Abstract
The small heat shock protein, HSPB6, is a 17-kDa protein that belongs to the small heat shock protein family. HSPB6 was identified in the mid-1990s when it was recognized as a by-product of the purification of HSPB1 and HSPB5. HSPB6 is highly and constitutively expressed in smooth, cardiac, and skeletal muscle and plays a role in muscle function. This review will focus on the physiologic and biochemical properties of HSPB6 in smooth, cardiac, and skeletal muscle; the putative mechanisms of action; and therapeutic implications.
Keywords: Smooth muscle, Skeletal muscle, Cardiac muscle, HSPB6
Introduction
HSPB6 (also known as HSP20, protein accession no. O14558) is a 17-kDa member of the small heat shock family of proteins (Fig. 1). HSPB6 was first identified in 1994 by Kato et al. when it was isolated from rat and human skeletal muscle as a complex with HSPB1 (also known as HSP27) and HSPB5 (also known as αB-crystallin; Kato et al. 1994a, b). In humans, 11 crystallin-related small heat shock proteins have been recognized, termed HSB1–HSP11 (Kampinga et al. 2009). Members of the small heat shock protein family have molecular masses between 15 and 30 kDa and share considerable sequence homology at the HSPB5 domain of 80–100 amino acid residues located at the C-terminal half of the molecule (Garcia-Ranea et al. 2002). HSPB6 is expressed in multiple tissues; however, HSPB6 is most highly and constitutively expressed in different types of muscle including vascular (Beall et al. 1997), airway (Komalavilas et al. 2008), colonic (Gilmont et al. 2008), bladder (Batts et al. 2005), and uterine (Cross et al. 2007) smooth muscle; cardiac muscle (Pipkin et al. 2003; Fan et al. 2005a, b); and skeletal muscle (Kato et al. 1994a, b). Research in the recent years has demonstrated specific functions for HSPB6 in vasodilation, platelet function, and insulin resistance. Recent reviews by Salinthone et al. (2008) and Fan et al. (2005a, b) have highlighted the specific functions of HSPB6 in the smooth and cardiac muscle, respectively.
Fig. 1.
Rat and human HSPB6 amino acid sequence and domains. Activation of cyclic nucleotide signaling pathways leads to increases in the phosphorylation of HSPB6 on serine 16 (bold italic). The minimal peptide sequence surrounding the phosphorylation site that induces relaxation is identified with a solid line (phosphorylation motif). The HSPB5 domain that is homologous in all small heat shock proteins is indicated with a dashed line. The troponin 1 binding motif is represented with a dotted line. Human HSPB6 does not contain serine 157 that is present in the rat sequence
Small heat shock proteins characteristically function as molecular chaperones assisting in the assembly, disassembly, stabilization, and internal transport of intracellular proteins. van de Klundert et al. examined the chaperone activity of HSPB6 by measuring the ability of recombinant HSPB6 to prevent aggregation of insulin B chains. HSPB6 had poor chaperone activity compared to HSPB5 (van de Klundert et al. 1998). Citrate synthase chaperone assays also demonstrated greater chaperone activity with HSPB5 compared to HSPB6 (van de Klundert et al. 1998). In a study comparing the in vivo thermoprotective ability of HSPB6 and HSPB5 after heat shock, Chinese hamster ovary cells stably overexpressing HSPB6 survived equally as well as HSPB5 expressing cells. In addition, transient overexpression of HSPB6 led to enhanced recovery of coexpressed firefly luciferase after heat shock (van de Klundert et al. 1999). Thus, HSPB6 has limited chaperone activity compared to HSPB5, but nonetheless is able to protect cells against injury.
Small heat shock proteins also form macromolecular associates (Fontaine et al. 2005). A yeast two-hybrid screen study demonstrated that HSPB6 phosphorylation can trigger conformational changes (Sun et al. 2006). In rat cardiac myocytes, chromatography with molecular sieving columns revealed that HSPB6 and HSPB5 associated in aggregates of approximately 200 kDa (Pipkin et al. 2003). Furthermore, phosphorylation of HSPB6 led to dissociation of the macromolecular HSPB6 aggregates in bovine carotid artery smooth muscle tissue (Brophy et al. 1999). Hence, phosphorylation of HSPB6 may alter biologic functions through changes in protein–protein interactions.
HSPB6 and smooth muscle
Cyclic nucleotide signaling pathway and HSPB6
Activation of the cyclic nucleotide signaling pathway leads to vasorelaxation of isolated smooth muscle (Lincoln and Cornwell 1991; Fig. 2). Nitric oxide (NO) and NO donors, such as sodium nitroprusside and nitroglycerin, interact with a heme-containing moiety in soluble guanylyl cyclase leading to increases in cyclic guanosine monophosphate (cGMP) (Lincoln 1989). cGMP in turn activates a protein kinase, cGMP-dependent protein kinase (PKG; Lincoln et al. 1996). Activation of adenylyl cyclase by isoproterenol, prostaglandin, and forskolin leads to increases in cyclic adenosine monophosphate (cAMP) and activation of cAMP-dependent protein kinase (PKA; Murray 1990). PKG and PKA phosphorylate specific substrate proteins including HSPB6. HSPB6 phosphorylation on serine 16 has been demonstrated to mediate smooth muscle relaxation (Beall et al. 1997, 1999; Brophy et al. 1997; Jerius et al. 1999; Woodrum et al. 1999; Rembold et al. 2000; Tessier et al. 2004a, b; Flynn et al. 2007).
Fig. 2.
