Abstract
Exposure to 17β-estradiol prior to induction of apoptosis protects skeletal muscle cells against damage. The mechanism involved in this protective action of the hormone is poorly understood. In the present study, using the murine muscle cell line C2C12, evidence was obtained that inhibition of H2O2-induced apoptosis by the estrogen requires the participation of heat shock protein 27 (HSP27). Reverse transcriptase polymerase chain reaction, Western blot, and immunocytochemistry assays showed that 17β-estradiol induces a time-dependent (5–60 min) increase in the expression of HSP27. In addition, in presence of quercetin, an inhibitor of HSPs, the antiapoptotic effect of the hormone was diminished. More specifically, blockage experiments with short interference RNA targeting HSP27 confirmed the role of this chaperone in the protective effect of the steroid. 17β-Estradiol abolished caspase-3 cleavage elicited by H2O2. Coimmunoprecipitation assays suggested physical interaction of HSP27 with caspase-3 in presence of estradiol. Furthermore, we observed that this chaperone interacts with estrogen receptors (ER) β in mitochondria. Then, this study suggests that HSP27 plays a new role in the antiapoptotic action triggered by 17β-estradiol by modulating caspase-3 activity and stabilizing ERβ in skeletal muscle cells.
Keywords: Apoptosis, Caspase-3, 17β-Estradiol, ERβ, HSP27, Skeletal muscle
Introduction
The protective action of estrogens on tissues is currently receiving increased attention. It is well known that estrogen therapy has beneficial effects on neurons, bone, and the cardiovascular system (Persky et al. 2000; Jia et al. 2008). Skeletal muscle is also a target tissue for estrogens where they play an important regulatory action on proliferation and differentiation of skeletal myoblasts (Kahlert et al. 1997). Moreover, muscle performance diminishes during the postmenopausal years, leading to sarcopenia, and several myopathies are caused in part by decreased estrogen levels (Dionne et al. 2000). In agreement with these observations, it has been established that human skeletal muscle contains estrogen receptors (ERs) α and β (Lemoine et al. 2003; Wiik et al. 2003). In previous work, we demonstrated that 17β-estradiol (E2) inhibits apoptosis in C2C12 skeletal muscle cells through ERs with non-classical localization and involving the PI3K/Akt pathway (Vasconsuelo et al. 2008).
Apoptosis is a central homeostatic process that regulates cell number in living organisms. Diverse stimuli trigger apoptosis by activating one or more signal transduction pathways, which converge in the activation of a conserved family of cysteine proteases known as caspases (Steller 1995). They are constitutively expressed as zymogens and become active by proteolytic cleavage (Cohen 1997). These pathways for apoptosis induction have been classified as intrinsic and extrinsic. The hallmarks of the intrinsic pathway are mitochondrial involvement with direct participation of mitochondrial proteins and the formation of the “apoptosome”. Apoptosome formation results in activation of the caspase cascade (Saleh et al. 2000; Jiang and Wang 2000). In contrast, the extrinsic pathway is initiated through the stimulation of the transmembrane death receptors and formation of the death-inducing signal complex and in turn caspase activation (Kischkel et al. 1995; Ashkenazi and Dixit 1998). Recently, a correlation between the expression of heat shock proteins (HSPs) and increased cell survival was shown, pointing them as regulatory agents of components of apoptotic pathways (Mehlen et al. 1996; Samali and Cotter 1996).
Heat shock proteins are a family composed of ubiquitous and conserved proteins that according to their molecular weight include high molecular mass HSPs (≥100 kDa), HSP90 (81 to 99 kDa), HSP70 (65 to 80 kDa), HSP60 (55 to 64 kDa), HSP40 (35 to 54 kDa), and small HSPs (≤34 kDa; Hartl 1996). HSPs are known for controlling cell homeostasis, proper folding of proteins, and translocation through cell membranes, acting as molecular chaperones (Welch 1992; Muchowski et al. 1997; Arrigo 1998). Moreover, this family of proteins supplies an intrinsic mechanism to defend the cell against external physiological stresses. As mentioned before, high expression levels of HSPs imply increased cell survival. Specifically, the HSP involved in cytoprotective actions is the small (24–28 kDa) HSP, referred to as HSP25 or HSP27. HSP27 is expressed constitutively in many mammalian tissues and cell lines (Arrigo and Landry 1994). Of relevance for our work, HSP27 also appears to be involved in the suppression of apoptosis (Arrigo 1998; Bruey et al. 2000; Pandey et al. 2000). Interestingly, HSP27 is induced by estrogens in various cells such as platelets (Mendelsohn et al. 1991) as well as breast and endometrial tumors (Ciocca et al. 1993). Furthermore, related to its antiapoptotic role, high levels of HSP27 are a marker for increased malignancy in breast cancer (Hansen et al. 1999). Regarding skeletal muscle, it has been observed that the expression of HSP27 is induced by a variety of stimuli (Welch 1992). However, its functions in muscle cells in connection to the effects of estrogens in this tissue have not been studied yet.
In view of our recent evidence (Vasconsuelo et al. 2008), in this work, we investigated whether HSP27 plays a role in the antiapoptotic action of 17β-estradiol on skeletal muscle cells.
