Abstract
Background
Adjuvant radiotherapy is common for uterine corpus cancer patients, yet the long-term carcinogenic effects of different types of radiotherapy have not been studied adequately.
Methods
Second primary cancer risks were quantified in a cohort of 60,949 individuals surviving one or more years of uterine corpus cancer diagnosed 1973–2003 in Surveillance, Epidemiology and End Results Program cancer registries. Incidence Rate Ratios (IRR) were estimated by comparing patients treated with surgery plus various types of radiotherapy with patients receiving surgery only.
Results
The IRRs of a second cancer were increased among irradiated patients compared with patients having surgery only (combination radiotherapy, IRR=1.26, 95% confidence interval [CI] 1.16–1.36; external beam therapy, IRR=1.15, CI 1.08–1.22; brachytherapy, IRR=1.07, CI 1.00–1.16). IRRs were highest for heavily-irradiated sites (i.e., colon, rectum, and bladder) and for leukemia following any external beam therapy, with the largest risks for solid cancers among ten-year survivors. Any external beam therapy had a 44% higher cancer risk at heavily-irradiated sites than brachytherapy when the two treatments were directly compared (five-year survivors: IRR=1.44, CI 1.19–1.75). We estimated that of 2012 solid cancers developing five or more years after irradiation, 213 (11%) could be explained by radiotherapy.
Conclusions
Radiotherapy for uterine cancer increases the risk of leukemia and second solid cancers at sites in close proximity to the uterus, emphasizing the need for continued long-term surveillance for new malignancies. The overall risk of a second cancer was lower following brachytherapy compared with any external beam radiotherapy.
Keywords: endometrial adenocarcinomas, epidemiology, gynecologic malignancy, radiation, radiotherapy
INTRODUCTION
Uterine corpus cancer (UCC) is the most common gynecologic malignancy in the U.S. and the fourth most common cancer among women.1 The disease is rare before the age of 45 years and the incidence peaks between 60 and 70 years of age.2 A high percentage of women are diagnosed with disease confined to the uterus resulting in overall five-year relative survival rates of 80 to 90%.2 More than 90% of UCCs are endometrial adenocarcinomas or carcinomas of the uterine body; sarcomas and other subtypes are uncommon.3 Approximately 90% of patients are treated surgically with removal of the uterus, cervix, ovaries, and fallopian tubes.3,4 UCC patients often receive adjuvant radiotherapy to the pelvis, typically including external beam radiation alone, radioactive implants (brachytherapy) alone, or a combination of both types.3,4
High-dose radiotherapy to the pelvis increases the risk of second primary cancers, particularly for cancers of the colon, rectum, bladder, and leukemia.5–9 However, data on second cancer risk for different types of radiotherapy are sparse, and, to our knowledge, no studies directly compare the risks among UCC patients treated with external beam therapy versus brachytherapy. External beam therapy to the pelvis delivers substantially greater radiation doses to abdominal organs than brachytherapy, especially to organs not immediately adjacent to the uterus. We evaluated the risks of second malignancies in a large population-based cohort of women diagnosed with carcinoma of the uterine corpus and quantified second cancer risk associated with radiation treatment compared to surgery alone. Further, we estimated the radiation dose to specific organs for typical treatments from each type of radiotherapy and quantified risks of second primary cancers of organs receiving relatively high, moderate, and low doses.
PATIENTS AND METHODS
Study population
Women with a first primary invasive adenocarcinoma or carcinoma of the uterine corpus (excluding sarcomas and other cell types) diagnosed between 1973 and 2003 at ages 15 to 79 years were identified (excluding women with prior malignancies) from nine population-based cancer registries reporting to the U.S. National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program.10 The SEER Program is a set of geographically defined, population-based tumor registries in the United States, operated by local nonprofit organizations under contract to the National Cancer Institute (NCI). Each registry annually submits its cases to the NCI on a computer tape. These computer tapes are then edited by the NCI and made available for analysis. The SEER9 registries include the states of Connecticut, Iowa, New Mexico, Utah, and Hawaii, and the metropolitan areas of Atlanta, Detroit, San Francisco-Oakland, and Seattle-Puget Sound which together represent approximately 10% of the U.S. population. The SEER Program records all treatment given for the first course of therapy in broad categories, i.e., surgery, radiotherapy, chemotherapy and hormonal therapy; therapy given subsequent to the first course is not recorded.
Our study was restricted to women who survived one or more years following their initial diagnosis, and who were initially treated with surgery only or with surgery and radiotherapy. The radiotherapy group was subdivided into external beam therapy, brachytherapy, and combination radiotherapy (external beam and brachytherapy).
The SEER files were searched for second primary cancers (excluding non-melanoma skin cancers and female genital tract cancers) that were diagnosed at least one year after the initial uterine corpus cancer diagnosis. Higher-order cancers (third, fourth, etc) occurring subsequent to the second cancer were not included.
Statistical methods
External comparisons
Standardized incidence ratios (SIR) were calculated as the ratio of the observed number of second cancer cases occurring among women who survived one or more years divided by the expected number. For each patient, person-years at risk were accumulated from one year after UCC diagnosis until the date of death, date of last follow-up, date of diagnosis of second primary tumor, or end of the study (December 31, 2003), whichever came first. Site-specific cancer incidence rates for females were computed by registry, five-year age and calendar year groups, and race (white, black, and other) and multiplied by the person-years at risk to estimate the expected number of second cancers in the general population. Poisson-based 95% confidence intervals (CI) were calculated.11
Internal comparisons
Because there are unmeasured risk factors related to UCC (obesity, exogenous hormones, etc), we assessed the effect of radiotherapy by internally comparing the risk of second cancers among patients treated with surgery and radiotherapy to those treated with surgery alone using Poisson regression methods for grouped survival data.12 Incidence rate ratios (IRR) and 95% CI were estimated with stratification by five-year age and calendar year, cancer registry, and race.
Other studies have indicated that the minimal time between radiation exposure and occurrence of a new cancer is approximately 5–15 years for solid cancer7,13 and about 1–5 years for leukemia.6,14 Therefore, analyses of solid cancers focused on the period five or more years from the initial diagnosis and analyses of second lymphoma/leukemia cases were limited to the period one or more years after initial diagnosis. We also assessed log-linear trends in latency-specific IRRs by incorporating a log-linear interaction between the treatment variable and four categories of time since first diagnosis (5–9, 10–14, 15–19, and ≥20 years).
The number of excess second cancers associated with the specific radiation modalities was calculated as [(IRR−1)/IRR] × (number of observed cases in the exposure group of interest). The total number of excess cases was obtained by summing over the three radiotherapy groups. The proportion of cancer cases attributed to a specific type of radiotherapy was calculated as (IRR−1)/IRR].