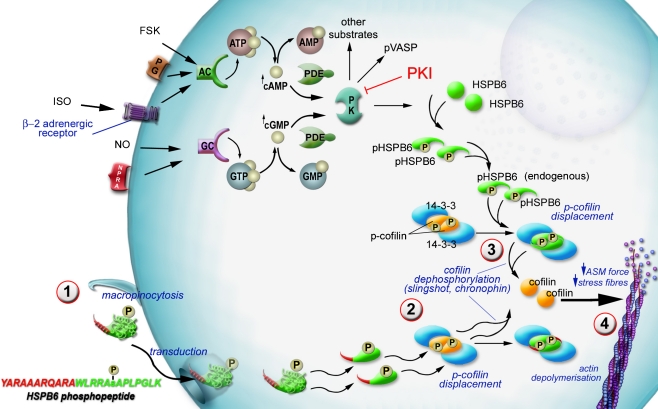
Proposed mechanism of HSPB6-induced smooth muscle relaxation. Activation of adenylate cyclase (AC) by prostaglandin (PG), forskolin (FSK), or isoproterenol (ISO) increases the levels of cyclic adenosine monophosphate (cAMP). Activation of guanylate cyclase (GC) by nitric oxide (NO), sodium nitroprusside (SNP), or natriuretic peptides leads to increased levels of cyclic guanosine monophosphate (cGMP). Cyclic AMP and GMP can be degraded by phosphodiesterases (PDEs). Increased cAMP and cGMP levels activate cAMP-dependent protein kinase and cGMP-dependent protein kinase (PK), respectively. HSPB6 is phosphorylated by cGMP-dependent or cAMP-dependent protein kinase on serine 16 (pHSPB6). Phosphorylation of HSPB6 on serine 16 is associated with vasorelaxation. The HSPB6 phosphopeptide (YARAAARQARAWLRRApSAPLPGLK) treatment of precontracted isolated smooth muscle tissue circumvents the cyclic nucleotide signaling pathway by first directly crossing the cell membrane (1). Endogenous phosphorylated HSPB6 or the HSPB6 phosphopeptide displaces phosphocofilin from a complex with 14-3-3 forming phospho-HSPB6-14-3-3 complex (2). The displaced phosphocofilin is dephosphorylated by phosphatases such as slingshot or chronophin (3). Dephosphorylated cofilin induces actin depolymerization resulting in stress fiber disruption in smooth muscle cells and induces relaxation in smooth muscle (4)
HSPB6 phosphorylation and smooth muscle tone
The role of HSPB6 as a mediator of vasorelaxation was recognized initially in full-term human umbilical artery smooth muscle (HUASM) tissue (Brophy et al. 1997). Unlike other vascular muscle tissues, HUASM is refractory to cyclic nucleotide-dependent vasorelaxation (Renowden et al. 1992; Bergh et al. 1995; Brophy et al. 1997; Flynn et al. 2005). Furthermore, activation of the cyclic nucleotide-dependent signaling pathway does not lead to increased phosphorylation of HSPB6 in HUASM. Impaired relaxation of umbilical artery was associated with decreased expression of HSPB6 (Bergh et al. 1995; Flynn et al. 2005). Rabbit bladder smooth muscle is also refractory to cyclic nucleotide-dependent relaxation and contains low levels of immunoreactive HSPB6 (Batts et al. 2005). Precontracted cerebral arteries removed from a subarachnoid hemorrhage (SAH) rat model had impaired relaxation that was also associated with decreased expression and phosphorylation of HSPB6 (Macomson et al. 2002). These data support a role for HSPB6 in mediating relaxation via changes in HSPB6 expression and phosphorylation levels and provide model systems for the study of impaired cyclic nucleotide-dependent relaxation.
On the other hand, increases in the phosphorylation of HSPB6 have been associated with cyclic nucleotide-dependent vasorelaxation in bovine carotid, porcine carotid, bovine airway, and porcine coronary smooth muscle tissues (Woodrum et al. 1999; Rembold et al. 2001; Tessier et al. 2004a, b; Komalavilas et al. 2008). Increased HSPB6 phosphorylation has also been correlated with cyclic nucleotide-dependent inhibition of contraction and endothelial-dependent vasodilation in isolated perfused carotid arteries (Jerius et al. 1999). This suggests that the physiologic levels of NO released by the endothelium are associated with increased HSPB6 phosphorylation (Jerius et al. 1999). Sildenafil, a phosphodiesterase inhibitor, induced vasorelaxation of precontracted coronary artery in a dose-dependent manner which is associated with an increase in HSPB6 phosphorylation (Tessier et al. 2004b). HSPB6 phosphorylation in response to hypoxia in porcine coronary artery may account for hypoxia-induced vasodilation (Frobert et al. 2005). Isoproterenol-induced relaxation of serotonin precontracted bovine airway smooth muscle strips has also been associated with the phosphorylation of HSPB6 (Komalavilas et al. 2008). Taken together, these data support an association between increases in the phosphorylation of HSPB6 and smooth muscle relaxation or inhibition of smooth muscle contraction.
Recombinant HSPB6 and HSPB6 phosphopeptides
In order to determine if increases in the phosphorylation of HSPB6 are necessary for smooth muscle relaxation, two approaches have been undertaken, genetic engineering and protein transduction. Transfection of contractile mesangial cells with a construct containing enhanced green fluorescence protein and HSPB6 inhibited the ability of the cells to form wrinkles on the substrate (contract) in response to serum (Woodrum et al. 2003). Site-directed mutagenesis which changed serine 16 to an alanine (S16A-HSPB6) inhibited cyclic nucleotide-dependent decreases in wrinkles (relaxation) in transfected mesangial cells (Woodrum et al. 2003).
Recombinant fusion proteins which contained the protein transduction domain, TAT (YGRKKKRRQRR), linked to HSPB6 phosphorylated by the catalytic subunit of PKA in vitro (rTAT-pHSPB6) inhibited norepinephrine-induced contraction of rabbit aorta and human saphenous vein (McLemore et al. 2004). Bacterial expression systems have been engineered to coexpress rTAT-HSPB6 and the catalytic subunit of PKG (Flynn et al. 2007). The ensuing recombinant expression protein was shown to be phosphorylated using two-dimensional gel electrophoresis, and the phosphorylation site was identified as serine 16 by mass spectrometric analysis. Pretreatment of isolated HUASM with rTAT-pHSPB6 restored physiologic levels of the protein and inhibited serotonin-induced contraction (Flynn et al. 2005). These data suggested that impaired relaxation of HUASM may result from decreased levels of phosphorylated HSPB6. Hence, rTAT-pHSPB6 can be used to restore the intracellular expression levels of HSPB6 and the associated physiological response.
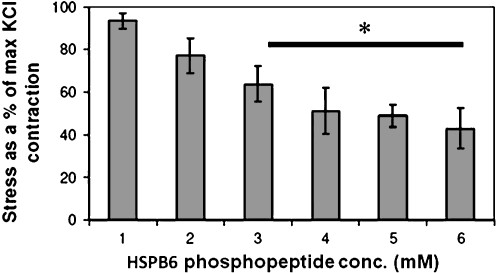
A short HSPB6 phosphopeptide sequence encompassing the serine 16 phosphorylation site (WLRRApSAPLPGLK), introduced into bovine carotid artery smooth muscle using transient permeabilization, inhibited agonist-induced contraction (Beall et al. 1999). In order to bypass the tissue permeabilization step, the HSPB6 phosphopeptide sequence was linked to a protein transduction domain (PTD) (YARAAARQARA), which enabled intracellular delivery of the HSPB6 phosphopeptide sequence. Precontracted strips of porcine coronary artery smooth muscle treated with transducible HSPB6 phosphopeptide led to a dose-dependent decrease in contractile force (Flynn et al. 2003). Peptides containing a scrambled HSPB6 motif linked to a PTD or the PTD alone had no significant effect on the contractile force. The transducible HSPB6 phosphopeptide also induced relaxation of human saphenous vein rings precontracted with norepinephrine (Tessier et al. 2004a, b). Recently, data has demonstrated that the HSPB6 phosphopeptide also induced vasorelaxation of isolated rat aortic rings in a dose-dependent manner (Fig. 3). These data demonstrate that the HSPB6 phosphopeptide, containing the short peptide sequence encompassing the HSPB6 phosphoserine 16 residue linked to a transduction domain, can mimic the effect of the entire molecule. The HSPB6 phosphopeptide has been demonstrated to induce vasorelaxation in multiple tissues and species (Table 1).
Fig. 3.