Materials and methods
Materials
Estrogen receptor β goat polyclonal Y-19 (mapping at the N terminus) and antiactin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Fluorescein-short interference RNA (siRNA) transfection control and TransPass R2 transfection reagent were from New England Biolabs (Beverly, MA, USA). HSP27 siRNA, polyclonal anticaspase-3, and monoclonal anti-HSP27 and anti-HSP90 antibodies were from Cell Signaling Technology, Inc (Danvers, MA, USA). DAPI and MitoTracker Red (MitoTracker Red CMXRos) dyes and Alexa 488-conjugated secondary antibody were from Molecular Probes (Eugene, OR, USA). 17β-Estradiol and quercetin were purchased from Sigma-Aldrich (St Louis, MO, USA). TRIZOL Reagent and primers were obtained from Invitrogen Corporation (Carlsbad, CA, USA). Reverse Transcription System was from Promega Corporation (Madison, WI). All the other reagents used were of analytical grade.
Cell culture and treatment
C2C12 murine skeletal muscle cells, kindly donated by Dr. Enrique Jaimovich (Universidad de Chile, Santiago, Chile), were cultured in growth medium (Dulbecco’s modified Eagle’s medium) supplemented with 10% heat-inactivated (30 min, 56°C) fetal bovine serum, 1% nistatine, and 2% streptomycin. Cells were incubated at 37°C in a humid atmosphere of 5% CO2 in air. Cultures were passaged every 2 days with fresh medium. The treatments were performed with 70–80% confluent cultures in medium without serum by adding 10−8 M 17β-estradiol or vehicle 0.001% isopropanol (control), approx. 45 min before induction of apoptosis with hydrogen peroxide (H2O2) during 8 h. H2O2 was diluted in culture medium without serum at a final concentration of 0.5 mM in each assay. Cells were preincubated with quercetin at the times indicated in each set of experiments. Quercetin was solubilized in 1 mM NaOH and then diluted in culture medium without serum at a final concentration of 100 μM in each assay. After treatments, cells were lysed using a buffer composed of 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.2 mM Na2VO4, 2 mM EDTA, 25 mM NaF, 1 mM PMSF, 1% NP40, 20 μg/ml leupeptin, and 20 μg/ml aprotinin. Lysates were collected by aspiration and centrifuged at 12,000×g during 15 min. Protein concentration from the supernatant was estimated by the method of Bradford (1976), using bovine serum albumin (BSA) as standard. Unless otherwise noted, cells were cultured in chamber slides for microscopy.
Western blot analysis
Protein samples (25 μg) were mixed with one fourth of the sample buffer (400 mM Tris/HCl pH 6.8, 10% sodium dodecyl sulfate (SDS), 50% glycerol, 500 mM DTT, and 2 mg/ml bromophenol blue), boiled for 5 min, and resolved by 10% SDS-polyacrylamide gel electrophoresis (PAGE) according to the method of Laemmli (1970). Fractionated proteins were electrotransferred to polyvinylidene fluoride membranes (Immobilon-P; PVDF) and then blocked for 1 h at room temperature with 5% non-fat dry milk in phosphate-buffered saline (PBS) containing 0.1% Tween-20 (PBS-T). Blots were incubated for 1 h with the appropriate dilution of the primary antibodies: anticaspase-3 (1:1,000) and anti-HSP27 (1:1,000) using, for both of them, antirabbit secondary antibodies. The membranes were repeatedly washed with PBS-T prior incubation with horseradish peroxidase-conjugated secondary antibodies. The enhanced chemiluminescence (ECL) blot detection kit (Amersham, Buckinghamshire, England) was used as described by the manufacturer to visualize reactive products. Relative migration of unknown proteins was determined by comparison with molecular weight markers (Amersham). For actin loading control, membranes were stripped with stripping buffer (62.5 mM Tris–HCl pH 6.7, 2% SDS, 50 mM β-mercaptoethanol) and then blocked for 1 h with 5% non-fat dry milk in PBS-T. The blots were then incubated 1 h with a 1:20,000 dilution of antiactin polyclonal antibody (A-5060) as primary antibody. After several washings with PBS-T, membranes were incubated with antirabbit (1:10,000) conjugated to horseradish peroxidase. The corresponding immunoreactive bands were developed by means of ECL.
Coimmunoprecipitation
Total homogenates from the C2C12 cell line containing 100 μg of protein were immunoprecipitated with 10 μl of a 50% suspension of protein A–agarose after incubating the extracts with the antibody indicated in each experiment. The immunoprecipitates were washed three times with wash buffer (50 mM Tris–HCl, pH 7.4; 1 mM EDTA; 1% Triton X-100; protease inhibitors: 2 mM PMSF, 20 μg/ml leupeptin, 20 μg/ml aprotinin, and 10 μg/ml of trypsin inhibitor). The final pellets were obtained by centrifugation for 3 min at 10,000×g, resuspended then in electrophoresis sample buffer without dithiothreitol, boiled for 5 min, and resolved by SDS-PAGE. Fractionated proteins were electrotransferred to PVDF membranes and then blocked for 1 h with 5% non-fat dry milk in PBS-T. The blots were incubated overnight at 4°C with primary monoclonal antibody against the protein of interest. After several washings with PBS-T, the membranes were incubated with the secondary antibody conjugated to horseradish peroxidase. Immunoreactive proteins were developed by means of enhanced chemiluminescence. The apparent molecular weight of reactive bands was estimated by reference to a wide size range of protein markers.
