Abstract
Chinese hamster lung fibroblasts V79 cells were treated with heat stress for 4 weeks with short duration (15 min) heat shock every alternate day in culture. It was observed that Hsp 70 and the antioxidant enzyme MnSOD became overexpressed during the chronic heat stress period. Both p38 MAPK and Akt became phosphorylated by chronic heat stress exposure. Simultaneous exposure to SB203580, a potent and specific p38MAPK inhibitor drastically inhibited the phosphorylation of p38MAPK and Akt. Furthermore, exposure to SB203580 also blocked the increase in Hsp70 and MnSOD levels and the elevated SOD activity brought about by chronic heat stress. Heat shock factor 1 (HSF1) transcriptional activity and nuclear translocation of HSF1 were prominently augmented by chronic heat stress, and this amplification is markedly reduced by concomitant exposure to SB203580. Also, activations of p38MAPK and Akt and upregulations of Hsp70 and MnSOD were observed on exposure to heat shock for a single exposure of longer duration (40 min). siRNA against p38MAPK notably reduced Akt phosphorylation by single exposure to heat stress and drastically diminished the rise in Hsp70 and MnSOD levels. Similarly, siRNA against Akt also eliminated the augmentation in Hsp70 and MnSOD levels but p38MAPK levels remained unaffected. Heat stress produced reactive oxygen species (ROS) in V79 fibroblasts. N-acetyl cysteine blocked the increase in phosphorylation of p38MAPK, amplification of Hsp70, and MnSOD levels by heat stress. Therefore, we conclude that heat stress-activated p38MAPK which in turn activated Akt. Akt acted downstream of p38MAPK to increase Hsp70 and MnSOD levels.
Concise summary: Thermal injury of the skin over a long period of time has been associated with development of cancerous lesions. Also, in many cancers, the cytoprotective genes Hsp70 and MnSOD have been found to be overexpressed. Therefore, we considered it important to identify the signaling elements upstream of the upregulated survival genes in heat stress. We conclude that heat stress activated p38MAPK which in turn activated Akt. Akt mediated an augmentation in Hsp70 and MnSOD levels working downstream of p38MAPK.
Keywords: Akt, Heat shock, HSF, Hsp70, MnSOD, p38MAPK
Introduction
Animals respond to environmental stress with a series of reactions depicted as the “fight-or-flight” response. Similar responses are also elicited by cells when they are dealing with stresses in their microenvironment. Studies with chronic heat stress (CHS) have demonstrated altered physiological, metabolic, biochemical, and cellular response in animal models and poultry birds. Reports also demonstrate that CHS treatment negatively affects the cell-mediated immune response in animals (Goligorsky 2001; Hu et al. 2007b; Jolly and Morimoto 2000; Lu et al. 2007; Odongo et al. 2006). However, information on the cellular response to chronic heat stress in relation to signaling pathways is insufficient and scattered.
The heat shock response (HSR) is a coordinated genetic response to a wide range of physiological and environmental stressors culminating in the induction of genes encoding molecular chaperones, proteases, and other proteins essential for maintaining cellular homeostasis to offset the damage associated with the stress. The stress response is initiated by the activation and/or de-repression of the transcription factor HSF1 that binds to the heat-shock element (HSE) in the promoter of most of the genes expressing the heat shock family proteins such as Hsp70 and Hsp90 (Jolly and Morimoto, 2000; Pirkkala et al. 2001). Hsp70 is one of the major chaperones of the cell which plays a crucial role in guiding conformational status of the proteins during folding and translocation (Arya et al. 2007). Abnormal heat shock response coupled with Hsp70 overexpression is observed in different malignancies and may be responsible for the survival advantage of these cells (Calderwood and Ciocca 2008; Calderwood et al. 2006;Chakraborty et al. 2008a; Mayer and Bukau 2005). The mechanisms underlying the expression of survival genes by heat stress remains largely undiscovered. Heat shock has been shown to produce ROS by some investigators, and quite the reverse has been demonstrated by others who did not detect ROS increase by heat shock (Ilangovan et al. 2006; Maridonneau-Parini et al. 1988; Maridonneau-Parini et al. 1993; Mattson et al. 2004; Shin et al. 2008; Zhang et al. 2003). Activation of p38MAPK by heat stress is also known (Dorion et al. 2002; Shin et al. 2008). However, whether ROS generation is a prerequisite for the activation of p38MAPK by heat shock remains ambiguous (Dorion et al. 2002; Shin et al. 2008). Furthermore, the connection between heat-induced production of ROS and activation of p38MAPK by heat stress has not been adequately probed. Studies depicting signaling pathways upstream of stresses or stimuli other than heat are more readily available (Kim et al. 2005a; b; Rafiee et al. 2006; Stathopoulou et al. 2006; Uehara et al. 1999). Human esophageal microvascular endothelial cells induce Hsp70 by the Akt and p38MAPK pathways when subjected to low pH (Rafiee et al. 2006). However, in alkalosis, p38MAPK activation is not necessary for induction of Hsp70 (Stathopoulou et al. 2006). Also, overexpression of Akt1 leads to enhancement in expression of Hsp70 through negative regulation of the FOXO3a forehead transcription factors (Kim et al. 2005b). Hypoxia or carbon monoxide exposure has been shown to activate p38MAPK upstream of Hsp70 augmentation (Kim et al 2005a; Uehara et al 1999).
ROS generation is often triggered by various forms of stress including oxidative stress, heavy metal stress, hyperthermia, etc. (Dröge 2002). The unfavorable effects of ROS are counteracted by the antioxidant defense of the cells which includes various antioxidant enzymes and ROS scavenging molecules. Of the various antioxidant enzymes, Cu/ZnSOD and MnSOD are primarily responsible for the conversion of O2− to H2O2 which is then degraded by catalase or peroxidases (Turrens 2003). Several studies have revealed overexpressed levels of MnSOD in various forms of cancer. Higher metabolic rates and mitochondrial dysfunction contribute to the presence of elevated ROS levels in the cancerous cells which is controlled by the presence of high endogenous levels of MnSOD in these cells (Hu et al. 2007a). It has been reported that heat stress produced moderate upregulation of MnSOD in cardiac myocytes conferring protection against hypoxia–reoxygenation injury (Yamashita et al. 1997). On the contrary, lack of increase in MnSOD activity in rat heart after hyperthermia has also been reported (Tekin et al. 2001).