Cell permeable phosphopeptide analogs of HSPB6 relax rat aortic smooth muscle. Transverse rings of freshly isolated rat aorta were suspended in muscle bath chambers and were equilibrated in Krebs bicarbonate buffer for 1 h with 95% oxygen/5% carbon dioxide at 37°C. Rings were contracted twice with 70 mM potassium chloride (KCl) and then washed repeatedly prior to challenging with 10 µM phenylephrine (10 min) followed by 0, 1, 2, 3, 4, or 6 mM HSPB6 phosphopeptide. Force measurements were obtained with Panlab force transducers interfaced with PowerLab from AD Instruments and data were recorded with Chart software. At the end of the experiments, arterial rings were washed then contracted with KCl to verify tissue viability. The force was converted to stress and decrease in stress minus that of the untreated control was converted to a percentage of the maximal KCl (70 mM) contraction [(mean ± SEM), n = 9, *P < 0.05]
Table 1.
Recombinant HSPB6 and HSPB6 phosphopeptide-induced vasorelaxation
| Species | Vessel | Citation |
|---|---|---|
| Human | Saphenous vein | Tessier et al. 2004a, b; McLemore et al. 2004 |
| Umbilical artery | Flynn et al. 2005 | |
| Anal sphincter | Unpublished findings | |
| Porcine | Coronary artery | Tessier et al. 2004a, b; Flynn et al. 2003 |
| Lower esophageal sphincter | Unpublished findings | |
| Airway smooth muscle | Unpublished findings | |
| Bovine | Carotid artery | Woodrum et al. 2003 |
| Airway smooth muscle | Komalavilas et al. 2008 | |
| Pulmonary artery | Unpublished findings | |
| Coronary artery | Unpublished findings | |
| Rat | Aorta | See Fig. 3 |
| Rabbit | Aorta | McLemore et al. 2004 |
The recombinant HSPB6 fusion protein and HSPB6 phosphopeptide analogs induce vasorelaxation in various isolated smooth muscle tissues from different species
HSPB6 phosphopeptide mechanism of action
The HSPB6 phosphopeptide has been used to elucidate the potential mechanism of action of phosphorylated HSPB6 in smooth muscle. Pull-down assays with cell lysates demonstrated that the HSPB6 phosphopeptide (but not scrambled or nonphosphorylated HSPB6 peptides) interacted with the intracellular scaffolding protein 14-3-3 (Dreiza et al. 2005). 14-3-3 proteins are abundant, ubiquitous proteins that bind to various proteins, including a large group of proteins that regulate cytoskeletal architecture (Jin et al. 2004). The 14-3-3 proteins typically recognize specific phosphopeptide motifs with consensus sequences RSXpSXP (X = any amino acid) and RXY/FXpSXP. 14-3-3 proteins typically form stable homodimers and heterodimers as a result of reciprocal contacts between their N-terminal α-helices. The site surrounding serine 16 in HSPB6 has such a consensus sequence for 14-3-3 binding (RRApSAP). Size exclusion chromatography and chemical cross-linking studies have demonstrated that human 14-3-3 gamma dimers form a tight complex with dimers of phosphorylated HSPB6. However, nonphosphorylated HSPB6 peptides and HSPB6 peptides in which the phosphorylated serine is replaced with an alanine do not interact with 14-3-3 (Chernik et al. 2007). It has been shown that immunoprecipitation of 14-3-3 followed by spiking with the HSPB6 phosphopeptide in tissue lysates leads to dissociation of cofilin from 14-3-3 (Fig. 4). Cofilin is an actin-depolymerizing protein that has been shown to interact with 14-3-3 proteins upon phosphorylation (Birkenfeld et al. 2003; Dreiza et al. 2005). Displacement of cofilin from 14-3-3 leads to dephosphorylation of cofilin and activation as an actin-depolymerizing protein (Gohla and Bokoch 2002). Treatment of NIH 3T3 cells, keloid dermal fibroblasts, and human airway smooth muscle cells with the HSPB6 phosphopeptide leads to loss of central actin stress fibers (Dreiza et al. 2005; Komalavilas et al. 2008). These data suggest that phosphorylated HSPB6 may modulate actin cytoskeletal dynamics by competing with the actin-depolymerizing protein cofilin for binding to the scaffold protein 14-3-3. This represents a unique thin filament mechanism of action for HSPB6 that leads to smooth muscle relaxation.
Fig. 4.
HSPB6 phosphopeptide prevents the association of 14-3-3 and phosphocofilin in a dose-dependent manner. Western blot is shown for bovine coronary artery tissue lysates immunoprecipitated with 14-3-3 antibodies. Following immunoprecipitation, lysates were spiked with the HSPB6 phosphopeptide (0, 10, and 100 μM) for 2 h. Supernatants were collected and separated on 12% SDS-PAGE gel. Lysates were transferred to nitrocellulose membrane and probed with antiphosphocofilin antibodies. Proteins were visualized with secondary IR antibodies using an Odyssey scanner (Li-cor)
HSPB6 and cardiac muscle
HSPB6 has been demonstrated to have a role in cardiac myocyte contractility. Treatment of cardiomyocytes with the HSPB6 phosphopeptide increased myocyte shortening rate through increased calcium uptake and more rapid myocyte lengthening compared to scrambled and nonphosphorylated HSPB6 peptide analogs (Pipkin et al. 2003). Since the NO–cGMP pathway increases beat rate by stimulating the hyperpolarization-activated pacemaker current, If (Musialek et al. 1997), these results suggest that the mechanism of NO stimulation of heart rate via If may involve phosphorylation of HSPB6 and that phosphorylated HSPB6 may have a direct effect on If channels. The increased rate of shortening was also related to increases in the rate of relaxation of the myocytes. Although there was no change in the magnitude of the Ca2+ transient after treatment with the HSPB6 phosphopeptide, there was an increase in the rate of decline of the Ca2+ transient, suggesting that the HSPB6 phosphopeptide facilitates the increased rate by stimulating a more rapid uptake of Ca2+ by the sarcoplasmic reticulum. β-Agonist stimulation of cardiac myocytes was also associated with increased myocyte shortening and increased relaxation rates, as well as increased HSPB6 phosphorylation (Chu et al. 2004). Adenoviral gene transfer of HSPB6 increased myocyte contractility and calcium transient peaks in adult rat cardiomyocytes (Chu et al. 2004).
The intracellular localization of HSPB6 changes in response to cardiac stress. In cultured rat neonatal cardiomyocytes, heat stress leads to partial redistribution of HSPB6 from the cytoplasm to the nucleus and sacromeric-HSPB6 association (van de Klundert and de Jong 1999). HSPB6 also redistributes to the myofibrils in ischemic adult rat hearts (Golenhofen et al. 1998, 2004). Immunohistochemistry data suggested that HSPB6 localized to the Z/I-area of ischemic myofibrils (Golenhofen et al. 2004). Inhibition of proteosomes, multisubunit protease complexes that degrade proteins, led to intracellular redistribution of HSPB6 in rat cardiac myoblasts (Verschuure et al. 2002). Treatment of adult rat cardiomyocytes with isoproterenol resulted in HSPB6 redistribution to the cytoskeleton (Fan et al. 2004). Together, these data support a role for HSPB6 in myocyte contractility and demonstrate that HSPB6 has an active role in cardiac stress responses.
