Abstract
Although heat shock (stress) proteins are typically regarded as being exclusively intracellular molecules, it is now apparent that they can be released from cells in the absence of cellular necrosis. We and others have reported the presence of Hsp60 (HSPD1) and Hsp70 (HSPA1A) in the circulation of normal individuals and our finding that increases in carotid intima-media thicknesses (a measure of atherosclerosis) in subjects with hypertension at a 4-year follow-up are less prevalent in those having high serum Hsp70 (HSPA1A) levels at baseline suggests that circulating Hsp70 (HSPA1A) has atheroprotective effects. Given that circulating Hsp70 (HSPA1A) levels can be in the range which has been shown to elicit a number of biological effects in vitro, and our preliminary findings that Hsp70 (HSPA1A) binds to and is internalised by human endothelial cell populations, we speculate on the mechanisms that might be involved in the apparent atheroprotective properties of this protein.
Keywords: Cardiovascular disease, Hsp70, Endothelial cells, Cell surface binding, Endocytosis, Flow cytometry
Note on nomenclature
A recent consensus document has attempted to arrive at a consistent and clear nomenclature for the heat shock protein and related chaperone genes in the human database (Kampinga et al. 2009). The majority of studies into levels of Hsp70 in the peripheral circulation have used enzyme immunoassays, for which the capture antibody is C92F3A-5 monoclonal antibody. Although this monoclonal antibody was originally described as being reactive with human Hsp72 (HSPA1A; Welch and Suhan 1986), it has since been referred to as anti-DAQB 147D11.1 001 antibody, anti-heat shock 70-kDa protein 1 antibody, anti-heat shock 70-kDa protein 1A antibody, anti-heat shock 70-kDa protein 1B antibody, anti-heat shock protein 70 antibody, anti-HSP70 1 antibody, anti-HSP70 2 antibody, anti-HSP70.1 antibody, anti-HSP72 antibody, anti-HSPA1 antibody, anti-HSPA1A antibody, and anti-HSPA1B antibody. Unfortunately, in some instances, it is difficult to ascertain the precise identity of the heat shock (stress) proteins to which investigators refer in the literature. Although it is therefore not possible to use the recommended nomenclature in all instances, this has been included whenever possible.
Introduction
Observations that the majority of myocardial infarctions are caused by atherosclerotic coronary lesions which obstruct less than 50% of the lumen (Falk et al. 1995; Mann and Davies 1996) have prompted a major re-evaluation of the pathogenesis of atherosclerosis and its complications. The biology of the plaque rather than its size is now regarded as being the most important determinant of its vulnerability (Libby 1995). The stability of the fibrous plaque depends on the balance between collagen synthesis and breakdown, and this balance is maintained by inflammatory signals (Libby 1995). If a pro-inflammatory state in which interferon gamma halts collagen synthesis by smooth muscle cells and activated macrophages secrete collagen-degrading matrix metalloproteinases dominates, then fibrous cap thinning, rupture and an acute ischaemic is likely (Blake and Ridker 2001).
We have identified a new biomarker for the progression of atherosclerosis, levels of which are, in contrast to the more accepted markers such as high-sensitivity C-reactive protein, inversely related to the progression of atherosclerosis (Pockley et al. 2003). This finding has potentially important implications for monitoring the susceptibility of individuals to and treating individuals with cardiovascular disease. Our finding that circulating levels of the 70-kDa heat shock (stress) proteins Hsp70 (HSPA1A) in some way influence atherogenesis and the progression to atherosclerosis (Pockley et al. 2003) has prompted us to consider the mechanism(s) via which such effects might be mediated. It is on this aspect of Hsp70 biology that this chapter focuses.
Circulating stress proteins and cardiovascular disease
Although for many years the perception has been that stress proteins are intracellular molecules that are only released from non-viable (necrotic) cells, it is now known that these molecules can be released from a variety of viable (non-necrotic) mammalian cells, including endothelial cells (ECs; Hightower and Guidon 1989; Child et al. 1995; Bassan et al. 1998; Liao et al. 2000). Furthermore, Hsp60 (HSPD1) and Hsp70 (HSPA1A) have been shown to be present in the peripheral circulation of normal individuals by a number of investigators (Pockley et al. 1998, 1999, 2000, 2002; Xu et al. 2000; Rea et al. 2001; Lewthwaite et al. 2002; Njemini et al. 2003). The impact that circulating stress proteins have on pathophysiological processes, if any, is currently unclear.