Immunocytochemistry
Semiconfluent (60–70%) monolayers were washed with PBS and fixed with 2% paraformaldehyde in PBS. After fixation, cells were rinsed three times with PBS, and non-specific sites were blocked for 1 h in 2% BSA. Cells were incubated with anti-HSP27 (1:200 in 2% BSA) for 1 h at 4°C. The primary antibody was recognized by Alexa 488-conjugated secondary antibody. Finally, the stained cells were analyzed with a confocal laser scanning microscope.
MitoTracker Red staining
Coverslips with adherent cells were stained with MitoTracker Red, which was prepared in dimethyl sulfoxide and then added to the cell culture medium at a final concentration of 1 µmol/l. After 15- to 30-min incubation at 37°C, the cells were washed with PBS and fixed with methanol at −20°C for 30 min. Finally, the coverslips were analyzed by confocal microscopy as described before. Images were collected using a digital camera
Confocal microscopy
The samples used for confocal microscopy were processed as described above, and confocal scanning laser microscopy was performed with a Leica TCS SP2 AOBS microscope, using a ×63 objective. The specificity of the labeling techniques was proven by the absence of labeling when the primary or the secondary antibodies were omitted.
Transfection of short interfering RNA
Transfection was performed with a culture cellular density reaching 40–60% confluence with HSP27 siRNA according to the manufacturer’s (NEB) instructions. Briefly, TransPass R2 transfection reagent was mixed with HSP27 siRNAs. The mix was maintained 20 min at room temperature. The C2C12 cells were incubated with the transfection mixture for 24 and 48 h at room temperature. To estimate the transfection efficiency of siRNA, 10–30 pmol of fluorescein-siRNA was used following NEB's protocol. Cells were then visualized in a conventional fluorescence microscope. To evaluate the effective silencing of HSP27, total protein lysates from cells transfected with HSP27 siRNA or TransPass R2 reagent alone (controls) were tested for HSP27 expression by Western blot analysis as described above using anti-HSP27 monoclonal antibody. The specificity of the siRNA probe was also checked by immunoblotting with anti-HSP90 monoclonal antibody.
Reverse transcription polymerase chain reaction
Semiconfluent cells in 75-cm2 culture flasks were rinsed once with 1× PBS (pH 7.4) and then harvested by scraping using Trizol Reagent, for total RNA isolation, according to manufacturer's instructions. The RNA pellet was air-dried for 5 min and subsequently dissolved in 50 μl of deionized water. The concentration and purity of the RNA preparation were determined by measuring the absorbance of RNA at 260 and 280 nm. The RNA integrity was analyzed on a 1% native agarose gel in 1× TBE buffer. cDNA was prepared with AMV reverse transcriptase using random primer hexamers. The reaction was performed in a total volume of 20 μl containing 5 μg of total RNA, according to manufacturer's instructions. The reaction mixture was incubated at 42°C for 1 h.
For the polymerase chain reaction (PCR), the total volume (20 μl) from the reverse transcription reaction was amplified in a final volume of 100 μl containing 0.5 μM of each primer (forward and reverse). Amplification was performed on an Eppendorf Mastercycler® personal 5332 for 35 cycles with denaturation at 94°C (30 s), annealing at 58°C (30 s) and extension at 72°C (30 s).
PCR primers were specifically designed to amplify mouse HSP27 employing the Mouse BLASTN program of the Gen Bank Sequence database. The program was used to analyze the specificity of primer annealing. No matching was obtained with the mouse gene sequence of other chaperones.
Statistical analysis
Statistical treatment of the data was performed using the Student’s t test (Snedecor and Cochran 1967). Data are means ± standard deviation (SD) of not less than three independent experiments. The data were considered statistical significant when p < 0.05.
Results
17β-Estradiol increases levels of HSP27 in C2C12 cells
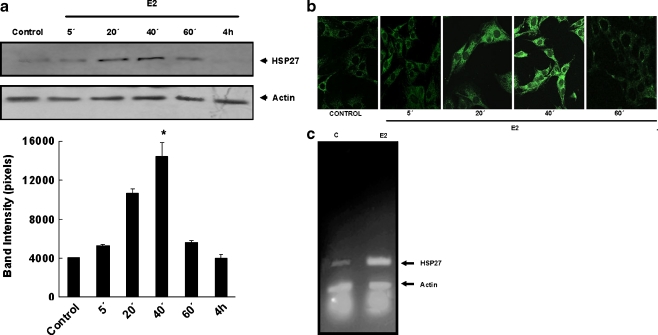
As a first approach to evaluate the role of HSP27 in the regulation of apoptosis by 17β-estradiol in skeletal muscle cells, we investigated the chaperone expression levels in response to the hormone. C2C12 cell cultures were incubated with the steroid hormone (10−8 M) during different times (5, 20, 40, and 60 min and 4 h) followed by measurement of HSP27 levels by immunoblot analysis. As observed in Fig. 1a, Western blots using an anti-HSP27 monoclonal antibody revealed a time-dependent increase in the expression of HSP27 in response to E2, the effects being very marked after estrogen treatment for 20 min (167%) and 40 min (271%). In agreement with these results, immunocytochemistry studies using confocal microscopy and the same antibody showed that after 20–40 min of treatment with E2 (10−8 M), the fluorescence intensity was higher than control conditions (Fig. 1b). Using semiquantitative reverse transcriptase (RT)-PCR assays with specific primers (forward: 5′cccaccctctatcacggc 3′; reverse: 5′gatagagcagctcgaacccg 3′; amplified HSP27 fragment: 290 pb), HSP27 mRNA levels were examined in E2-treated (10−8 M 17β-estradiol, 40 min) and control (0.001% of isopropanol, 40 min) C2C12 cells. The levels of mRNA of control and E2-treated cells were compared using β-actin as an internal control (primers for actin fragment of 151 pb: forward: 5′ ctccctggagaagagctatg 3′, reverse: 5′cttcatgatggaattgaatg 3′). Figure 1c shows increased levels of HSP27 mRNA after E2 treatment of C2C12 cells.