HSPB6 also has cardioprotective properties. Cardiac myocytes transfected with a mutated HSPB6 in which serine 16 is replaced with aspartic acid (S16D, mimicking a phosphoserine residue) prevented β-agonist-induced cardiac apoptosis (Fan et al. 2004). Conversely, a nonphosphorylated mutant in which serine 16 is replaced with an alanine (S16A) exhibited no antiapoptotic properties. Isolated transgenic mouse hearts overexpressing HSPB6 exhibited improved recovery of contractile performance after ischemia/reperfusion in Langendorff preparations (Fan et al. 2005a, b). The extent of infarction and apoptotic cell death was reduced in the HSPB6 transgenic hearts. Levels of phosphorylated HSPB6 were also increased in the ischemia/reperfusion transgenic hearts. Adenoviral gene transfer of HSPB6 protected against ischemia/reperfusion injury in a left anterior descending coronary artery ligation model (Zhu et al. 2005). Islamovic et al. (2007) demonstrated that overexpression of full-length HSPB6 protected cultured adult and neonatal rat cardiomyocytes from simulated ischemia/reperfusion injury when compared to overexpressed HSPB6 lacking the C terminus. These data suggest that HSPB6, specifically the HSPB6 C terminus, is able to confer cardioprotection.
Congestive heart failure results from the inability of the heart to pump blood to the organs. Analysis of tissue lysates collected from the left ventricle of normal and tachycardia-induced congestive heart failure dogs demonstrated that phosphorylated and nonphosphorylated HSPB6 increased significantly in a cardiac heart failure cohort compared to a normal cohort (Dohke et al. 2006). Other proteomic studies demonstrated that chronic exercise trained rats (6 weeks, 5 days/week of treadmill work) had improved heart health and increased levels of HSPB6 expression and phosphorylation compared to sedentary rats (Boluyt et al. 2006). Increases in phosphorylated HSPB6 have also been demonstrated in a proteomic analysis in a rodent model of intensity-controlled endurance exercise (Morton et al. 2009). These data suggest that elevated HSPB6 expression and HSPB6 phosphorylation level changes may be involved in regulating cardiac function. Gaining an understanding of HSPB6 expression and phosphorylation patterns during adverse cardiac events may improve our ability to treat such conditions.
HSPB6 and skeletal muscle
The role of HSPB6 in skeletal muscle is less understood than in cardiac and smooth muscle. Some early investigations seem to suggest the possible mechanistic action of HSPB6 as protective against atrophy, ischemia, hypertensive stress, and metabolic dysfunction. A comparison of HSPB6 expression in anterior tibialis (fast twitch) and soleus (slow twitch) of a muscular dystrophy mouse model demonstrated increased HSPB6 expression in anterior tibialis and reduced HSPB6 expression in soleus compared to normal mice (Sakuma et al. 1998). The muscular dystrophy model is pathophysiological, suggesting that mechanical and neurological control of HSPB6 could alter the function and metabolic processes in muscular dystrophy. However, in a spinal cord isolation model where muscle inactivity occurred even when the neuromuscular connection remained intact, HSPB6 content in predominantly slow twitch soleus and adductor longus was two or three times higher than in plantaris and tibialis anterior fast twitch muscles. With and without neurological synapses from the brain to skeletal muscle the HSPB6 expression remained unchanged in tibialis anterior (slow twitch) muscle (Huey et al. 2004). The dystrophy and spinal cord isolation models are contradictory to that of sarcopenia where muscles succumb to age and a lack of normal constructive muscular regeneration. Studies of gastrocnemius muscle in a rat model of aging suggest that HSPB6 expression increased in skeletal muscle with age. Of the four troponin T isoforms, two were upregulated, while α-actin and CapZ-B (an actin capping protein) were downregulated (Piec et al. 2005). Interestingly, HSPB6 has a binding domain to troponin 1 (Rembold et al. 2000) and it could be that HSPB6 can affect skeletal muscle contraction through troponin 1 and the troponin complex.
Furthermore, under ischemic conditions, HSPB6 may function either directly or indirectly with myofibrils through arteriole myogenic constriction. An increase in intraluminal pressure from skeletal muscle contraction could cause induced pressure and produce a shear stress release of NO in arteriole smooth muscle, elevating cGMP levels. Increased cGMP levels may result in increased phosphorylation of HSPB6 through PKG and a decrease in calcium sensitivity (Ungvari and Koller 2001). It was demonstrated that denervation led to decreased HSPB6 expression levels in the soleus, adductor longus (fast), and plantaris (slow) muscles. HSPB6 translocated rapidly to the Z/I-area of myofibrils of isolated ischemic skeletal muscle compared to nonischemic control tissue (Golenhofen et al. 2004). This ischemic process of increasing the muscle blood pressure and sequestering of HSPB6 to the Z/I-area of myofibrils could alleviate impeding stress and further promote activity of the muscle. Alternatively, in obese subjects with decreased insulin sensitivity, HSPB6 may function as a blood flow regulator in muscle arterioles, promoting blood flow and increase glucose utilization during exercise (Wang et al. 1999a, b). HSPB6 may play multiple roles in the muscle from contractile processes associated with troponin complexes to metabolic responses due to muscle activity or inactivity. These data demonstrate that muscle fiber type, aging, denervation, or disease can alter HSPB6 expression levels and function in skeletal muscle and associated tissues.
Myometrium
While the mechanisms underlying uterine quiescence during pregnancy and activation during parturition remain poorly understood, recent data supports a role for HSPB6 in modulating this process. In a rat model of normal pregnancy and labor, the expression of HSPB6 significantly decreased during late gestation (both mRNA and protein; Cross et al. 2007). Others have demonstrated that HSPB6 phosphorylation occurs during cyclic nucleotide-mediated myometrial relaxation (MacIntyre et al. 2008). Since phosphorylation of HSPB6 has been associated with inhibition of agonist-induced contraction (Beall et al. 1999; Rembold et al. 2000), phosphorylated HSPB6 may contribute to maintaining uterine quiescence during gestation. It is not known whether HSPB6 plays a role in pathologic processes such as preterm labor.
