Abstract
In recent years, it has been hypothesised that a new signalling system may exist in vertebrates in which secreted molecular chaperones form a dynamic continuum between the cellular stress response and corresponding homeostatic physiological mechanisms. This hypothesis seems to be supported by the finding that many molecular chaperones are released from cells and act as extracellular signals for a range of cells. However, this nascent field of biological research seems to suffer from an excessive criticism that the biological activities of molecular chaperones are due to undefined components of the microbial expression hosts used to generate recombinant versions of these proteins. In this article, a number of the proponents of the cell signalling actions of molecular chaperones take this criticism head-on. They show that sufficient evidence exists to support fully the hypothesis that molecular chaperones have cell–cell signalling actions that are likely to be part of the homeostatic mechanism of the vertebrate.
Keywords: Heat shock proteins, Endotoxin, Innate immunity, Adaptive immunity, Inflammation, Immunoregulation
Introduction
The movers and shakers of Biology are the proteins, and a large part of current biological research owes its existence to the pioneering molecular biologists who have enabled us to clone specific genes into appropriate expression vector systems and to generate recombinant proteins in a range of expression hosts. Pick up any molecular biology catalogue and peruse the variety of proteins that are offered for sale. It is likely that most, if not all of them (antibodies apart), will be recombinant proteins produced in one of three of four expression hosts. The most common expression hosts for a recombinant protein will be Escherichia coli, a yeast such as Pichia pastoris or a eukaryotic cell. The simplest and cheapest expression host is E. coli, and many recombinant proteins, including many mammalian proteins, are expressed in this Gram-negative bacterium. For most researchers, the nature of the expression host is irrelevant. If a favourite protein is a connective tissue component, and you want to show that it interacts with other specific components, then the nature of the host in which it is made is irrelevant—as long as it is properly folded and appropriately glycosylated.
However, if a recombinant protein is to be tested as an exogenous stimulator of eukaryotic cells, then the nature of the host may become more important. If proteins are expressed in E. coli, the individual protein may be contaminated with Gram-negative pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS). The term PAMPs was introduced by Janeway and refers to evolutionarily conserved microbial components that are recognised by the host through binding to pattern-recognition receptors such as CD14 or the Toll-like receptors (TLRs) (Medzhitov and Janeway 2000). It has to be assumed that most proteins expressed in E. coli will be contaminated with some LPS unless steps are taken to remove it. Most people working with recombinant proteins fail to recognise this as a problem; therefore, some of our literature of the analysis of the biological actions of E. coli-expressed recombinant proteins on eukaryotic cells is likely to be artefactual. However, it is possible to remove contaminating PAMPS from recombinant proteins. The best example of this is Kineret®, a drug now used to treat patients with rheumatoid arthritis (Fearon and Fearon 2008). Kineret® is a recombinant form of interleukin-1 receptor antagonist (IL-1ra) expressed in E. coli and purified to remove all contaminating PAMPs. Initial scale-up purification involved mechanical lysis of cells followed by chromatographic purification, initially on an SP Streamline column, a strong cation exchange expanded bed adsorbent used for dealing with crude extracts of proteins. This is followed by anion exchange chromatography on Q-Sepharose. The final material is passed through a polymyxin B column yielding IL-1ra which is >95% pure by SDS-PAGE with purity confirmed by MALDI-TOF MS and ESI-MS. LPS contamination is estimated to be 10 pg/µg protein. Administration of up to 300 µg/kg of IL-1ra to Cynomolgus monkeys showed no evidence of endotoxaemia (Zanette et al. 1998). This protein is generally administered to patients daily (with minimal side effects), thus, showing that it is possible to generate large amounts of recombinant proteins in E. coli with minimal contamination with pro-inflammatory PAMPs (Fearon and Fearon 2008).
Although many studies using recombinant proteins that have been generated in E. coli have failed to recognise that contamination with PAMPs is a potential problem, those working on the cell signalling actions of molecular chaperones have recognised that microbial contaminants might be a problem, and steps have been taken to avoid such contamination. Unfortunately, this has not failed to silence critics who have come up with other apparent problems. We started off with the problems of LPS contamination of Hsp60 and Hsp70 recombinant proteins (Gao and Tsan 2003a, b). Following this was the suggestion that flagellin contamination of Hsp70 was responsible for its biological actions (Ye and Gan 2007). The latest contaminant responsible for the cellular activation of Hsp70 proteins is, apparently, nucleotides (Bendz et al. 2008). This review will concentrate mainly on the subject of LPS contamination, and these other contaminants will be dealt with briefly. The flagellin contamination of Hsp70 is countered by the work of Figueiredo and co-workers who have expressed Hsp70 in mammalian cells and have generated LPS-free protein that still stimulates T lymphocytes (Figueiredo et al. 2009). The possibility that the leukocyte-stimulating activity of Mycobacterium tuberculosis Hsp70 is due to nucleotides introduced by affinity purification of the Hsp70 is unlikely because: (1) the recombinant Hsp70 protein is subject to further chromatography which would remove nucleotides and (2) the C-terminal fragment of M. tuberculosis Hsp70, purified on a nickel affinity column, with no involvement of nucleotides, was biologically active (Wang et al. 2001)
We are currently living on a cusp in Biology where the hypothesis that cell stress proteins have one set of biological functions within the cell, and a second set of functions when they are released from cells, and that both are part of a physiological homeostatic system is either a testable hypothesis or will be snuffed out due to a failure to fund the work required to test the hypothesis. This lack of funding is, increasingly, the experience of those trying to study the signalling actions of cell stress proteins. Without funding, this nascent field of Biology will be lost, and we will not know if we are abandoning a very important arena of biological control.
This short review brings together a group of people who are studying the cell–cell signalling actions of molecular chaperones. Each has been asked to review the literature on the reported biological and immunological properties of their protein of interest and to describe how they ensure that the signalling actions of the molecular chaperones on which they work are not due to some form of microbial contamination. The aim of this article is to draw up some form of Koch’s postulates to prove that if a protein is described as having a particular biological activity, then it can only be due to the inherent activity of the protein and is not due to some contaminants in the expression system. The reader is asked to remember the modern casting of William of Occam’s philosophical principal that ‘the simplest of two or more competing theories is preferable and that an explanation for unknown phenomena should first be attempted in terms of what is already known’. In other words, the simplest explanation for the extracellular biological actions of molecular chaperones is that they have inherent biological activity.
Beyond the lowly PAMP (S.K.C.)
The finding of heat shock proteins in the extracellular microenvironment and blood is no longer a cause for incredulity. Indeed, it is clear that the strict rules of protein localisation within and without cells have become loosened and proteins traffic between membrane-bounded zones much more freely than we originally thought. The heat shock proteins, which are proteins without a canonical signal sequence, are able to negotiate the plasma membrane even when such membranes remain intact (Mambula et al. 2007). Perhaps more surprising is the finding that heat shock proteins of the Hsp60, Hsp70 and Hsp90 families can traffic in large concentrations to the outside, even though their intracellular functions require large ATP concentrations, which are unlikely to be found outside the cell (Lindquist and Craig 1988; Bukau and Horwich 1998).
The finding of extracellular roles for heat shock proteins was first greeted with some enthusiasm (Srivastava 2002). Extracellular heat shock proteins were envisaged as being multifunctional extracellular messengers indeed for some the very “Swiss Army knives” of intercellular signalling (Srivastava and Amato 2001). However, the fact that some of these functions included the induction of innate immune responses (Asea et al. 2000; 2002) which involved processes that resemble the effects of PAMPs, aroused scepticism (Tsan and Gao 2004). For some, heat shock proteins were merely carriers of contaminating PAMPs such as E. coli LPS that can bind to recombinant heat shock proteins that had been generated in this organism (Tsan and Gao 2004). Opinion seems divided between the advocates of a signalling role for heat shock proteins and proponents of the contamination theory (Wallin et al. 2002; Tsan and Gao 2004, 2009).
As with most controversies, the answer is likely to be located somewhere between the extremes. Clearly, much care needs to be devoted to quality control in heat shock protein preparations, particularly those that are isolated from prokaryotes, and results need to be interpreted with considerable caution. For studies on mammalian heat shock proteins, the Calderwood laboratory expresses proteins in insect cells from baculoviral vectors prior to purification or uses proteins isolated from mouse organs in order to minimise the contamination with prokaryotic PAMPs. However, in this author’s humble opinion, the way forward for the discriminating extracellular heat shock protein biologist is to obtain a better understanding of the mechanisms underlying heat shock protein-mediated cell–cell signalling. This includes:
Mechanisms of heat shock protein release from intact cells
Non-canonical secretion of intracellular proteins is still, after many years of study, something of an enigma. Even for the most venerable member of the inflammatory cytokines, IL-1β, controversy continues regarding pathways of secretion [reviewed in (Mambula and Calderwood 2006; Mambula et al. 2007)]. We, therefore, need to expand our efforts to understand mechanisms of heat shock protein secretion. The Henderson laboratory is attempting to devise new approaches and materials for this purpose.
The issue of heat shock protein receptors
One disappointment for the Calderwood laboratory is that it has failed to detect a dedicated heat shock protein receptor. Instead, heat shock proteins appear to interact with cell surface proteins that have quite promiscuous binding habits. In addition, many such cell surface receptor types recognise heat shock proteins including, at a minimum: scavenger receptors, CD91/LRP, TLR family members and c-type lectins (Binder et al. 2000; Asea et al. 2002; Delneste et al. 2002; Berwin et al. 2004; Rosser et al. 2004; Mambula and Calderwood 2006; Thériault et al. 2006). Discerning the signalling networks emanating from such an array of receptors will, thus, be challenging; however, this is essential if sense is to be made of the heat shock protein signal input. For scavenger receptors alone, heat shock proteins can interact with family members SRA1, SREC-1, LOX-1 and FEEL-1 (Calderwood et al. 2007). In addition, heat shock protein binding to SRA1 is immunosuppressive, whereas LOX-1 mediates Hsp70 immunogenicity and antigen cross-presentation (Wang et al. 2007). Detailed studies of each receptor subtype and its interaction with others are essential in order to determine the sequelae of cellular interactions with heat shock proteins. There also remains some controversy over whether such an encounter is likely to be pro- or anti-inflammatory (Pockley et al. 2008).
A convincing solution to this dilemma requires a more detailed understanding of the basic questions of receptor-ligand (heat shock protein) affinity and pathways of signalling through the intracellular domains of heat shock protein receptors.
Heat shock proteins as intracellular message bearers
The use of heat shock proteins in anti-cancer vaccines is predicated on their ability to carry antigenic peptides as cargo, to protect the peptides from intracellular proteases after entering antigen presenting cells (APCs) and to deliver the peptide to MHC class I molecules. However, the mechanisms underlying this fascinating process are almost completely unclear (Tamura et al. 2007). Again, further detailed study is indicated.