Radiation dose assessment
We estimated typical organ-specific radiation doses in gray (Gy) and weighted doses to the active bone marrow (Table 1). Doses were estimated separately for each type of radiotherapy, based on techniques typically used during the study period. Dose calculations were performed with a three-dimensional mathematical phantom and measurements in water, using methods previously described.15 We did not have detailed information to individualize the brachytherapy dose based on placement of the radioactive source. Typical doses for brachytherapy were based on the assumption that sources were placed in the uterus and vagina16 which will overestimate the site-specific doses compared to using sources placed in vaginal only. For brachytherapy, a rapid fall-off in dose occurs with distance from the implant; organs adjacent to the sources such as urinary bladder, colon and rectum receive high doses. External beam therapy results in high doses of radiation to the same organs in the pelvic region (about 40 to 50 Gy), whereas the stomach and pancreas receive moderate radiation doses in the range of 0.4 to 5.0 Gy. The doses to colon were heterogeneous with lower doses to the transverse colon compared to the ascending colon. Using the estimated doses, we evaluated the risk of second cancers for three separate groups: heavily- (≥5 Gy), moderately- (0.4–5 Gy), and lightly-irradiated sites (<0.4 Gy).
Table 1.
Estimated organ specific doses (in gray) for specific locations after radiotherapy for uterine corpus cancer.
| Location | Combination Radiotherapy* | External beam therapy† | Location | Brachytherapy‡ | |||
|---|---|---|---|---|---|---|---|
| Average | Range | Average | Range | Average | Range | ||
| Heavily-irradiated sites§ | Heavily-irradiated sites§ | ||||||
| Small intestine | 45 | 11 – 70 | 40 | 10 – 50 | Small intestine | 5.0 | 1.0 – 20 |
| Colon | Colon | ||||||
| Ascending | 45 | 10 – 54 | 43 | 9.0 – 50 | Cecum | 5.0 | 4.0 – 6.5 |
| Descending | 24 | 1.5 – 54 | 23 | 1.2 – 50 | Sigmoid | 20 | 15 – 35 |
| Transverse | 9.4 | 4.2 – 18 | 8.2 | 3.6 – 16 | Rectum | 35 | 20 – 60 |
| Sigmoid | 25 | 20 – 40 | 50 | 49 – 51 | Ureter | 5.2 | 1.0 – 9.0 |
| Cecum | 55 | 53 – 57 | 50 | 49 – 51 | Urinary bladder | 35 | 20 – 60 |
| Rectum | 40 | 25 – 65 | 50 | 49 – 51 | Bone¶ | 10 | 5 – 20 |
| Ureter | 45 | 9.7 – 59 | 40 | 8.7 – 50 | Soft tissue# | 6.5 | 0.50 – 20 |
| Urinary bladder | 40 | 25 – 65 | 50 | 49 – 51 | |||
| Bone¶ | 60 | 55 – 70 | 50 | 49 – 51 | Moderately-irradiated sites§ | ||
| Soft tissue# | 40 | 2.2 – 70 | 34 | 1.7 – 50 | Colon | ||
| Active bone marrow | 20 | 19 – 22 | 19 | 18 – 20 | Ascending | 1.8 | 1.0 – 3.7 |
| Descending | 1.2 | 0.30 – 3.7 | |||||
| Moderately-irradiated sites§ | Transverse | 1.2 | 0.60 – 1.7 | ||||
| Stomach | 4.1 | 0.80 – 25 | 3.4 | 0.60 – 23 | Stomach | 0.70 | 0.20 – 1.8 |
| Liver | 2.3 | 1.9 – 4.9 | 1.9 | 1.7 – 4.0 | Liver | 0.40 | 0.20 – 0.90 |
| Gall bladder | 3.3 | 1.7 – 4.8 | 2.6 | 1.3 – 3.7 | Gall bladder | 0.70 | 0.40 – 1.1 |
| Pancreas | 2.0 | 1.5 – 3.7 | 1.5 | 1.2 – 3.0 | Pancreas | 0.50 | 0.30 – 0.70 |
| Kidney | 3.4 | 1.4 – 6.3 | 2.8 | 1.1 – 5.4 | Kidney | 0.60 | 0.30 – 0.85 |
| Renal pelvis | 3.2 | 3.0 – 3.4 | 2.6 | 2.5 – 2.7 | Renal pelvis | 0.60 | 0.50 – 0.70 |
| Bone** | 0.52 | 0.10 – 2.5 | 0.40 | 0.06 – 2.0 | Active bone marrow | 1.3 | 1.0 – 1.6 |
| Lightly-irradiated sites§ | Lightly-irradiated sites§ | ||||||
| Buccal cavity, pharynx | 0.37 | 0.31 – 0.42 | 0.35 | 0.30 – 0.40 | Buccal cavity, pharynx | 0.02 | 0.01 – 0.02 |
| Lung and bronchus | 0.30 | 0.16 – 0.45 | 0.20 | 0.10 – 0.30 | Lung and bronchus | 0.10 | 0.06 – 0.15 |
| Breast | 0.30 | 0.22 – 0.37 | 0.20 | 0.15 – 0.25 | Breast | 0.09 | 0.07 – 0.12 |
| Thyroid | 0.08 | 0.06 – 0.10 | 0.05 | 0.04 – 0.06 | Bone** | 0.12 | 0.04 – 0.50 |
| Thyroid | 0.03 | 0.02 – 0.04 | |||||
Combination therapy (external beam therapy plus brachytherapy) doses were estimated assuming 50 gray (Gy) Tumor Dose (TD) to midline of the pelvis with Anterior-Posterior/Posterior-Anterior (AP/PA) fields 20 × 20 cm2, with central blocking and with 5000 mgh radium equivalent Tandem or Heyman capsules with ovoids brachytherapy.
External Beam therapy doses were estimated assuming 50 Gy TD to midline of the pelvis, AP/PA fields 20 × 20 cm2 (open field) 6 megavoltage (MV) photons.
Brachytherapy doses were estimated assuming 5000 mgh Radium equivalent, Tandem or Heyman capsules with ovoids.
Heavily-, moderately-, and lightly-irradiated sites were defined as those sites with estimated radiation doses ≥5, 0.4–5, and <0.4 Gy, respectively.
Heavily-irradiated bone sites include the pelvic bones, sacrum, coccyx and associated joints.
Heavily-irradiated soft tissue sites include the abdomen; pelvis; and trunk soft tissues.
Include the ribs, sternum, and clavicle.
RESULTS
We observed 7,428 second cancers in the cohort of 60,949 women who survived at least one year following UCC and who received surgery with or without radiotherapy as their first course of treatment. In total, 62% received surgery alone and these patients were similar to those treated with radiotherapy in terms of age, survival, race, and initial treatment with chemotherapy (Table 2). Women treated with surgery alone were more likely to have tumors diagnosed at a localized stage of disease and were more frequently diagnosed in later calendar years compared with irradiated women. Patients treated with brachytherapy had longer follow-up (data not shown) and more localized uterine tumors compared with those in other radiotherapy groups.
Table 2.