Platelet function
HSPB6 appears to have extracellular functions. Platelet aggregation induced by thrombin or botrocetin but not ADP is inhibited by HSPB6 purified from skeletal muscle (Matsuno et al. 1998). Purified HSPB6 also inhibited thrombin-induced and collagen-induced calcium influx without affecting thrombin-induced calcium release from intracellular calcium stores. These data suggest that HSPB6 inhibits the receptor-mediated calcium influx of platelets leading to its antiplatelet activity. HSPB6 has also been shown to inhibit thrombin-induced phosphoinositide hydrolysis by phospholipase C in human platelets (Kozawa et al. 2002). Scatchard plot analysis indicates that HSPB6 binds to the surface of human platelets and that there is a single class of binding sites (Kozawa et al. 2002). In these studies, it was a dissociated form of HSPB6 but not an aggregated form that inhibited platelet aggregation. Recombinant HSPB6 has also been shown to inhibit collagen-induced aggregation of human platelets (McLemore et al. 2004). A synthetic nine-amino-acid peptide derived from HSPB6 (WIRRASAPI) inhibited platelet aggregation in response to thrombin, botrocetin, and ristocetin (but not collagen or ADP; Matsuno et al. 2003). This peptide sequence surrounds the serine 16 phosphorylation site; however, the normal leucine residues were replaced with an isoleucine. Taken together, these studies suggest that HSPB6 binds to a specific receptor on platelets, inhibiting activation of cascades that lead to platelet aggregation. HSPB6 is present in serum (0.06 ng/ml in hamsters; Kozawa et al. 2002) and hence may be an endogenous modulator of platelet function.
HSPB6 and pathologic processes
Research in the field of HSPB6 in the last several years provides strong evidence that phosphorylated HSPB6 mediates smooth muscle relaxation and it is a downstream effector of the cyclic nucleotide pathway. Thus, phosphorylated HSPB6 or agents that act on the same target proteins as phosphorylated HSPB6 have been explored as therapeutics to treat vasospasm and other smooth muscle diseases (Fig. 2). Phosphorylation of HSPB6 also leads to the disruption of the actin cytoskeleton which opens other therapeutic uses in diseases such as wound healing.
Subarachnoid hemorrhage
SAH causes a delayed vasospasm of the cerebral arteries and can lead to severe stroke and/or death. HSPB6 downregulation has been associated with SAH in a noncraniotomy rat model (Macomson et al. 2002). This downregulation of HSPB6 correlated with the time course of vasospasm demonstrated by laser Doppler and may mediate cerebral vasospasm after SAH. Thus, cerebral vessels may be refractory to relaxation induced by activation of upstream receptors or enzymes since the downstream effector molecule of relaxation (HSPB6) is downregulated. In support of this hypothesis, the HSPB6 phosphopeptide administered by single injection (intrathecal or intravenous) 24 h after induction of SAH completely inhibited vasospasm in this rat model. Furthermore, HSPB6 was effective at reversing vasospasm by administration after the onset of vasospasm 48 and 72 h after SAH (Brophy et al., unpublished data). These data suggest that the HSPB6 phosphopeptide may offer a putative therapeutic for SAH-induced vasospasm.
Asthma
β-Agonists are the most commonly used therapy for relief of acute bronchospasm in asthmatics. β-Agonists mediate their effect primarily by increasing cAMP concentration through activation of β2-adrenergic receptor–adenylyl cyclase pathway. Several mediators of inflammation and therapies such as β-agonists themselves can cause alterations in β2-adrenergic receptor responsiveness in a time-dependent and cell-specific manner (Penn and Benovic 1998). The loss of prophylactic bronchoprotection and deterioration of asthma control observed in patients who use β-agonists regularly may be due to agonist-specific desensitization of the receptor. Airway inflammation or cytokine treatment also contributes to β2-adrenergic receptor dysfunction and a loss of the relaxant effect of β-agonists in airway smooth muscle cells, tissues, and in vivo models (Motojima et al. 1989; Hakonarson et al. 1997; Moore et al. 2001; Callaerts-Vegh et al. 2004). Recent studies have identified adverse effects with inhaled β2-adrenergic agonist in patients with a genetic polymorphism that results in homozygosity for arginine, rather than glycine at amino acid residue 16 of the β2-adrenergic receptor (Israel et al. 2004). Hence, regulation of proximal transmembrane signaling events in the β2-adrenergic receptor–Gs-adenylyl cyclase pathway may limit the efficacy of β-agonist therapy. Recent muscle bath-based studies have demonstrated that β-agonist-induced relaxation of airway smooth muscle is associated with increases in the phosphorylation of HSPB6 (Komalavilas et al. 2008). Treatment of bovine airway smooth muscle with HSPB6 phosphopeptide also relaxed airway smooth muscle similar to β-agonist activation. Studies in naive beagles challenged with an inhaled dose of methacholine that induced a 200–300% increase in airway resistance, followed by an inhaled vehicle or HSPB6 phosphopeptide (1, 3, 10, 30, 300, and 1200 µg/kg) and methacholine, demonstrated attenuated methacholine-induced bronchoconstriction at HSPB6 phosphopeptide doses 1, 3, 10, 30, and 300 µg/kg (unpublished data). The HSPB6 phosphopeptide may be relevant for the treatment of bronchoconstriction in patients who are refractory to β-agonists. Furthermore, these data suggest that the HSPB6 phosphopeptide acts in the same manner as the physiologic downstream PKA effector HSPB6, representing a potential treatment approach to bronchospasm (Komalavilas et al. 2008).
Intimal hyperplasia
Intimal hyperplasia is the thickening of the tunica intima of a blood vessel. Intimal hyperplasia represents a response of the vessel to injury and is the leading cause of bypass graft failure. Balloon induced injury of the common carotid artery of hamsters led to a rapid decrease in the amount of HSPB6 in the vessel wall (Kozawa et al. 2002). Kozawa et al. demonstrated that the injury is associated with the release of HSPB6 from the vessel wall. In an organ culture model of intimal hyperplasia, treatment of human saphenous vein segments with the HSPB6 phosphopeptide prevented the development of intimal hyperplasia (Tessier et al. 2004a). Treatment of cultured vascular smooth muscle cells with HSPB6 inhibited migration but not proliferation, two processes thought to be relevant to the development of intimal hyperplasia. The inhibition of migration suggests that HSPB6 may play a role in other cytokinetic processes. HSPB6 may represent a target as a therapeutic approach for enhancing vascular graft patency by preventing intimal hyperplasia.
Wound healing
A growing body of evidence suggests the involvement of connective tissue growth factor (CTGF) in the development and maintenance of fibrosis and excessive scarring. CTGF has been proposed to mediate some of the profibrotic effects of TGF-β including fibroblast proliferation and the production of extracellular matrix proteins such as collagen and fibronectin (Duncan et al. 1999; Leask and Abraham 2004; Leask and Abraham 2006). The expression of CTGF requires an intact actin cytoskeleton and actin depolymerization or increased monomeric actin leads to decreases in the expression of CTGF (Ott et al. 2003; Muehlich et al. 2007). In addition, increased levels of cAMP have been shown to abolish TGF-β-induced reorganization of the actin cytoskeleton as well as TGF-β-induced CTGF expression (Kothapalli and Grotendorst 1998; Santibanez et al. 2003). The HSPB6 phosphopeptide is known to disrupt the actin cytoskeleton and leads to increases in monomeric actin (Dreiza et al. 2005; Komalavilas et al. 2008). The HSPB6 phosphopeptide significantly decreased the expression of CTGF and type I collagen in human keloid fibroblasts induced by mediators TGF-β1, endothelin, and lysophosphatidic acid (Lopes et al. 2009). Staining of the actin cytoskeleton with Alexa 450 phalloidin revealed that the HSPB6 phosphopeptide treatment decreased stress fiber formation and altered the morphology of the cells that were either untreated or stimulated with TGF-β1. Consistent with the in vitro observations, the HSPB6 phosphopeptide significantly improved collagen organization in vivo in a Siberian hamster scarring model (Lopes et al. 2009). Therefore, HSPB6 phosphopeptide may be useful as a novel approach to reduce the expression of CTGF and prevent/treat fibrotic skin disorders such as keloids.