The Calderwood laboratory has focused most of its attention on the Hsp70 protein. It is now appreciated that humans have at least 12 genes encoding Hsp70 proteins. Each of these proteins might have very different signalling actions which need to be detailed to fully understand the potential physiological and pathological activity of these family members.
Mycobacterial Hsp60 proteins as host signals (A.R.M.C.)
Much of Coates’ work has focused on the Hsp60.1 (chaperonin 60.1) protein of M. tuberculosis, which is the second HSP60 gene in this bacterium and which was cloned and sequenced in 1993 (Kong et al. 1993). Our initial studies revealed that M. tuberculosis Hsp60.1 activated myeloid cells (Lewthwaite et al. 2001; Tormay et al. 2005). The HSP60.1 gene is expressed in E. coli as a 6xHis recombinant protein and is purified by Ni-NTA affinity chromatography followed by ion exchange. To reduce LPS contamination the recombinant Hsp60 on the Ni-NTA column is washed with an excess of polymyxin B (an LPS-binding antibiotic) which lowers the LPS contamination to <2 pg/µg recombinant protein (Tormay et al. 2005). Digestion of Hsp60.1 with proteinase K destroys the protein and also obliterates its ability to induce eukaryotic cells to secrete pro-inflammatory cytokines (Lewthwaite et al. 2001; Tormay et al. 2005). In contrast, the monocyte-activating ability of LPS is not affected by proteinase K treatment, and this approach thereby demonstrates that the monocyte-activating ability of Hsp60.1 is not due to LPS contamination (Lewthwaite et al. 2001). Cloning and expression of the three separate domains of M. tuberculosis HSP60.1 reveals that the monocyte-modulating activity is present only in the equatorial domain. The other two recombinant domains, which have an equal chance of being contaminated with LPS or other proteinaceous PAMPs, are inactive (Tormay et al. 2005). Applying Occam’s razor to this question of PAMP contamination would surely lead to the conclusion that M. tuberculosis Hsp60.1 is a monocyte-modulating protein in its own right. We have also generated small peptides from the equatorial domain sequence and have found some of these to be active and others inactive (unpublished). This role of synthetic peptides will be discussed again later in this review.
Deletion of the entire HSP60.1 gene in M. tuberculosis produces a mutant which grows normally in culture within monocytes and in mice. However, this isogenic mutant fails to induce granulomas (Hu et al. 2008). Complementation of this mutant with HSP60.1 restores granuloma-inducing activity. This indicates that it is the expressed protein itself which induces inflammation in vivo and not a contaminant. An important finding is that the M. tuberculosis HSP60.1 gene cannot complement an E. coli GroEL-deficient mutant. Applying Occam’s razor would suggest the hypothesis that M. tuberculosis Hsp60.1 is not a molecular chaperone but a directly acting virulence determinant of this bacterium.
The initial studies of M. tuberculosis Hsp60.1 revealed a protein which activates pro-inflammatory cytokine synthesis by human monocytes. This study was prompted by the findings of Brian Henderson that the potent bone resorbing protein released by the oral bacterium, Aggregatibacter (Actinobacillus) actinomycetemcomitans was the Hsp60 protein of this organism (Kirby et al. 1995). It was then shown that E. coli Hsp60 (GroEL) was a potent stimulator of the formation of the myeloid cell population which is responsible for the normal homeostatic breakdown of bone—the osteoclast (Reddi et al. 1998). However, testing of the Hsp60.2 proteins from mycobacteria revealed that they did not stimulate bone resorption (Kirby et al. 1995). BH and ARMC then showed that the Hsp60.1 and Hsp60.2 proteins from M. tuberculosis had no effect on bone resorption (Meghji et al. 1997). Both recombinant forms of the M. tuberculosis Hsp60 proteins are contaminated with LPS to an almost identical level. Bone is exquisitely sensitive to picogramme per millilitre concentrations of E. coli LPS, which cause osteoclast-driven resorption (Reddi et al. 1995). This suggests that these recombinant mycobacterial proteins have an insufficient level of contaminating LPS or other PAMPs to promote bone breakdown in vitro. Further evaluation of the effect of the M. tuberculosis Hsp60 proteins on bone and bone cells has revealed that Hsp60.2 neither promotes nor inhibits bone resorption. In contrast, the highly homologous Hsp60.1 protein is a potent inhibitor of bone resorption and of osteoclast formation, acting, at least in part, by inhibiting the transcription of the key osteoclast transcription factor, NFATc1 (Winrow et al. 2008). Such biological differences cannot be due to differences in contaminants. Interestingly, administration of Hsp60.1 or Hsp60.2 to rats with adjuvant arthritis, a model of rheumatoid arthritis with a characteristic florid production of osteoclasts, reveals major differences in tissue responses. The Hsp60.2 has no effect on joint inflammation or on radiographically monitored bone damage. In contrast, Hsp60.1 completely suppresses the bone damage without influencing the joint inflammation (Winrow et al. 2008). The ability of M. tuberculosis Hsp60.1 to inhibit bone resorption both in vitro and in vivo—a process that would be promoted by pro-inflammatory PAMPs—again rules out the possibility that the actions of this protein are due to contaminants such as LPS.
In earlier studies, it was also shown that M. tuberculosis Hsp60.1, but not Hsp60.2, could inhibit experimental allergic asthma in mice (Riffo-Vasquez et al. 2004). Both proteins have equivalent levels of LPS contamination (Lewthwaite et al. 2001; Tormay et al. 2005) but completely different biological actions both in vitro and in vivo with different leukocyte populations. Again, Occam’s razor would suggest that what we are seeing is the natural biological activity of these proteins and not the consequence of some contaminant which should be present equally in both protein preparations.
To confront directly the problem of LPS contamination in Hsp60.1 preparations, we have recently conducted some simple experiments. Using fluorescently labelled LPS, we have shown that Hsp60.1, in huge (105) excess, fails to compete with the labelled LPS for binding to monocytes. Tenfold excess concentration of unlabelled LPS inhibits the binding of labelled LPS. This shows that the Hsp60 preparations we used contain almost no functional LPS. Escherichia coli LPS alone at 1 ng/ml induces monocyte activation (unpublished observations). To determine what effect Hsp60.1 has on the activation state of LPS-stimulated monocytes, we have cultured human peripheral blood monocytes in the presence of concentrations of Hsp60.1 ranging from picogramme per millilitre to microgram per millilitre for 1 h followed by the addition of a fixed concentration of LPS that causes significant monocyte activation. We have used Affymetrix microarrays to determine the activation state of the monocytes. The activation of monocytes by LPS is completely blocked at very low concentrations of Hsp60.1. At higher concentrations, there is still a blockade of some genes, but others are unaffected. This is a completely unexpected effect which suggests that the Hsp60.1 protein is acting through more than one receptor. Again, these results rule out LPS as a contaminating component. The inhibitory action we are seeing also tends to rule out other PAMPs which generally induce a pro-inflammatory/monocyte activating profile of cell activation.
Reflections on Hsp60 (I.C.)
Innate immune effects attributed to Hsp60 might be really caused by LPS
There are three compelling reasons for ruling out LPS contamination as a cause for innate immune effects in vitro and in vivo associated with Hsp60 (or with other heat shock protein molecules; we have worked mostly with human Hsp60, so I will use the term Hsp60 generically):
Most recombinant Hsp60 is generated in bacteria, so LPS is, therefore, likely to be present.
LPS is a strong stimulus of innate immune signalling.
Hsp60 complexes strongly with LPS and other innate stimulatory molecules of bacteria and can enhance the effects of these bacterial contaminants.
Molecular exclusion of LPS and other bacterial molecules
Various molecular measures are routinely used to exclude the functions of bacterial contaminants such as sensitivity to boiling, specific antibodies, adsorptions of various kinds and measurement of the actual amounts of possible contamination. These are standard to most work in the field and have been described at length by others in this review. There is, therefore, no need for additional elaboration here (Quintana and Cohen 2005).
Functional exclusion of LPS and other bacterial molecules
Our studies have uncovered functional reasons why effects we attribute to human Hsp60 are not likely to result from LPS or other bacterial contaminants:
Human T cell adhesion responses via TLR2 can be 1,000-fold more sensitive to mammalian Hsp60 than they are to LPS (Zanin-Zhorov et al. 2003). There is simply not enough contaminant in the Hsp60 to do the job; the Hsp60 is the only active factor.
Cytokine and contact-dependant suppression mediated by human CD4+CD25+ immunoregulatory T cells via TLR2 can be produced by a synthetic peptide of Hsp60 as well as by whole, recombinant Hsp60 (Zanin-Zhorov et al. 2006). In fact, the immunoregulatory T cells do not manifest cytokine responses to LPS. Thus, LPS contamination, even if present, could not account for the Hsp60 effects on immunoregulatory T cells.
Mouse B cell responses via TLR4 can be produced by mammalian Hsp60, but not at all by GroEL or by M. tuberculosis Hsp60.2 (Hsp65) (Cohen-Sfady et al. 2005). Thus, LPS and other bacterial contamination, even if present, could not account for the observed effects of Hsp60 on mouse B cells.
Why innate effects of heat shock proteins are rejected so zealously
It has been taught classically, and is still taught, that the immune system must be purged of antigen receptors that are capable of strongly binding to self-molecules; autoimmunity in health is forbidden (Cohen 2000). This teaching, by association, has been carried over to innate immune receptors; TLRs and others are called pathogen-related receptors. Therefore, if such receptors appear to be activated by self-molecules such as Hsp60, it must be due to bacterial contamination. Scientists, like most people, tend to stick to their beliefs, so self-recognition is resisted, whether innate or adaptive. Unlike most people, scientists are influenced by controlled experiments and ultimately do change their minds. It is, therefore, hoped that this review will help change the minds of the scientific community.
Recombinant molecular chaperones suppress inflammatory diseases (W. Van E.)
An important aspect of molecular chaperones is their prominent capacity to signal antigen-specific elements of the adaptive immune system. Microbial Hsp60 was already known as the so-called common antigen of Gram-negative bacteria before the first heat shock proteins were molecularly defined (Young et al. 1987). Later on, experimental immunizations with recombinant heat shock proteins demonstrated these proteins to be among the most immunogenic microbial proteins. This was not due to contaminating impurities, because epitope mapping studies reproduced the reactivity of the responding antigen specific T and B cells with synthetic peptides, such as in inflammatory diseases, as discussed below.
The first studies of recombinant Hsp60 in experimental arthritis were performed in the rat model of mycobacteria-induced adjuvant arthritis. T cell lines and clones had been developed in the model, which had either disease-inducing or disease-suppressing activities (Cohen et al. 1985; van Eden et al. 1985). These T cells had been raised against whole mycobacteria. The specificity of these T cells for mycobacterial Hsp60 was discovered using the first cloned M. tuberculosis-derived recombinant Hsp60 (van Eden et al. 1988). The exact specificity of these T cells for linear epitopes of Hsp60 was extensively documented using synthetic peptides (van der Zee et al. 1989). In addition, in various studies, the capacity of recombinant Hsp60 to interfere with arthritis was reproduced in experiments using synthetic peptides, which were administered either parenterally with a suitable adjuvant (Anderton et al. 1995) or intranasally in PBS (Prakken et al. 1998).