Characteristics of women with uterine corpus cancer (UCC*), ages 15 to 79 years, diagnosed 1973 to 2003, who were initially treated with surgery only or with surgery and radiotherapy, SEER Program.†
| Characteristics | Surgery only | Surgery and radiotherapy |
|||
|---|---|---|---|---|---|
| Any radiotherapy | Combination therapy‡ | External beam therapy | Brachytherapy | ||
| Persons at risk | 37 534 | 23 415 | 5 650 | 10 464 | 6 605 |
| Person-years at risk | 386 284 | 254 791 | 51 845 | 106 812 | 89 356 |
| Mean survival time after UCC diagnosis (yrs) | 10 | 11 | 9 | 10 | 14 |
| Mean age at UCC diagnosis (yrs) | 60 | 62 | 62 | 63 | 61 |
| Age at UCC diagnosis (yrs) | |||||
| <45 | 2 980 (8%) | 1 135 (5%) | 297 (5%) | 454 (4%) | 339 (5%) |
| 45–54 | 7 653 (20%) | 3 833 (16%) | 912 (16%) | 1 490 (14%) | 1 320 (20%) |
| 55–64 | 12 995 (35%) | 8 456 (36%) | 1 987 (35%) | 3 609 (34%) | 2 635 (40%) |
| 65–79 | 13 906 (37%) | 9 991 (43%) | 2 454 (43%) | 4 911 (47%) | 2 311 (35%) |
| Calendar year of UCC diagnosis | |||||
| <1980 | 7 534 (20%) | 7 653 (33%) | 1 320 (23%) | 2 910 (28%) | 3 245 (49%) |
| 1980–1989 | 11 168 (30%) | 7 971 (34%) | 1 989 (35%) | 3 671 (35%) | 2 115 (32%) |
| 1990–1999 | 14 133 (38%) | 6 052 (26%) | 1 735 (31%) | 3 131 (30%) | 932 (14%) |
| ≥2000 | 4 699 (13%) | 1 739 (7%) | 606 (11%) | 752 (7%) | 313 (5%) |
| Race | |||||
| White | 34 030 (91%) | 21 426 (92%) | 5 049 (89%) | 9 481 (91%) | 6 258 (95%) |
| Black | 1 278 (3%) | 958 (4%) | 280 (5%) | 437 (4%) | 207 (3%) |
| Other | 2 226 (6%) | 1 031 (4%) | 321 (6%) | 546 (5%) | 140 (2%) |
| Chemotherapy | |||||
| Yes | 1 018 (3%) | 946 (4%) | 247 (4%) | 504 (5%) | 126 (2%) |
| No | 36 511 (97%) | 22 456 (96%) | 5 401 (96%) | 9 953 (95%) | 6 475 (98%) |
| Unknown | 5 (0%) | 13 (0%) | 2 (0%) | 7 (0%) | 4 (0%) |
| Tumor stage | |||||
| Localized | 34 238 (91%) | 16 628 (71%) | 3 170 (56%) | 7 365 (70%) | 5 604 (85%) |
| Regional | 1 774 (5%) | 4 707 (20%) | 1 951 (35%) | 2 005 (19%) | 628 (10%) |
| Distant | 987 (3%) | 1 401 (6%) | 406 (7%) | 791 (8%) | 146 (2%) |
| Unknown | 535 (1%) | 679 (3%) | 123 (2%) | 303 (3%) | 227 (3%) |
| Persons with a second cancer diagnosis§ | 4 136 | 3 292 | 734 | 1 421 | 1 048 |
| Mean time between 1st and 2nd cancer (yrs) | 10 | 10 | 10 | 10 | 12 |
Includes patients with endometrial adenocarcinoma or carcinoma (sarcomas and other cell types excluded) who survived one or more years. The numbers of specific radiotherapy do not match exactly to numbers of any radiotherapy because of unknown specific radiotherapy (N=696).
National Cancer Institute’s Surveillance, Epidemiology, and End Results Program.
Combination therapy is external beam therapy and brachytherapy.
Includes all primary second cancers (excluding female genital system and non-melanoma skin cancer).
The risk of developing a second cancer was lower than that expected in the general population among women treated with surgery only for their first course of the therapy (SIR=0.89 for all second cancers combined; SIR=0.90 for second solid cancers) (Table 3). Overall, women receiving radiotherapy did not differ from the general population in their second cancer risk, either for all cancers combined (SIR=1.02) or second solid cancers (SIR=1.02). However, significant elevations in risk were observed for several sites, including cancers of the colon (SIR=1.25), rectum (SIR=1.27), urinary bladder and ureter (SIR=1.78), and soft tissue sarcomas (SIR=1.76). While the risk of non-chronic lymphocytic leukemia was significantly elevated following radiotherapy (non-CLL, SIR=1.55), a significant deficit in risk was observed for chronic lymphocytic leukemia (CLL, SIR=0.65). Lower than expected risks were also found for smoking-related second cancer sites (buccal cavity and pharynx, esophagus, and the respiratory system) among irradiated and non-irradiated women.
Table 3.
Observed numbers (N), standardized incidence ratios (SIR), and 95 percent confidence intervals (CI) of second primary cancers by treatment for uterine corpus cancer.
| Second cancer | Surgery only | Surgery and radiotherapy | ||||
|---|---|---|---|---|---|---|
| N | SIR | 95% CI | N | SIR | 95% CI | |
| All cancers* | 4136 | 0.89 | 0.86–0.91 | 3292 | 1.02 | 0.99–1.06 |
| Solid cancers† | 3667 | 0.90 | 0.87–0.93 | 2871 | 1.02 | 0.98–1.06 |
| Buccal cavity and pharynx | 65 | 0.70 | 0.55–0.89 | 41 | 0.63 | 0.46–0.85 |
| Digestive system | 1074 | 0.91 | 0.85–0.96 | 978 | 1.14 | 1.07–1.22 |
| Esophagus | 17 | 0.52 | 0.32–0.84 | 13 | 0.56 | 0.32–0.96 |
| Stomach | 55 | 0.66 | 0.51–0.86 | 66 | 1.07 | 0.84–1.37 |
| Small intestine | 24 | 1.30 | 0.87–1.94 | 18 | 1.44 | 0.91–2.29 |
| Colon | 562 | 0.95 | 0.87–1.03 | 540 | 1.25 | 1.15–1.36 |
| Rectum | 110 | 0.92 | 0.76–1.11 | 109 | 1.27 | 1.05–1.53 |
| Liver | 18 | 0.64 | 0.40–1.01 | 17 | 0.89 | 0.55–1.43 |
| Gallbladder | 17 | 0.67 | 0.42–1.08 | 20 | 1.07 | 0.69–1.66 |
| Pancreas | 144 | 0.90 | 0.77–1.06 | 114 | 0.99 | 0.83–1.20 |
| Respiratory system | 534 | 0.68 | 0.63–0.74 | 443 | 0.84 | 0.77–0.92 |
| Lung and bronchus | 516 | 0.68 | 0.63–0.75 | 430 | 0.85 | 0.77–0.93 |
| Larynx | 9 | 0.42 | 0.22–0.80 | 9 | 0.60 | 0.31–1.16 |
| Breast | 1542 | 1.02 | 0.97–1.07 | 964 | 0.95 | 0.89–1.02 |
| Urinary system | 228 | 0.86 | 0.75–0.98 | 266 | 1.44 | 1.28–1.63 |
| Urinary bladder and ureter | 139 | 0.86 | 0.73–1.02 | 203 | 1.78 | 1.55–2.04 |
| Kidney | 79 | 0.88 | 0.71–1.10 | 44 | 0.73 | 0.55–0.99 |
| Renal pelvis | 8 | 0.69 | 0.34–1.37 | 13 | 1.56 | 0.90–2.68 |
| Bone | 5 | 1.10 | 0.46–2.63 | 6 | 1.91 | 0.86–4.26 |
| Soft tissue | 19 | 0.86 | 0.54–1.37 | 25 | 1.76 | 1.19–2.61 |
| Melanoma | 107 | 0.96 | 0.80–1.16 | 75 | 1.06 | 0.84–1.33 |
| Thyroid | 25 | 0.54 | 0.37–0.80 | 26 | 0.94 | 0.64–1.38 |
| Lymphatic and hematopoietic | 335 | 0.82 | 0.74–0.92 | 303 | 1.07 | 0.95–1.19 |
| Hodgkin lymphoma | 7 | 0.63 | 0.30–1.33 | 9 | 1.18 | 0.61–2.26 |
| Non-Hodgkin lymphoma | 172 | 0.81 | 0.70–0.94 | 155 | 1.07 | 0.91–1.25 |
| Multiple Myeloma | 59 | 0.86 | 0.66–1.11 | 44 | 0.90 | 0.67–1.21 |
| Non-CLL leukemia‡ | 60 | 0.92 | 0.71–1.19 | 71 | 1.55 | 1.23–1.96 |
| ALL | 3 | 0.83 | 0.27–2.59 | 4 | 1.65 | 0.62–4.39 |
| ANLL | 46 | 1.03 | 0.77–1.38 | 51 | 1.63 | 1.24–2.15 |
| CML | 11 | 0.65 | 0.36–1.17 | 16 | 1.32 | 0.81–2.16 |
| CLL | 37 | 0.73 | 0.53–1.01 | 24 | 0.65 | 0.44–0.98 |
Include all second cancers (excluding female genital system and non-melanoma skin cancer).
