Abstract
Increasing evidence indicates that carbon monoxide (CO) may protect against several diseases including sepsis. The ability of CO pre-treatment to provide good pre-conditioning against lipopolysaccharide (LPS)-induced injury was tested using an in vitro model of primary culture of porcine aortic endothelial cells (pAEC). pAEC were exposed to CO (250 ppm) or air for 1 h prior to the addition of LPS (10 μg/ml). Hsp70, HO-1, and Egr-1 protein levels were determined as well as vascular endothelial growth factor (VEGF) secretion after 4, 7, and 15 h. The effect of CO on LPS-induced apoptosis was also detected at 15 h. CO pre-treatment before the addition of LPS, significantly reduced LPS-induced apoptosis. LPS induced an increase in the level of VEGF in culture media after 7 and 15 h, and a larger increase was detected in CO pre-treated cells. In addition, CO pre-treatment reduced LPS-induced Hsp70, HO-1, and Egr-1 protein expression. In conclusion, CO treatment seems to provide a good pre-conditioning for the prevention of LPS-induced endothelial injury.
Keywords: Carbon monoxide, Pig, Endothelial cells, Heat shock proteins, Egr-1, VEGF
Introduction
Lipopolysaccharide (LPS) is a proactive molecule that causes systemic inflammatory response syndrome (SIRS), and endothelial injury is one of the critical stages in the progression of this disease (Schouten et al. 2008; Matsuda and Hattori 2007). Several studies demonstrated that LPS binds mCD14 and sCD14 causing structural and functional alterations converting a quiescent endothelial phenotype into an activated state resulting in endothelial cell death (Lloyd-Jones et al. 2008). The deleterious cellular effects of LPS are mediated by induction of multiple transcription factors, among which early growth response (Egr)-1 factor is a pluripotent inducer of several pro-inflammatory endothelial genes (Khachigian and Collins 1997; Mechtcheriakova et al. 2001; Okada et al. 2001; Schabbauer et al. 2007). However, the role of Egr-1 in endothelial cells is very complex and not fully understood, in fact it may regulate other transcriptional regulators, signaling proteins, and growth factors, including vascular endothelial growth factor (VEGF), heme oxygenase-1 (HO-1) (Fu et al. 2003) and heat shock protein 70 (Hsp70) (Li et al. 2007). In vitro studies demonstrated the ability of LPS to induce apoptosis in endothelial cells in different species (Koshi et al. 1993; McCuskey et al. 1996; Meyrick 1986). Our previous research (Bernardini et al. 2005) indicated that LPS induces apoptosis in primary porcine aortic endothelial cells (pAEC) and evokes “the heat shock response” and the expression of the specific growth factor VEGF. We also demonstrated that the LPS-induced apoptosis was partially reversed by Hemin pre-treatment that induced an increase in HO-1 expression, suggesting a probable involvement of HO-1 activity specific products: carbon monoxide, biliverdin, and Fe2+. Among these products increasing evidence indicates that carbon monoxide (CO), if used at low doses, may protect against several diseases including sepsis (Otterbein 2002; Choi and Otterbein 2002; Morse et al. 2003; Ryter and Otterbein 2004; Sarady et al. 2004; Zuckerbraun et al. 2005; Nakao et al. 2006; Hoetzel et al. 2007). We showed the positive effect of CO administration in a porcine hyperacute endotoxic shock model (Mazzola et al. 2005). In particular, our study demonstrated that a CO pre-treatment could ameliorate the LPS-induced alteration in pulmonary gas exchange.
Taking into account all these considerations, using our in vitro model of pAEC, we verified if CO pre-treatment could represent a model of pre-conditioning against LPS-induced apoptosis testing the ability of CO to influence the expression level of stress markers (Hsp70, HO-1), specific endothelial growth factor (VEGF), and the pro-inflammatory transcription factor Egr-1.
Materials and methods
Cell culture and treatment
Porcine aortic endothelial cells were isolated and maintained as previously described (Bernardini et al. 2005).
All experiments were performed with cells from the third to the eighth passage.