Antibodies and T cells reactive with chaperones were also frequently detected in patients with inflammatory diseases (van Eden et al. 2005). In many cases, the relevant peptide epitopes were defined, proving true specificity for the chaperones themselves. We have been involved in studies with patients suffering from juvenile idiopathic arthritis. T-cell responses to recombinant human Hsp60 have been found to correspond with good prognosis and especially with the self-remitting oligo-articular forms of this disease (de Graeff-Meeder et al. 1991, 1995). Similar to what has been done in the experimental models, most of the immune reactivities in these patients have been reproduced with synthetic peptides, again demonstrating the self-heat shock protein-reactive focus of the T-cell reactivity to endogenous chaperones (Kamphuis et al. 2005).
The effects of recombinant mycobacterial Hsp60 have been reproduced with synthetic peptides representing a dominant T cell epitope in other disease models. A recent example is the study by van Puijvelde and co-workers in which experimental atherosclerosis was suppressed by oral administration of either whole Hsp60 or a defined Hsp60 T cell epitope in the form of a synthetic peptide (van Puijvelde et al. 2006).
Studies have also been performed with recombinant Hsp70. Recombinant mycobacterial Hsp70 was documented to immunise against adjuvant arthritis in rats in a manner which was very similar to that seen with Hsp60. Furthermore, in the case of Hsp70, T cells responding to conserved synthetic peptide-defined sequences of Hsp70 were found to be responsible for the arthritis-protective effects (Wendling et al. 2000). Thus, both Hsp60 and Hsp70, being sequentially conserved and highly immunogenic microbial proteins, have the capacity to activate T cells which cross-recognise endogenously expressed self-homologues of these molecular chaperones. Such self-homologue cross-recognised cells have arthritis-suppressive effects, possibly by producing immune modulatory cytokines, such as IL-10 (Prakken et al. 2001).
In recent experiments by Wieten and co-workers, experimental arthritis has been prevented by the mere oral administration of a stress protein co-inducing compound (unpublished data). Very much in line with the Hsp60- and Hsp70-induced protective effect, the up-regulation of endogenous chaperones was found to trigger heat shock protein-reactive T cells with inflammation suppressive activity. This, in the author’s opinion, is a convincing aspect of the nature of chaperones as effective signalling molecules for the immune system.
Most of the recombinant mycobacterial proteins used in our studies were produced in the Utrecht University-based centralised facility for the production and purification of recombinant heat shock proteins. This facility was initiated under the umbrella of a previous Concerted Action (EU FP5), which consisted of a large number of European laboratories and focussed on the role of Hsp60 and Hsp70 in autoimmune inflammatory diseases. Due to its focussed aim of elucidating the immunology of these proteins, this facility made maximal efforts and collected ample experience for the production of proteins with extremely low levels of residual contaminants. Nonetheless, especially in the in vivo studies, a significant degree of contaminating LPS would have had an arthritis promoting pro-inflammatory effect, which is incompatible with the arthritis suppressive outcome of these experiments.
M. tuberculosis Hsp70 and host immunity (T.L.)
LPS, a highly active biological molecule, with a time-honoured history of extensive investigations, is found in all Gram-negative organisms in the intestinal tract, and in health, and is prevented from invading the body. The large family of heat shock proteins and molecular chaperones have a shorter pedigree of investigations and are found both in Gram-positive and Gram-negative bacteria and in eukaryotic cells. Human intracellular Hsp70 molecules share about 60% homology with microbial Hsp70 (Bardwell and Craig 1984), and the author believes that they are critical for our understanding of immunology in health and disease. This applies to the basic immunological mechanism of differentiating immunity from tolerance, response to microbial infections, and immunopathology of autoimmune diseases. Investigators of heat shock proteins have faced the criticism that the effects demonstrated by heat shock proteins are due to contamination with LPS (Gao and Tsan 2003a) and more recently, flagellin (Ye and Gan 2007) or nucleotides (Bendz et al. 2008). This refers largely to recombinant heat shock proteins, which are generated in E. coli, from which the removal of traces of LPS has proved difficult. Heat shock proteins are fairly sticky proteins, and it would be a challenging exercise to reverse the trend and study contamination with heat shock proteins of the host of recombinant proteins that are expressed in E. coli.
To demonstrate that the immunological activity under investigation is due to the recombinant heat shock protein (and not some E. coli contaminant), we would advocate the application of three or more of the five procedures outlined below, which have been carried out with microbial Hsp70 (Wang et al. 2001, 2005a, b). These should be applied after preparation of purified heat shock proteins by ion exchange chromatography, using Q-Sepharose resin, followed by ATP affinity chromatography (Mehlert and Young 1989) and treatment (×2) with polymyxin B-coated beads.
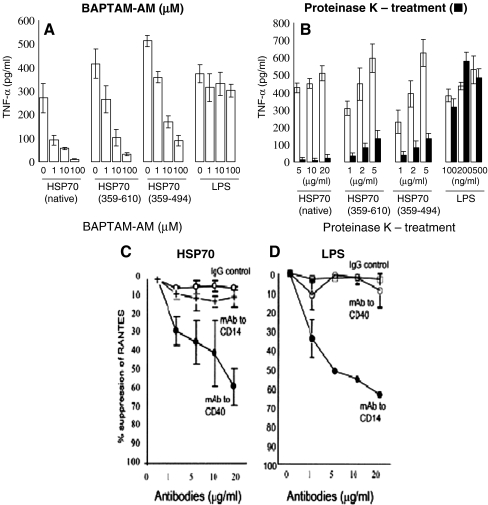
Biochemical tests (Fig. 1). Heat shock protein activation is dependent on intracellular calcium flux, unlike LPS (McLeish et al. 1989; Asea et al. 2002), and the intracellular calcium chelator BAPTA-AM has proven to be an effective inhibitor of this process. Using the cytokine, TNF-α, as a read out, its production is inhibited in a dose-dependent manner with the calcium chelator when monocytes are stimulated with Hsp70 but not when stimulated with LPS (Fig 1a). Proteinase K has an inhibitory effect on TNF-α production by Hsp70 but does not affect LPS (Fig. 1b). Similar results have been found when production of the chemokine CCL-5 is measured.
Antibodies and/or siRNA inhibition. Antibodies to the M. tuberculosis Hsp70 receptor CD40 inhibit CCL-5 (Rantes) production in a human monocyte-derived cell line (THP1) stimulated with Hsp70 in a dose-dependent manner (Fig. 1c) but failed to affect the generation of CCL-5 elicited by LPS (Fig. 1d). Conversely, antibodies to CD14 inhibited CCL-5 production in LPS-stimulated cells but had no effect on the Hsp70-stimulated cells. siRNA may also interfere differentially between heat shock proteins and LPS receptors but not between common receptors, such as TLR2 and TLR4.
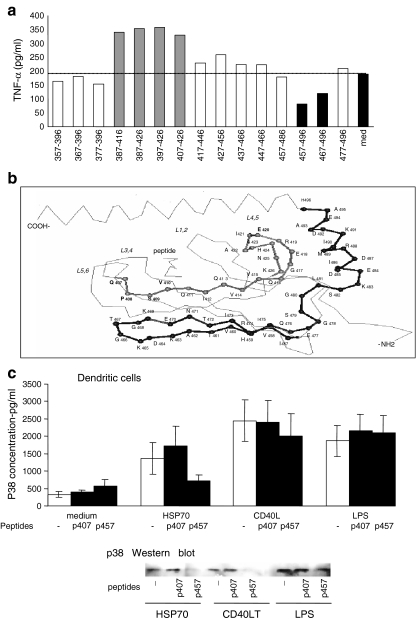
Heat shock protein-derived synthetic peptide epitopes. Synthetic peptides are free of any detectable LPS, flagellin or nucleotides which should rule out the criticism that their function can be accounted for by any of these contaminants. A stimulating peptide epitope (aa407–426) at the base of the peptide-binding groove of Hsp70 and an inhibitory epitope (aa457–496) downstream from the groove have been identified (Fig. 2a, b; Wang et al. 2005a, b). The critical residues identified were Q407, P408, S409 and V410, and alanine substitutions significantly reduced the stimulation of IL-12 and TNF-α production. IL-12p40 production by dendritic cells (DCs) stimulated with p407–426 was increased 10-fold compared with the unstimulated control (275.4 ± 41.2 vs. 23.1 ± 10.3 pg/ml). Similar results have been found with respect to TNF-α production. However, native Hsp70 stimulates higher concentrations of these cytokines than the peptide alone. The effect of these peptides on Hsp70 or CD40 ligand (trimer)-induced maturation of human monocyte-derived immature DCs was then studied. The proportion of CD83+ DCs was significantly increased by p407–426, whereas it was inhibited by p457–496. LPS-stimulated DCs were not inhibited by p457–496, although p407–426 increased the proportion of CD83+ DCs (Table 1).
Fig. 1.
The effect of intracellular calcium chelator BAPTA-AM (a) or treatment with proteinase K (b) on the production of TNF-α stimulated by Hsp70, its two fragments or LPS. Suppression of RANTES (CCL-5) production by mAb to CD40 or CD4 in THP1 cells stimulated with Hsp70 (c) or LPS (d)
Fig. 2.
a Identification of a stimulating epitope within peptide 387–426 and inhibiting epitope within peptide 457–496 using DCs co-stimulated with Hsp70 and with two adjacent or overlapping 20-mer peptides. b Stimulating epitope p407–426 at the base of L3, 4 and L4, 5 loops and the suppressive epitope (p457–496) in the β sheet. c Effect of peptides 407–426 and 457–496 on p38 MAP kinase phosphorylation stimulated by Hsp70 or LPS of lysed DCs by enzyme immunoassay or by Western blotting
Table 1.