Insulin resistance
Insulin is primarily required to maintain blood glucose homeostasis and direct energy storage. Insulin acts by stimulating glucose uptake in muscle and adipocytes, while reducing hepatic glucose production. Wang et al. (1999a, b) have demonstrated that HSPB6 is phosphorylated on serine 157 and serine 16 in response to insulin in skeletal muscle. On the other hand, insulin antagonists, epinephrine and calcitonin gene-related peptide, decreased the phosphorylation at serine 157 and increased phosphorylation at serine 16, suggesting that HSPB6 could be a potential modulator of insulin's functions (Wang et al. 1999a, b).
Future directions
There are many questions remaining about the HSPB6 molecule. The crystal structure has not been determined; the putative promoter sequence of HSPB6 is known from the databases, however, the functionality has not been tested and the effect of decreasing the expression of HSPB6 with siRNA or transgenic approaches is not known. While putative mechanisms of action have been identified, further work is needed to confirm the experimental results described in this review and identify other possible mechanisms of action. However, the development of cell permeant phosphopeptide analogs of this molecule represents a powerful tool to continue to investigate the physiology and biology of HSPB6. In addition, these peptide analogs may represent a therapeutic approach for the treatment of disorders involving spasm of smooth muscles and possibly diseases related to other muscles.
Summary
In summary, HSPB6 shares structural and functional characteristics with other small heat shock proteins. However, studies in the last 10 years have demonstrated distinct roles for HSPB6 in muscle physiology. Although the exact role of HSPB6 in these physiological processes remains to be elucidated, evidence suggests that changes in HSPB6 expression and phosphorylation levels are important in regulating HSPB6's functions. The identification of a cell permeant HSPB6 phosphopeptide analog has led to an increased understanding of the mechanism of action of HSPB6. Furthermore, this HSPB6-based peptide may prove to have important therapeutic implications in the treatment of various medical conditions including fibrosis and asthma.
Acknowledgements
This work was supported by National Institute of Health RO1HL58027 and RO1HL70715 and a Veterans Administration Merit Review award to C.M. Brophy.
Disclosures The patents for the HSPB6 phosphopeptide are owned by Arizona State University and the Veteran's Administration. The intellectual property has been licensed to Capstone Therapeutics, Tempe, AZ, USA and the authors (C.D., P.K., E.F., C.F., M.S., and C.M.B.) have financial interest in this technology.
References
- Batts TW, Walker JS, et al. Absence of force suppression in rabbit bladder correlates with low expression of heat shock protein 20. BMC Physiol. 2005;5:16. doi: 10.1186/1472-6793-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall A, Bagwell D, et al. The small heat shock-related protein, HSP20, is phosphorylated on serine 16 during cyclic nucleotide-dependent relaxation. J Biol Chem. 1999;274(16):11344–11351. doi: 10.1074/jbc.274.16.11344. [DOI] [PubMed] [Google Scholar]
- Beall AC, Kato K, et al. Cyclic nucleotide-dependent vasorelaxation is associated with the phosphorylation of a small heat shock-related protein. J Biol Chem. 1997;272(17):11283–11287. doi: 10.1074/jbc.272.17.11283. [DOI] [PubMed] [Google Scholar]
- Bergh CM, Brophy CM, et al. Impaired cyclic nucleotide-dependent vasorelaxation in human umbilical artery smooth muscle. Am J Physiol. 1995;268(1 Pt 2):H202–H212. doi: 10.1152/ajpheart.1995.268.1.H202. [DOI] [PubMed] [Google Scholar]
- Birkenfeld J, Betz H, et al. Identification of cofilin and LIM-domain-containing protein kinase 1 as novel interaction partners of 14-3-3 zeta. Biochem J. 2003;369(Pt 1):45–54. doi: 10.1042/BJ20021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluyt MO, Brevick JL, et al. Changes in the rat heart proteome induced by exercise training: Increased abundance of heat shock protein hsp20. Proteomics. 2006;6(10):3154–3169. doi: 10.1002/pmic.200401356. [DOI] [PubMed] [Google Scholar]
- Brophy CM, Beall A, et al. Small heat shock proteins and vasospasm in human umbilical artery smooth muscle. Biol Reprod. 1997;57(6):1354–1359. doi: 10.1095/biolreprod57.6.1354. [DOI] [PubMed] [Google Scholar]
- Brophy CM, Dickinson M, et al. Phosphorylation of the small heat shock-related protein, HSP20, in vascular smooth muscles is associated with changes in the macromolecular associations of HSP20. J Biol Chem. 1999;274(10):6324–6329. doi: 10.1074/jbc.274.10.6324. [DOI] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Evans KL, et al. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci U S A. 2004;101(14):4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernik IS, Seit-Nebi AS, et al. Small heat shock protein Hsp20 (HspB6) as a partner of 14-3-3 gamma. Mol Cell Biochem. 2007;295(1–2):9–17. doi: 10.1007/s11010-006-9266-8. [DOI] [PubMed] [Google Scholar]
- Chu G, Egnaczyk GF, et al. Phosphoproteome analysis of cardiomyocytes subjected to beta-adrenergic stimulation: identification and characterization of a cardiac heat shock protein p20. Circ Res. 2004;94(2):184–193. doi: 10.1161/01.RES.0000107198.90218.21. [DOI] [PubMed] [Google Scholar]
- Cross BE, O'Dea HM, et al. Expression of small heat shock-related protein 20 (HSP20) in rat myometrium is markedly decreased during late pregnancy and labour. Reproduction. 2007;133(4):807–817. doi: 10.1530/REP-06-0291. [DOI] [PubMed] [Google Scholar]
- Dohke T, Wada A, et al. Proteomic analysis reveals significant alternations of cardiac small heat shock protein expression in congestive heart failure. J Card Fail. 2006;12(1):77–84. doi: 10.1016/j.cardfail.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Dreiza CM, Brophy CM, et al. Transducible heat shock protein 20 (HSP20) phosphopeptide alters cytoskeletal dynamics. FASEB J. 2005;19(2):261–263. doi: 10.1096/fj.04-2911fje. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Frazier KS, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13(13):1774–1786. [PubMed] [Google Scholar]