Although circulating Hsp60 (HSPD1) levels are not associated with cardiovascular risk factors such as body mass index, blood pressure and smoking status (Pockley et al. 2000), levels are elevated in a subpopulation of patients with acute coronary syndromes (Zal et al. 2004). Levels are also associated with early atherosclerosis (on the basis of carotid intima-media thicknesses; Pockley et al. 2000; Xu et al. 2000) and with serum concentrations of the pro-inflammatory cytokine tumour necrosis alpha (TNF-α) and other markers of inflammation in overtly healthy individuals (Lewthwaite et al. 2002). Circulating Hsp60 (HSPD1) is higher in individuals exhibiting an unfavourable lipid profile (low high-density lipoprotein cholesterol and high total/high-density lipoprotein cholesterol ratio; Lewthwaite et al. 2002) and levels are associated with very-low-density lipoprotein and triglyceride concentrations (Pockley et al. 2000). In contrast, Hsp70 (HSPA1A) levels are not associated with very-low-density lipoprotein and triglyceride concentrations (Pockley et al. 2000), and there is no relationship between Hsp70 (HSPA1A) levels and intima-media thicknesses in normal subjects, subjects with established hypertension or subjects with borderline hypertension (Pockley et al. 2000, 2002)
An interesting and potentially important observation has been made in a study of 218 subjects with established hypertension which has shown that increases in carotid intima-media thicknesses (a measure of atherosclerosis) over a 4-year follow-up period are significantly less prevalent (odds ratio 0.42; p < 0.008) in individuals having high serum Hsp70 (HSPA1A) levels (75th percentile, >300 ng/ml) at baseline (Pockley et al. 2003). The relationship between Hsp70 (HSPA1A) levels and changes in intima-media thickness was independent of age, smoking habits and blood lipids. Another study of 421 individuals evaluated for coronary artery disease has found that serum Hsp70 (HSPA1A) levels were significantly higher in disease-free patients (Zhu et al. 2003). Furthermore, healthy end arteries secrete more Hsp70 than carotid atherosclerotic plaques, and low plasma levels of Hsp70 (HSPA1A) are found in patients with atherosclerosis (Martin-Ventura et al. 2007). The source of circulating Hsp70 (HSPA1A), the mechanism(s) leading to its release and the association between circulating Hsp70 (HSPA1A) and atherosclerosis are unknown. How, then, might extracellular Hsp70 (HSPA1A) influence the development and progression of cardiovascular disease?
Influence of Hsp70 on inflammatory status
Although Hsp70 is typically regarded as being a pro-inflammatory activator of innate immune cells (Asea et al. 2000, 2002; Gastpar et al. 2005), the induction of T cell reactivity to self-Hsp70 epitopes downregulates inflammatory events in experimental models by a mechanism which involves the generation of immunoregulatory (Th2) cells producing the regulatory cytokine interleukin (IL)-10 (Kingston et al. 1996; Tanaka et al. 1999; Wendling et al. 2000). More recently, mycobacterial Hsp70 has been shown to suppress inflammation and tissue damage in proteoglycan-induced arthritis via a mechanism which involves an enhanced regulatory response mediated by antigen-specific IL-10 production (Wieten et al. 2009). The bias of immune reactivity towards a Th2 phenotype is relevant, as the promotion of Th2 reactivity reduces atherogenesis in the apoE−/− mice (Laurat et al. 2001). Although the precise mechanism underlying the anti-inflammatory properties of Hsp70 has yet to be elucidated, it might involve the recruitment/activation of immunoregulatory T cell (Treg) populations. This is possible given that Hsp70 and another stress protein, Hsp60 (HSPD1), are putative ligands for Toll-like receptors (TLRs; Binder et al. 2004), that TLRs are expressed on naturally occurring CD4+CD25+ regulatory T cells (Caramalho et al. 2003) and that the ligation of TLRs on CD4+CD25+ T cells induces a tenfold increase in their suppressive activity (Caramalho et al. 2003). Indeed, human Hsp60 (HSPD1) co-stimulates and activates CD4+CD25+ Treg cell populations via interactions with TLR2 and enhances their capacity to regulate CD8+ T cell activity (Zanin-Zhorov et al. 2006). It is not currently known whether Hsp70 has similar properties, although we have shown that Hsp70 (HSPA1A) binds to and preferentially activates CD4+CD25+ Treg cell populations (manuscript in preparation). These studies support our proposition that Hsp70 (HSPA1A) influences the progression of atherosclerosis as such Treg cell populations have been shown to control its development and progression (Mallat et al. 2007).