The effect of inhibitory peptide (457–496) and stimulating peptide (407–426) on immature DC stimulated with HSP70, CD40LT or LPS
| Percent change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Immature DC | HSP70 | CD40LT | LPS | ||||||||
| Nil | p457–496 | p407–426 | Nil | p457–496 | p407–426 | Nil | p457–496 | p407–426 | Nil | p457–496 | p407–426 | |
| CD83 | ||||||||||||
| Mean ± SEM | 5.1 ± 0.8 | 4.8 ± 0.4 | 4.9 ± 1.1 | 21.9 ± 1.8 | 15.4 ± 1.8 | 26.6 ± 3.1 | 20.5 ± 2.5 | 11.5 ± .2 | 22.3 ± 2.4 | 17.0 ± 1.6 | 15.8 ± 2.4 | 20.2 ± 1.9 |
| T | 0.6 | 0.18 | 3.56 | 3.49 | 5.68 | 9.28 | 1.15 | 3.69 | ||||
| P | 0.5 | 0.8 | 0.024 | 0.033 | 0.004 | 0.0008 | 0.31 | 0.02 | ||||
Three concentrations of each peptide were used, and the optimum effect on CD83 is presented as mean percent ± SEM and analysed in comparison with untreated (nil) by the paired Student t test (n = 5)
The p38 MAP kinase signalling pathway is critical in Hsp70 activation of monocytes via binding to CD40 molecules and is inhibited by the 457–496 inhibitory peptide and enhanced by the 407–426 stimulating peptide, when assessed by enzyme immunoassay and Western blot analysis (Fig. 2c; Wang et al. 2005a, b). Furthermore, the dose-dependent inhibition of HIV-1 infection of human CD4+ T cell lines by Hsp70 has been reproduced by p407–426 (Babaahmady et al. 2007). The mechanism involves the stimulation of CCL-3, CCL-4 and CCL-5 chemokines, which bind and down-modulate CCR5, thereby preventing HIV-1 binding to the CCR5 co-receptors. It might be of interest to note that when the manuscript on stimulating and inhibitory peptide epitopes of Hsp70 was submitted for publication, one of the referees commented, “reproducing the Hsp70 functions by synthetic peptides will finally rule out that LPS contamination is responsible for the effects of Hsp70”!
LPS contaminant quantitative and dose-response studies. Any LPS in the purified Hsp70 should be determined by the Limulus amoebocyte lysate assay, which is, in our experience, typically about 0.001 U/µg or 10 pg/µg of the Hsp70 preparation. LPS dose-response studies have shown that the lowest concentration of LPS which is required to stimulate IL-12p40 is 10,000 pg/ml, which is 1,000-fold higher than the contaminating LPS (10 pg/ml) in the Hsp70 preparation. Any enhancing effect on IL-12p40 production was not found by complexing up to 1,000 pg/ml LPS with Hsp70 (20 µg/ml), Hsp70359–609 or CD40L. A 2-fold increase in IL-12p40 concentration was observed with all three complexes only after adding 10,000 pg/ml LPS, and so it is unlikely that the minute amount of LPS complexed with Hsp70 is responsible for the observed activity of this protein (Wang et al. 2005a, b).
The dual Hsp70 receptor functions of CD40 and CCR5 have been utilised in the generation of the anti-viral factor APOBEC3G (A3G) by stimulating human CD4+ T cells with Hsp70, which up-regulates A3G and inhibits HIV-1 infectivity (Babaahmady et al. 2007). A comparative experiment in which GFP-labelled pseudovirions are treated with Hsp70, and LPS showed that the LPS-treated cells were 5- to 6-fold higher than Hsp70-treated counterparts. Hsp70 also increased A3G expression in CD8+ and CD4+ T cells and DCs to a greater extent than LPS, and LPS had no detectable effect on A3G expression at concentrations of 2.5 to 20 ng/ml (Babaahmady et al. 2007).
Stress-induced heat shock protein reproducibility studies. Under stressful conditions, such as heat treatment, inducible Hsp70 is up-regulated. We have demonstrated that heat treatment (42°C) of human monocyte-derived DCs up-regulates inducible Hsp70 expression and that this is inhibited by the KNK437 HSP inhibitor. Co-culture of heat-treated DCs with autologous CD4+ T cells induces cytokine production, such as IFN-γ, and elicits other responses similar to those of exogenous Hsp70. Contamination with LPS, flagellin or nucleotides can be ruled out under these conditions, although other cellular changes might be induced. Thus, extensive quantitative, specificity and inhibitory studies stimulating the production of cytokines, chemokines, antiviral factors and DC maturation have demonstrated that the purified Hsp70 is responsible for these functions. Any further criticism is ruled out by the ability to reproduce the responses to Hsp70 using synthetic peptides derived from the amino acid sequence of Hsp70 or by heat- and other stress-induced conditions.
The Janus-like properties of HSPC4 (gp96) (A.G.P.)
Gp96, also previously known as glucose-regulated protein, endoplasmin and 99 kDa endoplasmic reticulum protein Erp99, is a 94–96-kDa member of the Hsp90 family which resides within the lumen of the endoplasmic reticulum. A recent consensus document has attempted to arrive at a consistent and clear nomenclature for the heat shock protein and related chaperone genes in the human database, and this has suggested that this member of the 90-kDa family of stress proteins should be referred to as HSPC4 (Kampinga et al. 2009). This proposed nomenclature will be used here.
The immunological properties of HSPC4 first became apparent in studies by Pramod Srivastava and colleagues in the 1980s, which demonstrated that the administration of a 96-kDa protein fractionated from a murine tumour cell lysate (which was subsequently shown to be HSPC4) to mice-induced resistance to the same tumour from which the protein had been originally isolated (Srivastava et al. 1986). It was proposed that the specificity of the response was defined by tumour-derived peptides which were associated with the administered HSPC4 (Chandawarkar et al. 1999). However, Liu and colleagues have reported problems with identifying peptides that are associated with HSPC4 (Liu et al. 2004). Furthermore, the observation that the yield of peptides extracted from HSPC4 is highly sub-stoichiometric, with an estimated occupancy of between 0.1% and 0.4%, appears to argue against HSPC4 having a role as a peptide chaperone in antigen processing (Demine and Walden 2005). Nicchitta and colleagues believe that HSPC4 does not interact with tumour peptides but instead interacts with polypeptides (Nicchitta et al. 2004).
Although HSPC4 has been reported to have pro-inflammatory effects and to induce peptide-specific protective immunity, it has been demonstrated that high-dose HSPC4 does not induce protective anti-tumour immunity (Chandawarkar et al. 1999), indeed it can attenuate inflammatory disease and delay skin transplant rejection (Chandawarkar et al. 1999, 2004; Kovalchin et al. 2006). These anti-inflammatory properties appear to be mediated by the induction and/or activation of immunoregulatory CD4+ T-cell populations (Chandawarkar et al. 1999). More recently, the Pockley laboratory has demonstrated that high-dose HSPC4 can prolong the survival of rat cardiac allografts (Slack et al. 2007) and induce a systemic state of T-cell hypo-responsiveness (Mirza et al. 2006).
APCs such as DCs have been reported to spontaneously internalise HSPC4 by receptor-mediated endocytosis (Arnold-Schild et al. 1999) via a number of receptors including the α2-macroglobulin receptor (CD91 molecule) (Binder et al. 2000; Binder and Srivastava 2004), the LPS receptor (Vabulas et al. 2002c) and members of the Toll-like receptor family (Ramirez et al. 2005) (TLR-2, TLR-4), the latter either alone or in combination with CD14 (Vabulas et al. 2002c). HSPC4 internalisation by human APCs has also been found to take place via scavenger receptor A (Berwin et al. 2003).
The interactions of HSPC4 with receptors that are also used by LPS, especially members of the TLR family, have led to the proposition that the immunological properties of this stress protein are also mediated by contaminating LPS or other PAMPs which interact with pattern recognition receptors.
The purification of HSPC4 typically involves a sequence of ammonium sulphate precipitation, affinity chromatography on Concanavalin A-Sepharose, passage through a P10 size exclusion column and a final purification using anion affinity chromatography (Wearsch and Nicchitta 1996; Srivastava 1997; Fairburn et al. 2006). It has been reported that gp96 purified using a Concanavalin A affinity purification step contains sub-stoichiometric amounts of Concanavalin A which can promote the formation of HSPC4-ConA oligomers, enhance the immunological activity of HSPC4 as a consequence (Monks et al. 2005) and itself activate T cells due to its lectin-mediated mitogenic activity (Arnold-Schild et al. 2000). However, the Pockley laboratory has not been able to demonstrate the presence of such contamination (Fairburn et al. 2006). HSPC4 has also been purified using a one-step affinity chromatography approach involving single chain Fv recombinant antibodies (Arnold-Schild et al. 2000).
Although the use of preparations of HSPC4 that have been purified from tumours, cancer cell lines or other tissues might circumvent the potential involvement of E. coli PAMP contamination that is associated with the use of recombinant proteins generated using E. coli expression systems, the potential for a bacterial contaminant-mediated effects nevertheless exists and must be considered. It is, therefore, essential that all of the laboratory equipment which is used in the purification procedure is baked for a minimum of 4 h at 220°C and that all solutions are prepared using autoclaved ultrapure pyrogen-free water (Fairburn et al. 2006). The potential endotoxin content of purified HSPC4 preparations can also be reduced using a detergent wash, as has been described by Reed and colleagues (2003). Endotoxin is not a single component but is an admixture of LPS and other components (proteins, lipoproteins, teichoic acids, etc.) that can be removed by simple extraction procedures applied to Gram-negative bacteria.
Reed and colleagues have shown that HSPC4 binds endotoxin in a high affinity, saturable and specific manner (Reed et al. 2003). The same study demonstrated that ‘low’ endotoxin preparations of HSPC4 which had been purified from porcine pancreas rough microsomes using an approach which includes a detergent wash and containing <0.27 EU endotoxin/mg protein do not stimulate NF-κB activation or NO release in macrophages, but do induce ERK phosphorylation (Reed et al. 2003). Other studies which have attributed immunological properties to HSPC4 have used preparations having endotoxin levels of <50 EU endotoxin/mg protein (Vabulas et al. 2002b) and an absolute quantity of <0.02 EU endotoxin (Basu et al. 2000). Studies in the Pockley laboratory which have shown HSPC4 purified from rat livers (Fairburn et al. 2006) to prolong rat cardiac allograft survival (Slack et al. 2007) and to activate T cells, but not bone marrow-derived DCs (Mirza et al. 2006) have used preparations containing <1 EU endotoxin (0.1 ng)/mg protein. Warger and colleagues (2006) have reported that ‘endotoxin-free’ (<0.5 U/mg) preparations of HSPC4 can stimulate DCs at concentrations greater that 50 µg/ml (containing <5 pg/ml LPS—a concentration not able to activate cells). Pre-incubation of low amounts of HSPC4 with low concentrations of LPS which are, on their own, incapable of activating DCs resulted in DC activation and an up-regulation in their T-cell stimulatory capacity (Warger et al. 2006). The interpretation of these data is that there is cooperation between HSPC4 and TLR ligands and that the former amplify the effects of the latter (Warger et al. 2006).
Notwithstanding the ability to generate preparations of HSPC4 which have low levels of endotoxin, it is clearly important to ensure that exogenous endotoxin is excluded from the experimental system and that appropriate controls are included in the experimental design. A typical control for this field is to determine the functional properties of stress proteins in the presence of the LPS-binding antibiotic polymyxin B. However, this is not possible in the case of HSPC4, as this protein binds to polymyxin (Reed et al. 2003). In addition, a caveat to the general use of polymyxin B as such a control is the report that polymyxin B itself can induce several functional and molecular modifications in monocyte-derived human DCs that are characteristic of DCs undergoing a maturation process (Valentinis et al. 2005). This might, therefore, confuse the interpretation of experiments that include such controls.