Fig. 1.
17β-Estradiol increases the expression of HSP27 in C2C12 skeletal muscle cells. C2C12 cells were treated with 10−8 M of 17β-estradiol (E2) for the times indicated. Controls were exposed to vehicle isopropanol (0.001%). The cells were harvested and lysed as described in “Materials and methods”. The lysates were then subjected to SDS-PAGE and blotted with anti-HSP27 antibody. Actin levels were measured as protein loading controls. a Representative immunoblot (upper panel) of three independent experiments with comparable results and the corresponding densitometric analysis (bottom panel) showing the increase of total Hsp27 during E2 treatment. Bars are means ± SD; *p < 0.05 and **p < 0.01 with respect to the control. b Confocal microscopy of HSP27 expression. HSP27 (green fluorescence) was stained by using anti-HSP27 primary antibody and Alexa 488-conjugated secondary antibody. Control cells treated with vehicle, E2 cells incubated with 10−8 M 17β-estradiol for the times indicated. Magnification, ×63. Images are representative of at least three independent experiments. c Total RNA was extracted from C2C12 cells after treatments indicated (E2 10−8 M of 17β-estradiol at the times indicated, Control untreated cells). RNA samples were subjected to RT and subsequently to PCR as in “Materials and methods”. β-Actin is used as an internal control. Equal amounts of the reaction products were analyzed on agarose gels and stained with ethidium bromide. The photograph is representative of at least three independent experiments
Quercetin and HSP27 siRNA inhibit the protective effects of 17β-estradiol in C2C12 muscle cells
To examine the role of HSP27 as mediator of the antiapototic effects of 17β-estradiol, C2C12 cells were preincubated with quercetin, an inhibitor of HSPs, or alternatively with HSP27 siRNA, and then HSP27 expression and apoptosis were evaluated after challenging the muscle cells with H2O2 in the absence and presence of E2.
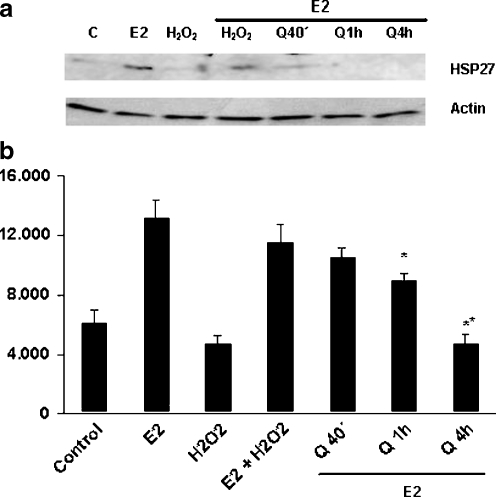
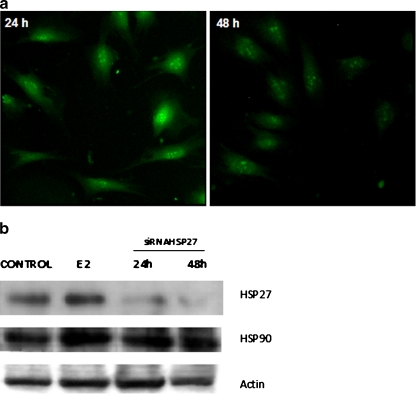
The effects of quercetin on E2-mediated expression of HSP27 in C2C12 cells were investigated measuring HSP27 levels by immunoblotting of lysates of muscle cells preincubated with 100 μM quercetin for 40 min, 1 h, or 4 h and then treated with the hormone during 40 min. As shown in Fig. 2, attenuation of HSP27 expression levels was observed after 1-h treatment with quercetin, larger inhibition being detected after 4 h of preincubation with the flavonoid. Considering this evidence, C2C12 cells were pretreated with quercetin for 1 h, followed by incubation with E2 (10−8 M for 40 min) and then challenged with H2O2 (0.5 mM for 8 h) to induce apoptosis. Morphological changes typical of apoptosis such as nuclear fragmentation/condensation (pyknotic nuclei) and cytoskeleton disorganization using the nuclear dye DAPI and antiactin antibody were investigated. Table 1 shows that quercetin inhibits the protective action of E2. In addition, we analyzed these changes and the effect of the hormone thereupon in C2C12 cells transfected with specific siRNAs to induce silencing of HSP27. First, optimum transfection conditions were established using fluorescein-siRNA (≥70% after 24-/48-h incubation with 20 pmol of fluorescent probe; Fig. 3a). To verify the silencing efficiency and specificity of siRNA effects, we examined by Western blot analysis the expression levels of HSP27 and HSP90 after transfection with siRNAs (24/48 h with 10/20 pmol of HSP27 siRNA). Figure 3b shows that the siRNA probe significantly inhibited the expression of HSP27. However, HSP90 protein levels were not affected, evidencing that the siRNA used was both highly efficient and specific. The efficiency of HSP27 silencing was further demonstrated by immunocytochemistry assays (data not shown). As indicated in Table 1, HSP27 silencing caused a reduction of the antiapoptotic effects of E2 although to a lesser degree than quercetin.