Include all solid second cancers (excluding female genital system and non-melanoma skin cancer)
Non-chronic lymphocytic leukemia (CLL) includes acute lymphocytic leukemia (ALL), acute non-lymphocytic leukemia (ANLL), and chronic myeloid Leukemia (CML). Four patients with non CLL-leukemia in the surgery only group and two patients in the surgery and radiotherapy group were initially treated with chemotherapy.
Note: P <0.05 are in bold.
Table 4 shows the risks of second cancers among women receiving radiotherapy compared to women treated with surgery only. Among women who survived one or more years, the IRRs of developing a second cancer were increased among irradiated patients compared with patients having surgery only (combination external beam and brachytherapy, IRR=1.26; external beam therapy alone, IRR=1.15; brachytherapy alone, IRR=1.07). External beam therapy alone was associated with a two-fold risk of leukemia excluding CLL (IRR=2.03) and combination radiotherapy with a similarly elevated risk of non-Hodgkin lymphoma (IRR=1.79). Among five-year survivors, elevated risks for second solid cancers were concentrated in heavily-irradiated organs, specifically the colon, rectum, and pelvic area of soft tissues, following combination radiotherapy and external beam therapy alone. Excess cancers of the bladder/ureter were found after all three radiation modalities (IRR=2.66, combination radiotherapy; IRR=2.30, external beam therapy; IRR=1.73, brachytherapy). Elevated risks of stomach cancer and cancers of the liver and thyroid (based on small numbers), were limited to the brachytherapy group. Results for non-CLL and non-Hodgkin lymphoma were similar for one- and five-year survivors but with lower precision for five-year survivors due to the smaller number of survivors.
Table 4.
Incidence rate ratios* (IRR) and 95 percent confidence intervals (CI) of selected second primary cancers among uterine corpus cancer patients, by type of radiotherapy.
| Second cancer and latency time | Surgery only† | Surgery and radiotherapy |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination radiotherapy | External beam therapy | Brachytherapy | Total Excess Cases§ | |||||||||||
| N‡ | N‡ | IRR | 95% CI | Excess cases§ | N‡ | IRR | 95% CI | Excess cases§ | N‡ | IRR | 95% CI | Excess cases§ | ||
| ≥ 1 year latency: | ||||||||||||||
| All cancers¶ | 4136 | 734 | 1.26 | 1.16–1.36 | 151 | 1421 | 1.15 | 1.08–1.22 | 185 | 1048 | 1.07 | 1.00–1.16 | 69 | 405 |
| Solid cancers# | 3667 | 639 | 1.24 | 1.13–1.35 | 124 | 1237 | 1.14 | 1.06–1.21 | 152 | 914 | 1.06 | 0.98–1.14 | 52 | 327 |
| Lymphatic and hematopoietic | 335 | 67 | 1.57 | 1.20–2.05 | 24 | 115 | 1.24 | 1.00–1.55 | 22 | 92 | 1.23 | 0.95–1.60 | 17 | 64 |
| Hodgkin lymphoma | 7 | 2 | 1.82 | 0.37–9.00 | 1 | 2 | 1.06 | 0.22–5.20 | 0 | 4 | 1.89 | 0.46–7.83 | 2 | 3 |
| Non-Hodgkin lymphoma | 172 | 43 | 1.79 | 1.27–2.52 | 19 | 56 | 1.06 | 0.79–1.44 | 3 | 54 | 1.21 | 0.86–1.70 | 9 | 32 |
| Myeloma | 59 | 7 | 0.80 | 0.36–1.78 | −2 | 21 | 1.03 | 0.61–1.71 | 1 | 16 | 0.97 | 0.53–1.77 | 0 | −2 |
| Non-CLL leukemia** | 60 | 15 | 1.67 | 0.94–2.97 | 6 | 36 | 2.03 | 1.34–3.10 | 18 | 18 | 1.56 | 0.87–2.80 | 6 | 31 |
| ALL | 3 | 0 | N/A | N/A | 3 | 3.55 | 0.69–18.24 | 2 | 1 | 0.95 | 0.09–10.00 | 0 | 2 | |
| ANLL | 46 | 12 | 1.80 | 0.94–3.43 | 5 | 24 | 1.77 | 1.07–2.92 | 10 | 13 | 1.43 | 0.72–2.83 | 4 | 20 |
| CML | 11 | 3 | 1.73 | 0.47–6.34 | 1 | 9 | 2.80 | 1.15–6.85 | 6 | 4 | 2.49 | 0.67–9.26 | 2 | 9 |
| CLL | 37 | 6 | 1.03 | 0.43–2.47 | 0 | 8 | 0.63 | 0.29–1.36 | −5 | 9 | 0.82 | 0.37–1.80 | –2 | –6 |
| ≥ 5 year latency: | ||||||||||||||
| All cancers | 2946 | 524 | 1.30 | 1.18–1.43 | 121 | 1001 | 1.14 | 1.06–1.23 | 123 | 799 | 1.04 | 0.96–1.14 | 31 | 275 |
| Solid cancers | 2 604 | 453 | 1.28 | 1.15–1.42 | 99 | 870 | 1.13 | 1.05–1.22 | 100 | 689 | 1.02 | 0.93–1.12 | 14 | 213 |
| Buccal cavity and pharynx | 52 | 8 | 1.09 | 0.51–2.32 | 1 | 10 | 0.64 | 0.32–1.26 | −6 | 13 | 1.07 | 0.55–2.08 | 1 | −4 |
| Digestive system | 763 | 178 | 1.67 | 1.41–1.97 | 71 | 300 | 1.29 | 1.12–1.47 | 67 | 241 | 1.15 | 0.98–1.35 | 31 | 170 |
| Esophagus | 10 | 2 | 1.35 | 0.29–6.32 | 1 | 2 | 0.61 | 0.13–2.80 | −1 | 5 | 1.83 | 0.54–6.18 | 2 | 2 |
| Stomach | 40 | 9 | 1.48 | 0.71–3.09 | 3 | 19 | 1.42 | 0.82–2.46 | 6 | 23 | 1.95 | 1.10–3.48 | 11 | 20 |
| Small intestine | 18 | 3 | 0.99 | 0.28–3.45 | 0 | 8 | 1.50 | 0.65–3.48 | 3 | 4 | 0.80 | 0.25–2.57 | −1 | 2 |