Another control which is typically included in such experiments involves the heat treatment of the molecular chaperone, on the basis that endotoxin is heat stable (Vabulas et al. 2002a). However, this has been reported to be not necessarily the case by one laboratory (Gao and Tsan 2003a). This contradicts over 100 years experience with the marked heat stability of LPS. Experience in the Pockley laboratory is that boiling of purified HSPC4 preparations results in a marked aggregation of HSPC4 as observed by silver staining of electrophoretic gels (Mirza et al. 2006). It is possible that such aggregated proteins retain immunological activities, and again, the data deriving from such controls should be considered in the context of this. Another approach is to incubate samples for 3 h at 37°C with proteinase K acrylic beads (which can be obtained from the Sigma–Aldrich Company Ltd.) (Mirza et al. 2006). No protein bands are observed following this treatment, and all activity is lost (Mirza et al. 2006). By far, the best approach for minimising the potential contamination of HSPC4 preparations with endotoxin is to reduce the potential for such contamination to occur. This can be achieved using purified proteins and procedures which prevent the introduction of endotoxin (which are possible) or by using experimental approaches which exclude the possibility of such contamination. With respect to the latter, there are a number of reports in which the immunological properties of HSPC4 have been described, none of which can be attributed to endotoxin contamination. These primarily include experimental systems involving synthesised HSPC4 peptides or HSPC4 which is secreted and/or expressed in a cell culture system. For instance, an HSPC4-IgG fusion protein secreted by tumour cells induces CD8+ T cell activation and has the capacity to elicit tumour rejection (Yamazaki et al. 1999) and can activate NK cells in vivo (Strbo et al. 2003). Truncated forms of the HSPC4 molecules secreted into conditioned medium by transfected cells have also been shown to mediate tumour rejection in mice and to induce DC maturation (Baker-LePain et al. 2002). Furthermore, cell-surface-expressed HSPC4 has been shown to induce the activation of DCs and the development of autoimmune disease in mice (Liu et al. 2003), and HSPC4-secreting fibroblasts lead to in vivo activation of CD11b+ and CD11c+ APCs (Baker-LePain et al. 2004). Although it has recently been argued that the in vivo effects of HSPC4 are mediated by chaperoned peptides rather than the HSPC4 itself, due to the reports that highly purified HSPC4 which is free of pathogen-derived contaminants does not have immunostimulatory properties (Tsan and Gao 2009), this does not take into account the stimulatory capacity of HSPC4 which has been demonstrated using the in vitro culture systems described above.
It is clear that the debate continues concerning the involvement of endotoxin in the biological and immunological properties of stress proteins. The majority of these concerns can be addressed by undertaking two simple experimental steps:
Generate recombinant stress proteins in endotoxin-free expression systems
Purify stress proteins using an endotoxin-free system
Moonlighting proteins
As we approach the end of the first decade of the twenty-first century we see an ever-expanding role for molecular chaperones in biological processes and realise that in spite of significant sequence conservation, individual chaperone family members can have very distinct functions. Proteins with more than one function are now termed moonlighting proteins (Jeffery 2003), and the number of moonlighting functions for molecular chaperones is growing rapidly, as has been reviewed elsewhere (Henderson and Pockley 2005; Novartis Foundation Symposium 2008). Examples of these moonlighting functions include the finding that murine spermatozoa have Hsp60 on their cell surface and that this protein has to be phosphorylated to enable the sperm to be capacitated (Asquith et al. 2004). It would naturally be assumed that if this is the case in mice, then other mammals would utilise Hsp60 similarly. In fact, human sperm do not have Hsp60 on their cell surface; therefore, this protein cannot be used in sperm capacitation (Mitchell et al. 2007). A cell-surface location for molecular chaperones is increasingly indicated and one surprising finding has been that the receptor complex for LPS includes, among a number of proteins, the molecular chaperones Hsp70 and Hsp90 (Triantafilou et al. 2001a), and there is direct evidence for the role for these proteins in the activation of monocytes by LPS (Triantafilou et al. 2001b). Indeed, there are a growing number of reports of bacteria utilising Hsp60 and Hsp70 as adhesins (Henderson et al. 2006). Perhaps the most outlandish moonlighting example is of the bacterium Listeria monocytogenes which was identified to have a major adhesin termed Listeria adhesion protein (LAP). It was known that LAP bound to cell surface Hsp60 on human cells. Further analysis of LAP has revealed it to be the metabolic enzyme, alcohol acetaldehyde dehydrogenase, and surface plasmon resonance analysis has revealed that the Ka of binding between LAP and Hsp60 is 5.4 × 108 M−1 s−1, which is a high affinity interaction and similar to the affinity of antibodies used to detect Hsp60 in immunoassays (Kim et al. 2006). Thus, this is a moonlighting protein binding to a moonlighting protein.
Many more examples could be given of the moonlighting actions of molecular chaperones, and some of these can be found in recent books and reviews (Henderson and Pockley 2005; Henderson et al. 2006; Henderson and Henderson 2009; Pockley et al. 2008). They have been included to show that molecular chaperones have a wide-ranging set of biological activities in addition to those that are associated with the activation, or inhibition, of leukocytes. Indeed, much of the controversy about the extracellular signalling actions of molecular chaperones has focused on their published actions as activators of monocytes and lymphocytes (Gao and Tsan 2003a, b; Ye and Gan 2007; Tsan and Gao 2009). However, there is increasing evidence that molecular chaperones can inhibit the functions of immune cells. Evidence has been presented that Hsp10 (Johnson et al. 2005), Hsp27 (Miller-Graziano et al. 2008), human Hsp60 (Zanin-Zhorov et al. 2003, 2006; Cohen-Sfady et al. 2005), the endoplasmic reticulum Hsp70 family member, BiP (Corrigall et al. 2001, 2004) and HSPC4 (Chandawarkar et al. 1999, 2004; Kovalchin et al. 2006; Slack et al. 2007), inhibit monocyte or lymphocyte activation. Indeed, Hsp10 was actually discovered in human blood, as an immunosuppressive factor, 1 year (1977) before the terminology of molecular chaperones was introduced (Morton et al. 1977; Noonan et al. 1979). Not only are some molecular chaperones immunosuppressive but they are also in clinical trial for the treatment of chronic inflammatory diseases. Thus, human Hsp10 has been reported to have some therapeutic effect in patients with rheumatoid arthritis (Vanags et al. 2006) and in those with psoriasis (Williams et al. 2008). BiP is currently entering clinical trials for the treatment of rheumatoid arthritis in the UK (Panayi, personal communication). Furthermore, the human Hsp60 peptide DiaPep277 is in a clinical trial for diabetes (Huurman et al. 2008).
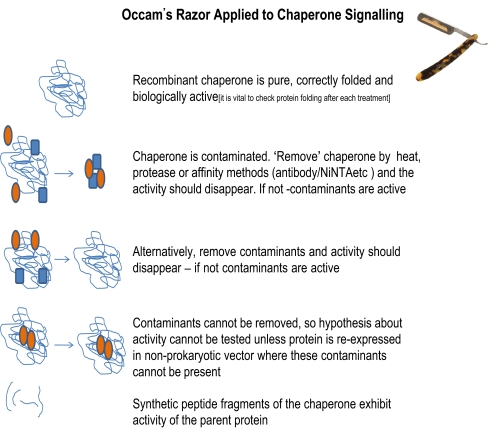
The wide-ranging biological actions of cell surface molecular chaperones, which are independent of PAMP contamination, plus the evidence for immunosuppressive/anti-inflammatory actions of the heat shock proteins, again clearly actions that are not associated with PAMP contamination, combined with the evidence that has been presented earlier supports the hypothesis that the moonlighting functions of molecular chaperones are inherent properties of these proteins. To establish this hypothesis, it is important that future work in the field should be carried out to the highest standards of molecular chaperone purification, and a set of recommendations as to how to achieve this are provided in Table 2. As E. coli expression is likely to remain popular with those generating recombinant molecular chaperones, it would be useful to characterise the PAMPs that could contaminate recombinant molecular chaperones that are expressed in the common expression strains of E. coli. Such proteins are likely to be found on the cell surface and to get there have to pass through the periplasm. We have used E. coli strain M607 (derived from the K12 strain JM83 F-ara Δ(lac-proAB) rpsL (Strr) [φ80 dlacΔ(lacZ)M15] in which the gene encoding the acyltransferase responsible for transferring the acyl chains to the 3′ position in the lipid A moiety of LPS has been mutated (Somerville et al. 1996). The LPS from this isogenic mutant is inactive and thereby allows the cytokine-inducing activity of the periplasmic proteins to be measured. We found that these periplasmic proteins could stimulate human monocytes to generate pro-inflammatory cytokines in the microgramme per millilitre concentration range. Surprisingly, although all of this activity was blocked by polymyxin B, it was not due to LPS. We used polymyxin B columns to remove the cytokine-inducing proteins and on elution of these columns with deoxycholate, two proteins were identified: flagellin and peptidylprolyl cis,trans isomerase (Sharp et al. 2009). These appear to be the major E. coli PAMPs which contribute to recombinant protein cytokine-inducing activity. Importantly, their activity is relatively low (compared to LPS) both proteins can be removed and/or activity blocked by polymyxin B, and they can be removed using antibodies. In addition, the E. coli strain M607 is a useful one for expressing recombinant molecular chaperones, as it has almost a non-endotoxic LPS. A schematic representation of the tests of the hypothesis that molecular chaperones have cell signalling activity is shown in Fig. 3. Finally, Table 3 compares and contrasts the literature on Hsp60 that covers both the papers which claim that the activity of this protein is due to contaminants and those in which evidence is produced to show that contaminants could not generate the published results.
Table 2.