To analyze the Hsp70, HO-1, Egr-1, and VEGF expression pAEC were placed in T-25 tissue culture flasks (3 × 105 cells/flask) (T25-Falcon, Beckton-Dickinson, Franklin Lakes, NJ, USA) and grown until confluence. Thereafter cells were exposed to CO (250 ppm) or air for 1 h according to the published method by Otterbein et al. (2000). After 1 h of CO treatment, LPS 10 (μg/ml; Escherichia coli 055:B5, Sigma-Aldrich Co, St Louis, MO, USA) was added to the culture medium for 4, 7, and 15 h. At the end of each experiment point, cells and culture media were collected and stored until analysis expression. For apoptosis detection pAEC (approximately 4 × 104 cells/well) were grown till confluence in a flat bottom 24-well assay plate (Falcon Beckton-Dickinson), and exposed to CO (250 ppm) or air for 1 h prior to the addition of LPS 10 μg/ml for 15 h.
Apoptosis detection
A sandwich enzyme-linked immunosorbent assay for histone-associated DNA fragments (1774425, Roche Diagnostics GmbH, 82372 Penzberg, Germany) was used according to the manufacturer’s instruction and as described previously (Bernardini et al. 2005). Briefly, cells were lysed directly in the well and the cytoplasmatic and nuclear fractions were separated by centrifugation at 200×g. Twenty microliters of cytoplasmatic fraction were added to a streptavidin-coated microtiter plate. The biotin-labeled anti-histone antibody was added, followed by a horseradish peroxidase (HRP)-conjugated anti-DNA antibody. The reaction of the nucleosome-bound DNA fragments was quantified by a photometric analysis.
VEGF detection
VEGF concentration in culture media was determined by an enzyme-linked immunoabsorbent assay (ELISA, Quantikine; R&D Systems, MN, USA) as previously described (Bernardini et al. 2005).
Western blot
Cells were lysed in SDS solution (Tris–HCl 50 mM pH 6.8; SDS 2%, 5% glycerol). Protein content of cellular lysates was determined by a Protein Assay Kit (TP0300, Sigma). Aliquots containing 15 μg of proteins were separated on NuPage 4–12% bis–Tris Gel (Gibco-Invitrogen Paisley, UK) for 50 min at 200 V. Proteins were then electrophotoretically transferred onto a nitrocellulose membrane. Blots were washed in PBS and protein transfer was checked by staining the nitrocellulose membranes with 0.2% Ponceau Red. After blocking treatment the membranes were incubated with primary rabbit anti-HO-1 (1: 2,000, SPA-896 Stressgen Biotechnologies Corp, Victoria, BC, Canada) or mouse anti-Hsp70 (1:1,000, SPA-810 StressGen) or rabbit anti Egr-1 (1:1,000, Sc-110 Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) in Tris-Buffered Saline-T20 (TBS-T20 20 mM Tris–HCl, pH 7.4, 500 mM NaCl, 0.1% Tween-20). After several washings with PBS-T20 the membranes were incubated with the secondary biotin-conjugate antibody and then with a 1:1,000 dilution of an anti-biotin horseradish peroxidase-linked antibody. The western blots were developed using chemiluminescent substrate (Super Signal West Pico Chemiluminescent Substrate, Pierce Biotechnology, Inc, Rockford, IL, USA) according to the manufacturer’s instructions. The intensity of the luminescent signal of the resultant bands was acquired by Fluor-S™ Multimager using the Quantity One Software (Bio-Rad Laboratories Inc., California, USA).
In order to normalize the data on housekeeping protein membranes were stripped and re-probed for the housekeeping β-tubuline (1:500 sc-5274 Santa Cruz). The relative protein content (protein of interest/β-tubulin) was expressed as arbitrary units (AU).
Statistical analysis
Each treatment was replicated six times for apoptosis detection and three times for protein expression. Data are presented as mean ± SEM. Apoptosis results were analyzed using the one-way analysis of variance (ANOVA; SPSS Inc, Chicago, IL, USA). The variation of protein expression was subjected to a two-way ANOVA. On the basis of significant differences observed by two-way ANOVA, the mean comparisons, among group of treatment (CTR, CO, LPS, CO + LPS) or among time (4, 7, and 15 h) of each treatment, have been performed by one-way ANOVA followed by the Duncan’s post-hoc test.
