 min/37 °C) in 1 ml of PBS 1×, followed where appropriate by incubation with leptin (30 min/37 °C) in the same volume. At the same time, a control sample was kept under the same conditions but was treated neither with inhibitors nor leptin. After two washes with PBS 1×, monocytes were transferred as 200-μl samples to wells in a 96-well black plate and fluorescence was measured in an LS-55 Perkin-Elmer fluorescence spectrometer with the excitation and emission wavelengths set at 490 and 567 nm, respectively, as suggested in a previous study (Zhao et al. 2005).
Superoxide dismutase activity measurement
A modified version of a previously described method was utilized (Peskin and Winterbourn 2000). The method is based on measuring the inhibitory effect of SOD on the reduction of WST-1 by superoxide, which is produced through the action of xanthine oxidase (XO). In brief, monocytes were treated with leptin (30 min and 1 and 2 h/37 °C) and washed with cold PBS 1×. Cells were then transferred to clean Eppendorf tubes and incubated on ice for 20 min with lysis buffer 1× (500 μl per 106 cells) which contained protease inhibitors (10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethanesulfonylfluoride (PMSF)). After centrifugation (12,000×g/10 min/4 °C), 20 μl from the supernatant of each sample were transferred to wells of a 96-well plate. In separate wells, 20-μl samples of known concentrations of SOD were added in order to be able later to plot a standard curve. Additionally, controls (A: w/o SOD, with WST-1, w/o XO and B: w/o SOD, with WST-1, with XO) necessary for result interpretation were also included. This was followed by addition of 200 μl of the reagent mixture (50 mM Tris–HCl, 0.1 mM DTPA, 0.1 mM hypoxanthine, 51.5 μΜ WST-1, 4.5 U/ml xanthine oxidase, pH 8.0) in every well that was used. The 96-well plate was incubated 20 min/RT with gentle agitation and then absorbance was measured at 450 nm in an enzyme-linked immunosorbent assay plate reader. Absorbance measurements were converted into SOD units per milliliter through the use of the standard curve and then SOD activity was calculated through the equation: SOD activity (inhibition%) = {[(control B − control A) − (sample − control A)] / (control B − control A)} × 100.
Immunoprecipitation of NHE1 and Western blotting
In brief, monocytes were incubated with the pharmacological inhibitors and leptin, followed by two washes with ice-cold PBS 1×. They were then lysed with lysis buffer (1% Nonidet P-40, 0.5% deoxycholic acid, 0.05% sodium dodecyl sulfate (SDS), 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 3 mM KCl, 1 mM EDTA, protease inhibitors [10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF]) at a ratio of 100 μl lysis buffer per 106 cells for 1 h/4 °C under rotation. Lysates were transferred to clean Eppendorf tubes and kept continually on ice. They were then precleared for 30 min/4 °C under rotation with the addition of 1-μg rabbit control IgG and 20-μl Protein A/G PLUS-Agarose in each sample. There followed centrifugation at 1,000×g/5 min/4 °C and transfer of the supernatants to clean Eppendorf tubes, always on ice. The protein concentration in each sample was measured with bicinchoninic acid (BCA) and, with the addition of appropriate volumes of lysis buffer, the samples were calibrated to the same protein concentration. Next, 1–10 μl of anti-NHE1 primary antibody were added to 100–500 μg of protein in each sample and incubation ensued for 1 h/4 °C. After this, 20 μl Protein A/G PLUS-Agarose were added to each sample followed by incubation overnight/4 °C under rotation. The following day, the samples were centrifuged at 1,000×g/5 min/4 °C and the pellets were washed three times with lysis buffer. After the final wash, protein concentration was assayed with BCA again and the samples were diluted with loading buffer not containing dithiothreitol. Thirty microliters from each sample were separated in four 8% SDS-polyacrylamide gel electrophoresis gels. After electrophoresis, one gel was set aside for silver staining in order to reevaluate protein concentrations, while the other three gels were used for the semi-dry blotting procedure. After the transfer, the nitrocellulose membrane was blocked for 1 h/RT, followed by overnight incubation with anti-NHE1 primary antibody or anti-Hsp70 primary antibody or anti-GSH primary antibody under gentle agitation. After three washes with Tris-buffered saline (TBS)/Tween, the membrane was incubated for 1 h/RT with the appropriate HRP-conjugated secondary antibodies (anti-rabbit for NHE1, anti-goat for Hsp-70, anti-mouse for GSH, anti-biotin for the markers). After three more washes with TBS/Tween, the protein bands were visualized on photographic film using an ECL kit.