Recommendations for generating recombinant molecular chaperones with minimal contamination with PAMPs
| Before considering the points below, it is important to check that a final recombinant chaperone is properly folded using circular dichroism (CD) or NMR. Improperly folded proteins might lack activity. |
|---|
| 1. Express proteins in eukaryotic hosts to avoid suggestions of PAMP contamination. |
| 2. Even if generated in eukaryotic hosts, great care must be taken with protein purification to ensure no bacterial contamination occurs in buffers, piping, columns etc. |
| 3. Always check final preparation of recombinant chaperone with the Limulus assay to ensure that there is no LPS contamination. |
| 4. Check the purity of recombinant chaperones by mass spectrometry as well as SDS-PAGE. |
| 5. If chaperones must be expressed in E. coli, then use the M607 strain, as this has less endotoxic LPS. |
| 6. Chaperones must be treated with polymyxin B to remove LPS and other polymyxin B-binding PAMPs. As some chaperones are very sticky, particularly to polymyxin B, it is best to have the chaperones on some matrix (Ni-NTA, GST etc.). It is better to wash proteins with an excess of polymyxin B than to pass them through a polymyxin B column, as this leads to protein loss. |
| 7. Monitor LPS content in chaperone preparations using the Limulus assay, and decrease LPS content as much as possible. |
| 8. Check that the activity of E. coli expressed proteins is heat sensitive (80–100°C for 10–30 min). Compare with LPS from the strain of E. coli used to express the chaperone. |
| 9. Check that the activity of E. coli expressed proteins is sensitive to proteinase K. Again use E. coli LPS as a control. |
| 10. Check that LPS-neutralising monoclonal antibodies have no effect on the activity of recombinant chaperones. |
| 11. Treat recombinant chaperone with acyloxyacyl hydrolase, which removes 3′acyl groups from LPS and decreases endotoxic activity. Is activity lost? |
| 12. Can the level of LPS contamination in the chaperone preparation account for any of the activity being measured? i.e. if the LPS level is 10 pg/µg chaperone do the cells you are using respond to such levels of LPS? |
| 13. If antibodies to the chaperone are available, use these to immunoprecipitate and remove the chaperone from the potential admixture of moieties being tested or, if antibody is neutralising, add to purified recombinant chaperone and to LPS as a specificity control. If results are positive can these antibodies be used to purify the chaperone? |
| 14. Can chaperone activity be replicated using peptides derived from the chaperone sequence? Do these peptides block the binding of the chaperone to the cell surface? |
| 15. Do the intracellular signalling activities in response to your chaperone and LPS (or other PAMPs) differ? This can be further explored in 16. |
| 16. Use microarray analysis to compare the cellular global transcriptional response to the recombinant chaperone compared to an equivalent activating concentration of LPS. |
| 17. Generate truncation or site-directed mutants of chaperone of interest. If all of the activity of the chaperone is due to contaminants, then this should not influence activity, unless truncation modifies binding to PAMPS. This should be checked. |
Fig. 3.
Possible explanations and tests for the cytokine-inducing actions of chaperones, especially if these have been expressed in E. coli
Table 3.
Evidence for and against Hsp60 having cell signalling activity
| Hsp60 (evidence against) |
|---|
| Human Hsp60 activation of murine monocytes is due to LPS contamination (Gao and Tsan 2003a, b) |
| Human Hsp60 fails to induce expression of 96 common cytokine genes in murine macrophages (Gao and Tsan 2004) |
| Murine Hsp60 fails to activate murine splenocytes (Wang et al. 2005a) |
| Hsp60 (evidence for) |
| Human recombinant Hsp60 purified so that LPS contamination was below assay sensitivity, activity of protein blocked by boiling (not same for LPS), polymyxin B had no effect. LPS stimulates NF-κB but Hsp60 did not (Kol et al. 2000) |
| Human Hsp60 truncation mutants identify equatorial domain as having murine bone resorbing activity (this is not the LPS-binding region of Hsp60) (Meghji et al. 2003) |
| Human Hsp60 equatorial domain peptides promote activation of murine monocytes (Habich et al. 2004) |
| NOTE T cells are generally unresponsive to LPS but LPS stimulates T cells to adhere to fibronectin via TLR4 signalling (Zanin-Zhorov et al. 2007) |
| Human Hsp60 promotes T lymphocyte adhesion to fibronectin even in TLR4-ve cells (Zanin-Zhorov et al. 2003) |
| Human Hsp60 downregulates T cell signalling and cytokine release and blocks induction of Con A-induced hepatitis (Zanin-Zhorov et al. 2005a) |
| Human Hsp60 downregulates T cell chemotaxis in vitro and in vivo and selected T cell signalling—effects could not possibly be due to LPS or lipoproteins (Zanin-Zhorov et al. 2005b) |
| Human Hsp60 but not GroEL or M. tuberculosis Hsp60.2 modulate B cell signalling—again, the effects seen could not be due to LPS (Cohen-Sfady et al. 2005) |
| Human Hsp60 up-regulates T regulatory cell activity in a TLR2-dependent manner (Zanin-Zhorov et al. 2006) |
| Peptide p277, 24-amino acid fragment of Hsp60 inhibits (like intact Hsp60) T cell chemotaxis (Nussbaum et al. 2006) |
| Peptide 277 is beneficial in treating early diabetes (Raz et al. 2007; Huurman et al. 2008)) |
| M. tuberculosisHsp60.2 stimulates a non-classical form of macrophage activation distinct from LPS (Peetermans et al. 1994) |
| M. tuberculosis Hsp60.2 stimulates endothelial cell adhesion protein synthesis distinct from that of LPS (Verdegaal et al. 1996) |
| M. tuberculosis Hsp60.1 and Hsp60.2 proteins fail to stimulate human monocyte cytokine after proteinase K treatment (Lewthwaite et al. 2001) |
| M. tuberculosis equatorial domain, but not apical or intermediate domains contain monocyte-activating activity (Tormay et al. 2005) |
| Inactivation of hsp60.1 gene in M. tuberculosis generates an isogenic mutant that fails to induce a pro-inflammatory response (Hu et al. 2008) |
| M. tuberculosis Hsp60.2 protein fails to activate or inhibit bone resorption or osteoclast formation [LPS is a potent osteolytic agent] (Winrow et al. 2008) |
| M. tuberculosis Hsp60.1 protein is a potent inhibitor of osteoclast formation and bone breakdown in vitro and in vivo. Activity cannot be due to LPS contamination as LPS is a potent osteolytic agent (Winrow et al. 2008) |
| M. tuberculosis Hsp60.1 (but not Hsp60.2 which has equal LPS contamination) inhibits experimental allergic asthma in mice (Riffo-Vasquez et al. 2004) |
| M. leprae Hsp60.2 but not M. tuberculosis Hsp60.2 or Hsp60 proteins from Streptococcus pneumoniae, Helicobacter pylori or bacillus Calmette-Guérin inhibits experimental asthma in mice (Rha et al. 2002) [note the M. leprae and M. tuberculosis proteins exhibit 95% sequence identity] |
| A. actinomycetemcomitans Hsp60 stimulates bone breakdown in explants of C3H/HeJ (TLR4-ve) mouse bone (LPS inactive) (Kirby et al. 1995) |
| Purified H. pylori Hsp60 stimulates macrophage cytokine synthesis via a TLR2/TLR4/Myd88-independent mechanism (LPS acts via a TLR4/Myd88 mechanism) (Gobert et al. 2004). |
| Recombinant Rhizobium leguminosarum Hsp60.3, but not recombinant Hsp60.1 (70% sequence identity and both with similar (low) LPS levels), stimulates human monocyte pro-inflammatory cytokine synthesis (Lewthwaite et al. 2002) |
Conclusion
Although the potential for contaminating LPS to account for the activities of recombinant molecular chaperones that have been prepared using E. coli expression systems, or which have been poorly purified, does exist, a wealth of evidence indicates that these proteins do indeed have a number of biological and immunological properties that are not induced by associated contaminants. Proteins produced in eukaryotic expression systems have been shown to have a number of biological/immunological properties, as have heat shock protein derived peptides and proteins expressed on the surfaces of cells. Furthermore, the ‘moonlighting’ profile of heat shock proteins is far greater than that of LPS. This provides another strong argument against those suggesting that all of the activities that have been attributed to heat shock proteins are due to the presence of contaminating LPS. Whereas the conclusions of experimental studies that do not include the appropriate controls should be interpreted with caution, it is important that the findings from studies that have been performed properly should be accepted and considered on their merit.
Acknowledgements
BH and ARMC wish to thank the Medical Research Council, Wellcome Trust, British Heart Foundation and Arthritis Research Campaign for funding their studies.
References
- Anderton SM, Zee R, Prakken B, Noordzij A, Eden W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J Exp Med. 1995;181:943–952. doi: 10.1084/jem.181.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee H-G, Salle H, Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- Arnold-Schild D, Kleist C, Welschof M, Opelz G, Rammensee HG, Schild H, Terness P. One-step single-chain Fv recombinant antibody-based purification of gp96 for vaccine development. Cancer Res. 2000;60:4175–4178. [PubMed] [Google Scholar]
- Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nature Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70. Role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117:3645–3657. doi: 10.1242/jcs.01214. [DOI] [PubMed] [Google Scholar]
- Babaahmady K, Oehlmann W, Singh M, Lehner T. Inhibition of human immunodeficiency virus type 1 infection of human CD4+ T cells by microbial HSP70 and the peptide epitope 407–426. J Virol. 2007;81:3354–3360. doi: 10.1128/JVI.02320-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–1459. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Sarzotti M, Nicchitta CV. Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo. J Immunol. 2004;172:4195–4203. doi: 10.4049/jimmunol.172.7.4195. [DOI] [PubMed] [Google Scholar]
- Bardwell JC, Craig EA. Major heat shock gene of Drosophila and the Escherichia coli heatinducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984;81:848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activates the NF-κB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Bendz H, Marincek BC, Momburg F, Ellwart JW, Issels RD, Nelson PJ, Noessner E. Calcium signaling in dendritic cells by human or mycobacterial Hsp70 is caused by contamination and is not required for Hsp70-mediated enhancement of cross-presentation. J Biol Chem. 2008;283:26477–26483. doi: 10.1074/jbc.M803310200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J Biol Chem. 2004;279:51250–51257. doi: 10.1074/jbc.M406202200. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci USA. 2004;101:6128–6133. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/S0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Thériault J, Gray PJ, Gong J. Cell surface receptors for molecular chaperones. Methods. 2007;43:199–206. doi: 10.1016/j.ymeth.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J Exp Med. 1999;189:1437–1442. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandawarkar RY, Wagh MS, Kovalchin JT, Srivastava P. Immune modulation with high-dose heat-shock protein gp96: therapy of murine autoimmune diabetes and encephalomyelitis. Int Immunol. 2004;16:615–624. doi: 10.1093/intimm/dxh063. [DOI] [PubMed] [Google Scholar]