Thermal injury of the skin over a long period of time has been associated with development of cancerous lesions (Aziz et al. 1998). Also, the cytoprotective genes Hsp70 and MnSOD have been found to be overexpressed in many cancers (Chakraborty et al 2008a; Hu et al 2007a; Mayer and Bukau 2005). Therefore, in the present study, we considered it meaningful to identify the signaling elements upstream of the upregulated survival genes in heat stress. In the current study, we establish that the upregulation of MnSOD and Hsp70 levels, two important pillars of cellular defense and survival, is due to the p38MAPK-mediated activation of the Akt pathway in both chronic low-grade and moderate single exposure heat stress. The present study also proposes a chronic heat stress fibroblast cell model where we establish an important link between the progressive upregulation of cytoprotective genes and the key signaling kinases at the cellular and molecular levels.
Materials and methods
Materials
Antibodies against Phospho Akt (ser473, cat.no.4051, CST), Akt (cat. No. 9272, CST), Phospho p38MAPK (cat. no. 612280, BD_Pharmingen), p38MAPK (cat no. M0800, Sigma), Hsp70 (cat. no. 610607, BD_Pharmingen, against amino acids 429–640 of human Hsp70), Hsp72 (cat. no. 1428, Abcam, synthetic peptide surrounding amino acid 560 of human Hsp70, specific for the inducible form of Hsp70–Hsp72), ab2923 Abcam for HSF1, MnSOD (cat. no. 611580, BD-Pharmingen) were purchased from well-known vendors. β-Actin antibody was purchased from Imgenex (Cat. no. IMG-5142A), and propidium iodide was bought from Sigma Chemicals, USA.
Cell culture and drug treatment conditions
Chinese Hamster lung fibroblast (V79) cells were maintained in exponential growth in minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin in humidified atmosphere of 5% CO2 at 37°C. SB-203580 (Santa-Cruz Biotechnology) was dissolved in DMSO at a concentration of 5 mM and added to cells at the indicated concentrations.
Schedule for chronic heat stress
For development of chronic heat stress, V79 fibroblasts were exposed to thermal stress at 43°C for 15 min in culture every alternate day for a period of 4 weeks. The p38MAPK inhibitor SB-203580 (5 µM) was added in the culture medium of both inhibitor control cells (cells receiving no heat shock) and stressed cells which received heat shock for 4 weeks. Untreated controls were subcultured normally and received no other treatment for 4 weeks. The inhibitor was freshly added during subculturing every 48 h.
Measurement of ROS
To measure the ROS production due to heat shock in the presence of 5 mM N-acetyl cysteine (NAC), intracellular H2O2 was determined with the fluorescent dye 2′,7′-dichlorodihydrofluorescein (H2DCF). Cells were grown on coverslips and first incubated with NAC for 2 h. After that, the cells were given three washes with fresh medium and were subjected to heat shock at 43°C for 40 min. Thereafter, the cells were incubated with the ester form of the dye for 15 min at 37°C in Hanks buffer. Cells were visualized under confocal microscope for determining extent of ROS production at the excitation wavelength of 506 nm.
Western blot analysis
Protein extracts, 20–50 µg, were first separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then transferred onto polyvinylidene fluoride membrane. After blocking with 5% bovine serum albumin (BSA), membranes were incubated overnight at 4°C with respective primary antibodies. The membranes were incubated with 1:5,000 dilutions of the appropriate peroxidase-conjugated secondary antibodies and or alkaline phosphatase-conjugated antibodies and developed for detection by chemiluminescence or colorimetry. Western blots were scanned by HP scanjet 4370 Scanner, and the bands were quantified by using ImageJ software
Reverse transcriptase polymerase chain reaction
Total RNA (2.5 μg) was reverse transcribed with oligodT primer using 0.2 mM of dNTP mix, 25 U of RNase inhibitor, and 100 U of Moloney murine leukemia virus reverse transcriptase. For polymerase chain reaction (PCR), 1 μl of cDNA from the RT reaction mixture was taken to amplify in a 25-μl reaction volume containing 10 pmol of each primer, 200 μM of each dNTP, in buffer containing 1.5 mM MgCl2, and 5 U of Taq polymerase. A 10-μl portion of PCR product was electrophoresed in a 1.0% agarose gel. The following primers were used for Hsp70 (FWD 5′ACCCTGTCGTCCAGCACCCA3′; REV 5′CTGCGTCTGCTTGGTGGGGA3′) MnSOD (FWD 5′ACCACAGCAAGCACCACGG 3′ REV 5′CCCCAGCAGCGGAATAAGGC3′) and β-actin (FWD 5′GAAGCA TTTGCGGTGGACCAT3′; REV 5′TCCTGTGGCATCCACCAAACT3′).
Electrophoretic mobility shift assay
Protein was extracted using the NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (Pierce Biotechnology, Rockford, IL, USA). Western blot analysis for HSF1 quantification was performed with the cytoplasmic fraction. The nuclear fraction was used for EMSA for HSF1 and HSE binding. Oligonucleotides bearing the sequence of the heat shock element (HSE, 5′GAT CCT CGA AGG TTC GAG GAT CCT CGA AGG TTC GAG 3′ and 5′GAT CCT CGA ACC TTC GAG GAT CCT CGA ACC TTC GAG 3′ Integrated DNA Technologies) were end-labeled with [γ-32P] dATP by incubating annealed oligonucleotides with 5× reaction buffer and 10 U T4 polynucleotide kinase (Invitrogen). Then, annealing of the labeled oligos was done at room temperature for 30 min. An equal amount of protein (20 μg) from each sample was used in 25-μl binding reactions, which consisted of 1 μg poly dI-dC, 5× binding buffer (20 mM Tris pH 7.5, 100 mM NaCl, 2 mM EDTA, 10% glycerol, and 10 mg/mL BSA), labeled probe; protein lysate volumes were made to equal 5 μL by addition of buffer (20 mM HEPES pH 7.8, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, and 0.5 mM PMSF). To determine specificity of DNA binding, unlabeled competitor DNA was added to the binding reactions and allowed to incubate for 10 min at room temperature. Labeled probe was then added, and samples were further incubated for 20 min at room temperature. Electrophoresis of the samples was carried out on a 5% nondenaturing polyacrylamide gel in 0.5× TBE for 1.5 h at 120 V, and final visualization was done by autoradiography.