Fig. 2.
Effect of quercetin on 17β-estradiol-induced HSP27 expression in C2C12 skeletal muscle cells. C2C12 cells were preincubated with 100 μM quercetin for 40 min, 1 h, or 4 h and then treated with 10−8 M 17β-estradiol for 40 min. Control untreated cells, E2 cells treated with 17β-estradiol. Cell lysates were then prepared followed by SDS-PAGE and blotting with anti-HSP27 antibody as described in “Materials and methods”. Actin levels were measured as protein loading control. a Representative immunoblot. b Densitometric quantification of blots from three independent experiments; averages ± SD are given. *p < 0.05; **p < 0.01
Table 1.
Role of HSP27 in the antiapoptotic action of 17β-estradiol in C2C12 cells
| Conditions | Apoptotic cells (%) |
|---|---|
| Control | 23 ± 7.2 |
| Quercetin | 37 ± 3.7 |
| E2 | 14.5 ± 7.3 |
| H2O2 | 77 ± 7.5* |
| E2 + H2O2 | 27.5 ± 10.4 |
| Quercetin + E2 + H2O2 | 69 ± 3.2* |
| HSP27 siRNA | 31 ± 9 |
| HSP27 siRNA + E2 + H2O2 | 42 ± 8* |
Morphological analysis of C2C12 cells after treatments were performed using antiactin antibody and DAPI by epifluorescence microscopy as described in “Materials and methods”. Results are presented as percentages of apoptotic cells of three independent experiments ± SD. At least 10 fields per dish were examined. Each value represents the mean of three independent experiments ± SD
Control untreated cells, Quercetin cells treated with 100 μM quercetin for 1 h, E2 cells incubated with 10−8 M 17β-estradiol for 45 min, H2O2 cells treated with 0.5 mM H2O2 during 8 h, Quercetin + E2 + H2O2 cells preincubated with 100 μM of quercetin for 1 h, next with 10−8 M 17β-estradiol for 45 min and then treated with 0.5 mM H2O2 during 8 h, HSP27 siRNA cells without HSP27 protein expression, HSP27siRNA + E2 + H2O2 cells without HSP27 protein expression and treated with E2 and H2O2 as before
*p < 0.05 with respect to the control
Fig. 3.
Blockage of HSP27 expression in C2C12 cells. Transfections of C2C12 cells with siRNA were performed as described under “Materials and methods”. a Transfection efficiency of siRNA was estimated using fluorescein-siRNA transfection control and TransPass R2 transfection reagent. Cells were then visualized in a conventional microscope employing an adequate filter for green fluorescence. A typical pattern of fluorescence with fluorescein-siRNA is shown (more than 70% cells present green fluorescence after 24-h/48-h incubation with fluorescent probe). Original magnification, ×400. b C2C12 cells were transiently transfected with 20 pmol of HSP27 siRNA. Expression of HSP27 was analyzed 24- and 48-h posttransfection by Western blot analysis as described in “Materials and methods”. Immunoblots of total cell lysates employing anti-HSP27 or anti-HSP90 monoclonal antibodies are shown. Actin loading control was detected using antiactin polyclonal antibody. A representative blot from three independent experiments is shown. Control vehicle isopropanol (0.001%), E2 cells treated with 10−8 M 17β-estradiol for 40 min
HSP27 interacts with ERβ in C2C12 muscle cells
In order to study whether HSP27 interacts with the ERs, confocal microscopy was first performed to detect colocalization of these proteins. In a previous work, we demonstrated that in C2C12 muscle cells, ERα localizes in the cytosol and perinuclear region (Milanesi et al. 2008), whereas ERβ is mainly associated to mitochondria (Milanesi et al. 2009). C2C12 cells were treated with 10−8 M E2 during 40 min. The ERs were recognized by immunofluorescence with two different antibodies specific for each isoform and a highly selective monoclonal antibody for HSP27 was used. Immunocytochemistry assays demonstrated colocalization of HSP27 with ERβ in the mitochondria. Colocalization of anti-HSP27 and anti-ERβ is shown in the merged images as yellow fluorescence, which was more intense in cells exposed to E2 (Fig. 4a). To assess whether this colocalization may imply physical interaction between ERs and HSP27, coimmunoprecipitation assays using both anti-ERβ and anti-ERα antibodies with lysates from control and estrogen-treated (10−8 M, 40 min) C2C12 cells were performed. The precipitates were then analyzed by Western blot with the anti-HSP27 antibody. As shown in Fig. 4b, the anti-ERβ antibody immunoprecipitated ERβ associated to HSP27, indicating the interaction between both proteins. This interaction was also observed when the coimmunoprecipitation assay was performed using the same antibodies in reverse order (data no shown). On the other hand, the interaction of the chaperone with ERα was not significant (Fig. 4b).