| Colon | 398 | 103 | 1.81 | 1.45–2.26 | 46 | 178 | 1.44 | 1.21–1.73 | 54 | 117 | 1.03 | 0.82–1.29 | 3 | 104 |
| Rectum | 80 | 22 | 2.06 | 1.27–3.35 | 11 | 34 | 1.46 | 0.97–2.19 | 11 | 24 | 1.08 | 0.65–1.78 | 2 | 24 |
| Liver | 13 | 0 | N/A | N/A | 5 | 1.40 | 0.49–3.96 | 1 | 6 | 4.42 | 1.40–13.97 | 5 | 6 | |
| Gallbladder | 12 | 1 | 0.67 | 0.09–5.21 | 0 | 4 | 1.11 | 0.36–3.48 | 0 | 8 | 2.27 | 0.81–6.36 | 4 | 4 |
| Pancreas | 101 | 24 | 1.76 | 1.12–2.78 | 10 | 28 | 0.91 | 0.59–1.38 | –3 | 30 | 1.18 | 0.75–1.86 | 5 | 12 |
| Respiratory system | 383 | 75 | 1.57 | 1.22–2.02 | 27 | 128 | 1.14 | 0.93–1.40 | 16 | 89 | 1.08 | 0.83–1.38 | 7 | 50 |
| Lung and bronchus | 371 | 73 | 1.58 | 1.23–2.04 | 27 | 126 | 1.17 | 0.95–1.43 | 18 | 86 | 1.08 | 0.83–1.40 | 6 | 51 |
| Larynx | 6 | 2 | 2.34 | 0.44–12.48 | 1 | 1 | 0.52 | 0.62–4.34 | –1 | 2 | 1.04 | 0.18–6.12 | 0 | 0 |
| Breast | 1096 | 132 | 0.88 | 0.73–1.05 | −18 | 285 | 0.89 | 0.78–1.02 | −35 | 236 | 0.80 | 0.69–0.94 | −59 | −112 |
| Urinary system | 155 | 45 | 2.04 | 1.45–2.88 | 23 | 85 | 1.83 | 1.40–2.39 | 39 | 63 | 1.57 | 1.13–2.18 | 23 | 84 |
| Urinary bladder and ureter | 98 | 38 | 2.66 | 1.81–3.93 | 24 | 69 | 2.30 | 1.68–3.14 | 39 | 44 | 1.73 | 1.16–2.58 | 19 | 81 |
| Kidney | 51 | 6 | 0.90 | 0.38–2.12 | −1 | 13 | 0.91 | 0.49–1.69 | −1 | 10 | 0.82 | 0.39–1.72 | −2 | −4 |
| Renal pelvis | 6 | 1 | 1.21 | 0.14–10.35 | 0 | 3 | 1.53 | 0.38–6.22 | 1 | 7 | 2.71 | 0.83–8.83 | 4 | 6 |
| Bone†† | 0 | 0 | N/A | N/A | 5 | N/A | N/A | 0 | N/A | N/A | N/A | |||
| Soft tissue‡‡ | 2 | 1 | 2.48 | 0.12–50.90 | 1 | 7 | 10.96 | 2.23–53.89 | 6 | 0 | N/A | N/A | 7 | |
| Melanoma | 75 | 6 | 0.65 | 0.28–1.51 | −3 | 22 | 1.09 | 0.67–1.75 | 2 | 20 | 1.18 | 0.68–2.06 | 3 | 2 |
| Thyroid | 11 | 1 | 0.78 | 0.10–6.22 | 0 | 5 | 2.05 | 0.69–6.06 | 3 | 10 | 4.22 | 1.56–11.46 | 8 | 10 |
| Non-Hodgkin lymphoma | 127 | 35 | 2.00 | 1.36–2.95 | 18 | 42 | 1.06 | 0.74–1.51 | 2 | 44 | 1.09 | 0.75–1.59 | 4 | 24 |
| Non-CLL leukemia** | 42 | 7 | 1.15 | 0.51–2.59 | 1 | 24 | 1.91 | 1.15–3.18 | 11 | 16 | 1.80 | 0.94–3.44 | 7 | 19 |
Poisson regression analysis stratified for age at UCC diagnosis, calendar year of diagnosis, cancer registry, and race.
The “surgery only” category (no radiotherapy) is the reference group.
N = number of observed second cancers. The sum of the observed second cancers for each type of radiotherapy does not necessarily match exactly the total number of cases for any radiotherapy because there are cases with unknown type of radiotherapy.
The number of excess second primary cancers cases associated with each specific radiotherapy was calculated as: [(IRR−1)/IRR] × number of observed cases in the exposure group of interest. Deficits are also presented to equal to the sum of excess.
All second cancers (excluding female genetic tract and non-melanoma skin cancer).
All solid second cancers (excluding female genital system and non-melanoma skin cancer)
Non-chronic lymphocytic leukemia (CLL) includes acute lymphocytic leukemia (ALL), acute non-lymphocytic leukemia (ANLL), and chronic myeloid Leukemia (CML). Four patients in the surgery only group were initially treated with chemotherapy (SIR=7.08, 95% CI=2.66–18.87); 2 patients in the radiotherapy group were initially treated with chemotherapy (SIR=3.63, 95% CI=0.91–14.52).
The bone category includes: vertebral column; rib, sternum, clavicle and associated joints; pelvic bones, sacrum, coccyx and associated joints.
The soft tissue category includes: connective, subcutaneous and other soft tissues of abdomen; connective, subcutaneous and other soft tissues of pelvis; connective, subcutaneous and other soft tissues of trunk.
Note: P <0.05 are in bold.
In a direct comparison among radiation modalities (using brachytherapy alone as referent group), women treated with any external beam therapy (combination or beam therapy alone) had significantly higher risks of second cancers. The IRRs were 1.10 (CI=1.02–1.20) for cancers in one-year survivors and 1.44 (CI=1.19–1.75) for heavily-irradiated sites in five-year survivors. Combination radiotherapy had the overall highest estimated organ-specific doses and the strongest association with new solid malignancies, with significantly elevated risks observed for heavily-irradiated segments of the colon (IRR=1.62, CI=1.18–2.22), and bladder/ureter (IRR=2.15, CI=1.34–3.46), compared with women receiving brachytherapy.