- Fan GC, Chu G, et al. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005a;15(4):138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fan GC, Chu G, et al. Small heat-shock protein Hsp20 phosphorylation inhibits beta-agonist-induced cardiac apoptosis. Circ Res. 2004;94(11):1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- Fan GC, Ren X, et al. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005b;111(14):1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- Flynn C, Smoke C, et al. In vivo activation of a transducible recombinant form of human HSP20 in Escherichia coli. Protein Expr Purif. 2007;52(1):50–58. doi: 10.1016/j.pep.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn CR, Brophy CM, et al. Transduction of phosphorylated heat shock-related protein 20, HSP20, prevents vasospasm of human umbilical artery smooth muscle. J Appl Physiol. 2005;98:1836–1845. doi: 10.1152/japplphysiol.01043.2004. [DOI] [PubMed] [Google Scholar]
- Flynn CR, Komalavilas P, et al. Transduction of biologically active motifs of the small heat shock- related protein, HSP20, leads to relaxation of vascular smooth muscle. FASEB J. 2003;10:1358–1360. doi: 10.1096/fj.02-1028fje. [DOI] [PubMed] [Google Scholar]
- Fontaine JM, Sun X, et al. Interactions of HSP22 (HSPB8) with HSP20, alphaB-crystallin, and HSPB3. Biochem Biophys Res Commun. 2005;337(3):1006–1011. doi: 10.1016/j.bbrc.2005.09.148. [DOI] [PubMed] [Google Scholar]
- Frobert O, Buus CL, et al. HSP20 phosphorylation and interstitial metabolites in hypoxia-induced dilation of swine coronary arteries. Acta Physiol Scand. 2005;184(1):37–44. doi: 10.1111/j.1365-201X.2005.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ranea JA, Mirey G, et al. p23 and HSP20/alpha-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett. 2002;529(2–3):162–177. doi: 10.1016/S0014-5793(02)03321-5. [DOI] [PubMed] [Google Scholar]
- Gilmont RR, Somara S, et al. VIP induces PKA-mediated rapid and sustained phosphorylation of HSP20. Biochem Biophys Res Commun. 2008;375(4):552–556. doi: 10.1016/j.bbrc.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohla A, Bokoch GM. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr Biol. 2002;12(19):1704–1710. doi: 10.1016/S0960-9822(02)01184-3. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Ness W, et al. Ischemia-induced phosphorylation and translocation of stress protein alpha B-crystallin to Z lines of myocardium. Am J Physiol. 1998;274(5 Pt 2):H1457–H1464. doi: 10.1152/ajpheart.1998.274.5.H1457. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Perng MD, et al. Comparison of the small heat shock proteins alphaB-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem Cell Biol. 2004;122(5):415–425. doi: 10.1007/s00418-004-0711-z. [DOI] [PubMed] [Google Scholar]
- Hakonarson H, Herrick DJ, et al. Autocrine role of interleukin 1beta in altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1997;99(1):117–124. doi: 10.1172/JCI119122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey KA, Thresher JS, et al. Inactivity-induced modulation of Hsp20 and Hsp25 content in rat hindlimb muscles. Muscle Nerve. 2004;30(1):95–101. doi: 10.1002/mus.20063. [DOI] [PubMed] [Google Scholar]
- Islamovic E, Duncan A, et al. Importance of small heat shock protein 20 (hsp20) C-terminal extension in cardioprotection. J Mol Cell Cardiol. 2007;42(4):862–869. doi: 10.1016/j.yjmcc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Israel E, Chinchilli VM, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364(9444):1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- Jerius H, Karolyi DR, et al. Endothelial-dependent vasodilation is associated with increases in the phosphorylation of a small heat shock protein (HSP20) J Vasc Surg. 1999;29(4):678–684. doi: 10.1016/S0741-5214(99)70314-9. [DOI] [PubMed] [Google Scholar]
- Jin J, Smith FD, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004;14(16):1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Goto S, et al. Purification and characterization of a 20-kDa protein that is highly homologous to alpha B crystallin. J Biol Chem. 1994a;269(21):15302–15309. [PubMed] [Google Scholar]
- Kato K, Hasegawa K, et al. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J Biol Chem. 1994b;269(15):11274–11278. [PubMed] [Google Scholar]
- Komalavilas P, Penn RB, et al. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L69–L78. doi: 10.1152/ajplung.00235.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothapalli DHN, Grotendorst GR. Inhibition of TGF-beta-stimulated CTGF gene expression and anchorage-independent growth by cAMP identifies a CTGF-dependent restriction point in the cell cycle. FASEB J. 1998;12(12):1151–1161. doi: 10.1096/fasebj.12.12.1151. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Matsuno H, et al. HSP20, low-molecular-weight heat shock-related protein, acts extracellularly as a regulator of platelet functions: a novel defense mechanism. Life Sci. 2002;72(2):113–124. doi: 10.1016/S0024-3205(02)02144-6. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(Pt 23):4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lincoln TM. cGMP and mechanisms of vasodilation. Pharmacol Ther. 1989;41:479–502. doi: 10.1016/0163-7258(89)90127-7. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 1991;28(1–3):129–137. doi: 10.1159/000158852. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL, et al. Cyclic GMP-dependent protein kinase in nitric oxide signaling. Methods Enzymol. 1996;269:149–166. doi: 10.1016/S0076-6879(96)69017-X. [DOI] [PubMed] [Google Scholar]
- Lopes LB, Furnish EJ, et al. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-beta1-induced CTGF expression in keloid fibroblasts. J Invest Dermatol. 2009;129(3):590–598. doi: 10.1038/jid.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre DA, Tyson EK, et al. Contraction in human myometrium is associated with changes in small heat shock proteins. Endocrinology. 2008;149(1):245–252. doi: 10.1210/en.2007-0662. [DOI] [PubMed] [Google Scholar]
- Macomson SD, Brophy CM, et al. Heat shock protein expression in cerebral vessels after subarachnoid hemorrhage. Neurosurgery. 2002;51(1):204–210. doi: 10.1097/00006123-200207000-00029. [DOI] [PubMed] [Google Scholar]
- Matsuno HIA, Nakajima K, Kato K, Kozawa O. A peptide isolated from alpha B-crystallin is a novel and potent inhibitor of platelet aggregation via dual prevention of PAR-1 and GPIb/V/IX. J Thromb Haemost. 2003;1(12):2636–2642. doi: 10.1111/j.1538-7836.2003.00502.x. [DOI] [PubMed] [Google Scholar]
- Matsuno H, Kozawa O, et al. A heat shock-related protein, p20, plays an inhibitory role in platelet activation. FEBS Lett. 1998;429:327–329. doi: 10.1016/S0014-5793(98)00626-7. [DOI] [PubMed] [Google Scholar]
- McLemore EC, Tessier DJ, et al. Transducible recombinant small heat shock-related protein, HSP20, inhibits vasospasm and platelet aggregation. Surgery. 2004;136(3):573–578. doi: 10.1016/j.surg.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Moore PE, Lahiri T, et al. Selected contribution: synergism between TNF-alpha and IL-1 beta in airway smooth muscle cells: implications for beta-adrenergic responsiveness. J Appl Physiol. 2001;91(3):1467–1474. doi: 10.1152/jappl.2001.91.3.1467. [DOI] [PubMed] [Google Scholar]