Statistical analysis
Values are expressed as the arithmetic means ± standard deviations (SDs) of at least six experiments. Comparison between groups was conducted through the two-tailed paired t test and the one-way analysis of variance with the Student–Newman–Keuls test. P < 0.05 was used as the minimum accepted significance level. For the statistical evaluation, the statistical software GraphPad InStat version 3.00 was used (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
Results
Effect of leptin on intracellular pH
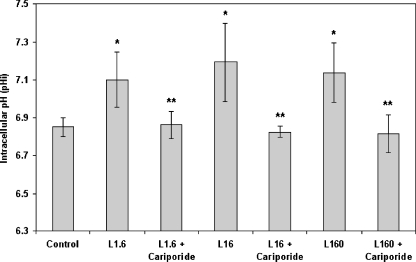
Leptin was found to increase pHi at every concentration that was used (Fig. 1). In more detail, leptin at concentrations of 1.6, 16, and 160 ng/ml caused pHi values of 7.09 ± 0.12, 7.18 ± 0.22, and 7.11 ± 0.14, respectively, compared to a control value of 6.85 ± 0.05. Pharmacological inhibition of NHE1 abolished the pHi-raising effect of leptin. As described in the “Materials and methods” section, appropriate inhibitory measures were taken in order to eliminate any interference from other pHi-regulating mechanisms. The effect of H+–ATPase on pHi should be considered negligible as the measurement involves circulating monocytes and not fully differentiated macrophages (Heming and Bidani 2003). Consequently, all the observed changes in pHi must be attributed exclusively to NHE1 action.
Fig. 1.
Effect of leptin on intracellular pH (pHi) as measured with the fluorescent probe BCECF-AM. Monocytes were incubated with carioporide for 15 min/37 °C followed by incubation with leptin (1.6, 16, and 160 ng/ml) for 30 min/37 °C. The arithmetic means of at least six experiments are shown. Error bars indicate standard deviations. The level of significance of the differences between the samples was calculated by ANOVA with a Student–Newman–Keuls post-test. *P < 0.05 versus the control sample. **P < 0.05 versus the respective leptin sample
Effect of leptin on general oxidative species production
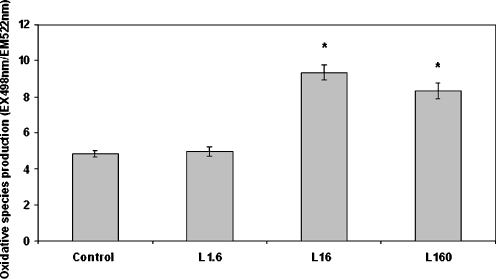
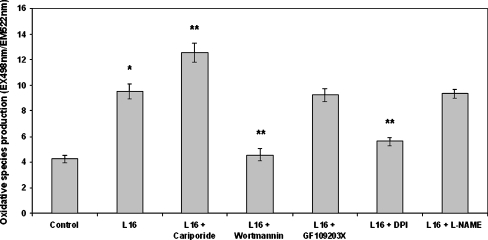
The fluorescent probe H2DCFDA was used in order to assess the effect of leptin on oxidative species production. This probe was in the past thought to be a good indicator of H2O2. However, it is today considered a more general marker of oxidative stress since it does not only interact with H2O2 but also with a variety of species (both ROS and RNS) such as OH∙, ROO∙, NO∙, and ONOO− to name but a few (Gomes et al. 2005). Leptin, at concentrations of 16 and 160 ng/ml, was found to increase oxidant species production (Fig. 2). Since the effect peaked at 16 ng/ml, this concentration was chosen in order to investigate the effect of specific pharmacological inhibition on leptin-induced oxidative species production. Leptin (16 ng/ml) increased oxidant species production (2.26-fold compared to the control) and this was further enhanced when NHE1 was inhibited (2.97-fold and 1.32-fold compared to the control and the leptin sample, respectively; Fig. 3). Inhibition of either PI3K or NADPH oxidase attenuated the effect of leptin (Fig. 3). Inhibition of either NHE1, PI3K, or NADPH oxidase in the absence of leptin did not affect basal oxidant species production (Table 1). Inhibition of PKC and NO synthase did not alter the leptin effect (Fig. 3).