Of the 3,203 second cancers observed in women treated with radiotherapy, 405 excess cancer cases overall (including 327 solid cancers) occurred among one-year survivors and 275 cases overall (including 213 solid cancers) among five-year survivors could be explained by radiotherapy (Table 4). Of the 151 cancers of the bladder/ureter occurring among irradiated patients surviving five years or more, 81 cases or 54% were estimated as attributed to radiation therapy. External beam therapy (either combination or external beam alone) accounted for 100 excess colon cases (36%) and 22 excess rectal cancer (39%), compared to only five excess colorectal cancer cases following brachytherapy (4%).
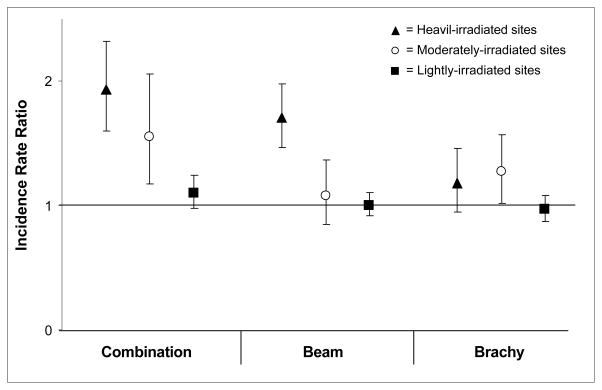
Figure 1 shows the IRRs of second solid cancers among five-year survivors by type of radiation and according to three categories of estimated radiation dose to specific organ sites. Statistically significant increased risks following combination radiotherapy were found for second solid cancers occurring at heavily- (IRR=1.93, CI=1.60–2.32) and moderately-irradiated sites (IRR=1.55, CI=1.17–2.06). Increased risks for heavily-irradiated sites were also seen following external beam therapy alone (IRR=1.71, CI=1.47–1.98), however, elevated risks were not evident for moderately-irradiated sites. Risks of second solid cancers following brachytherapy alone were increased for both heavily (not significant)- and moderately-irradiated sites, but were overall lower than the risks observed for any external beam therapy.
Figure 1.
Incidence rate ratios* and 95% confidence intervals of second solid cancers among ≥5 year survivors of uterine corpus cancer (UCC). Secondary tumor sites are categorized by estimated irradiated dose† (heavily-, moderately-, and lightly-irradiated sites) following UCC radiotherapy.
* Incidence Rate Ratios are from Poisson regression analysis stratified for age at UCC diagnosis, calendar year of diagnosis, cancer registry, and race. Patients treated with surgery only form the reference group
† Heavily-irradiated sites (estimated radiation doses ≥5 Gy)includes: bone (pelvic bones, sacrum, coccyx and associated joints), colon (ascending, descending, cecum, sigmoid, and transverse), rectum, small intestine, soft tissue (connective, subcutaneous and other soft tissues of abdomen, connective, subcutaneous, and other soft tissues of pelvis, connective, subcutaneous, and other soft tissues of trunk), urinary bladder and ureter. Moderately-irradiated sites (0.4–5 Gy) includes: bone (vertebral column, and rib, sternum, clavicle, and associated joints), colon (other sites than in heavy irradiated sites), gall bladder, kidney, liver, pancreas, renal pelvis, and stomach. Lightly-irradiated sites (<0.4 Gy) includes: all solid sites except the sites defined in the heavily- and moderately-irradiated sites above. The brachytherapy analysis has a different site exposure classification compared to the other radiotherapy groups (ascending, descending, and transverse colon are classified as moderately-irradiated sites, and vertebral column and rib, sternum, clavicle, and associated joints are classified as lightly-irradiated sites).
Table 5 shows IRRs by latency (time since initial diagnosis) for heavily-, moderately- and lightly-irradiated solid cancer sites. For both combination and beam therapy significantly increased IRRs of second cancers were detected for most latency intervals among the heavily-irradiated sites (P-trends<0.01 over 5–9, 10–14, 15–19, ≥20 years) with women followed ≥10 years having more than two-fold increases in risk. For brachytherapy, radiation-related risks were substantially lower and a statistically significant elevated risk (RR=1.36) was seen only for the 10–19 year time interval (P-trend=0.02). The IRRs of second solid cancers were lower among the moderately-irradiated sites compared to the heavily-irradiated sites, but statistically significant risks were detected for the 10–19 year latency interval for women receiving either combination radiotherapy (IRR=1.87) or external beam therapy (IRR=1.39). P-trends over the latency periods ≥5 years were significant for each radiation modality. Among patients treated with brachytherapy, there was only a modest non-significant elevation in second solid cancers risks at moderately-irradiated sites. For cancer sites that received low radiation exposures, we observed a significantly elevated risk only for the time period 1–4 years after brachytherapy.
Table 5.
Incidence rate ratios* (IRR) and 95 percent confidence interval (CI) of second solid cancers by time after uterine corpus cancer diagnosis. Solid sites are categorized by estimated irradiated dose following radiotherapy of uterine corpus cancer.
| Solid sites and latency interval (yrs) | Surgery only† | Combination radiotherapy | External beam therapy | Surgery only‡ | Brachytherapy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N§ | N§ | IRR | 95% CI | N§ | IRR | 95% CI | N§ | N§ | IRR | 95% CI | |
| Heavily-irradiated sites¶ | |||||||||||
| 1–4 | 226 | 53 | 1.49 | 1.09–2.02 | 85 | 1.22 | 0.94–1.57 | 174 | 37 | 1.06 | 0.72–1.57 |
| 5–9 | 236 | 52 | 1.48 | 1.09–2.01 | 95 | 1.31 | 1.03–1.67 | 181 | 36 | 0.92 | 0.62–1.36 |
| 10–19 | 231 | 77 | 2.29 | 1.76–2.99 | 144 | 2.04 | 1.65–2.52 | 171 | 77 | 1.36 | 1.01–1.83 |
| ≥20 | 67 | 20 | 2.42 | 1.42–4.12 | 40 | 2.10 | 1.41–3.12 | 53 | 22 | 1.13 | 0.65–1.97 |
| Trend (P) # | <0.01 | <0.01 | 0.02 | ||||||||
| Moderately-irradiated sites** | |||||||||||
| 1–4 | 118 | 16 | 0.83 | 0.49–1.40 | 42 | 1.21 | 0.85–1.74 | 170 | 38 | 1.01 | 0.69–1.49 |
| 5–9 | 102 | 17 | 1.26 | 0.75–2.13 | 26 | 0.85 | 0.55–1.31 | 157 | 38 | 1.23 | 0.83–1.82 |
| 10–19 | 137 | 36 | 1.87 | 1.29–2.73 | 58 | 1.39 | 1.02–1.90 | 197 | 72 | 1.28 | 0.95–1.73 |
| ≥20 | 46 | 6 | 1.23 | 0.52–2.90 | 10 | 0.69 | 0.34–1.37 | 60 | 28 | 1.18 | 0.71–1.95 |
| Trend (P) # | <0.01 | <0.01 | <0.01 | ||||||||
| Lightly-irradiated sites†† | |||||||||||
| 1–4 | 719 | 117 | 1.12 | 0.92–1.34 | 240 | 1.15 | 0.99–1.33 | 719 | 150 | 1.25 | 1.03–1.52 |
| 5–9 | 793 | 127 | 1.16 | 0.96–1.41 | 226 | 1.00 | 0.86–1.16 | 793 | 133 | 0.82 | 0.68–1.01 |
| 10–19 | 809 | 94 | 0.86 | 0.69–1.07 | 230 | 0.96 | 0.83–1.12 | 809 | 209 | 0.96 | 0.81–1.13 |
| ≥20 | 183 | 24 | 1.28 | 0.83–1.98 | 41 | 0.76 | 0.54–1.07 | 183 | 74 | 1.08 | 0.80–1.46 |
| Trend (P) # | 0.47 | 0.24 | 0.03 | ||||||||
Poisson regression analysis stratified for age at UCC diagnosis, calendar year of diagnosis, cancer registry, and race.