Another level at which Hsp70 might influence inflammatory events is at the level of gene transcription for inflammatory cytokines such as IL-6. Hsp70 functions as a wide spectrum negative regulator and restrains a range of processes by inhibiting the activities of protein kinases and transcription factors (Nollen and Morimoto 2002; Pratt and Toft 2003). Indeed, elevated Hsp70 levels inhibit a plethora of intracellular processes and Hsp70 levels are strictly regulated via negative control of its transcription factor heat shock factor 1 (HSF1) and its destabilisation at the messenger RNA level (Feder et al. 1992; Chu et al. 1996; Zhao et al. 2002; Wang et al. 2003). HSF1 itself can negatively regulate the promoters of cytokine genes and genes involved in cell proliferation (Xie et al. 2002, 2003). Stress proteins such as Hsp70 have the potential to foster an anti-inflammatory environment which might attenuate atherosclerosis (George et al. 1998; Zhou et al. 2001; Binder et al. 2002; Hansson 2002). Hsp70 could also influence atherosclerosis via direct effects on ECs.
Influence of Hsp70 on leucocyte adhesive events
The recruitment and transmigration of inflammatory cells plays a central role in atheroma development and the pathogenesis of atherosclerosis (Price and Loscalzo 1999; Blankenberg et al. 2003; de Boer et al. 2003). The vascular cell adhesion molecule 1 (VCAM-1) is rapidly expressed in pro-atherosclerotic conditions and plays a critical role in atherosclerosis (O'Brien et al. 1993; Nakai et al. 1995; Nakashima et al. 1998; Cybulsky et al. 2001; Dansky et al. 2001). A monoclonal antibody against VCAM-1 profoundly reduces neointimal formation after carotid injury in a mouse model (Oguchi et al. 2000), and, in a primate model, antibody blockade of the ligand for VCAM-1 (α4β1 integrin) reduces intimal hyperplasia in endarterectomised carotid arteries (Lumsden et al. 1997). In addition, anti-CD40L treatment of hyperlipidaemic mice reduces atherosclerosis by reducing the expression of VCAM-1 and thereby inhibiting the accumulation of inflammatory cells (Mach et al. 1998b).
It is known that elevations of intracellular Hsp70 decrease TNF-α-induced intercellular adhesion molecule (ICAM-1) expression (Kohn et al. 2002) and inhibit in vivo leukocyte adhesion to mesenteric endothelium in response to a potent inflammatory stimulus (House et al. 2001), as well as their secretion of IL-6 (Kim et al. 2005). The induction of intracellular Hsp70 expression also attenuates TNF-α-mediated induction of adhesion molecule expression on human ECs (Nakabe et al. 2007). This anti-adhesive effect of exogenous and intracellular Hsp70 might be explained by the observation that the induction of Hsp70 decreases TNF-α-induced ICAM-1 expression via an inhibition of I kappa kinase activity (Kohn et al. 2002). Hsp70 might therefore maintain/induce an anti-adhesive phenotype in the vascular endothelium and thereby an anti-inflammatory/anti-atherogenic state. Hsp70 might also modify non-immunological events via direct interactions with vascular endothelial cells.
Influence of Hsp70 on endothelial necrosis and apoptosis?
Apoptosis of endothelial cells and smooth muscle cells is a consequence of vascular injury and contributes to atherosclerosis and the acute events that trigger myocardial infarction, including plaque rupture (MacLellan and Schneider 1997; Dimmeler et al. 1998; Dimmeler and Zeiher 2000; Stoneman and Bennett 2004; Viles-Gonzales et al. 2004). Hsp70 prevents caspase-3 and SAPK/JNK activation in heat-shock- or ceramide-induced apoptosis (Mosser et al. 1997; Ahn et al. 1999). Although the cytoprotective effects of intracellular Hsp70 are established (Jäättelä et al. 1992; Simon et al. 1995; Samali and Cotter 1996; Lasunskaia et al. 1997; Mosser et al. 1997), exogenous Hsp70 protects heat-stressed cynomolgus macaque aortic cells (Johnson et al. 1990) and serum-deprived rabbit arterial smooth muscle cells (Johnson and Tytell 1993), the latter by a mechanism which involves cell association but not internalisation. The mechanism by which such protection is afforded is currently unknown, and the cell surface interactions involved have not been identified. Hsp70 might have some impact on atheroma denudation, and atherothrombotic events by preventing endothelial cell death and apoptosis.
Influence of Hsp70 on endothelial function/dysfunction?