Fig. 4.
HSP27 interacts with ERβ in C2C12 skeletal muscle cells. a Immunocytochemistry. C2C12 cells treated with 0.001% isopropanol (Control) or 10−8 M 17β-estradiol during 40 min (E2) were double-labeled using polyclonal antibodies anti-ERβ, Y19 (red fluorescence), and anti-HSP27 (green fluorescence) as described in “Materials and methods”. Laser confocal microscopy shows merge of staining with the two antibodies as yellow fluorescence. The photograph is representative of at least three independent experiments. Magnification, ×63. b Coimmunoprecipitation. Lysates from C2C12 cells treated as indicated above were immunoprecipitated using anti-ERβ or anti-ERα antibody and immunoblotted with anti-HSP27 antibody. Control cells treated with vehicle, E2 cells treated with 10−8 M 17β-estradiol during 40 min. The blot is representative of three independent experiments
HSP27 associates with caspase-3 in C2C12 muscle cells
Recent evidence has shown that HSP27 regulates apoptosis through its ability to interact with various components of the apoptotic cascade, in particular, blocking caspase activation (Havasi et al. 2008; Concannon et al. 2003). Western blot analysis performed using an anticaspase-3 antibody showed that estrogen prevented the activation of caspase-3 induced by H2O2. In the presence of the HSP's inhibitor quercetin, this steroid inhibition is not observed (data not shown). Quantitative analysis of immunoreactive bands showed a significant decrease in the levels of full-length caspase-3 in lysates from C2C12 cells induced to apoptosis with H2O2 as described in “Materials and methods”, compared to lysates from muscle cells treated with 17β-estradiol or vehicle isopropanol alone (control; Fig. 5). To obtain further evidence that the chaperone plays a role in the steroid protective action at this level of the apoptotic cascade, HSP27 and caspase-3 interaction was investigated. Coimmunoprecipitation assays of lysates from C2C12 cells treated with 17β-estradiol in the absence and presence of H2O2 were performed. Figure 6 shows that coimmunoprecipitation with anti-HSP27 followed by Western blot using an anticaspase-3 antibody precipitated a complex formed by at least HSP27 and caspase-3. This interaction was detected in all conditions tested except in cells treated with H2O2 without preincubation with E2. A similar result was obtained when the same antibodies were used in reverse order (data not shown).
Fig. 5.
17β-Estradiol abrogates H2O2-induced caspase-3 activation in C2C12 skeletal muscle cells. C2C12 cells were treated as follows. Control vehicle isopropanol alone, E2 10−8 M 17β-estradiol for 8 h, H2O2 0.5 mM H2O2 during 8 h, H2O2+ E2 10−8 M 17β-estradiol for 45 min followed by 0.5 mM H2O2 during 8 h. Cell lysate proteins (25 μg) from each condition were fractionated by SDS-PAGE and then immunoblotted with anticaspase-3 antibody as described in “Materials and methods”. The band detected represents uncleaved (inactive) caspase-3 (35 kDa). Actin levels were measured as protein loading controls. Experiments were repeated at least three times with essentially identical results. Representative immunoblots and densitometric analysis are shown
Fig. 6.
HSP27 associates with caspase-3 in C2C12 skeletal muscle cells. Lysates from C2C12 cells treated as follows were immunoprecipitated with anti-HSP27 antibody before immunoblotting with anticaspase-3 antibody. Control vehicle, E2 10−8 M 17β-estradiol for 40 min, H2O2 0.5 mM H2O2 for 8 h, H2O2 + E2 10−8 M 17β-estradiol for 40 min and then H2O2 for 8 h. Experiments were repeated at least three times with essentially identical results
Discussion
Evidence showing that E2 can sustain survival or alternatively induce apoptosis of cells depending on their biological context has been reported (Choi et al. 2001; Okasha et al. 2001; Florian and Magder 2008; Seli et al. 2007). In earlier experiments, we demonstrated that E2, at physiological concentrations, abrogates H2O2-induced apoptosis in C2C12 skeletal muscle cells involving both ERα and ERβ and acting at least at two different levels. One of them is inducing PI3K/Akt activation and then BAD phosphorylation, a process in which both ER isoforms participate. The other relates to a protective effect on mitochondria integrity and mainly involves ERβ (Vasconsuelo et al. 2008). In the present work, we found that HSP27 is another intermediate of the protective action of 17β-estradiol. This is in agreement with previous reports, which suggested an antiapoptotic role of HSP27 by interaction with components of the apoptotic cascade (Arrigo 1998; Bruey et al. 2000; Pandey et al. 2000).