Fig. 2.
Dose-dependent effect of leptin on oxidative species production as measured with the fluorescent probe H2DCFDA. Monocytes were incubated with leptin (1.6, 16, and 160 ng/ml) for 30 min/37 °C. The arithmetic means of at least six experiments are shown. Error bars indicate standard deviations. The level of significance of the differences between the samples was calculated by ANOVA with a Student–Newman–Keuls post-test. *P < 0.05 versus the control sample
Fig. 3.
Effect of leptin on oxidative species production as measured with the fluorescent probe H2DCFDA. Monocytes were incubated with the inhibitors for 15 min/37 °C followed by incubation with leptin (16 ng/ml) for 30 min/37 °C. The arithmetic means of at least six experiments are shown. Error bars indicate standard deviations. The level of significance of the differences between the samples was calculated by ANOVA with a Student–Newman–Keuls post-test. *P < 0.05 versus the control sample. **P < 0.05 versus the leptin sample. Inhibitor concentrations are mentioned in the “Materials and methods” section
Table 1.
Oxidative species production assessment as measured with H2DCFDA (control vs inhibitors)
| Sample | Measurement EX498 nm/EM522 nm |
|---|---|
| Control | 4.529 ± 0.136 |
| Leptin (16 ng/ml) | 9.034 ± 0.532 |
| Cariporide (20 nM) | 4.765 ± 0.280 |
| Wortmannin (50 nM) | 4.443 ± 0.310 |
| DPI (10 μΜ) | 4.632 ± 0.407 |
Effect of leptin on superoxide anion (O−∙2) production
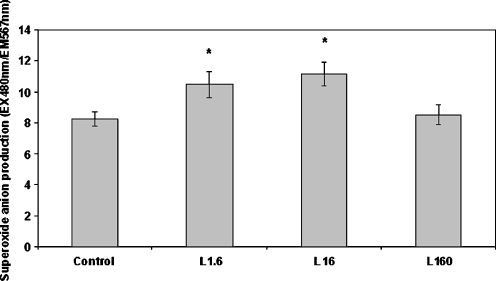
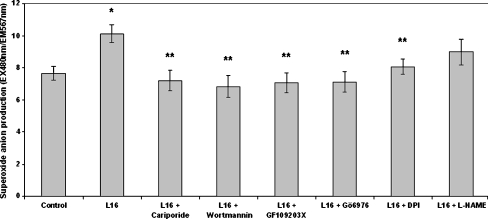
We next measured the effect of leptin on superoxide anion production. H2DCFDA is not a good marker of superoxide anions; thus, we used DHE in order to estimate the levels of this free radical (Wardman 2007). Dose–response results indicated that leptin increased superoxide anion production at concentrations of 1.6 and 16 ng/ml, with the latter being the peak concentration (Fig. 4). Leptin (16 ng/ml) caused a 1.32-fold increase in superoxide anion production (Fig. 5). This increase was completely prevented when NHE1, PI3K, PKC (α-, βi- or βii-isoforms), or NADPH oxidase were pharmacologically inhibited prior to leptin addition (Fig. 5). Inhibition of NO synthase did not appear to have an effect. Sole inhibition of each one of these signaling components in the absence of leptin had no significant effect on basal superoxide anion levels (Table 2).
Fig. 4.
Dose-dependent effect of leptin on superoxide anion (O–.2) production as measured with the fluorescent probe dihydroethidium. Monocytes were incubated with leptin (1.6, 16, and 160 ng/ml) for 30 min/37 °C. The arithmetic means of at least six experiments are shown. Error bars indicate standard deviations. The level of significance of the differences between the samples was calculated by ANOVA with a Student–Newman–Keuls post-test. *P < 0.05 versus the control sample
Fig. 5.
Effect of leptin on superoxide anion (O−.2) production as measured with the fluorescent probe dihydroethidium (DHE). Monocytes were incubated with the inhibitors for 15 min/37 °C followed by incubation with leptin (16 ng/ml) for 30 min/37 °C. The arithmetic means of at least six experiments are shown. Error bars indicate standard deviations. The level of significance of the differences between the samples was calculated by ANOVA with a Student–Newman–Keuls post-test. *P < 0.05 versus the control sample. **P < 0.05 versus the leptin sample. Inhibitor concentrations are mentioned in the “Materials and methods” section
Table 2.