Confocal microscopy for localization of HSF1
Chronically heat stressed or control cells with or without inhibitors were grown on coverslips and then washed with PBS, fixed with 4% p-HCHO in PBS, permeabilized with 0.1% TritonX-100, blocked with 3% BSA, and 100 ug/ml RNAaseA. Subsequently, the cells were incubated with HSF1 antibody. Afterwards, anti-rabbit mouse fluorescein isothiocyanate (FITC) was used for staining along with 5 µg propidium iodide (PI). Then, the cells were washed, and HSF1 localization was visualized under Zeiss confocal laser microscope.
Short interfering RNA transfection
Fifty percent confluent V79 fibroblasts were treated with siRNA of p38MAPK (p38MAPK short-cut short interfering RNA (siRNA) and siRNA of Akt mix (New England Biolabs), by the procedure mentioned in the New England Biolab protocol for the kit. Transpass R1 transfection reagent from New England Biolabs was used for transfection. Cells were subjected to heat shock at 43°C for 40 min after 36 h of transfection, and cells were harvested after 9 h of heat shock treatment. Polylinker siRNA (New Englad Biolabs), a heterogeneous mixture of 21–23 bp siRNAs was used as a nonspecific control for the siRNA experiments. Both polylinker siRNA control and only Transpass R1 reagent controls were included as controls for the siRNA experiments.
SOD assay
Whole cell extract was prepared, and 50 µg of protein samples were loaded in 10% native PAGE gel, (constant 100 V, ∼1 h). The gel was soaked in 10 ml Riboflavin (1 mg)–NBT (2.5 mg) solution. The gel was incubated at room temperature for 15 min in dark and then removed from the Riboflavin–NBT solution. Of 0.1% TEMED, 2–5 ml was added and further incubated at room temperature for 15 min in dark. The solution was removed, and the gel was developed in light when gel color turned into blue-purple, and SOD bands appeared white. Gel was dried in the dark and scanned.
Results
Enhancement of MnSOD and Hsp70 protein expression and phosphorylation of p38MAPK and Akt by chronic heat shock
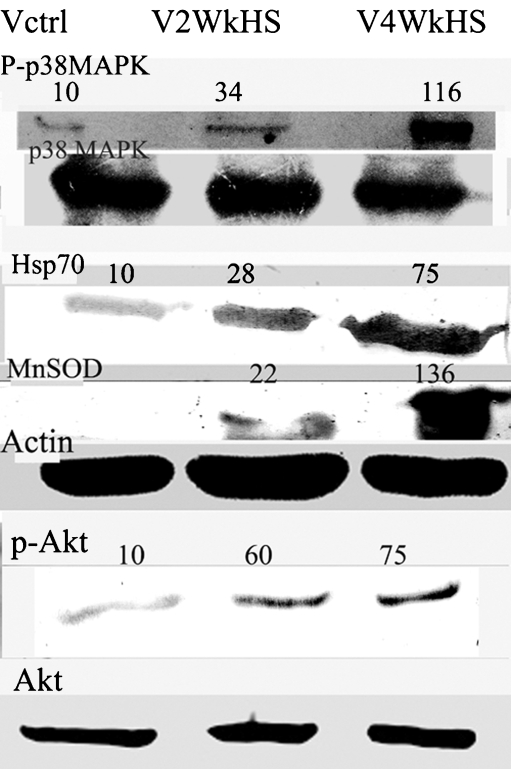
Chronic heat shock (Fig. 1) for 2 weeks resulted in the increase in protein levels of two important survival-related genes Hsp70 and MnSOD which was further bolstered by the prolongation of the chronic heat shock period to 4 weeks (about seven- to eightfold for Hsp70 and (>tenfold for MnSOD). However, another antioxidant enzyme catalase was not found to be upregulated by heat stress (data not shown).While checking for the upstream elements, it was found that the protein kinases p38MAPK and Akt phosphorylations were enhanced considerably by chronic heat shock at 2 weeks, and further increase was observed at 4 weeks(>tenfold for p38MAPK and about sevenfold for Akt).
Fig. 1.
Chronic heat stress causes increases in phosphorylation of p38MAPK and Akt, and induces enhancement of protein levels of Hsp70 and MnSOD in V79 fibroblasts. Cells were treated with chronic heat stress for a period of 4 weeks. Thermal stress was applied at 43°C for 15 min in culture every alternate day. Cells were harvested after either a 2-week or a 4-week experimental period. Western blotting of the cell lysates was performed. The Western blots are representative of two–three independent experiments. The numbers on the top of the blots are indicative of the respective band densities when the control is normalized to 10. Cells were either left untreated (Vctrl), treated with chronic heat stress (V2WkHS) for 2 weeks, or treated with chronic heat stress (V4WkHS) for 4 weeks. Details of the experimental procedure are given in the materials and methods section
Inhibition of p38MAPK by the pharmacological inhibitor SB203580 leads to blockage of the induction of Hsp70/Hsp72 and MnSOD during chronic heat shock
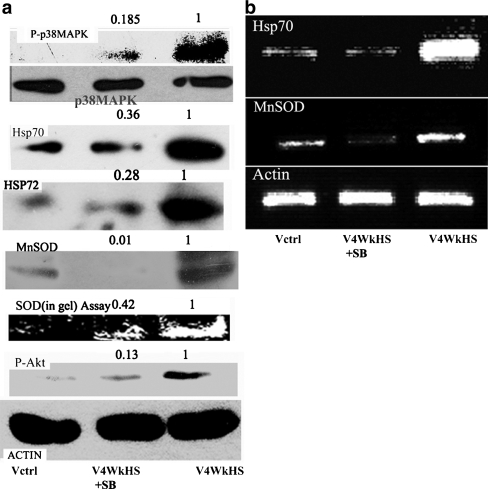
To decipher the signaling pathway upstream of increased expression of Hsp70 and MnSOD, we looked at the activation status of the MAPK family enzymes. Phosphorylation of Jun terminal kinase (JNK) was not increased by chronic heat shock and a minor increase in phosphorylation of ERK1/2 was detected which was not sensitive to the presence of p38MAPK inhibitor SB203580 (data not shown). Figure 2A shows that the p38MAPK inhibitor, SB203580, severely inhibits the enhancement in the phosphorylation status of p38MAPK (>80% reduction). Both Hsp70 and the inducible Hsp72 levels also undergo strong declines (close to 70%). The levels of MnSOD protein (>95% reduction) and SOD enzyme activity are also heavily curtailed by the presence of SB203580 during the period of chronic heat stress. Moreover, the presence of SB203580 during the chronic heat shock period also severely constrained Akt phosphorylation (>80%). Figure 2B demonstrates that the mRNA levels of both MnSOD and Hsp70 are drastically reduced by the exposure to SB203580.