The vascular endothelium is an active autocrine and paracrine organ which regulates vascular tone and maintains blood circulation and fluidity. It also regulates platelet activation, thrombosis and inflammatory responses by producing several locally active substances. Key to the maintenance of a normal haemorheological status is the control of vasomotion via the balanced production of potent vasodilators such as nitric oxide (NO) and/or adenosine and vasoconstrictors such as endothelin-1 and angiotensin. Endothelial cells also play an important role in chronic inflammatory disease by co-ordinating the onset, progression, and resolution of inflammation by promoting or attenuating leukocyte extravasation at inflammatory sites (Krieglstein and Granger 2001) and by expressing pro-inflammatory gene products that further augment the activation of infiltrating leucocytes (Kinlay et al. 2001). NO mediates many of the functions exerted by the intact endothelium and, in addition to being a potent vasodilator, it inhibits platelet aggregation, vascular smooth muscle cell migration and proliferation, monocyte adhesion and adhesion molecule expression. All of these are key events in the development and progression of atherosclerosis.
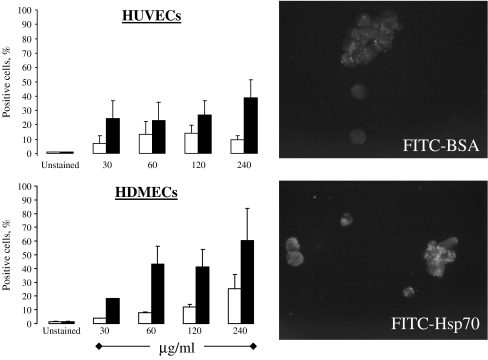
Role of the endothelium in atherosclerosis and atherothrombosis A deterioration of endothelial vasodilator function can manifest as a decreased secretion of vasodilatory mediators, an increased production of vasoconstrictors, an increased sensitivity to vasoconstrictors and/or a resistance of vascular smooth muscle to the effects of endothelial vasodilators. The endothelium plays a central role in the development and progression of atherosclerotic lesions, and its dysfunction is one of the earliest disease markers in patients with atherogenic risk factors in the absence of angiographic evidence of disease (Vita et al. 1990; Egashira et al. 1993).In the early stages of the disease, the endothelium appears to be morphologically normal, but functionally impaired, in that there is a decreased bioavailability of NO. In more advanced disease, endothelial cell apoptosis compromises the integrity of the endothelial layer which in turn results in plaque destabilisation, denudation and the development of atherothrombotic events (Valgimigli et al. 2003). Vascular endothelial cells express or can be induced to express the TNF-α family protein CD40 (Hollenbaugh et al. 1995; Karmann et al. 1995; Yellin et al. 1995; Mach et al. 1998a; Lienenlüke et al. 2000; Ahmed-Choudhury et al. 2003), oxidised low-density lipoprotein receptor-1 (LOX-1; Sawamura et al. 1997; Li and Mehta 2000; Hu et al. 2003; Li et al. 2003; Masaki 2003) and TLR-2 and -4 (Faure et al. 2000, 2001; Bulut et al. 2002; Edfeldt et al. 2002; Hijiya et al. 2002; Zeuke et al. 2002; de Kleijn and Pasterkamp 2003). Although not typically thought to constitutively express membrane CD14 (Goyert et al. 1986; Haziot et al. 1988; Wright et al. 1990; Kirkland et al. 1993), one study has reported endothelial cells to express this molecule (Jersmann et al. 2001). Hsp70 has been reported to bind to and activate innate immune cells via these receptors (Wang et al. 2001; Asea et al. 2002; Becker et al. 2002; Delneste et al. 2002; Vabulas et al. 2002), and, importantly, the concentrations of Hsp70 that are required to induce such in vitro responses (0.1–5 μg/ml (Asea et al. 2000, 2002; Vabulas et al. 2002)) are present and in some cases are below those that have been reported to be present in the circulation (Pockley et al. 1998, 2000, 2002, 2003; Rea et al. 2001; Njemini et al. 2003, 2004). Hsp70 might therefore interact with and influence the functional phenotype of endothelial cells in vivo.The Calderwood laboratory has shown that human Hsp70 binds to human umbilical vein endothelial cells (HUVECs), although the receptors involved and the biological consequences have yet to be determined (Thériault et al. 2005). Although the Calderwood laboratory could demonstrate significant binding of Hsp70 to the C-type lectin receptor LOX-1, this did not account for the total binding capacity of these cells. Binding of Hsp70 to the “more established” receptors CD91, CD40, CD14, TLR2 or TLR4 could not be demonstrated (Thériault et al. 2005). It therefore appears that endothelial cells express as yet unidentified receptor(s) for Hsp70 that are distinct to those that have already been identified on antigen-presenting cells such as monocytes/macrophages and dendritic cells.We have recently confirmed that recombinant human Hsp70 (HSPA1A) binds to HUVECs, and also shown that it is rapidly internalised and localised to defined intracellular organelles (Fig. 1). The features of Hsp70 (HSPA1A) internalisation and its intracellular localisation are distinct to those of bovine serum albumin (BSA; Fig. 1). Importantly, Hsp70 (HSPA1A) binds more prevalently to primary human dermal microvascular endothelial cells (Fig. 1), suggesting that the biological and physiological effects of Hsp70 (HSPA1A)–endothelial cell interactions might be vascular compartment dependent.