Superoxide measurement (control vs inhibitors)
| Sample | Measurement EX480 nm/EM567 nm |
|---|---|
| Control | 7.954 ± 0.424 |
| Leptin (16 ng/ml) | 10.283 ± 0.754 |
| Cariporide (20 nM) | 7.645 ± 0.675 |
| Wortmannin (50 nM) | 7.776 ± 0.499 |
| GF109203X (10 μΜ) | 8.034 ± 0.578 |
| Gö6976 (500 nM) | 8.213 ± 0.534 |
| DPI (10 μΜ) | 8.001 ± 0.489 |
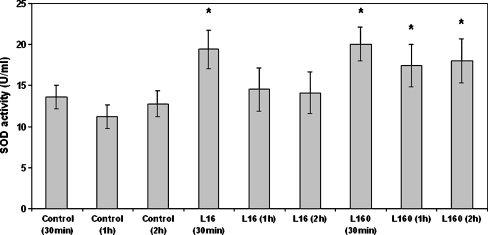
Effect of leptin on superoxide dismutase activity
Considering the increase in oxidant species levels caused by leptin, we investigated the effect of this cytokine on the activity of the enzyme SOD, a well-known anti-oxidant mechanism. SOD activity is targeted mainly towards eliminating superoxide anions but at the same time results in increased concentrations of H2O2. Its mechanism of action is, perhaps crudely but adequately, described by the following reaction: 2O−∙2 + 2H+ → H2O2 + O2 (Hancock et al. 2001). Dose–response experiments showed that leptin at a low concentration (16 ng/ml) caused a transient 1.37-fold increase of SOD activity within 30 min, which then diminished rapidly, reaching control sample levels within 1 h (Fig. 6). A higher leptin concentration (160 ng/ml) again caused a rapid increase in SOD activity within 30 min (1.47-fold), but this was longer lasting, still being significantly higher than the control after 2 h (Fig. 6).
Fig. 6.
Effect of leptin on superoxide dismutase (SOD) activity. Monocytes were incubated with leptin (16 ng/ml and 160 ng/ml) for 30 min and 1 and 2 h/37 °C, lysed and assayed for SOD activity. The arithmetic means of at least six experiments are shown. Error bars indicate standard deviations (SDs). The level of significance of the differences between the samples was calculated by ANOVA with a Student–Newman–Keuls post-test. *P < 0.05 versus the respective control sample of the appropriate time point
Detection of NHE1-Hsp70 co-immunoprecipitation and Hsp70 glutathionylation
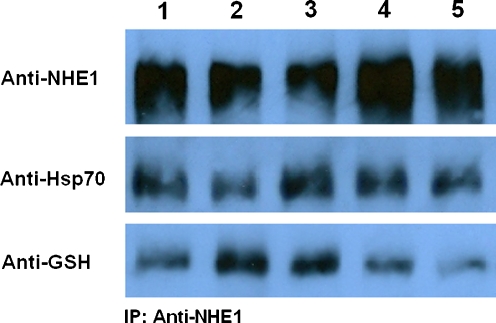
Since leptin could mediate a signal to NHE1 (resulting in its activation and subsequent changes in pHi), we studied the effect of leptin-induced oxidative stress on NHE1 interactions with the anti-oxidant factors Hsp70 and glutathione. Leptin (16 ng/ml) did not appear to increase Hsp70 attachment to NHE1, but it did increase the glutathionylation levels of the already attached Hsp70 molecules. This was concluded as it was observed that anti-GSH blots corresponded exactly to the position of the anti-Hsp70 blots (that is at approximate molecular weight of 70 kDa). NHE1 inhibition prior to leptin addition increased both Hsp70 attachment to NHE1 and its glutathionylation levels. PI3K or PKC inhibition prior to leptin addition reversed the leptin effect by increasing Hsp70–NHE1 attachment levels but reducing Hsp70 glutathionylation levels (Fig. 7).
Fig. 7.