Fig. 2.
Effect of p38MAPK inhibitor SB203580 on the phosphorylation status of p38MAPK and Akt and on the mRNA and protein levels of Hsp70 and MnSOD in V79 fibroblasts subjected to chronic heat stress. a phosphorylation status of p38MAPK (uppermost panel) and Akt (last but one panel from top) and protein expression of Hsp70 (third panel from top), Hsp72 (fourth panel from top), MnSOD (fifth panel from top). SOD enzyme activity is presented in the sixth panel from top. The last panel from top shows beta actin to indicate protein loading. b mRNA level of Hsp70 (uppermost panel), MnSOD (second panel from top), and beta-actin (last panel from top to indicate loading). The numbers on the top of the blots are indicative of the respective band densities when the control is normalized to 1 Details of the experimental procedure are given in the materials and methods section. Cells were either left untreated (Vctrl), treated with chronic heat stress (V4WkHS) or treated simultaneously with chronic heat shock and 5 µM SB203580 (V4WkHS + SB). The results are representative of two-three experiments
Chronic heat shock influences heat shock factor 1 activity
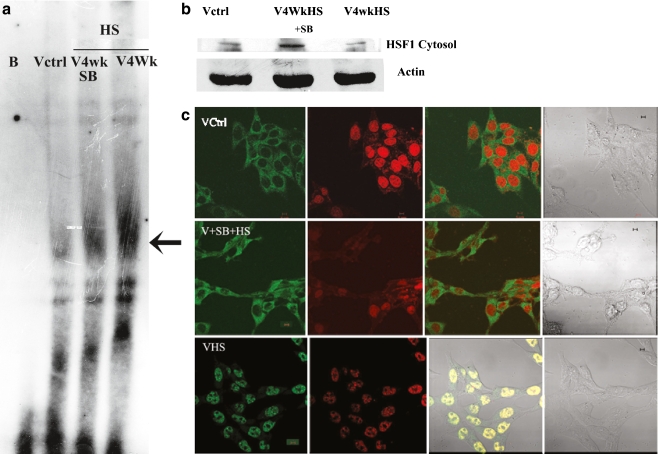
Figure 3A shows by EMSA that the heat shock factor 1 (HSF1) DNA-binding activity was prominently augmented by exposure to chronic heat stress. This enhancement is markedly reduced by the exposure to SB203580. Figure 3B demonstrates that the presence of HSF1 in cytosol is reduced by chronic heat stress, but exposure to SB203580 during chronic heat stress stimulates an increase of HSF1 in the cytosol. In Fig. 3C, it is demonstrated that HSF1 is predominantly localized in the cytoplasm of the control cells (upper panel). After chronic heat shock, the localization pattern of HSF1 changed to an exclusively nuclear location (lower panel). In cells subjected to SB203580 exposure along with chronic heat stress, HSF1 again became localized to the cytoplasm similar to control cells (middle panel).
Fig. 3.
Effect of SB203580 on the DNA binding activity and cellular localization of heat shock factor 1 (HSF1) in V79 fibroblasts exposed to chronic heat stress. a Electrophoretic Mobility Shift Assay (EMSA) showing HSF1 DNA-binding activity in V79 fibroblasts is provided. Cells were either left untreated (Vctrl), treated with chronic heat stress (V4WkHS), or treated simultaneously with chronic heat shock and 5 µM SB203580 (V4WkHS + SB). Nuclear extracts from these differently treated cells were used for the EMSA. BL Blank without nuclear extract. Arrow indicates HSF1-DNA binding. b Cytosolic localization of HSF1.Upper panel shows HSF1 bands from cytosolic extracts of differently treated cells. Lower panel displays beta-actin to indicate protein loading. Cells were either left untreated (Vctrl), treated with chronic heat stress(V4WkHS) or treated simultaneously with chronic heat shock and 5 µM SB203580 (V4WkHS + SB). c Cellular localization of HSF1 in chronic heat stressed V79 cells by confocal microscopy. Upper panel—Vctrl-untreated V79 cells, middle panel VHS + SB–chronic heat stressed V79 cells treated simultaneously with 5 µM SB203580, lower panel—VHS-chronic heat-stressed cells; micrographs on the left hand side of all panels show cells processed for visualization of HSF1 with secondary antibody–FITC conjugate. The second from left micrographs on all panels show cells processed for visualization of the nucleus with the DNA stain propidium iodide (PI).The third from left micrograph on all panels depict a merger of the FITC and propidium iodide signal. The fourth from left micrograph on all panels show differential interference contrast (DIC) from the same fields. After permeabilization and fixation, cells were treated with anti-HSF1 antibody and subsequently with FITC tagged secondary antibody and PI. The bar is 10 µm. The micrographs are representative of two separate experiments. The cells were treated with chronic heat stress for 4 weeks or were left untreated (control). The chronic heat stressed cells received either no other treatments or received simultaneous treatments with 5 µM SB203580. Experimental details are provided in the “Materials and methods” section