- Cohen IR. Tending Adam’s Garden: evolving the cognitive immune self. London: Academic Press; 2000. [Google Scholar]
- Cohen IR, Holoshitz J, Eden W, Frenkel A. T lymphocyte clones illuminate pathogenesis and affect therapy of experimental arthritis. Arthritis Rheum. 1985;28:841–845. doi: 10.1002/art.1780280802. [DOI] [PubMed] [Google Scholar]
- Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin-Zhorov A, Lider O, Cohen IR. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Bodman-Smith MD, Fife MS, Canas B, Myers LK, Wooley P, Soh C, Staines NA, Pappin DJ, Berlo SE, Eden W, Zee R, Lanchbury JS, Panayi GS. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001;166:1492–1498. doi: 10.4049/jimmunol.166.3.1492. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Bodman-Smith MD, Brunst M, Cornell H, Panayi GS. Inhibition of antigen-presenting cell function and stimulation of human peripheral blood mononuclear cells to express an antiinflammatory cytokine profile by the stress protein BiP: relevance to the treatment of inflammatory arthritis. Arthritis Rheum. 2004;50:1164–1171. doi: 10.1002/art.20134. [DOI] [PubMed] [Google Scholar]
- Graeff-Meeder ER, Zee R, Rijkers GT, Schuurman HJ, Kuis W, Bijlsma JWJ, Zegers BJM, Eden W. Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet. 1991;337:1368–1372. doi: 10.1016/0140-6736(91)93057-G. [DOI] [PubMed] [Google Scholar]
- Graeff-Meeder ER, Eden W, Rijkers GT, Prakken BJ, Kuis W, Voorhorst Ogink MM, Zee R, Schuurman HJ, Helders PJ, Zegers BJ. Juvenile chronic arthritis: T-cell reactivity to human HSP60 in patients with a favorable course of arthritis. J Clin Invest. 1995;95:934–940. doi: 10.1172/JCI117801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/S1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Demine R, Walden P. Testing the role of gp96 as peptide chaperone in antigen processing. J Biol Chem. 2005;280:17573–17578. doi: 10.1074/jbc.M501233200. [DOI] [PubMed] [Google Scholar]
- Fairburn B, Muthana M, Hopkinson K, Slack LK, Mirza S, Georgiou AS, Espigares E, Wong C, Pockley AG. Analysis of purified gp96 preparations from rat and mouse livers using 2-D gel electrophoresis and tandem mass spectrometry. Biochimie. 2006;88:1165–1174. doi: 10.1016/j.biochi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Fearon WF, Fearon DT. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–2579. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, Wittmann M, Wang D, Dressel R, Seltsam A, Blasczyk R, Eiz-Vesper B. Heat shock protein 70 (Hsp70) induces cytotoxicity of T-helper cells. Blood. 2009;113:3008–3016. doi: 10.1182/blood-2008-06-162727. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Endotoxin contamination in recombinant human Hsp70 preparation is responsible for the induction of TNFα release by murine macrophages. J Biol Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor a from murine macrophages. J Biol Chem. 2003;278:22523–22529. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Induction of cytokines by heat shock proteins and endotoxin in murine macrophages. Biochem Biophys Res Commun. 2004;317:1149–1154. doi: 10.1016/j.bbrc.2004.03.160. [DOI] [PubMed] [Google Scholar]
- Gobert AP, Bambou JC, Werts C, Balloy V, Chignard M, Moran AP, Ferrero RL. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J Biol Chem. 2004;279:245–250. doi: 10.1074/jbc.M307858200. [DOI] [PubMed] [Google Scholar]
- Habich C, Kempe K, Burkart V, Zee R, Lillicrap M, Gaston H, Kolb H. Identification of the heat shock protein 60 epitope involved in receptor binding on macrophages. FEBS Lett. 2004;568:65–69. doi: 10.1016/j.febslet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Henderson B, Henderson S. Unfolding the relationship between secreted molecular chaperones and macrophage activation states. Cell Stress Chaperones. 2009;14:329–341. doi: 10.1007/s12192-008-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Pockley AG. Molecular chaperones and cell signalling. New York: Cambridge University Press; 2005. [Google Scholar]
- Henderson B, Allan E, Coates AR. Stress wars: the direct role of host and bacterial molecular chaperones in bacterial infection. Infect Immun. 2006;74:3693–3706. doi: 10.1128/IAI.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Henderson B, Lund PA, Tormay P, Ahmed MT, Gurcha SS, Besra GS, Coates AR. A Mycobacterium tuberculosis mutant lacking the groEL homologue cpn60.1 is viable but fails to induce an inflammatory response in animal models of infection. Infect Immun. 2008;76:1535–1546. doi: 10.1128/IAI.01078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci USA. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huurman VA, Meide PE, Duinkerken G, Willemen S, Cohen IR, Elias D, Roep BO. Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol. 2008;152:488–497. doi: 10.1111/j.1365-2249.2008.03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery CJ. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 2003;19:415–417. doi: 10.1016/S0168-9525(03)00167-7. [DOI] [PubMed] [Google Scholar]
- Johnson BJ, Le TT, Dobbin CA, Banovic T, Howard CB, Flores de Mayo F, Vanags D, Naylor DJ, Hill GR, Suhrbier A. Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J Biol Chem. 2005;280:4037–4047. doi: 10.1074/jbc.M411569200. [DOI] [PubMed] [Google Scholar]
- Kamphuis S, Kuis W, Jager W, Teklenburg G, Massa M, Gordon G, Boerhof M, Rijkers GT, Uiterwaal CS, Otten HG, Sette A, Albani S, Prakken BJ. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet. 2005;366:50–56. doi: 10.1016/S0140-6736(05)66827-4. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KP, Jagadeesan B, Burkholder KM, Jaradat ZW, Wampler JL, Lathrop AA, Morgan MT, Bhunia AK. Adhesion characteristics of Listeria adhesion protein (LAP)-expressing Escherichia coli to Caco-2 cells and of recombinant LAP to eukaryotic receptor Hsp60 as examined in a surface plasmon resonance sensor. FEMS Microbiol Lett. 2006;256:324–332. doi: 10.1111/j.1574-6968.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- Kirby AC, Meghji S, Nair SP, White P, Reddi K, Nishihara T, Nakashima K, Willis AC, Sim R, Wilson M, Henderson B. The potent bone-resorbing mediator of Actinobacillus actinomycetemcomitans is homologous to the molecular chaperone GroEL. J Clin Invest. 1995;96:1185–1194. doi: 10.1172/JCI118150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- Kong TH, Coates ARM, Butcher PD, Hickman CJ, Shinnick TM. Mycobacterium tuberculosis expresses two chaperonin-60 homologs. Proc Natl Acad Sci USA. 1993;90:2608–2612. doi: 10.1073/pnas.90.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchin JT, Mendonca C, Wagh MS, Wang R, Chandawarkar RY. In vivo treatment of mice with heat shock protein, gp96, improves survival of skin grafts with minor and major antigenic disparity. Transplant Immunol. 2006;15:179–185. doi: 10.1016/j.trim.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lewthwaite JC, Coates ARM, Tormay P, Singh M, Mascagni P, Poole S, Roberts M, Sharp L, Henderson B. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (hsp 65) and contains a CD14-binding domain. Infect Immun. 2001;69:7349–7355. doi: 10.1128/IAI.69.12.7349-7355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewthwaite JC, George R, Lund PA, Poole S, Tormay P, Sharp L, Coates ARM, Henderson B. Rhizobium leguminosarum chaperonin 60.3, but not chaperonin 60.1, induces cytokine production by human monocytes: activity is dependent on interaction with cell surface CD14. Cell Stress & Chaperones. 2002;7:130–136. doi: 10.1379/1466-1268(2002)007<0130:RLCBNC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Ann Rev Genetics. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z. Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proc Nat Acad Sci USA. 2003;100:15824–15829. doi: 10.1073/pnas.2635458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ewing N, DeFilippo M. Analytical challenges and strategies for the characterization of gp96-associated peptides. Methods. 2004;32:32–37. doi: 10.1016/S1046-2023(03)00185-3. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43:168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeish KR, Dean WL, Wellhausen SR, Stelzer GT. Role of intracellular calcium in priming of human peripheral blood monocytes by bacterial lipopolysaccharide. Inflammation. 1989;13:681–692. doi: 10.1007/BF00914312. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065X.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Meghji S, White P, Nair SP, Reddi K, Heron K, Henderson B, Zaliani A, Fossati G, Mascagni P, Hunt JF, Roberts MM, Coates AR. Mycobacterium tuberculosis chaperonin 10 stimulates bone resorption: a potential contributory factor in Pott's disease. J Exp Med. 1997;186:1241–1246. doi: 10.1084/jem.186.8.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghji S, Lillicrap M, Maguire M, Tabona P, Gaston JSH, Poole S, Henderson B. Human chaperonin 60 (Hsp60) stimulates bone resorption: Structure/function relationships. Bone. 2003;33:419–425. doi: 10.1016/S8756-3282(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Mehlert A, Young DB. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol Microbiol. 1989;3:125–130. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Miller-Graziano CL, De A, Laudanski K, Herrmann T, Bandyopadhyay S. HSP27: an anti-inflammatory and immunomodulatory stress protein acting to dampen immune function. Novartis Found Symp. 2008;291:196–208. doi: 10.1002/9780470754030.ch15. [DOI] [PubMed] [Google Scholar]
- Mirza S, Muthana M, Fairburn B, Slack LK, Hopkinson K, Pockley AG. The stress protein gp96 is not an activator of resting rat bone marrow-derived dendritic cells, but is a co-stimulator and activator of CD3+ T cells. Cell Stress Chaperones. 2006;11:364–378. doi: 10.1379/CSC-208.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Aitken RJ. Analysis of chaperone proteins associated with human spermatozoa during capacitation. Mol Hum Reprod. 2007;13:605–613. doi: 10.1093/molehr/gam043. [DOI] [PubMed] [Google Scholar]
- Monks SA, Gaibor FP, Hassan-Zahraee M, Sawlivich W, Liu C, Rottman JB, Zabrecky JR. The role of oligomeric structure in the biological activities of heat-shock protein, gp96. Immunology. 2005;114:148. [Google Scholar]
- Morton H, Rolfe B, Clunie GJ. An early pregnancy factor detected in human serum by the rosette inhibition test. Lancet. 1977;1:394–397. doi: 10.1016/S0140-6736(77)92605-8. [DOI] [PubMed] [Google Scholar]
- Nicchitta CV, Carrick DM, Baker-Lepain JC. The messenger and the message: gp96 (GRP94)-peptide interactions in cellular immunity. Cell Stress Chaperones. 2004;9:325–331. doi: 10.1379/CSC-62.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan FP, Halliday WJ, Morton H, Clunie GJ. Early pregnancy factor is immunosuppressive. Nature. 1979;278:649–651. doi: 10.1038/278649a0. [DOI] [PubMed] [Google Scholar]
- The Biology of Extracellular Molecular Chaperones. Chichester: Wiley; 2008. [PubMed] [Google Scholar]
- Nussbaum G, Zanin-Zhorov A, Quintana F, Lider O, Cohen IR. Peptide p277 of HSP60 signals T cells: inhibition of inflammatory chemotaxis. Int Immunol. 2006;18:1413–1419. doi: 10.1093/intimm/dxl074. [DOI] [PubMed] [Google Scholar]