Detection of NHE1, Hsp70, and GSH after immunoprecipitation with anti-NHE1. Monocytes were incubated with the inhibitors for 15 min/37 °C followed by incubation with leptin (16 ng/ml) for 30 min/37 °C and then lysed. Cell extracts were immunoprecipitated with anti-NHE1 and then analyzed through Western blot. Anti-Hsp70 and anti-GSH blots were observed at the same molecular weight marker (approximately 70 kDa) indicating that Hsp70 was glutathionylated. Samples: 1 control, 2 leptin 16 ng/ml, 3 leptin 16 ng/ml + cariporide 20 nM, 4 leptin 16 ng/ml + wortmannin 50 nM, 5 leptin 16 ng/ml + GF109203X 10 μΜ
Discussion
In a previous study, we had shown that leptin could activate NHE1 in human erythrocytes as represented by an increase in pHi (Konstantinou-Tegou et al. 2001). We observed the same effect in human monocytes derived from healthy subjects. Leptin activated NHE1 at all three concentrations that were tested and which ranged from normal to high hyperleptinemic levels (1.6, 16, and 160 ng/ml). The fact that NHE1 could be activated even by very low concentrations of leptin (1.6 ng/ml) indicates that NHE1 is particularly sensitive to leptin signaling.
We then proceeded to investigate the effect of leptin on oxidative species production (excluding superoxide anions) by human monocytes. Leptin was found to promote oxidative species production. This was observed at moderate and high hyperleptinemic concentrations (16 and 160 ng/ml, respectively) but not at a concentration which was within the normal range (1.6 ng/ml). A similar leptin-induced increase in oxidative stress was observed in a previous study in human umbilical vein endothelial cells (Bouloumie et al. 1999). Another study utilizing the same cells demonstrated an increase in oxidative stress in cases of hyperleptinemia accompanying obesity (Korda et al. 2008). During inflammation, monocytes and endothelial cells are in close proximity. Consequently, increases in oxidative species production by both types of cells could create microenvironments in which LDLs could be oxidized towards the more pro-atherosclerotic oxLDLs. Moreover, increased oxidative stress could cause irreversible damage to cells leading to their apoptosis (Dimmeler and Zeiher 2000). Foam cells formed due to increased cytoplasmic oxLDL accumulation, as well as apoptotic cells, are basic building blocks of atheromatous plaques, a typical event during atherosclerosis (Choudhury et al. 2005). Additionally, leptin might play a potentially pro-atherosclerotic role in vascular remodeling (Schroeter et al. 2008). Therefore, our results indicate that hyperleptinemic patients are more vulnerable to atheromatic plaque formation compared to people with normal leptin levels.
The signaling pathway initiated by leptin resulting in oxidative species production appeared to involve PI3K and NADPH oxidase since their inhibition abrogated the leptin effect. It did however appear to be PKC independent. An interesting finding was that inhibition of NHE1 further enhanced the effect of leptin indicating that NHE1 activity was protective against oxidative species formation. In a previous study, we observed a similar effect when NHE1 was inhibited in epithelial cancer cells (Konstantinidis et al. 2006). Although no agonist such as leptin was used in that study, we proposed that the detrimental effect of NHE1 inhibition occurred due to cellular stress resulting from the inability of cells to reach the necessary pHi requirements for mitosis. It is possible that the inability of leptin signaling to increase pHi through NHE1 activity, due to the latter’s complete inhibition, is translated into additional oxidative stress.
We continued our study by measuring the effect of leptin on superoxide anion production. Superoxide anions are particularly toxic and in combination with other molecules can give rise to other oxidative species (e.g., combined with NO it can produce peroxynitrite or ONOO−; Touyz 2003). Concentrations in the range of normal and moderate hyperleptinemic (1.6 and 16 ng/ml, respectively) increased superoxide anion production in human monocytes. Hyperleptinemic leptin levels had been shown in a previous study to induce superoxide anion production in murine cardiomyocytes (Dong et al. 2006). Appropriate pharmacological inhibition indicated the involvement of NHE1, PI3K, conventional isoforms of PKC (α-, βi-, βii-), and NADPH oxidase in this leptin-induced mechanism. NADPH oxidase is known to produce O−∙2; as a result, it is a potential terminal point of the signal initiated by leptin. It is also known in monocytes that PKCα, a conventional PKC, is necessary for NADPH oxidase assembly, hence the negative effect of PKC inhibition on O−∙2 production (Cathcart 2004). PI3K has also been shown to be activated by leptin in peripheral blood mononuclear cells (which include monocytes; Martin-Romero and Sanchez-Margalet 2001). Furthermore, NHE1 has previously been shown to be directly or indirectly regulated by both PI3K and PKC (Maly et al. 2002; Wu et al. 2004). The fact that superoxide anion production decreased after NHE1 inhibition indicates the existence of communication between NHE1 and NADPH oxidase. It is also worth noting that NO synthase did not appear to participate in signaling concerning leptin-induced oxidative stress since its inhibition had no significant effect on either general oxidative species production or superoxide anion production.