Effect of siRNA against p38MAPK and Akt on the induction of Hsp70 and MnSOD by heat shock
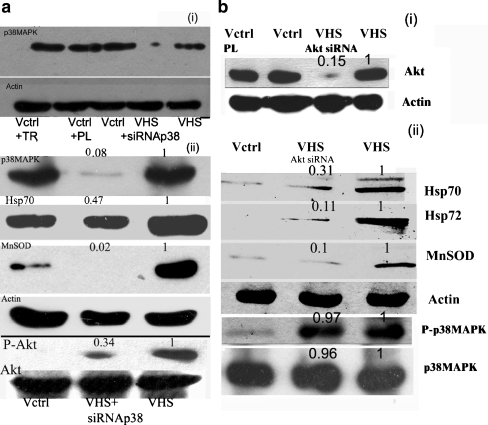
To delineate the role played by the upstream kinases in the induction of Hsp70/Hsp72 by heat shock, siRNA was used to silence either p38MAPK or Akt before applying heat shock. Transfection of polylinker siRNA, a nonspecific siRNA did not produce any change in the protein levels of either p38MAPK or Akt (Fig. 4) and did not bring about any noticeable change in viability or morphology of the cells (data not shown). Since long-term experiments were not possible with siRNA-transfected cells, one time heat shock of a greater strength was applied. Results obtained by p38MAPK siRNA experiments confirmed the data from chronic heat shock studies using the pharmacological inhibitor of p38MAPK. Silencing p38MAPK reduced prominently the increase in Hsp70 by heat shock. Increased MnSOD levels in heat shock-treated cells were drastically curtailed (>95% reduction) by p38MAPK siRNA (Fig. 4A). Akt protein levels were unchanged by the siRNA silencing of p38MAPK. Yet, phosphorylation status of Akt underwent a prominent change. p38MAPK siRNA-treated cells showed that the augmentation of Akt phosphorylation by heat shock could be severely blocked by p38MAPK siRNA (66% blockage). Use of Akt siRNA decreased Akt levels severely but p38MAPK levels remained unaltered. Akt siRNA drastically decreased the rise in Hsp70/Hsp72 and MnSOD levels brought about by heat shock (Fig. 4B). However, phosphorylation of p38MAPK upon heat shock remained at an elevated level in Akt siRNA-treated cells (Fig. 4B).
Fig. 4.
Inhibition of the overexpression of Hsp70 and MnSOD induced by heat shock through transfection of siRNA against p38MAPK and Akt. a Exposure to p38MAPK si RNA- (1) Uppermost panel depicts the protein level of p38MAPK. Second panel from top depicts beta actin protein level. (2) Uppermost panel of a series of Western blots depicts the protein level of p38MAPK. Second panel from the top depicts Hsp70. The third panel from the top shows MnSOD levels. The fourth panel from top shows beta actin levels. The fifth panel from top displays phospho-Akt levels. The last panel shows Akt protein levels; Vctrl + TR− control V79 fibroblasts + transfection reagent; VCtrl + PL—Control V79 fibroblasts + Polylinker siRNA; VCtrl- Control V79 fibroblasts; VHS—untransfected V79 fibroblasts treated with single dose heat shock of 43°C for 40 min. VHS + siRNA p38-p38MAPK siRNA transfected V79 fibroblasts treated with single dose heat shock of 43°C for 40 min. b Exposure to Akt siRNA—(1) Upper panel shows Akt protein levels, and second panel depicts actin levels. (2) Upper panel depicts Hsp70, the second panel from top depicts Hsp72, and the third panel from the top shows MnSOD immunoblot. The fourth panel from top shows actin immunoblot. The fifth and sixth panels from the top show phospho-p38MAPK and p38MAPK levels, respectively. VCtrl-control V79 fibroblasts; Vctrl + PL-polylinker siRNA treated control; VHS- untransfected V79 fibroblasts treated with single dose heat shock of 43°C for 40 min. VHS + siRNA Akt -siRNA transfected V79 fibroblasts treated with single dose heat shock of 43°C for 40 min. Details of the experimental procedures are described in the “Materials and methods” section. The numbers above the panels indicate band densities with respect to control bands normalized to 1. The blots are representative of two–three experiments
Heat produces reactive oxygen species in V79 fibroblasts
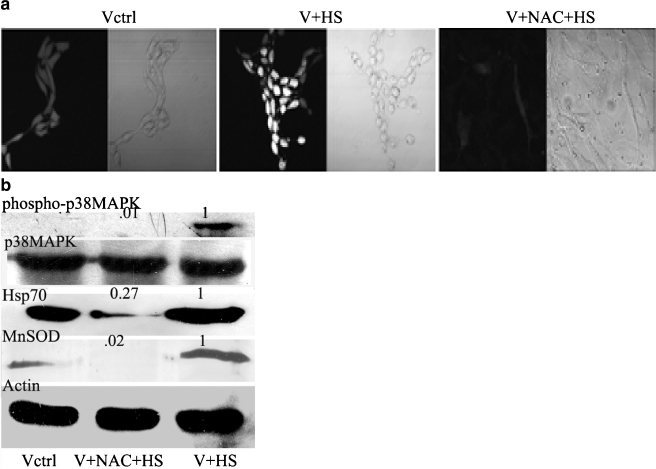
Figure 5A depicts that moderate heat shock (43°C for 40 min), produces reactive oxygen species (ROS) as measured with the fluorescent dye DCF-DA. This rise in ROS level was blocked by incubation of the cells with the potent antioxidant N-acetyl cysteine (NAC) prior to heat shock. Since, in heat-shocked V79 fibroblasts ROS are generated, we wanted to establish whether heat-induced ROS generation is responsible for the phosphorylation and activation of p38MAPK. We find that NAC almost completely (99% inhibition) blocked p38MAPK phosphorylation induced by heat shock (Fig. 5B). Similarly, increases in Hsp70 and MnSOD levels brought about by heat shock were repressed by NAC (Fig. 5B).
Fig. 5.