A probability of at least P < 0.05 was considered significant.
Results
CO effect on LPS-induced apoptosis
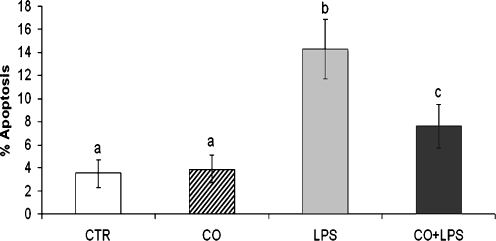
Apoptosis data are represented in Fig. 1. CO pre-treatment significantly reduced (P < 0.05) the LPS-induced apoptosis, CO alone did not modify the apoptosis rate.
Fig. 1.
Effect of CO pre-treatment on LPS-induced apoptosis. Data represent the mean ± SEM of three replicates. Different letters above bars indicate statistically significant differences (P < 0.05). CTR standard culture condition, CO CO pre-treatment (1 h) + standard culture condition (15 h), LPS LPS treatment (15 h), LPS + CO CO pre-treatment (1 h) + LPS treatment (15 h)
CO effect on VEGF secretion
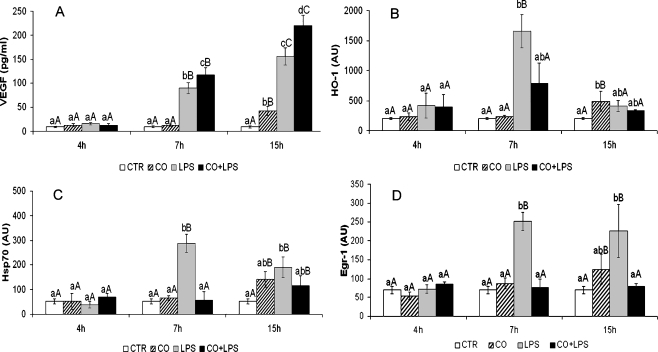
VEGF level detected in culture media is shown in Fig. 2a. Two-way ANOVA analysis demonstrated significant effects of treatment, time, and treatment × time interaction (Table 1). VEGF secretion was affected by LPS treatment with a significant increase after 7 h of continuous treatment. CO pre-treatment determined a larger increase of VEGF level in culture media after 7 and 15 h of LPS. CO alone determined a significant VEGF increase after 15 h of standard culture condition (Fig. 2a).
Fig. 2.
Effect of CO pre-treatment on protein expression. a VEGF secretion in culture medium (pg/ml). b–d HO-1, Hsp70, Egr-1 expression (AU arbitrary unit). Data are presented as the mean ± SEM of three replicates. Capital letters above bars indicate statistically significant differences (P < 0.05) within treatment during the time. Small letters above bars indicate statistically significant differences (P < 0.05) among treatment at the same time point. CTR standard culture condition, CO CO pre-treatment (1 h) + standard culture condition (4, 7, and 15 h), LPS LPS treatment (4, 7, and 15 h), LPS + CO CO pre-treatment (1 h) + LPS treatment (4, 7, and 15 h)
Table 1.
Two-way ANOVA P values
| Protein | Treatment | Time | Interaction (treatment × time) |
|---|---|---|---|
| VEGF | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| HO-1 | P < 0.0001 | P < 0.005 | P < 0.0001 |
| Hsp70 | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| Egr-1 | P < 0.0001 | P < 0.0001 | P < 0.0001 |
Normalized protein values (VEGF, Hsp70, HO-1 Egr-1; AU) were analyzed using two-way analysis of variance (SPSS Program Version 13.0, SPSS, Inc., Chicago, IL, USA). A probability level of P < 0.05 was considered as significant. As indicated by P values both factors (treatment, time) as well as their interaction. (treatment × time) influenced significantly the expression of all the proteins analyzed
CO effect on LPS-induced stress response and Egr-1 expression
Two-way ANOVA analysis demonstrated significant effects of treatment, time, and treatment × time interaction on Hsp70, HO-1, and Egr-1 protein levels (Table 1). CO pre-treatment resulted in a significant (P < 0.05) reduction of the LPS-induced HO-1 (Fig. 2b) and Hsp70 (Fig. 2c) increase.