First, we found that E2 at physiological concentrations increased mRNA and protein levels of HSP27 in muscle cells, this effect being quite significant after 40 min of treatment with the hormone. E2-induced increase of expression of HSP27 has been evidenced in other cell types (Cooper et al. 2000). Next, we investigated any possible correlation of E2 induction of the chaperone and the steroid protective effect. To this end, we evaluated the antiapoptotic action of E2 in the presence of quercetin. It is known that quercetin, one of the most widely distributed bioflavonoids in the plant kingdom, is an inhibitor of the expression of HSPs (Wei et al. 1994; Jakubowicz-Gil et al. 2002 and ref. therein). With regard to HSP27, the mechanism of inhibition by quercetin has not been clearly elucidated but the diminished HSP27 expression by this flavonoid may be explained by the ability of quercetin to inhibit the activity of HSP25 kinase, an enzyme necessary for the activation of small HSPs (Hayees and Benndorf 1997).
Here, we observed that treatment of C2C12 muscle cells with quercetin inhibited 17β-estradiol-induced HSP27 expression, which resulted in a blockage of the antiapoptotic effect of 17β-estradiol, as deduced from the count of apoptotic nuclei. However, since quercetin could affect other HSPs, to obtain specific evidence on the participation of HSP27 in estrogen downregulation of apoptosis, we selectively suppressed the expression of this chaperone by siRNA technology. The data obtained confirmed the participation of HSP27 although the possibility that another HSP also contributes to the antiapoptotic action of the steroid cannot be excluded.
Our experiments suggest that HSP27 could act at least at two different levels. One of them relates to the interaction of the chaperone with ERβ in mitochondria. This interaction might confer major stability to this isoform and then be more efficient in stress conditions and/or regulating the estrogen signal. In agreement with this contention, it has been observed that the interaction of HSP27 and ERβ leads to estrogen signaling in human coronary arteries (Miller et al. 2005). In our muscle cell system, the interaction observed appears to be specific for ERβ and HSP27 since it was not observed for the chaperone and the isoform α of the estrogen receptor. This may explain the fact that E2 involves mainly ERβ in its protective action (Vasconsuelo et al. 2008). Secondly, the chaperone may act at the caspase-3 level in the apoptotic cascade. In the present study, we showed that E2 prevents caspase-3 cleavage induced by H2O2 in C2C12 cells, and this antiapoptotic action is inhibited by quercetin treatment. In addition, we found that E2 induces HSP27 and caspase-3 interaction. This association is not observed in apoptotic muscle cells (C2C12 cells treated with hydrogen peroxide). Since there is evidence that HSP27 is capable of interacting with a number of components of the apoptotic pathway (Arrigo 1998; Bruey et al. 2000; Pandey et al. 2000), the possibility that HSP27 acts at levels other than caspase-3 in muscle cells cannot be excluded. Moreover, in view that one of the effect of estradiol is the induction of HSP27 expression in C2C12 cells, more molecules of the chaperone would increase the possibility of interaction with various apoptotic components, tilting the balance toward cell survival. Strengthening this hypothesis, in coimmunoprecipitation assays with anticaspase-3 followed by Western blot using an anti-HSP27 antibody, we observed in supernatants from cell lysates that a portion of HSP27s do no interact with caspase-3. These molecules may probably act on other apoptotic mediators. Clearly, further studies are necessary to identify other sites of action of HSP27 in muscle cells.
In conclusion, the results of this work indicate that HSP27 mediates the antiapoptotic action of 17β-estradiol at the levels of ERβ and caspase-3. These findings represent a new mechanism in which E2 promotes HSP27 interactions with ERβ and caspase-3 to negatively regulate H2O2-induced apoptosis in muscle cells. These findings improve our understanding of the basis, which underlie the myopathies associated to deficit of estrogen and/or deregulation of apoptosis (Di Giovanni et al. 2000; Dionne et al. 2000; Tews 2002).