Role of ROS on the induction of Hsp70 and MnSOD by heat shock. a Confocal micrographs of V79 fibroblasts treated with single dose heat shock of 43°C for 40 min in the presence and absence of NAC. Cells were grown on coverslips and first incubated with NAC for 2 h. After that, the cell-containing petridishes were given three washes with fresh medium and were subjected to heat shock at 43°C for 40 min. Thereafter, the cells were incubated with the ester form of the H2DCF dye for 15 min at 37°C in Hanks buffer. Cells were visualized under confocal microscope for determining extent of ROS production at the excitation wavelength of 506 nm. Vctrl- untreated cells; V + HS- V79 fibroblasts treated with single dose heat shock of 43°C for 40 min; V + NAC + HS- V79 fibroblasts pretreated with 5 mM NAC and subsequently treated with single dose heat shock of 43°C for 40 min; Vctrl—First picture from left is visualized by dye fluorescence and second picture from the left is differential interference contrast image of the same field. V + HS- Third picture from left is visualized by dye fluorescence and fourth picture from the left is differential interference contrast image of the same field. V + NAC + HS- fifth picture from left is visualized by dye fluorescence and sixth picture from the left is differential interference contrast image of the same field. b Western blot showing p38MAPK phosphorylation and Hsp70 and MnSOD protein levels of V79 fibroblasts treated with single dose heat shock of 43°C for 40 min in the presence and absence of NAC. Vctrl—untreated cells; V + HS- V79 fibroblasts treated with single dose heat shock of 43°C for 40 min; V + NAC + HS- V79 fibroblasts pretreated with 5 mM NAC subsequently treated with single dose heat shock of 43°C for 40 min; The numbers above the panels indicate band densities with respect to control bands normalized to 1. Details of the experimental procedures are described in the “Materials and methods” section
Discussion
ROS can induce both apoptotic as well as antiapoptotic effects in the cell depending upon the nature and degree of the stimulus and the character of the signaling pathways that ROS may trigger (Dröge 2002; Schimmel and Bauer 2002). The role of ROS may, therefore, be more multifaceted than previously anticipated. Cellular signaling pathways can be triggered by ROS which in turn decide the fate of the cell by activating a number of kinases and different transcription factors. Growing evidence suggests that cancer cells show an augmented inherent ROS stress, due in part to oncogenic stimulation, increased metabolic activity, and greater mitochondrial malfunction (Hu et al. 2007a; Pelicano et al. 2004; Schimmel and Bauer 2002). As the levels of molecular chaperones are overexpressed in various malignancies (Calderwood and Ciocca 2008;Calderwood et al 2006; Chakraborty et al. 2008a; Jolly and Morimoto 2000), and prolonged thermal injury of the skin may lead to malignant transformation (Aziz et al. 1998), the role of ROS in modulating chaperone activity in heat stress through upregulation of survival kinases gains importance. Previous reports have shown both ROS generation and lack of ROS production by heat shock (Ilangovan et al. 2006; Maridonneau-Parini et al. 1988; Maridonneau-Parini et al. 1993; Mattson et al. 2004; Shin et al. 2008; Zhang et al. 2003). Here, we clearly show that heat stress in V79 fibroblasts produces ROS which is preventable by the well-known antioxidant NAC.
Furthermore, application of NAC blocks heat shock induction of p38MAPK activation. Our data clearly pinpoints the production of ROS by heat as an inducer of p38MAPK activation. Earlier studies have either observed lack of connection between ROS production and p38MAPK activation by heat shock (Dorion et al. 2002) or have linked p38MAPK activation in hyperthermia with ROS production (Shin et al. 2008). Here, we offer clear evidence of the link between hyperthermia, ROS production and ROS-mediated activation of p38MAPK. Furthermore, the overexpression of Hsp70, in chronically heat-stressed fibroblasts is totally dependent upon the activation of p38MAPK. We clearly visualized by confocal imaging that HSF1 translocation to the nucleus, an obvious prerequisite for the increased expression of heat shock proteins is blocked by the inhibition of p38MAPK by SB203580. Also, higher HSF1 DNA-binding activity observed in chronically heat shocked cells could be reversed by the presence of p38MAPK inhibitor SB203580. Therefore, p38MAPK is definitely upstream of the augmented HSF1 activity. Stresses other than heat like hypoxia (Uehara et al. 1999) or low pH (Rafiee et al. 2006) have been observed to raise Hsp70 levels along with an increased activity of p38MAPK. Low pH causes human esophageal endothelial cells to overexpress Hsp70 by increasing HSF1 transcriptional activity through mediation by p38MAPK and Akt (Rafiee et al. 2006). However, in alkalosis, increase of Hsp70 levels were not accompanied by augmented p38MAPK activation (Stathopoulou et al. 2006). Carbon monoxide exposure of lung cells produce elevated Hsp70 levels and the involvement of HSF1 is indicated (Kim et al. 2005a). We provide here a comprehensive picture of the heat shock response beginning with the production of ROS and culmination with the overexpression of Hsp70 protein. The key intermediary players in this signaling cascade are p38MAPK and Akt, and p38MAPK is the upstream controller of Akt activation. Earlier studies by our group in V79 fibroblasts (Chakraborty et al. 2008b) have also emphasized the importance of p38MAPK activation in the induction of pro-survival effects by repetitive/chronic low-grade H2O2 stress. This finding has been very recently corroborated by Jiang et al. in a similar repetitive low-grade H2O2 stress model in human umbilical vein endothelial cells -HUVECs (Jiang et al. 2008). These authors have further shown that enhanced PPAR-β activity, down-stream of p38MAPK activation exerts anti-apoptotic effects on HUVECs under repetitive H2O2 stress (Jiang et al. 2008).