- Morton JP, Holloway K, et al. Exercise training-induced gender-specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve. 2009;39(2):230–233. doi: 10.1002/mus.21182. [DOI] [PubMed] [Google Scholar]
- Motojima S, Yukawa T, et al. Changes in airway responsiveness and beta- and alpha-1-adrenergic receptors in the lungs of guinea pigs with experimental asthma. Allergy. 1989;44(1):66–74. doi: 10.1111/j.1398-9995.1989.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Muehlich SCI, Garlichs CD, Krueger B, Posern G, Goppelt-Struebe M. Actin-dependent regulation of connective tissue growth factor. Am J Physiol Cell Physiol. 2007;292(5):C1732–C1738. doi: 10.1152/ajpcell.00552.2006. [DOI] [PubMed] [Google Scholar]
- Murray KJ. Cyclic AMP and mechanisms of vasodilation. Pharmacol Ther. 1990;47(3):329–345. doi: 10.1016/0163-7258(90)90060-F. [DOI] [PubMed] [Google Scholar]
- Musialek P, Lei M, et al. Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, I(f) Circ Res. 1997;81(1):60–68. doi: 10.1161/01.res.81.1.60. [DOI] [PubMed] [Google Scholar]
- Ott C, Iwanciw D, et al. Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton. J Biol Chem. 2003;278(45):44305–44311. doi: 10.1074/jbc.M309140200. [DOI] [PubMed] [Google Scholar]
- Penn RB, Benovic JL (1998) Regulation of G protein-coupled receptors. In Handbook of physiology, section 7: endocrinology, volume 1: Cellular endocrinology. Edited by PM Conn. Oxford University Press. Oxford, U.K. Sect 7, vol 1, p 125–164
- Piec I, Listrat A, et al. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005;19(9):1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- Pipkin W, Johnson JA, et al. Localization, macromolecular associations, and function of the small heat shock-related protein HSP20 in rat heart. Circulation. 2003;107(3):469–476. doi: 10.1161/01.CIR.0000044386.27444.5A. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Foster DB, et al. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol. 2000;524(Pt 3):865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold CM, O'Connor M, et al. Selected contribution: HSP20 phosphorylation in nitroglycerin- and forskolin-induced sustained reductions in swine carotid media tone. J Appl Physiol. 2001;91(3):1460–1466. doi: 10.1152/jappl.2001.91.3.1460. [DOI] [PubMed] [Google Scholar]
- Renowden S, Edwards DH, et al. Impaired cyclic nucleotide-mediated vasorelaxation may contribute to closure of the human umbilical artery after birth. Br J Pharmacol. 1992;106(2):348–353. doi: 10.1111/j.1476-5381.1992.tb14339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, et al. Pathological changes in levels of three small stress proteins, alphaB crystallin, HSP 27 and p20, in the hindlimb muscles of dy mouse. Biochim Biophys Acta. 1998;1406(2):162–168. doi: 10.1016/s0925-4439(97)00094-x. [DOI] [PubMed] [Google Scholar]
- Salinthone S, Tyagi M, et al. Small heat shock proteins in smooth muscle. Pharmacol Ther. 2008;119(1):44–54. doi: 10.1016/j.pharmthera.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santibanez JF, Olivares D, et al. Cyclic AMP inhibits TGFbeta1-induced cell-scattering and invasiveness in murine-transformed keratinocytes. Int J Cancer. 2003;107(5):715–720. doi: 10.1002/ijc.11457. [DOI] [PubMed] [Google Scholar]
- Sun X, Welsh MJ, Benndorf R. Conformational changes resulting from pseudophosphorylation of mammalian small heat shock proteins—a two-hybrid study. Cell Stress Chaperones. 2006;11(1):61–70. doi: 10.1379/CSC-149R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier DJ, Komalavilas P, et al. Transduction of peptide analogs of the small heat shock-related protein HSP20 inhibits intimal hyperplasia. J Vasc Surg. 2004a;40(1):106–114. doi: 10.1016/j.jvs.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Tessier DJ, Komalavilas P, et al. Sildenafil-induced vasorelaxation is associated with increases in the phosphorylation of the heat shock-related protein 20 (HSP20) J Surg Res. 2004b;118(1):21–25. doi: 10.1016/j.jss.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Koller A. Selected contribution: NO released to flow reduces myogenic tone of skeletal muscle arterioles by decreasing smooth muscle Ca(2+) sensitivity. J Appl Physiol. 2001;91(1):522–527. doi: 10.1152/jappl.2001.91.1.522. [DOI] [PubMed] [Google Scholar]
- Klundert FA, Jong WW. The small heat shock proteins, Hsp20 and aB-crystallin in cultured cardiac myocytes: differences in cellular localization and solubilization after heat stress. Eur J Cell Biol. 1999;78:567–572. doi: 10.1016/s0171-9335(99)80022-3. [DOI] [PubMed] [Google Scholar]
- Klundert FA, Smulders RH, et al. The mammalian small heat-shock protein Hsp20 forms dimers and is a poor chaperone. Eur J Biochem. 1998;258(3):1014–1021. doi: 10.1046/j.1432-1327.1998.2581014.x. [DOI] [PubMed] [Google Scholar]
- Klundert FAJM, IJssel PRLA, et al. Rat Hsp20 confers thermoresistance in a clonal survival assay, but fails to protect coexpressed luciferase in Chinese hamster ovary cells. Biochem Biophys Res Commun. 1999;254(1):164–168. doi: 10.1006/bbrc.1998.9917. [DOI] [PubMed] [Google Scholar]
- Verschuure P, Croes Y, et al. Translocation of small heat shock proteins to the actin cytoskeleton upon proteasomal inhibition. J Mol Cell Cardiol. 2002;34(2):117–128. doi: 10.1006/jmcc.2001.1493. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu A, et al. Phosphorylation of P20 is associated with the actions of insulin in rat skeletal and smooth muscle. Biochem J. 1999a;344:971–976. doi: 10.1042/0264-6021:3440971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu A, et al. Insulin and insulin antagonists evoke phosphorylation of P20 at serine 157 and serine 16 respectively in rat skeletal muscle. FEBS Lett. 1999b;462(1–2):25–30. doi: 10.1016/S0014-5793(99)01496-9. [DOI] [PubMed] [Google Scholar]
- Woodrum D, Pipkin W, et al. Phosphorylation of the heat shock-related protein, HSP20, mediates cyclic nucleotide-dependent relaxation. J Vasc Surg. 2003;37:874–881. doi: 10.1067/mva.2003.153. [DOI] [PubMed] [Google Scholar]
- Woodrum DA, Brophy CM, et al. Phosphorylation events associated with cyclic nucleotide-dependent inhibition of smooth muscle contraction. Am J Physiol. 1999;277(3 Pt 2):H931–H939. doi: 10.1152/ajpheart.1999.277.3.H931. [DOI] [PubMed] [Google Scholar]
- Zhu YH, Ma TM, et al. Gene transfer of heat-shock protein 20 protects against ischemia/reperfusion injury in rat hearts. Acta Pharmacol Sin. 2005;26(10):1193–1200. doi: 10.1111/j.1745-7254.2005.00139.x. [DOI] [PubMed] [Google Scholar]