Fig. 1.
Left: binding of FITC-BSA (white bars) and FITC-conjugated recombinant human Hsp70 (HSPA1A; black bars) to human umbilical vein endothelial cells (HUVECs; upper) and human dermal microvascular endothelial cells (HDMECs; lower) as determined by flow cytometry (means + SEM). Cells were incubated with FITC-Hsp70 (HSPA1A) and BSA for 30 min at 4°C and the binding of the fluorescently labelled proteins to viable (propidium iodide negative) cells was determined by multicolour flow cytometry. Right: Internalisation and intracellular localisation of FITC-BSA (upper) and FITC- Hsp70 (HSPA1A; lower) by HUVECs (1 h, 37°C). Cells were incubated with 240 μg/ml FITC-conjugated Hsp70 (HSPA1A) or BSA for 1 h at 37°C and the uptake of fluorescently labelled proteins was determined by fluorescence microscopy using Zeiss AxioVision digital image processing software. His-tagged Hsp70 protein was generated using a baculovirus expression system (multimmune GmbH, Munich, Germany) and was labelled with fluorescein isothiocyanate (FITC; F6434, Invitrogen, Eugene, OR, USA), according to the manufacturer’s instructions
Conclusion
The observations that high circulating levels of the 70-kDa stress protein Hsp70 (HSPA1A) appear to protect against cardiovascular disease prompt questions into the mechanism(s) that might be involved in the manifestation of such effects. These might include the inherent anti-inflammatory properties of Hsp70 and/or its direct effects on the biology and functional status of endothelial cells. The development of new molecular imaging techniques that are capable of imaging endothelial-expressed proteins such as Hsp60 (HSPD1) in vivo (Wick et al. 2008) might also provide new insight into real-time stress protein–endothelial interactions. In summary, studies are now revealing new mechanistic insights into the apparent atheroprotective effects of extracellular Hsp70, and these are likely to result in the development of new approaches for predicting the development and progression of cardiovascular disease and novel therapeutic strategies for its effective management.
Acknowledgements
We thank Dr. Mathias Gehrmann (Technische Universität München) for performing the fluorescent microscopy. We also thank Professor Nicola J. Brown (University of Sheffield) for providing primary human dermal microvascular endothelial cells.
References
- Ahmed-Choudhury J, Russell CL, Randhawa S, Young LS, Adams DH, Afford SC. Differential induction of nuclear factor-B and activator protein-1 activity after CD40 ligation is associated with primary human hepatocyte apoptosis or intrahepatic endothelial cell proliferation. Mol Biol Cell. 2003;14:1334–1345. doi: 10.1091/mbc.E02-07-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS. Suppression of ceramide-mediated apoptosis by HSP70. Mol Cells. 1999;9:200–206. [PubMed] [Google Scholar]
- Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70. Role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Giladi E, Davidson A, Wollman Y, Pitman J, Hauser J, Brenneman DE, Gozes I. The identification of secreted heat shock 60-like protein from rat glial cells and a human neuroblastoma cell line. Neurosci Lett. 1998;250:37–40. doi: 10.1016/S0304-3940(98)00428-5. [DOI] [PubMed] [Google Scholar]
- Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Novel clinical markers of vascular inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/S0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435–1440. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child DF, Williams CP, Jones RP, Hudson PR, Jones M, Smith CJ. Heat shock protein studies in type 1 and type 2 diabetes and human islet cell culture. Diabetic Med. 1995;12:595–599. doi: 10.1111/j.1464-5491.1995.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271:30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis M, Gutierrez-Ramos J-S, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- Boer OJ, Becker AE, Wal AC. T lymphocytes in atherogenesis—functional aspects and antigenic repertoire. Cardiovasc Res. 2003;60:78–86. doi: 10.1016/S0008-6363(03)00341-9. [DOI] [PubMed] [Google Scholar]