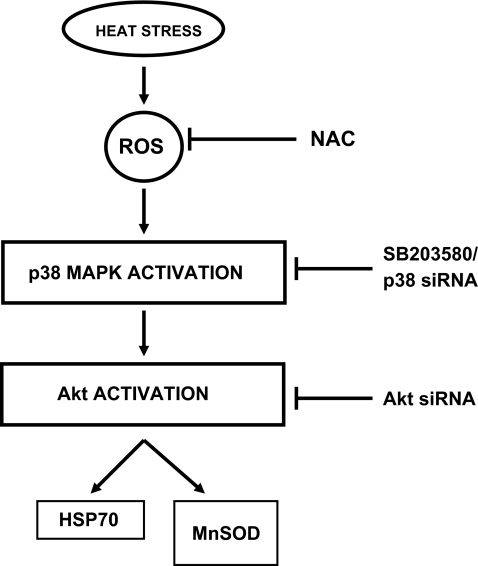
Mammalian p38 mitogen-activated protein kinases (MAPKs) are activated by a wide range of cellular stresses, both acute and chronic (Cuenda and Rousseau 2007; Johnson and Lapadat 2002). As p38MAPK activity is critical for normal immune and inflammatory responses, different components of this pathway have become potential targets for the treatment of autoimmune and inflammatory diseases (Zhang et al 2007). Then again, recent studies have shed light on the broad effect of p38MAPK activation on the regulation of many other aspects of cellular physiology. While a considerable number of studies describe proapototic roles of p38MAPK and its negative regulation in tumor formation (Han and Sun 2007), others have also connected p38MAPK-mediated signaling pathway with enhanced cell survival and tumor progression (Vega et al. 2004). In our present study, we demonstrate that the upregulation of p38MAPK in response to heat stress initiated the activation of a well-known survival kinase Akt and induced two survival –associated genes Hsp70 and MnSOD through mediation by Akt (Fig. 6). Earlier we have shown the upregulation of catalase levels by repetitive H2O2 stress (Sen et al. 2005). However, in chronic/repetitive heat stress catalase upregulation is not observed (data not shown) indicating perhaps the predominant generation of reactive oxygen species other than H2O2. Recent studies (Kiningham et al. 2008; Shilo et al. 2008) have demonstrated that MnSOD expression may be controlled by a number of transcription factors. The elements which control the transcription of MnSOD in heat-shocked cells and are downstream from Akt activation by heat shock remain to be elucidated. Akt/PKB is activated through phosphorylation on serine473 and threonine308 by upstream kinases (Sen et al. 2003). Akt activation by oxidative stress has been shown recently to be dependent on upstream activity of p38MAPK to a large extent (Chakraborty et al. 2008b). Here, we demonstrate that heat stress induction of Akt activation is also largely controlled by upstream p38MAPK. In summary, we firmly establish here the upstream network of signaling elements responsible for the upregulation of two cytoprotective genes by heat shock, namely, Hsp 70 and MnSOD.
Fig. 6.
Schematic representation of heat stress-induced signaling events leading to the increased expression of Hsp70 and MnSOD. Heat shock induces an enhancement of cellular ROS levels which in turn is responsible for the augmentation of p38MAPK activation. In heat-stressed V79 fibroblasts, Akt is also phosphorylated, and this is controlled by p38MAPK. Activated Akt functions upstream of the overexpression of both Hsp70 and MnSOD
Acknowledgement
We thank Dr. Swasti Roy Choudhuri, Structural Genomics section of our institute for his excellent help and guidance with the confocal microscopy. Partial financial support from grant no. SR/SO/HS-23/2003 from Department of Science and Technology, Government of India is acknowledged.
Abbreviations
- ROS
Reactive oxygen species
- Hsp
Heat shock protein
- MnSOD
Manganese superoxide dismutase
- HSF1
Heat shock factor 1
- NFKB
Nuclear factor kappa B
- CREB
cAMP response element-binding protein
- AP1
Activator protein 1
- MAPK
Mitogen-activated protein kinase
References
- Arya R, Mallik M, Lakhotia SC. Heat shock genes-integrating cell survival and death. J Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- Aziz SA, Hussain KS, Khan NA, Mushtaq A, Kharadi MY, Bhat JR. Profile of Kangari cancer: a prospective study. Burns. 1998;24:763–766. doi: 10.1016/S0305-4179(98)90094-8. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Ciocca DR. Heat Shock Proteins: Stress proteins with Janus-like properties in cancer. Int J Hypertherm. 2008;24:31–39. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer:chaperones of tumorigenesis. Trends Biochemic Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Chakraborty PK, Banerjee Mustafi S, Ganguly S, Chatterjee M, Raha S. Resveratrol induces apoptosis in K562 (Chronic myelogenous leukemia) cells by targeting a key survival protein Hsp70. Cancer Sc. 2008;99:1109–1116. doi: 10.1111/j.1349-7006.2008.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty PK, Banerjee Mustafi S, Raha S. Pro-survival effects of repetitive low-grade oxidative stress are inhibited by simultaneous exposure to Resveratrol. Pharmacol Res. 2008;58:281–289. doi: 10.1016/j.phrs.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rousseau S. p38 MAP-Kinases pathway regulation function and role in human diseases. Biochem Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Dorion S, Lambert H, Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J Biol Chem. 2002;277:30792–30797. doi: 10.1074/jbc.M203642200. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Goligorsky MS. The concept of cellular “fight-or-flight” reaction to stress. Am J Physiol Renal Physiol. 2001;280:F551–F561. doi: 10.1152/ajprenal.2001.280.4.F551. [DOI] [PubMed] [Google Scholar]
- Han J, Sun P. The pathways to tumor suppression via route p38. Trends Biochemic Sc. 2007;32:364–371. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Hu H, Luo ML, Du XL, Feng YB, Zhang Y, Shen XM, Xu X, Cai Y, Han YL, Wang MR. Up-regulated manganese superoxide dismutase expression increases apoptosis resistance in human esophageal squamous cell carcinomas. Chin Med J. 2007;120:2092–2098. [PubMed] [Google Scholar]
- Hu Y, Jin H, Du X, Xiao C, Luo D, Wang B, She R. Effects of chronic heat stress on immune responses of the foot-and-mouth disease DNA vaccination. DNA Cell Biol. 2007;26:619–626. doi: 10.1089/dna.2007.0581. [DOI] [PubMed] [Google Scholar]
- Ilangovan G, Venkatakrishnan CD, Bratasz A, Osinbowale S, Cardounel AJ, Zweier JL, Kuppusamy P. Heat shock-induced attenuation of hydroxyl radical generation and mitochondrial aconitase activity in cardiac H9c2 cells. Am J Physiol Cell Physiol. 2006;290:313–324. doi: 10.1152/ajpcell.00362.2005. [DOI] [PubMed] [Google Scholar]