Egr-1 expression was enhanced by LPS after 7 and 15 h of continuous treatment (Fig. 2d) while CO pre-treatment completely inhibited the LPS-induced Egr-1 protein expression.
CO alone determined a significant (P < 0.05) increase of the three proteins analyzed after 15 h of standard culture condition.
Discussion
Since endothelial injury is a key process in the pathogenesis of several diseases including sepsis (Grandel and Grimminger 2003), studies of the response of endothelial cells in simplified in vitro models are of great interest (Hotchkiss et al. 2002; Hemmer et al. 2008). Our previous research (Bernardini et al. 2005) demonstrated that the induction of HO-1 expression by Hemin partially protected against LPS-induced endothelial injury; the results of the present study indicate that the administration of the specific product of HO-1 activity carbon monoxide, had a positive effect on cellular vitality by reducing LPS-induced apoptosis, in agreement with the results obtained by Brouard et al. (2000) in an in vitro model of primary bovine aortic endothelial cells.
Our results also demonstrated that the reduction of LPS-induced apoptosis in CO pre-conditioned cells is positively correlated with an increase of VEGF, a specific endothelial growth factor with well-known anti-apoptotic and anti-inflammatory properties (Yilmaz et al. 2003; Bussolati et al. 2004). On the other hand CO alone slightly increased VEGF secretion after 15 h of standard culture conditions demonstrating the ability of CO to directly induce VEGF through HIF activation in agreement with Faleo et al. (2008).
Furthermore, CO pre-treatment influences the heat shock response by a reduction in LPS-induced Hsp70 and HO-1 expression, namely Hsp70 expression peak detected after 7 h of continuous LPS treatment was completely inhibited by CO, whereas HO-1 LPS-induced increase was strongly reduced by CO pre-treatment.
It is interesting to note that the effect of CO administration is different in the presence or absence of pro-inflammatory stimulus. CO alone induces Hsp70 and HO-1 expression after 15 h of recovery, and this effect does not result in cellular damage as shown by the analysis of apoptosis. The ability of CO to induce HO-1 is not surprising. CO is also the product of HO-1 activity, and therefore a positive feedback is possible, while the ability of CO to induce Hsp70 observed in our study is in agreement with data by Kim et al. (2005) in murine lung endothelial cells. It will be intriguing to further investigate the ability of CO to activate heat shock factors in the absence of evident cellular stress.
Considering that the heat shock response, well-known also as the stress response, is one of the highly conserved mechanisms activated by multiple types of stressors, the reduced stress response in LPS-exposed pAEC pre-treated with CO could mean that carbon monoxide induces a positive status of pre-conditioning necessary to counterbalance the deleterious effect of LPS treatment and could explain the positive effect of CO pre-treatment obtained by us (Mazzola et al. 2005) in a porcine hyperacute endotoxic shock model. Our results are in agreement with a recent study in vivo (Hoetzel et al. 2008) which demonstrated the anti-inflammatory ability of CO to prevent the induction of stress markers in a mouse model of mechanical ventilation lung injury via the inhibition of Egr-1. This transcription factor is involved in the progress of vascular disease (Khachigian and Collins 1997; Mechtcheriakova et al. 2001; Okada et al. 2001; Schabbauer et al. 2007) through the alteration of several genes including transcriptional factors, signaling proteins, growth factors, cytokines (Fu et al. 2003), and cellular apoptosis regulators (Thiel and Cibelli 2002).