We hypothesized that leptin could increase superoxide anion concentration by limiting its neutralization by the enzyme SOD. Consequently, we measured the effect of leptin on superoxide dismutase. Despite the fact that leptin increases superoxide production, we found that at moderate and high hyperleptinemic concentrations it also increases the activity of superoxide dismutase. This enzyme catalyzes the following reaction: 2O−∙2 + 2H+ → H2O2 + O2 (Hancock et al. 2001). Based on our results, leptin increases the concentration of superoxide anions, as well as the activity of superoxide dismutase, thus moving the reaction towards the right. This would result in higher concentrations of H2O2 and oxidative species derived from it. On the other hand, leptin activates NHE1 resulting in decreased intracellular H+ which thus limits the reaction. Perhaps this explains the reason for which inhibition of NHE1 amplifies leptin-induced oxidative species production. Inhibition of NHE1 moves the reaction to the right resulting in higher consumption of superoxide anions. This could account for the decrease in the observed leptin-induced superoxide anion production when NHE1 was inhibited. In summary, leptin at moderate hyperleptinemic levels appears to favor the movement of the equilibrium of the above reaction towards the right, leading to increased concentrations of H2O2. Compared to other oxidative species, H2O2 has many of the attributes required in order to serve as a signaling molecule such as higher stability and longer life span. Indeed, it appears to play that role as more and more of its intracellular targets are being revealed (Giorgio et al. 2007).
We next sought to investigate the effect of leptin-induced oxidative stress on NHE1 and its interactions with anti-oxidant mechanisms. Leptin at a moderate concentration (16 ng/ml) was not found to increase Hsp70 attachment to NHE1. It did, however, increase glutathionylation levels of Hsp70 molecules which had already been bound to NHE1. It was recently reported that Hsp70 can regulate glutathionylation through the modulation of the enzymes glutathione peroxidase and glutathione reductase (Guo et al. 2007). Inhibition of NHE1 prior to leptin addition increased both Hsp70 attachment to NHE1 and Hsp70 glutathionylation. It would appear that in response to the pro-oxidant action of leptin the cell increases glutathionylation levels of Hsp70 molecules already attached to NHE1 but does not increase the levels of Hsp70–NHE1 attachment themselves. As demonstrated in the results concerning general oxidative stress, it is possible that parallel leptin incubation and NHE1 inhibition cause oxidative stress levels to rise above a certain threshold, after which increased glutathionylation levels are not enough to prevent serious damage and thus the cell also increases Hsp70–NHE1 attachment levels. The effect of parallel leptin inhibition and PI3K or PKC inhibition during which, inversely to leptin action, Hsp70–NHE1 attachment levels increase but Hsp70 glutathionylation levels decrease could be due to similar regulatory effects of these two proteins on NHE1 and thus requires further study.
In conclusion, leptin at hyperleptinemic levels appears to induce oxidative stress in human monocytes. Moreover, through signaling proteins such as PI3K and conventional PKC isoforms (α-, βi-, βii-), the leptin signal reaches NHE1 and NADPH oxidase with the possibility of communication between the two hinted. The cell’s reaction to the leptin effect is to increase glutathionylation levels of Hsp70 bound to NHE1 but not the levels of Hsp70 binding to NHE1. The role of NHE1 in leptin-induced oxidative stress is ambiguous as its inhibition protects against superoxide anion production but at the same time it potentiates the effect of leptin on the production of other oxidative species.
Acknowledgements
The authors would like to thank Ms. Christina Befani and Ms. Konstantina Topouridou for their excellent technical and secretarial assistance.
Sources of funding This research project is co-financed by the European Union—European Social Fund (75%) and the Greek Ministry of Development—Greek Secretariat for Research and Technology (GSRT; 25%).