- Jiang B, Liang P, Zhang B, Huang X, Xio X. Enhancement of PPAR-β activity by repetitive low-grade H2O2 stress protects human umbilical vein endothelial cells from subsequent oxidative stress-induced apoptosis. Free Radical Biol Med. 2008;46:555–563. doi: 10.1016/j.freeradbiomed.2008.10.051. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1922. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J NatlCancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38β MAPK and heat shock factor-1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- Kim HS, Skurk C, Maatz H, Shiojima I, Ivashchenko Y, Yoon SW, Park YB, Walsh K. Akt/FOXO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. FASEB J. 2005;19:1042–1044. doi: 10.1096/fj.04-2841fje. [DOI] [PubMed] [Google Scholar]
- Kiningham KK, Cardozo ZA, Cook C, Cole MP, Stewart JC, Tassone M, Coleman MC, Spitz DR. All-trans-retinoic acid induces manganese superoxide dismutase in human neuroblastoma through NF-κB. Free Radical Biol Med. 2008;44:1610–1616. doi: 10.1016/j.freeradbiomed.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Wen J, Zhang H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci. 2007;86:1059–1064. doi: 10.1093/ps/86.6.1059. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I, Clerc J, Polla BS. Heat shock inhibits NADPH oxidase in human neutrophils. Biochem Biophys Res Commun. 1988;154:179–186. doi: 10.1016/0006-291X(88)90667-5. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I, Malawista SE, Stubbe H, Russo-Marie F, Polla BS. Heat shock in human neutrophils: superoxide generation is inhibited by a mechanism distinct from heat-denaturation of NADPH oxidase and is protected by heat shock proteins in thermotolerant cells. J Cell Physiol. 1993;156:204–211. doi: 10.1002/jcp.1041560127. [DOI] [PubMed] [Google Scholar]
- Mattson D, Bradbury MC, Bisht KS, Curry HA, Spitz DR, Gius D. Heat shock and the activation of AP-1and inhibition of NFҝB DNA binding activity. Possible role of intracellular redox status. Int J Hypertherm. 2004;20:224–233. doi: 10.1080/02656730310001619956. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odongo NE, AlZahal O, Lindinger MI, Duffield TF, Valdes EV, Terrell SP, McBride BW. Effects of mild heat stress and grain challenge on acid-base balance and rumen tissue histology in lambs. J Anim Sci. 2006;84:447–455. doi: 10.2527/2006.842447x. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Update. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- Rafiee P, Theriot ME, Nelson VM, Heidemann J, Kanaa Y, Horowitz SA, Rogaczewski A, Johnson CP, Ali I, Shaker R, Binion DG. Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am J Physiol Cell Physiol. 2006;291:931–945. doi: 10.1152/ajpcell.00474.2005. [DOI] [PubMed] [Google Scholar]
- Schimmel M, Bauer G. Proapoptotic and redox state-related signaling of reactive oxygen species generated by transformed fibroblasts. Oncogene. 2002;21:5886–5896. doi: 10.1038/sj.onc.1205740. [DOI] [PubMed] [Google Scholar]
- Sen P, Mukherjee S, Ray D, Raha S. Involvement of the Akt signaling pathway with disease processes. Mol Cell Biochem. 2003;253:241–246. doi: 10.1023/A:1026020101379. [DOI] [PubMed] [Google Scholar]
- Sen P, Chakraborty PK, Raha S. p38 mitogen-activated protein kinase (p38MAPK) upregulates catalase levels in response to low dose H2O2 treatment through enhancement of mRNA stability. FEBS Lett. 2005;579:4402–4406. doi: 10.1016/j.febslet.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Shilo S, Pardo M, Aharoni-Simon M, Glibter S, Tirosh O. Selenium supplementation increases liver MnSOD expression: molecular mechanism for hepato-protection. J Inorganic Biochem. 2008;102:110–118. doi: 10.1016/j.jinorgbio.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Shin MH, Moon YJ, Seo JE, Lee Y, Kim KH, Chung JH. Reactive oxygen species produced by NADH oxidase, Xanthine oxidase and mitochondrial electron transport system mediate heat shock- induced MMP-1 and MMP-9 expression. Free Radical Biol Med. 2008;44:635–645. doi: 10.1016/j.freeradbiomed.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Stathopoulou K, Gaitanaki C, Beis I. Extracellular pH changes activate the p38-MAPK signalling pathway in the amphibian heart. J Exp Biol. 2006;209:1344–1354. doi: 10.1242/jeb.02134. [DOI] [PubMed] [Google Scholar]
- Tekin D, Xi L, Zhao T, Tejero-Taldo ML, Atluri S, Kukreja RC. Mitogen-activated protein kinases mediate heat shock-induced delayed protection in mouse heart. Am J Physiol Heart Circ Physiol. 2001;281:H523–532. doi: 10.1152/ajpheart.2001.281.2.H523. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Kaneko M, Tanaka S, Okuma Y, Nomura Y. Possible involvement of p38MAP kinase in Hsp70 expression induced by hypoxia in rat primary astrocytes. Brain Res. 1999;823:226–230. doi: 10.1016/S0006-8993(99)01178-6. [DOI] [PubMed] [Google Scholar]
- Vega ML, Huerta-Yepaz S, Garban H, Zazirehi A, Emmanouilides C, Bonavida B. Rituximab inhibits p38MAPK activity in 2F7BNHL and decreases IL-10 transcription: pivotal role of p38MAPK in drug resistance. Oncogene. 2004;23:3530–3540. doi: 10.1038/sj.onc.1207336. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Nishida M, Igarashi J, Taniguchi N, Tada M, Kuzuya T, Hori M. Heat shock-induced manganese superoxide dismutase enhances the tolerance of cardiac myocytes to hypoxia–reoxygenation injury. J Mol Cell Cardiol. 1997;29:1805–1813. doi: 10.1006/jmcc.1997.0415. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Xu L, Drake VJ, Xie L, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J. 2003;17:2293–2295. doi: 10.1096/fj.03-0139fje. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shen B, Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacologic Sci. 2007;28:286–295. doi: 10.1016/j.tips.2007.04.008. [DOI] [PubMed] [Google Scholar]