- Kleijn D, Pasterkamp G. Toll-like receptors in cardiovascular diseases. Cardiovasc Res. 2003;60:58–67. doi: 10.1016/S0008-6363(03)00348-1. [DOI] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/S1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Reactive oxygen species and vascular cell apoptosis in response to angiotensin II and pro-atherosclerotic factors. Reg Pep. 2000;90:19–25. doi: 10.1016/S0167-0115(00)00105-1. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Hermann C, Zeiher AM. Apoptosis of endothelial cells. Contribution to the pathophysiology of atherosclerosis? Eur Cytokine Network. 1998;9:697–698. [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- Egashira K, Inou T, Hirooka Y, Yamada A, Maruoka Y, Kai H, Sugimachi M, Suzuki S, Takeshita A. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91:29–37. doi: 10.1172/JCI116183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-κB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-γ induce toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-κB activation. J Immunol. 2001;166:2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y, Harats D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–152. doi: 10.1016/S0021-9150(98)00015-X. [DOI] [PubMed] [Google Scholar]
- Goyert SM, Ferrero EM, Seremetis SV, Winchester RJ, Silver J, Mattison AC. Biochemistry and expression of myelomonocytic antigens. J Immunol. 1986;137:3909–3914. [PubMed] [Google Scholar]
- Hansson GK. Vaccination against atherosclerosis. Science or fiction? Circulation. 2002;106:1599–1601. doi: 10.1161/01.CIR.0000035275.64667.A3. [DOI] [PubMed] [Google Scholar]
- Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- Hightower LE, Guidon PT. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Hijiya N, Miyake K, Akashi S, Matsuura K, Higuchi Y, Yamamoto S. Possible involvement of toll-like receptor 4 in endothelial cell activation of larger vessels in response to lipopolysaccharide. Pathobiology. 2002;70:18–25. doi: 10.1159/000066000. [DOI] [PubMed] [Google Scholar]
- Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, Aruffo A. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995;182:33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House SD, Guidon PTJ, Perdrizet GA, Rewinski M, Kyriakos R, Bockman RS, Mistry T, Gallagher PA, Hightower LE. Effects of heat shock, stannous chloride, and gallium nitrate on the rat inflammatory response. Cell Stress Chaperones. 2001;6:164–171. doi: 10.1379/1466-1268(2001)006<0164:EOHSSC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Li D, Sawamura T, Mehta JL. Oxidized LDL through LOX-1 modulates LDL-receptor expression in human coronary artery endothelial cells. Biochem Biophys Res Commun. 2003;307:1008–1012. doi: 10.1016/S0006-291X(03)01295-6. [DOI] [PubMed] [Google Scholar]
- Jäättelä M, Wissing D, Bauer PA, Li GC. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jersmann HP, Hii CS, Hodge GL, Ferrante A. Synthesis and surface expression of CD14 by human endothelial cells. Infect Immun. 2001;69:479–485. doi: 10.1128/IAI.69.1.479-485.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Tytell M. Exogenous Hsp70 becomes cell associated, but not internalised by stressed arterial smooth muscle cells. In Vitro Cell Dev Biol. 1993;29A:807–812. doi: 10.1007/BF02634348. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Berberian PA, Bond MG. Effect of heat shock proteins on survival of isolated aortic cells from normal and atherosclerotic cynomolgus macaques. Atherosclerosis. 1990;84:111–119. doi: 10.1016/0021-9150(90)90080-3. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc Natl Acad Sci U S A. 1995;92:4342–4346. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Shin HM, Baek W. Heat-shock response is associated with decreased production of interleukin-6 in murine aortic vascular smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:27–33. doi: 10.1007/s00210-004-1007-5. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Hicks CA, Colston MJ, Billingham MEJ. A 71-kD heat shock protein (hsp) from Mycobacterium tuberculosis has modulatory effects on experimental rat arthritis. Clin Exp Immunol. 1996;103:77–82. doi: 10.1046/j.1365-2249.1996.929628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlay S, Libby P, Ganz P. Endothelial function and coronary artery disease. Curr Opin Lipidol. 2001;12:383–389. doi: 10.1097/00041433-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Kirkland TN, Finley F, Leturcq D, Moriarty A, Lee JD, Ulevitch RJ, Tobias PS. Analysis of lipopolysaccharide binding by CD14. J Biol Chem. 1993;268:24818–24823. [PubMed] [Google Scholar]
- Kohn G, Wong HR, Bshesh K, Zhao B, Vasi N, Denenberg A, Morris C, Stark J, Shanley TP. Heat shock inhibits TNF-induced ICAM-1 expression in human endothelial cells via I kappa kinase inhibition. Shock. 2002;17:91–97. doi: 10.1097/00024382-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertension. 2001;14:44S–54S. doi: 10.1016/S0895-7061(01)02069-6. [DOI] [PubMed] [Google Scholar]