In our model both LPS and CO treatments induced Egr-1 expression even if with a different extent and kinetic: LPS-induced Egr-1 increase was higher and persisted at 7 h and 15 h of continuous treatment while CO alone induced a slight increase of Egr-1 at 15 h of standard culture conditions. CO pre-conditioning induced the complete suppression of LPS-induced Egr-1 protein expression and this effect corresponded to an improvement in pAEC survival from LPS injury. Our data are in agreement with results obtained by Pritchard et al. (2007) that demonstrated reduced apoptosis and improved survival in Egr-1−/− mice after LPS administration. However, many reports indicated that Egr-1 exerts opposite effects on endothelial cells in relation to the intensity and the quality of stimuli (Fu et al. 2003; Lucerna et al. 2006).
All the genes studied in the present research could be influenced by Egr-1 (Fu et al. 2003; Li et al. 2007; Hoetzel et al. 2008); nevertheless, our results show an Egr-1-independent increase of VEGF and only a partial overlapping of Egr-1 activation with the Hsp70 and HO-1 induction. Our findings suggest that other cellular pathways may influence these genes and further investigations will be necessary to clarify the role of Egr-1 in endothelial cells during inflammatory response.
In conclusion, we demonstrated that 1 h of CO treatment induces a moderate stress response in PAEC together with VEGF and a low level of Egr-1 induction. The response is functional to cells survival and no induction of apoptosis was observed. This could act as a pre-conditioning treatment for a subsequent classical pro-inflammatory strong stimulus like LPS (10 ug/ml) by reducing cellular stress and endothelial apoptosis through the enhancement of a survival factor (VEGF) and through the reduction of the Egr-1 pro-inflammatory pathway.
Acknowledgments
This work was supported by RFO 60% UNIBO and MIUR-PRIN grants.
References
- Bernardini C, Zannoni A, Turba ME, Fantinati P, Tamanini C, Bacci ML, Forni M. Heat shock protein 70, heat shock protein 32 and vascular endothelial growth factor production and their effects on lipopolysaccharide-induced apoptosis in Porcine Aortic Endothelial Cells. Cell Stress Chaperones. 2005;10:340–348. doi: 10.1379/CSC-98R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192(7):1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati B, Ahmed A, Pemberton H, Landis RC, Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103(3):761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- Choi AM, Otterbein LE. Emerging role of carbon monoxide in physiologic and pathophysiologic states. Antioxid Redox Signal. 2002;4:227–228. doi: 10.1089/152308602753666271. [DOI] [PubMed] [Google Scholar]
- Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85(12):1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- Fu M, Zhu X, Zhang J, Liang J, Lin Y, Zhao L, Ehrengruber MU, Chen YE. Egr-1–1 target genes in human endothelial cells identified by microarray analysis. Gene. 2003;315:933–941. doi: 10.1016/S0378-1119(03)00730-3. [DOI] [PubMed] [Google Scholar]
- Grandel U, Grimminger F. Endothelial responses to bacterial toxins in sepsis. Crit Rev Immunol. 2003;23(4):267–299. doi: 10.1615/CritRevImmunol.v23.i4.20. [DOI] [PubMed] [Google Scholar]
- Hemmer CJ, Vogt A, Unverricht M, Krause R, Lademann M, Reisinger EC. Malaria and bacterial sepsis: similar mechanisms of endothelial apoptosis and its prevention in vitro. Crit Care Med. 2008;36(9):2562–2568. doi: 10.1097/CCM.0b013e31818441ee. [DOI] [PubMed] [Google Scholar]
- Hoetzel A, Dolinay T, Schmidt R, Choi AM, Ryter SW. Carbon monoxide in sepsis. Antioxid Redox Signal. 2007;9(11):2013–2026. doi: 10.1089/ars.2007.1762. [DOI] [PubMed] [Google Scholar]
- Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008;177(11):1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Tinsley KW, Swanson PE, Karl IE. Endothelial cell apoptosis in sepsis. Crit Care Med. 2002;30:S225–S228. doi: 10.1097/00003246-200205001-00009. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Collins T. Inducible expression of Egr-1-dependent genes. Circ Res. 1997;81:457–461. doi: 10.1161/01.res.81.4.457. [DOI] [PubMed] [Google Scholar]
- Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J Immunol. 2005;175(4):2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- Koshi R, Mathan VI, David S, Mathan MM. Enteric vascular endothelial response to bacterial endotoxin. Int J Exp Pathol. 1993;74:593–601. [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Ning W, Matthay MA, Feghali-Bostwick CA, Choi AM. MAPK pathway mediates EGR-1-HSP70-dependent cigarette smoke-induced chemokine production. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1297–L1303. doi: 10.1152/ajplung.00194.2006. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones KL, Kelly MM, Kubes P. Varying importance of soluble and membrane CD14 in endothelial detection of lipopolysaccharide. J Immunol. 2008;181(2):1446–1453. doi: 10.4049/jimmunol.181.2.1446. [DOI] [PubMed] [Google Scholar]
- Lucerna M, Pomyje J, Mechtcheriakova D, Kadl A, Gruber F, Bilban M, Sobanov Y, Schabbauer G, Breuss J, Wagner O, Bischoff M, Clauss M, Binder BR, Hofer E. Sustained expression of early growth response protein-1 blocks angiogenesis and tumor growth. Cancer Res. 2006;66(13):6708–6713. doi: 10.1158/0008-5472.CAN-05-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Hattori Y. Vascular biology in sepsis: pathophysiological and therapeutic significance of vascular dysfunction. J Smooth Muscle Res. 2007;43(4):117–137. doi: 10.1540/jsmr.43.117. [DOI] [PubMed] [Google Scholar]
- Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, Lavitrano M, Bach FH, Otterbein LE, Clement MG. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005;19(14):2045–2057. doi: 10.1096/fj.05-3782fje. [DOI] [PubMed] [Google Scholar]
- McCuskey RS, Urbaschek R, Urbaschek B. The microcirculation during endotoxemia. Cardiovasc Res. 1996;32:752–763. [PubMed] [Google Scholar]
- Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, Martin R, Binder BR, Hofer E. Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J. 2001;15(1):230–242. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- Meyrick BO. Endotoxin-mediated pulmonary endothelial cell injury. Fed Proc. 1986;45:19–24. [PubMed] [Google Scholar]
- Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, Otterbein SL, Otterbein LE, Choi AM. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278(39):36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in trasplantation. J Cell Mol Med. 2006;10(3):650–671. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Fujita T, Sakaguchi T, Olson KE, Collins T, Stern DM, Yan SF, Pinsky DJ. Extinguishing Egr-1-dependent inflammatory and thrombotic cascades following lung transplantation. FASEB J. 2001;15(14):2757–2759. doi: 10.1096/fj.01-0490fje. [DOI] [PubMed] [Google Scholar]
- Otterbein LE. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid Redox Signal. 2002;4:309–319. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide mediates anti-inflammatory effects via the mitogen activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, Roychowdhury S, McMullen MR, Guo L, Arteel GE, Nagy LE. Early growth response-1 contributes to galactosamine/lipopolysaccharide-induced acute liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1124–G1133. doi: 10.1152/ajpgi.00325.2007. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. BioEssays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 2004;18(7):854–856. doi: 10.1096/fj.03-0643fje. [DOI] [PubMed] [Google Scholar]
- Schabbauer G, Schweighofer B, Mechtcheriakova D, Lucerna M, Binder BR, Hofer E. Nuclear factor of activated T cells and early growth response-1 cooperate to mediate tissue factor gene induction by vascular endothelial growth factor in endothelial cells. Thromb Haemost. 2007;97(6):988–997. doi: 10.1160/th07-01-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten M, Wiersinga WJ, Levi M, Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83(3):536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193(3):287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Kliche S, Mayr-Beyrle U, Fellbrich G, Waltenberger J. p38 MAPK inhibition is critically involved in VEGFR-2-mediated endothelial cell survival. Biochem Biophys Res Commun. 2003;306(3):730–736. doi: 10.1016/S0006-291X(03)01064-7. [DOI] [PubMed] [Google Scholar]
- Zuckerbraun BS, McCloskey CA, Gallo D, Liu F, Ifedigbo E, Otterbein LE, Billiar TR. Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resuscitation. Shock. 2005;23(6):527–532. [PubMed] [Google Scholar]