List of Abbreviations
- BMI
(body mass index)
- JAK/STATs
(Janus kinases/signal transducers and activators of transcription)
- GSH
(glutathione)
- Hsp70
(heat shock protein 70 kDa)
- LDLs
(low-density lipoprotein)
- NHE1
(Na+–H+ exchanger isoform 1)
- ObRa–f
(Ob gene receptor a–f)
- oxLDLs
(oxidized low-density lipoproteins)
- PBMC
(peripheral blood mononuclear cells)
- PBS
(phosphate-buffered saline)
- PKC
(protein kinase C)
- RNS
(reactive nitrogen species)
- ROS
(reactive oxygen species)
- SOD
(superoxide dismutase)
Footnotes
Two- to three-sentence summary
Inhibition of NHE1 protects human monocytes against leptin-induced superoxide anion production, but at the same time it amplifies leptin-induced production of other oxidative species. Leptin-induced oxidative stress increases the glutathionylation levels of NHE1-bound Hsp70 but not Hsp70-binding to NHE1.
References
- Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81(2):223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Auwerx J, Staels B. Leptin. Lancet. 1998;351(9104):737–742. doi: 10.1016/S0140-6736(97)06348-4. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13(10):1231–1238. [PubMed] [Google Scholar]
- Bourikas D, Kaloyianni M, Bougoulia M, Zolota Z, Koliakos G. Modulation of the Na+–H+ antiport activity by adrenaline on erythrocytes from normal and obese individuals. Mol Cell Endocrinol. 2003;205(1–2):141–150. doi: 10.1016/S0303-7207(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- Choudhury RP, Lee JM, Greaves DR. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2(6):309–315. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriaucinas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Downey GP, Waddell TK, Grinstein S. Intracellular pH regulation spreading of human neutrophils. J Cell Biol. 1996;133(6):1391–1402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Reactive oxygen species and vascular cell apoptosis in response to angiotensin II and pro-atherosclerotic factors. Regul Pept. 2000;90(1–3):19–25. doi: 10.1016/S0167-0115(00)00105-1. [DOI] [PubMed] [Google Scholar]
- Dong F, Zhang X, Ren J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension. 2006;47(2):222–229. doi: 10.1161/01.HYP.0000198555.51645.f1. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8(9):722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–16. doi: 10.1016/S0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005;65(2–3):45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Devitt A. The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? Immunology. 2004;113(1):1–14. doi: 10.1111/j.1365-2567.2004.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Wharton W, Mosely P, Shi H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones. 2007;12(3):245–254. doi: 10.1379/CSC-265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria and oxidized lipids. The emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Desikan R, Neil SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29(Pt 2):345–350. doi: 10.1042/BST0290345. [DOI] [PubMed] [Google Scholar]
- Heming TA, Bidani A. Intracellular pH regulation in U937 human monocytes: roles of V-ATPase and Na+/H+ exchange. Immunobiology. 2003;207(2):141–148. doi: 10.1078/0171-2985-00224. [DOI] [PubMed] [Google Scholar]
- Incerpi S, Baldini P, Bellucci V, Zannetti A, Luly P. Modulation of the Na+/H+ antiport by insulin: interplay between protein kinase C, tyrosine kinase, and protein phosphatases. J Cell Physiol. 1994;159:205–212. doi: 10.1002/jcp.1041590203. [DOI] [PubMed] [Google Scholar]
- Koliakos G, Zolota Z, Paletas K, Kaloyianni M. High glucose concentrations stimulate human monocyte sodium/hydrogen exchanger activity and modulate atherosclerosis-related functions. Pflügers Arch. 2004;449(3):298–306. doi: 10.1007/s00424-004-1340-z. [DOI] [PubMed] [Google Scholar]
- Koliakos G, Paletas K, Kaloyianni M. NHE-1: a molecular target for signalling and cell matrix interactions. Connect Tissue Res. 2008;49(3):157–161. doi: 10.1080/03008200802151581. [DOI] [PubMed] [Google Scholar]
- Konstantinidis D, Koliakos G, Paletas K, Kaloyianni M. Signaling components involved in leptin-induced amplification of human monocyte atherosclerosis-related properties. J Vasc Res. 2009;46(3):199–208. doi: 10.1159/000161234. [DOI] [PubMed] [Google Scholar]