- Lasunskaia EB, Fridlianskaia II, Guzhova IV, Bozhkov VM, Margulis BA. Accumulation of major stress protein 70 kDa protects myeloid and lymphoid cells from death by apoptosis. Apoptosis. 1997;2:156–163. doi: 10.1023/A:1026460330596. [DOI] [PubMed] [Google Scholar]
- Laurat E, Poirier B, Tupin E, Caligiuri G, Hansson GK, Bariéty J, Nicoletti A. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- Lewthwaite J, Owen N, Coates A, Henderson B, Steptoe A. Circulating human heat shock protein 60 in the plasma of British civil servants. Circulation. 2002;106:196–201. doi: 10.1161/01.CIR.0000021121.26290.2C. [DOI] [PubMed] [Google Scholar]
- Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implication in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20:1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- Li D, Liu L, Chen H, Sawamura T, Mehta JL. LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:816–821. doi: 10.1161/01.ATV.0000066685.13434.FA. [DOI] [PubMed] [Google Scholar]
- Liao D-F, Jin Z-G, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- Lienenlüke B, Germann T, Kroczek RA, Hecker M. CD154 stimulation of interleukin-12 synthesis in human endothelial cells. Eur J Immunol. 2000;30:2864–2870. doi: 10.1002/1521-4141(200010)30:10<2864::AID-IMMU2864>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lumsden AB, Chen C, Hughes JD, Kelly AB, Hanson SR, Harker LA. Anti-VLA-4 antibody reduces intimal hyperplasia in the endarterectomized carotid artery in nonhuman primates. J Vasc Surg. 1997;26:87–93. doi: 10.1016/S0741-5214(97)70151-4. [DOI] [PubMed] [Google Scholar]
- Mach F, Schonbeck U, Libby P. CD40 signaling in vascular cells: a key role in atherosclerosis? Atherosclerosis. 1998;137(Suppl):S89–S95. doi: 10.1016/S0021-9150(97)00309-2. [DOI] [PubMed] [Google Scholar]
- Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signaling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- MacLellan WR, Schneider MD. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res. 1997;81:137–144. doi: 10.1161/01.res.81.2.137. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–118. doi: 10.1016/j.tcm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Mann JM, Davies MJ. Vulnerable plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation. 1996;94:928–931. doi: 10.1161/01.cir.94.5.928. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, Michel JB. Low plasma levels of HSP70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atherosclerosis. 2007;194:334–341. doi: 10.1016/j.atherosclerosis.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Masaki T. Endothelial dysfunction and LOX-1: forty years from muscle to endothelium. Circ Res. 2003;92:819–820. doi: 10.1161/01.RES.0000071523.67730.5F. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabe N, Kokura S, Shimozawa M, Katada K, Sakamoto N, Ishikawa T, Handa O, Takagi T, Naito Y, Yoshida N, Yoshikawa T. Hyperthermia attenuates TNF-α-induced up regulation of endothelial cell adhesion molecules in human arterial endothelial cells. Int J Hyperthermia. 2007;23:217–224. doi: 10.1080/02656730601143295. [DOI] [PubMed] [Google Scholar]
- Nakai K, Itoh C, Kawazoe K, Miura Y, Sotoyanagi H, Hotta K, Itoh T, Kamata J, Hiramori K. Concentration of soluble vascular cell adhesion molecule-1 (VCAM-1) correlated with expression of VCAM-1 mRNA in the human atherosclerotic aorta. Coron Artery Dis. 1995;6:497–502. [PubMed] [Google Scholar]
- Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Mets T. Elevated serum heat-shock protein 70 levels in patients with acute infection: use of an optimized enzyme-linked immunosorbent assay. Scand J Immunol. 2003;58:664–669. doi: 10.1111/j.1365-3083.2003.01341.x. [DOI] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2004;5:31–38. doi: 10.1023/B:BGEN.0000017684.15626.29. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing “heat shock” proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi S, Dimayuga P, Zhu J, Chyu KY, Yano J, Shah PK, Nilsson J, Cercek B. Monoclonal antibody against vascular cell adhesion molecule-1 inhibits neointimal formation after periadventitial carotid artery injury in genetically hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2000;20:1729–1736. doi: 10.1161/01.atv.20.7.1729. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton J. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1379/1466-1268(1999)004<0029:IOHHSP>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, Faire U, Frostegård J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815–1820. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faire U, Frostegård J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107:85–97. doi: 10.1016/S0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontology. 2001;36:341–352. doi: 10.1016/S0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, Jäättelä M, Schwarz T. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995;95:926–933. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci. 2004;107:343–354. doi: 10.1042/CS20040086. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kimura Y, Mitani A, Yamamoto G, Nishimura H, Spallek R, Singh M, Noguchi T, Yoshikai Y. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5565. [PubMed] [Google Scholar]
- Thériault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Merli E, Malagutti P, Soukhomovskaia O, Cicchitelli G, Macri G, Ferrari R. Endothelial dysfunction in acute and chronic coronary syndromes: evidence for a pathogenetic role of oxidative stress. Arch Biochem Biophys. 2003;420:255–261. doi: 10.1016/j.abb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Viles-Gonzales JF, Fuster V, Badimin JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J. 2004;25:1197–1207. doi: 10.1016/j.ehj.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kelly CG, Karttunen T, Whittall T, Lehner PJ, Duncan L, MacAry P, Younson JS, Singh M, Oehlmann W, Cheng G, Bergmeier L, Lehner T. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity. 2001;15:971–983. doi: 10.1016/S1074-7613(01)00242-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Grammatikakis N, Siganou A, Calderwood SK. Regulation of molecular chaperone gene transcription involves the serine phosphorylation, 14-3-3 epsilon binding, and cytoplasmic sequestration of heat shock factor 1. Mol Cell Biol. 2003;23:6013–6026. doi: 10.1128/MCB.23.17.6013-6026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Suhan JP. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986;103:2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling U, Paul L, Zee R, Prakken B, Singh M, Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Wick MC, Mayerl C, Backovic A, Zee R, Jaschke W, Dietrich H, Wick G. In vivo imaging of the effect of LPS on arterial endothelial cells: molecular imaging of heat shock protein 60 expression. Cell Stress Chaperones. 2008;13:275–285. doi: 10.1007/s12192-008-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieten L, Berlo SE, Ten Brink CB, Kooten PJ, Singh M, Zee R, Glant TT, Broere F, Eden W. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. PLoS ONE. 2009;4:e4186. doi: 10.1371/journal.pone.0004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Xie Y, Chen C, Stevenson MA, Hume DA, Auron PE, Calderwood SK. NF-IL6 and HSF1 have mutually antagonistic effects on transcription in monocytic cells. Biochem Biophys Res Commun. 2002;291:1071–1080. doi: 10.1006/bbrc.2002.6562. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhong R, Chen C, Calderwood SK. Heat shock factor 1 contains two functional domains that mediate transcriptional repression of the c-fos and c-fms genes. J Biol Chem. 2003;278:4687–4698. doi: 10.1074/jbc.M210189200. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- Yellin MJ, Brett J, Baum D, Matsushima A, Szabolcs M, Stern D, Chess L. Functional interactions of T cells with endothelial cells: the role of CD40L-CD40-mediated signals. J Exp Med. 1995;182:1857–1864. doi: 10.1084/jem.182.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal B, Kaski JC, Arno G, Akiyu JP, Xu Q, Cole D, Whelan M, Russell N, Madrigal JA, Dodi IA, Baboonian C. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation. 2004;109:1230–1235. doi: 10.1161/01.CIR.0000118476.29352.2A. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zeuke S, Ulmer AJ, Kusumoto S, Katus HA, Heine H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res. 2002;56:126–134. doi: 10.1016/S0008-6363(02)00512-6. [DOI] [PubMed] [Google Scholar]
- Zhao M, Tang D, Lechpammer S, Hoffman A, Asea A, Stevenson MA, Calderwood SK. Double-stranded RNA-dependent protein kinase (pkr) is essential for thermotolerance, accumulation of HSP70, and stabilization of ARE-containing HSP70 mRNA during stress. J Biol Chem. 2002;277:44539–44547. doi: 10.1074/jbc.M208408200. [DOI] [PubMed] [Google Scholar]
- Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1055–1059. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]