- Peetermans WE, Raats CJ, Langermans JA, Furth R. Mycobacterial heat-shock protein 65 induces proinflammatory cytokines but does not activate human mononuclear phagocytes. Scand J Immunol. 1994;39:613–617. doi: 10.1111/j.1365-3083.1994.tb03421.x. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory role of stress proteins. Trends Biochem Sci. 2008;3:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Wauben MHM, Kooten PJS, Anderton S, Zee R, Kuis W, Eden W. Nasal administration of arthritis related T cell epitopes of hsp60 as a promising way for immunotherapy in chronic arthritis. Biotherapy. 1998;10:205–211. doi: 10.1007/BF02678298. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Wendling U, Zee R, Rutten VPM, Kuis W, Eden W. Induction of IL-10 and inhibition of experimental arthritis are specific features of microbial heat shock proteins that are absent for other evolutionarily conserved immunodominant proteins. J Immunol. 2001;167:4147–4153. doi: 10.4049/jimmunol.167.8.4147. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- Ramirez SR, Singh-Jasuja H, Warger T, Braedel-Ruoff S, Hilf N, Wiemann K, Rammensee HG, Schild H. Glycoprotein 96-activated dendritic cells induce a CD8-biased T-cell response. Cell Stress Chaperones. 2005;10:221–229. doi: 10.1379/CSC-117R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, Cohen IR, Elias D. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev. 2007;23:292–298. doi: 10.1002/dmrr.712. [DOI] [PubMed] [Google Scholar]
- Reddi K, Meghji S, Wilson M, Henderson B. Comparison of the osteolytic activity of surface-associated proteins of bacteria implicated in periodontal disease. Oral Dis. 1995;1:26–31. doi: 10.1111/j.1601-0825.1995.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Reddi K, Meghji S, Nair SP, Arnett TR, Miller AD, Preuss M, Wilson M, Henderson B, Hill P. The Escherichia coli chaperonin 60 (groEL) is a potent stimulator of osteoclast formation. J Bone Miner Res. 1998;13:1260–1266. doi: 10.1359/jbmr.1998.13.8.1260. [DOI] [PubMed] [Google Scholar]
- Reed RC, Berwin B, Baker JP, Nicchitta CV. GRP94/gp96 elicits ERK activation in murine macrophages. A role for endotoxin contamination in NF-κB activation and nitric oxide production. J Biol Chem. 2003;278:31853–31860. doi: 10.1074/jbc.M305480200. [DOI] [PubMed] [Google Scholar]
- Rha YH, Taube C, Haczku A, Joetham A, Takeda K, Duez C, Siegel M, Aydintug MK, Born WK, Dakhama A, Gelfand EW. Effect of microbial heat shock proteins on airway inflammation and hyperresponsiveness. J Immunol. 2002;169:5300–5307. doi: 10.4049/jimmunol.169.9.5300. [DOI] [PubMed] [Google Scholar]
- Riffo-Vasquez Y, Spina D, Page C, Desel C, Whelan M, Tormay P, Singh M, Henderson B, Coates ARM. Differential effects of Mycobacterium tuberculosis chaperonins on bronchial eosinophilia and hyperresponsiveness in a murine model of allergic inflammation. Clin Exp Allergy. 2004;34:712–719. doi: 10.1111/j.1365-2222.2004.1931.x. [DOI] [PubMed] [Google Scholar]
- Rosser MF, Trotta BM, Marshall MR, Berwin B, Nicchitta CV. Adenosine nucleotides and the regulation of GRP94-client protein interactions. Biochemistry. 2004;43:8835–8845. doi: 10.1021/bi049539q. [DOI] [PubMed] [Google Scholar]
- Sharp L, Ward JM, Poole S, Beighton D, Wilkins JC, Homer KA, Nair SP, Henderson B (2009) The periplasm of Escherichia coli contains cytokine-inducing proteins whose activity can be blocked by the lipopolysaccharide-inhibiting antibiotic, polymyxin B. Immunology, in press
- Slack LK, Muthana M, Hopkinson K, Suvarna SK, Mirza S, Fairburn B, Pockley AG. Administration of the stress protein gp96 prolongs rat cardiac allograft survival, modifies rejection-associated inflammatory events and induces a state of peripheral T cell hyporesponsiveness. Cell Stress Chaperones. 2007;12:71–82. doi: 10.1379/CSC-237R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville JE, Jr, Cassiano L, Bainbridge B, Cunningham MD, Darveau RP. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Invest. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava PK. Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165–171. doi: 10.1006/meth.1997.0464. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Ann Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Amato RJ. Heat shock proteins: the 'Swiss Army Knife' vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–2597. doi: 10.1016/S0264-410X(00)00492-8. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strbo N, Oizumi S, Sotosek-Tokmadzic V, Podack ER. Perforin is required for innate and adaptive immunity induced by heat shock protein gp96. Immunity. 2003;18:381–390. doi: 10.1016/S1074-7613(03)00056-6. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kutomi G, Oura J, Torigoe T, Noriyuki Sato N. Piloting of exogenous antigen into cross-presentation pathway by heat shock proteins. In: Calderwood SK, Sherman MY, Ciocca DR, editors. Heat shock proteins in cancer. Dordrecht: Springer; 2007. pp. 383–396. [Google Scholar]
- Thériault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol. 2006;177:8604–8611. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
- Tormay P, Coates AR, Henderson B. The intercellular signaling activity of the Mycobacterium tuberculosis chaperonin 60.1 protein resides in the equatorial domain. J Biol Chem. 2005;280:14272–14277. doi: 10.1074/jbc.M414158200. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M, Ladha S, Mackie A, Dedrick RL, Fernandez N, Cherry R. Fluorescence recovery after photobleaching reveals that LPS rapidly transfers from CD14 to hsp70 and hsp90 on the cell membrane. J Cell Sci. 2001;114:2535–2545. doi: 10.1242/jcs.114.13.2535. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Cur Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- Valentinis B, Bianchi A, Zhou D, Cipponi A, Catalanotti F, Russo V, Traversari C. Direct effects of polymyxin B on human dendritic cells maturation. The role of IκB-alpha/NF-κB and ERK1/2 pathways and adhesion. J Biol Chem. 2005;280:14264–14271. doi: 10.1074/jbc.M410791200. [DOI] [PubMed] [Google Scholar]
- Zee R, Eden W, Meloen RH, Noordzij A, Embden JD. Efficient mapping and characterization of a T cell epitope by the simultaneous synthesis of multiple peptides. Eur J Immunol. 1989;19:43–47. doi: 10.1002/eji.1830190108. [DOI] [PubMed] [Google Scholar]
- Eden W, Holoshitz J, Nevo Z, Frenkel A, Klajman A, Cohen IR. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985;82:5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden W, Thole JER, Zee R, Noordzij A, Embden JDA, Hensen EJ, Cohen IR. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988;331:171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- Eden W, Zee R, Prakken B. Heat shock proteins induce T-cell regulation of chronic inflammation. Nat Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- Puijvelde GH, Hauer AD, Vos P, Heuvel R, Herwijnen MJ, Zee R, Eden W, Berkel TJ, Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- Vanags D, Williams B, Johnson B, Hall S, Nash P, Taylor A, Weiss J, Feeney D. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet. 2006;368:855–863. doi: 10.1016/S0140-6736(06)69210-6. [DOI] [PubMed] [Google Scholar]
- Verdegaal ME, Zegveld ST, Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;151:369–376. [PubMed] [Google Scholar]
- Wallin RPA, Lundqvist A, Moré SH, Bonin A, Kiessling R, Ljunggren H-G. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–135. doi: 10.1016/S1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kelly CG, Karttunen T, Whittall T, Lehner PJ, Duncan L, MacAry P, Younson JS, Singh M, Oehlmann W, Cheng G, Bergmeier L, Lehner T. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity. 2001;15:971–983. doi: 10.1016/S1074-7613(01)00242-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao B, Tsan MF. Induction of cytokines by heat shock proteins and concanavalin A in murine splenocytes. Cytokine. 2005;32:149–154. doi: 10.1016/j.cyto.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Whittall T, McGowan E, Younson J, Kelly C, Bergmeier LA, Singh M, Lehner T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;174:3306–3316. doi: 10.4049/jimmunol.174.6.3306. [DOI] [PubMed] [Google Scholar]
- Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-A negatively regulates antitumor immunity. Cancer Res. 2007;67:4996–5002. doi: 10.1158/0008-5472.CAN-06-3138. [DOI] [PubMed] [Google Scholar]
- Warger T, Hilf N, Rechtsteiner G, Haselmayer P, Carrick DM, Jonuleit H, Landenberg P, Rammensee HG, Nicchitta CV, Radsak MP, Schild H. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J Biol Chem. 2006;281:22545–22553. doi: 10.1074/jbc.M502900200. [DOI] [PubMed] [Google Scholar]
- Wearsch PA, Nicchitta CV. Purification and partial molecular characterization of GRP94, an ER resident chaperone. Protein Expr Purif. 1996;7:114–121. doi: 10.1006/prep.1996.0015. [DOI] [PubMed] [Google Scholar]
- Wendling U, Paul L, Zee R, Prakken B, Singh M, Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Williams B, Vanags D, Hall S, McCormack C, Foley P, Weiss J, Johnson B, Latz E, Feeney D. Efficacy and safety of chaperonin 10 in patients with moderate to severe plaque psoriasis: evidence of utility beyond a single indication. Arch Dermatol. 2008;144:683–685. doi: 10.1001/archderm.144.5.683. [DOI] [PubMed] [Google Scholar]
- Winrow VR, Mesher J, Meghji S, Morris CJ, Maguire M, Fox S, Coates AR, Tormay P, Blake DR, Henderson B. The two homologous chaperonin 60 proteins of Mycobacterium tuberculosis have distinct effects on monocyte differentiation into osteoclasts. Cell Microbiol. 2008;10:2091–2104. doi: 10.1111/j.1462-5822.2008.01193.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Nguyen T, Podack ER. Tumour secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol. 1999;163:5178–5182. [PubMed] [Google Scholar]
- Ye Z, Gan YH. Flagellin contamination of recombinant heat shock protein 70 is responsible for its activity on T cells. J Biol Chem. 2007;282:4479–4484. doi: 10.1074/jbc.M606802200. [DOI] [PubMed] [Google Scholar]
- Young DB, Ivanyi J, Cox JH, Lamb JR. The 65 kDa antigen of mycobacteria - a common bacterial protein? Immunol Today. 1987;8:215–219. doi: 10.1016/0167-5699(87)90168-X. [DOI] [PubMed] [Google Scholar]
- Zanette D, Dundon W, Soffientini A, Sottani C, Marinelli F, Akeson A, Sarubbi E. Human IL-1 receptor antagonist from Escherichia coli: large-scale microbial growth and protein purification. J Biotechnol. 1998;64:187–196. doi: 10.1016/S0168-1656(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–1569. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Bruck R, Tal G, Oren S, Aeed H, Hershkoviz R, Cohen IR, Lider O. Heat shock protein 60 inhibits Th1-mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J Immunol. 2005;174:3227–3236. doi: 10.4049/jimmunol.174.6.3227. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Tal G, Shivtiel S, Cohen M, Lapidot T, Nussbaum G, Margalit R, Cohen IR, Lider O. Heat shock protein 60 activates cytokine-associated negative regulator suppressor of cytokine signaling 3 in T cells: effects on signaling, chemotaxis, and inflammation. J Immunol. 2005;175:276–285. doi: 10.4049/jimmunol.175.1.276. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O, Cohen IR. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol. 2007;179:41–44. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]