- Konstantinidis D, Koliakos G, Vafia K, Liakos P, Bantekas C, Trachana V, Kaloyianni M. Inhibition of the Na+–H+ exchanger isoform-1 and the extracellular signal-regulated kinase induces apoptosis: a time course of events. Cell Physiol Biochem. 2006;18(4–5):211–222. doi: 10.1159/000097668. [DOI] [PubMed] [Google Scholar]
- Konstantinou-Tegou A, Kaloyianni M, Bourikas D, Koliakos G. The effect of leptin on Na+–H+ antiport (NHE1) activity of obese and normal subjects erythrocytes. Mol Cell Endocrinol. 2001;183(1–2):11–18. doi: 10.1016/S0303-7207(01)00639-6. [DOI] [PubMed] [Google Scholar]
- Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol. 2008;295(4):H1514–1521. doi: 10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo ME, Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can J Physiol Pharmacol. 2006;54(11):1081–1095. doi: 10.1139/Y06-065. [DOI] [PubMed] [Google Scholar]
- Maly K, Strese K, Kampfer S, Ueberall F, Baier G, Ghaffari-Tabrizi N, Grunicke HH, Leitges M. Critical role of protein kinase C alpha and calcium in growth factor induced activation of the Na+/H+ exchanger NHE1. FEBS Lett. 2002;521(1–3):205–210. doi: 10.1016/S0014-5793(02)02867-3. [DOI] [PubMed] [Google Scholar]
- Martin-Romero C, Sanchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212(2):83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- Peelman F, Waelput W, Iserentant H, Lavens D, Eyckerman S, Zabeau L, Tavernier J. Leptin: linking adipocyte metabolism with cardiovascular and autoimmune diseases. Prog Lipid Res. 2004;43(4):283–301. doi: 10.1016/j.plipres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clin Chim Acta. 2000;293(1–2):157–166. doi: 10.1016/S0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation and cellular actions. Annu Rev Pharmacol Toxicol. 2002;42:527–552. doi: 10.1146/annurev.pharmtox.42.092001.143801. [DOI] [PubMed] [Google Scholar]
- Ruete MC, Carrizo LC, Vallés PG. Na+/K+-ATPase stabilization by Hsp70 in the outer stripe of the outer medulla in rats during recovery from a low-protein diet. Cell Stress Chaperones. 2008;13(2):157–167. doi: 10.1007/s12192-008-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pozo C, Rodriguez-Bano J, Dominguez-Castellano A, Muniain MA, Goberna R, Sanchez-Margalet V. Leptin stimulates the oxidative burst in monocytes from HIV-infected patients. Clin Exp Immunol. 2003;134(3):464–469. doi: 10.1111/j.1365-2249.2003.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter MR, Leifheit M, Sudholt P, Heida NM, Dellas C, Rohm I, Alves F, Zientkowska M, Rafail S, Puls M, Hasenfuss G, Konstantinides S, Schäfer K. Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ Res. 2008;103(5):536–544. doi: 10.1161/CIRCRESAHA.107.169375. [DOI] [PubMed] [Google Scholar]
- Seager-Danciger J, Lutz M, Hama S, Cruz D, Castrillo A, Lazaro J, Phillips R, Premack B, Berliner J. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288(1–2):123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Maruyama S, Sato W, Morita Y, Sato F, Miki Y, Kato S, Katsuno M, Sobue G, Yuzawa Y, Matsuo S. Geranylgeranylacetone ameliorates ischemic acute renal failure via induction of Hsp70. Kidney Int. 2005;67:2210–2220. doi: 10.1111/j.1523-1755.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- Sweeney G. Leptin signalling. Cell Signal. 2002;14(8):655–663. doi: 10.1016/S0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species in vascular biology: role in arterial hypertension. Expert Rev Cardiovasc Ther. 2003;1(1):91–106. doi: 10.1586/14779072.1.1.91. [DOI] [PubMed] [Google Scholar]
- Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls and prospects. Free Radic Biol Med. 2007;43(7):995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem. 2004;279(25):26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- Xu L, Giffard RG. HSP70 protects murine astrocytes from glucose deprivation injury. Neurosci Lett. 1997;224(1):9–12. doi: 10.1016/S0304-3940(97)13444-9. [DOI] [PubMed] [Google Scholar]
- Zarkesh-Esfahani H, Pockley G, Metcalfe RA, Bidlingmaier M, Wu Z, Ajami A, Weetman AP, Strasburger CJ, Ross RJ. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–4599. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitation of fluorescence. Proc Natl Acad Sci U S A. 2005;102(16):5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]