The “surgery only” category (no radiotherapy) is the reference group for the combination and beam therapy analysis.
The “surgery only” category (no radiotherapy) is the reference group for the brachytherapy analysis. The brachytherapy analysis has a different site exposure classification compared to the other radiotherapy groups (ascending, descending, and transverse colon are classified as moderately irradiated sites, and bone sites: vertebral column and rib, sternum, clavicle, and associated joints are classified as lightly-irradiated sites).
N = number of observed second cancers.
Heavily-irradiated sites (dose ≥5 Gray)include: bone (pelvic bones, sacrum, coccyx and associated joints), colon (ascending, descending, cecum, sigmoid, and transverse), rectum, small intestine, soft tissue (connective, subcutaneous and other soft tissues of abdomen, connective, subcutaneous, and other soft tissues of pelvis, connective, subcutaneous, and other soft tissues of trunk), urinary bladder and ureter.
P for log-linear trend over latency time-specific IRRs were limited to four categories (5–9, 10–14, 15–20, and >20 years).
Moderately-irradiated sites (dose 0.4–5 Gray) include: bone (vertebral column, and rib, sternum, clavicle, and associated joints), colon (other sites than in heavy irradiated sites), gall bladder, kidney, liver, pancreas, renal pelvis, and stomach.
Lightly-irradiated sites (dose <0.4 Gray) include: all solid sites except the sites defined in the heavily- and moderately-irradiated sites above.
Note: P <0.05 are in bold.
We evaluated the effect of age at UCC diagnosis among ten-year survivors, comparing those treated with radiotherapy with those receiving surgery alone. There was no evidence of substantial differences in risk for heavily-irradiated solid cancer sites by age at diagnosis (<50, 50–59, 60–69, ≥70 years) (results not shown).
In a sensitivity analyses, we found no statistically significant heterogeneity by cancer registry-and results were similar to those reported above when analyses were restricted to women with localized stage. Excluding patients known to be treated with initial chemotherapy did not change the results.
DISCUSSION
Our study quantified the risk of radiation-related second cancers in a large cohort of UCC survivors treated with surgery, with or without radiotherapy, who have been followed for new malignancy over a 30 year period, and, for the first time, directly compared the risk of solid cancers among women treated with different radiotherapy modalities. We found that women initially treated with surgery and radiotherapy had significantly higher risks compared with those treated with surgery alone, with substantially lower risks following brachytherapy compared with any beam therapy. When we evaluated cancer risk for organ sites that were ranked according to estimated radiation dose received, the greatest risks were found for non-CLL leukemia and for heavily-irradiated solid organs sites (e.g., colon, rectum, and bladder). Among five-year survivors receiving radiotherapy, we estimate that 11% (213 of 2,012 cases) of the solid second cancer cases could be explained by radiotherapy with only 14 total excess cases related to brachytherapy as compared with 199 for any external beam therapy. Risk of radiation-related cancer did not depend on age at exposure in this population of mostly older women (>60 years).
Previous studies have reported an increased risk of second cancers among patients typically treated with pelvic external beam radiotherapy for cancers of the cervix, ovary, testis, and prostate.5,9,17–21 However, only sparse data exist on the carcinogenic effect of brachytherapy when given without external beam therapy, and no earlier investigation has evaluated solid cancer risk following this therapy for UCC. Brachytherapy alone has been used with increasing frequency to treat prostate cancer since the mid 1990s; however, numbers in the long term follow-up intervals remain small and evidence for differences in second cancer risk by radiation modality have been inconsistent.22–24
Our current investigation expands upon earlier SEER investigations19,25 by directly comparing risks of developing a new malignancy following three different radiation modalities for UCC compared to surgery alone, and by evaluating risk in groups of cancer sites defined by their average radiation doses. The previous two SEER investigations19,20 had somewhat different inclusion criteria and design (including age, calendar years, follow-up time, and definitions of radiation and second cancer) and therefore not directly comparable with our study but the overall second cancer results were similar. Our overall risk of second solid cancers among UCC five-year survivors was only slightly increased following brachytherapy, whereas risk was significantly elevated by 13% following external beam therapy and 28% after combination radiotherapy. We found a 44% higher risk for heavily irradiated solid organ sites following any beam therapy compared with brachytherapy alone. Differences in the carcinogenic effect of the two radiation modalities is not surprising since we estimated that radiation dose to organs in close proximity to the uterus is five- to ten-fold higher for beam therapy than brachytherapy. In addition, since today’s patients largely receive only vaginal brachytherapy26 risks would probably be even lower for current brachytherapy regimens.
Our results are in general agreement with studies of women treated with radiotherapy for cervical cancer, most of whom received only external beam therapy or combination radiotherapy.5,9,14 UCC survivors treated with any external beam therapy had significant excesses of second cancers of the colon, rectum, bladder, pancreas (combination therapy only) and soft tissues (beam therapy only). Risks for heavily-irradiated sites were elevated for both combination and external beam therapy alone and were highest among ten-year survivors. Similar to previous reports,6,19 risks of ANLL after UCC were elevated by 70–80% after external beam therapy. In a new finding, women treated with combination therapy in our study developed an increased risk of non-Hodgkin lymphoma, a cancer which has been infrequently associated with radiation in the literature.7
Although brachytherapy was associated with prominent excesses of second cancers of the bladder/ureter and stomach among five-year UCC survivors, little excess was seen for cancers of the rectum, pelvic soft tissues, parts of the colon, or for leukemia. Overall we found no evidence of an increased risk following brachytherapy to the group of heavily irradiated sites for the first ten years of follow-up, although risk appeared to rise thereafter. From previous studies, increased risks of cancers related to radiation have been observed among primarily younger women treated with brachytherapy given at lower doses for benign gynecologic bleeding disorders.27,28 Excess deaths were due to cancers of the uterus, bladder, colon, other genital sites, and leukemia, but not rectal cancer.28
Advantages of the current study include the large number of UCC women treated with radiation and with surgery alone in a population-based setting, and the availability of detailed information on type of radiotherapy received. However, we lacked informationon other risk factors for endometrial cancer, treatment subsequent to the first therapy, and individual organ doses. Because treatments have changed over time, some of the risks we observed are associated with old treatments. Moreover, increased medical surveillance of heavily irradiated organs, such as the colon, rectum and bladder, may have resulted in increased risks for irradiated compared to non-irradiated women.