Acknowledgments
This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
References
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Arrigo AP, Landry J. Expression and function of the low-molecular-weight heat shock proteins. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. New York: Cold Spring Harbor Laboratory Press; 1994. pp. 335–373. [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;172:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Choi KC, Kang SK, Tai CJ, Auersperg N, Leung PC. Estradiol up-regulates antiapoptotic Bcl-2 messenger ribonucleic acid and protein in tumorigenic ovarian surface epithelium cells. Endocrinology. 2001;142:2351–2360. doi: 10.1210/en.142.6.2351. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Oesterreich S, Chammess GC, McGuire WL, Fuqua SAW. Biological and clinical implications of heat shock protein 27000 (Hsp27): a review. J Natl Cancer Inst. 1993;85:1558–1569. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/A:1021601103096. [DOI] [PubMed] [Google Scholar]
- Cooper LF, Tiffee JC, Griffin JP, Hamano H, Guo Z. Estrogen-induced resistance to osteoblast apoptosis is associated with increased HSP27 expression. J Cell Physiol. 2000;185:401–407. doi: 10.1002/1097-4652(200012)185:3<401::AID-JCP10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Giovanni S, Mirabella M, D'Amico A, Tonali P, Servidei S. Apoptotic features accompany acute quadriplegic myopathy. Neurology. 2000;55:854–858. doi: 10.1212/wnl.55.6.854. [DOI] [PubMed] [Google Scholar]
- Dionne IJ, Kinaman KA, Poehlman ET. Sarcopenia and muscle function during menopause and horrmone-replacement therapy. J Nutr Health Aging. 2000;4:156–161. [PubMed] [Google Scholar]
- Florian M, Magder S. Estrogen decreases TNF-alpha and oxidized LDL induced apoptosis in endothelial cells. Steroids. 2008;73:47–58. doi: 10.1016/j.steroids.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Hansen RK, Parra I, Lemiux P, Oesterreich S, Hilsenbeck SG, Fuqua SAW. Hsp27 overexpression inhibits doxorubicin-induced apoptosis in human breast cancer cells. Breast Cancer Res Treat. 1999;56:187–196. doi: 10.1023/A:1006207009260. [DOI] [PubMed] [Google Scholar]
- Hartl F. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283:12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayees K, Benndorf R. Effect of protein kinase inhibitors on activity of mammalian small heat shock protein (Hsp25) kinase. Biochem Pharmacol. 1997;53:1239–1247. doi: 10.1016/S0006-2952(96)00877-5. [DOI] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Rzymowska J, Gawron A. Quercetin, apoptosis, heat shock. Biochem Pharmacol. 2002;64:1591–1595. doi: 10.1016/S0006-2952(02)01356-4. [DOI] [PubMed] [Google Scholar]
- Jia J, Guan D, Zhu W, Alkayed NJ, Wang MM, Hua Z, Xu Y. Estrogen inhibits Fas-mediated apoptosis in experimental stroke. Exp Neurol. 2008;215:48–52. doi: 10.1016/j.expneurol.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- Kahlert S, Grohe C, Karas RH, Lobbert K, Neyses L, Vetter H. Effects of estrogen on skeletal myoblast growth. Biochem Biophys Res Commun. 1997;232:373–378. doi: 10.1006/bbrc.1997.6223. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35:439–443. doi: 10.1249/01.MSS.0000053654.14410.78. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Zhu Y, O’Neill S. The 29-kDa proteins phosphorylated in thrombin-activated human platelets are forms of the estrogen receptor-related 27-kDa heat shock protein. PNAS. 1991;88:11212–11216. doi: 10.1073/pnas.88.24.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi L, Boland AR, Boland R. Expression and localization of estrogen receptor α in the C2C12 murine skeletal muscle cell line. J Cell Biochem. 2008;104:1254–1273. doi: 10.1002/jcb.21706. [DOI] [PubMed] [Google Scholar]
- Milanesi L, Vasconsuelo A, R. de Boland A, Boland R. Expression and subcellular distribution of native receptor beta in murine C2C12 cells and skeletal muscle tissue. Steroids. 2009;74:489–497. doi: 10.1016/j.steroids.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Miller H, Poon S, Hibbert B, Rayner K, Chen YX, O'Brien ER. Modulation of estrogen signaling by the novel interaction of HSP27, a biomarker for atherosclerosis, and estrogen receptor β. Vasc Biol. 2005;25:10–14. doi: 10.1161/01.ATV.0000156536.89752.8e. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Bassuk JA, Lubsen NH, Clark JI. Human alphaB-crystallin. Small heat shock protein and molecular chaperone. J Biol Chem. 1997;272:2578–2582. doi: 10.1074/jbc.272.4.2578. [DOI] [PubMed] [Google Scholar]
- Okasha SA, Ryu S, Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Evidence for estradiol-induced apoptosis and dysregulated T cell maturation in the thymus. Toxicol. 2001;163:49–62. doi: 10.1016/S0300-483X(01)00374-2. [DOI] [PubMed] [Google Scholar]
- Pandey P, Farber R, Nakazawa A. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19:1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- Persky AM, Green PS, Stubley L, Howell CO, Zaulyanov L, Brazeau GA, Simpkins JW. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proc Soc Exp Biol Med. 2000;223:59–66. doi: 10.1046/j.1525-1373.2000.22308.x. [DOI] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Seli E, Guzeloglu-Kayisli O, Kayisli UA, Kizilay G, Arici A. Estrogen increases apoptosis in the arterial wall in a murine atherosclerosis model. Fertil Steril. 2007;88:1190–1196. doi: 10.1016/j.fertnstert.2007.01.132. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. Ames: The Iowa State University Press; 1967. [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Tews DS. Apoptosis and muscle fibre loss in neuromuscular disorders. Neuromuscul Disord. 2002;12:613–622. doi: 10.1016/S0960-8966(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Vasconsuelo A, Milanesi LM, Boland RL. 17β-Estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol. 2008;196:385–397. doi: 10.1677/JOE-07-0250. [DOI] [PubMed] [Google Scholar]
- Wei YQ, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Induction of apoptosis by quercetin: involvement of heat shock. Cancer Res. 1994;54:4952–4957. [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Wiik B, Glenmark M, Ekman M, Esbjornsson-Liljedahl O, Johansson K, Bodin E, Enmark E, Jansson Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand. 2003;179:381–387. doi: 10.1046/j.0001-6772.2003.01186.x. [DOI] [PubMed] [Google Scholar]