In summary, radiotherapy for UCC increases the risk of second cancers, emphasizing the need for continued long-term medical surveillance of irradiated cancer patients. Our study shows that organs which receive high doses from any external beam radiation due to their close proximity to the uterus are particularly susceptible to iatrogenic cancers. The current results lend support to clinical studies emphasizing the more favorable toxicity profile of vaginal brachytherapy in early stage UCC,3,29 although we were unable to address the late effects of high dose rate brachytherapy, the latter being seen in wider use in the last decade.26 It is important that our findings be interpreted in light of the substantial benefits of adjuvant radiotherapy in reducing the risk of recurrence and possibly prolonging survival.29,30 Thus, physicians need to continually weigh the risk of developing a second cancer versus the benefits of radiotherapy.
Acknowledgments
This study was funded by the Intramural Research Program, National Cancer Institute, National Institutes of Health, USA.
We wish to thank Nathan Appel, Information Management Services, Rockville, MD, USA, for expert computer support and data management.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al. National Cancer Institute; Bethesda, MD: SEER Cancer Statistics Review, 1975–2005. based on November 2007 SEER data submission, posted to the SEER web site, 2008. http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 3.Chu CS, Lin LL, Rubin SC. Cancer of the Uterine Body. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer, Principals & Practice of Oncology. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. pp. 1543–1663. [Google Scholar]
- 4.Rose PG. Endometrial carcinoma. N Engl J Med. 1996;335:640–9. doi: 10.1056/NEJM199608293350907. [DOI] [PubMed] [Google Scholar]
- 5.Boice JD, Jr, Engholm G, Kleinerman RA, et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res. 1988;116:3–55. [PubMed] [Google Scholar]
- 6.Curtis RE, Boice JD, Jr, Stovall M, et al. Relationship of leukemia risk to radiation dose following cancer of the uterine corpus. J Natl Cancer Inst. 1994;86:1315–24. doi: 10.1093/jnci/86.17.1315. [DOI] [PubMed] [Google Scholar]
- 7.UNSCEAR (United Nations Scientific Committee on Effects of Atomic Radiation) UNSCEAR 2006 Report to the General Assembly, Volume 1 with scientific annexes. New York, United Nations; 2008. Effects of Ionizing Radiation. [Google Scholar]
- 8.Curtis RE, Freedman DM, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute; Bethesda, MD: 2006. NIH Publ. No. 05-5302. (available at http://seer.cancer.gov/publications) [Google Scholar]
- 9.Chaturvedi AK, Engels EA, Gilbert ES, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007;99:1634–43. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 10.Hankey BF, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results Program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–21. [PubMed] [Google Scholar]
- 11.Liddell FD. Simple exact analysis of the standardized mortality ratio. J Epidemiol Community Health. 1984;38:85–88. doi: 10.1136/jech.38.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breslow NE, Day NE. The design and analysis of cohort studies. II. Lyon (France): International Agency for Research on Cancer; 1987. Statistical methods in cancer research. IARC scientific publication No. 82. [PubMed] [Google Scholar]
- 13.Travis LB. Therapy-associated solid tumors. Acta Oncol. 2002;41:323–33. doi: 10.1080/028418602760169361. [DOI] [PubMed] [Google Scholar]
- 14.Kleinerman RA, Boice JD, Jr, Storm HH, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995;76:442–52. doi: 10.1002/1097-0142(19950801)76:3<442::aid-cncr2820760315>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–57. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 16.Perez CA, Knapp RC, Young RC. Gynecologic tumors. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer, Principals and Practice of Oncology. Philadelphia, PA: J. P. Lippincott company; 1982. pp. 849–56. [Google Scholar]
- 17.Boice JD, Jr, Day NE, Andersen A, et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst. 1985;74:955–75. [PubMed] [Google Scholar]
- 18.Travis LB, Curtis RE, Boice JD, Jr, et al. Second malignant neoplasms among long-term survivors of ovarian cancer. Cancer Res. 1996;56:1564–70. [PubMed] [Google Scholar]
- 19.Freedman DM, Curtis RE, Travis LB, et al. New Malignancies Following Cancer of the Uterine Corpus and Ovary. In: Curtis RE, Freedman DM, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute; Bethesda, MD: 2006. NIH Publ. No. 05-5302. (available at http://seer.cancer.gov/publications) [Google Scholar]
- 20.Brenner DJ, Curtis RE, Hall EJ, et al. Second malignancies after radiotherapy for prostate cancer. Cancer. 2000;88:398–406. doi: 10.1002/(sici)1097-0142(20000115)88:2<398::aid-cncr22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–65. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 22.Moon K, Stukenborg GJ, Keim J, et al. Cancer incidence after localized therapy for prostate cancer. Cancer. 2006;107:991–8. doi: 10.1002/cncr.22083. [DOI] [PubMed] [Google Scholar]
- 23.Kendal W, Eapen L, Nicholas G. Second primary cancers after prostatic irradiation: ensuring an appropriate analysis. Cancer. 2007;109:164. doi: 10.1002/cncr.22377. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Wahab M, Reis IM, Hamilton K. Second primary cancer after radiotherapy for prostate cancer -a SEER analysis of brachytherapy versus external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:58–68. doi: 10.1016/j.ijrobp.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Shah JP, Bryant CS, et al. Second neoplasms in survivors of endometrial cancer: Impact of radiation therapy. Gynecol Oncol. 2009;113:233–9. doi: 10.1016/j.ygyno.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 26.Small W, Jr, Erickson B, Kwakwa F. American Brachytherapy Society survey regarding practice patterns of postoperative irradiation for endometrial cancer: current status of vaginal brachytherapy. Int J Radiat Oncol Biol Phys. 2005;63:1502–7. doi: 10.1016/j.ijrobp.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Ryberg M, Lundell M, Nilsson B, et al. Malignant disease after radiation treatment of benign gynaecological disorders. A study of a cohort of metropathia patients. Acta Oncol. 1990;29:563–7. doi: 10.3109/02841869009090051. [DOI] [PubMed] [Google Scholar]
- 28.Inskip PD, Monson RR, Wagoner JK, et al. Cancer mortality following radium treatment for uterine bleeding. Radiat Res. 1990;123:331–44. [PubMed] [Google Scholar]
- 29.Cardenes HR, Look K, Michael H, et al. Endometrium. In: Halperin EC, Perez CA, Brady LW, editors. Perez and Brady’s Principles and Practice of Radiation Oncology. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. pp. 1610–28. [Google Scholar]
- 30.Lee CM, Szabo A, Shrieve DC, et al. Frequency and effect of adjuvant radiation therapy among women with stage I endometrial adenocarcinoma. JAMA. 2006;295:389–97. doi: 10.1001/jama.295.4.389. [DOI] [PubMed] [Google Scholar]