Abstract
Heat shock proteins belong to a conserved superfamily of molecular chaperones found in prokaryotes and eukaryotes. These proteins are linked to a myriad of physiological functions. In this study, we show that the N. crassa hsp70-1 (NCU09602.3) and hsp70-2 (NCU08693.3) genes are preferentially expressed in an acidic milieu after 15 h of cell growth in sufficient phosphate at 30°C. No significant accumulation of these transcripts was detected at alkaline pH values. Both genes accumulated to a high level in mycelia that were incubated for 1 h at 45°C, regardless of the phosphate concentration and extracellular pH changes. Transcription of the hsp70-1 and hsp70-2 genes was dependent on the pacC+ background in mycelia cultured under optimal growth conditions or at 45°C. The pacC gene encodes a Zn-finger transcription factor that is involved in the regulation of gene expression by pH. Heat shock induction of these two hsp genes in mycelia incubated in low-phosphate medium was almost not altered in the nuc-1− background under both acidic and alkaline pH conditions. The NUC-1 transcriptional regulator is involved in the derepression of nucleases, phosphatases, and transporters that are necessary for fulfilling the cell's phosphate requirements. Transcription of the hsp70-3 (NCU01499.3) gene followed a different pattern of induction—the gene was depressed under insufficient phosphate conditions but was apparently unaffected by alkalinization of the culture medium. Moreover, this gene was not induced by heat shock. These results reveal novel aspects of the heat-sensing network of N. crassa.
Keywords: Neurospora crassa, hsp70, Heat shock, Pi sensing, pH regulation, nuc-1, pacC
Introduction
The heat shock response was first described in the early 1960s when induction of new RNA synthesis was observed in the chromosomes of Drosophila melanogaster salivary glands after a temperature shift (Ritossa 1962, 1996). The current model on the function of the highly conserved heat shock system is that in a wide range of organisms, from prokaryotes to eukaryotes, heat shock proteins (HSP) act as molecular chaperones in the renaturation or degradation of damaged proteins; folding, assembly, and membrane translocation of newly synthesized proteins; activation of regulatory protein systems; and autoregulation. Furthermore, their expression is linked to many other stress conditions such as osmotic and oxidative stress, indicating the physiological complexity of their regulation in response to cellular stimuli (Bukau et al. 2006; Christis et al. 2008; Daugaard et al. 2007; Ellis 2006; Hohmann 2002; Kalmar and Greensmith 2009; Li et al. 2009; Mayer and Bukau 2005; Nicchitta 2009; Steel et al. 2004; Tokuriki and Tawfik 2009). However, certain related members of this conserved protein family are expressed in cell cultures under nonstress conditions (Hartl and Hayer-Hartl 2002; Leal et al. 2009).
Transcription of heat shock genes has been well documented in N. crassa (Britton and Kapoor 2002; Freitag et al. 1997; Kapoor et al. 1995; Plesofsky et al. 2008; Rensing et al. 1998; Tremmel et al. 2007; Ungermann et al. 1994) as well as in other filamentous fungi (Faircloth et al. 2009; Fox et al. 2007; Georg and Gomes 2007; Montero-Barrientos et al. 2008; Rezaie et al. 2000; Turkel et al. 2006; Xavier et al. 1999). However, little is known about the cell stress response to phosphate (Pi) deprivation and changes in extracellular pH. The molecular mechanism controlling the response to phosphorus deprivation in N. crassa consists of four regulatory genes—nuc-2, preg, pgov, and nuc-1, which are involved in a highly conserved hierarchical relationship (Metzenberg 1979). Pi shortage is sensed by the nuc-2 gene, the product of which inhibits the function of the PREG–PGOV complex. This allows NUC-1 to translocate into the nucleus (Peleg et al. 1996a, b). NUC-1 is a basic helix-loop-helix transcriptional regulator involved in the derepression of nucleases, phosphatases, and transporters that are necessary for fulfilling the cell's Pi requirements (Kang 1993; Kang and Metzenberg 1990; Ogawa et al. 2000). Regulation of gene expression by pH in N. crassa and Aspergillus nidulans, among other filamentous fungi, involves the conserved PacC signal transduction pathway that mediates many metabolic events at either acidic or alkaline pH values (Caddick et al. 1986; Freitas et al. 2007; Gras et al. 2009; Leal et al. 2009; Nahas et al. 1982; Silva et al. 2008; Tilburn et al. 1995). The pacC gene encodes a Zn-finger transcription factor that is proteolytically activated to a 27-kDa form at alkaline pH values by a conserved signaling cascade composed of six pal genes (Tilburn et al. 1995). In N. crassa and A. nidulans, PacC, in addition to its other functions, is required for the development and glycosylation of the Pi-repressible acid phosphatase that is secreted in an acidic milieu (Nozawa et al. 2003a, b). Expression of the Pi-repressible acid phosphatase is modulated by Pi and pH changes, and its expression is dependent on both NUC-1 and PACC transcription factors, suggesting possible interactions between the pH and Pi regulatory circuits (Silva et al. 2008). Our study aimed to evaluate the transcriptional level of the structurally related N. crassa genes NCU09602.3, NCU08693.3, and NCU01499.3, which are designated here as hsp70-1, hsp70-2, and hsp70-3, respectively, under various culture conditions. The expression of these genes was also assayed in the pacCko and nuc-1RIP strains. Transcription of the hsp70-1 and hsp70-2 genes was dependent on the pacC+ background in mycelia cultured under optimal culture conditions or at 45°C, which suggested that in N. crassa, the expression of these genes is under the control of the pH regulatory circuit. Transcription of the hsp70-3 gene followed a different pattern of induction—it was not induced by heat shock and was found to be independent of the pacC+ background.
Materials and methods
Strains, culture conditions, and heat shock treatment
Wild-type (control) N. crassa St.L.74.OR23-1VA (FGSC No 2489) and a strain with a pacC loss-of-function mutation (pacCKO, FGSC No 11397; Galagan et al. 2003) were obtained from the Fungal Genetic Stock Center, University of Missouri, Kansas City, MO (McCluskey 2003) and maintained on slants of Vogel's medium (1.5% agar). The pacCKO cultures were supplemented with hygromycin (450 µg/ml). The nuc-1RIP strain was generated by the repeat-induced point (RIP) mutation procedure (Selker and Garrett 1988) as previously described (Leal et al. 2009).
Conidia from each strain (about 106/ml cells) were grown for 15 h at 30°C in an orbital shaker (200 rpm), in both low- and high-Pi medium (0.1 or 10 mM Pi) adjusted to pH 5.4 (buffered with 50 mM sodium citrate) or pH 7.8 (buffered with 50 mM Tris-HCl), supplemented with 44 mM sucrose as the carbon source, and prepared as previously described (Rodrigues and Rossi 1985). To assay the effect of heat stress, mycelia from the strains grown for 15 h at 30°C were incubated in various culture conditions for 1 or 2 h at 45°C.
Northern analysis
To validate differential transcription of the hsp70 genes by Northern blot during adaptation to Pi, pH, and temperature, DNA probes specific to each hsp70 gene were obtained by PCR amplification using the following primers: 5′-TGGCTCCAACGACAACGA-3′ (forward) and 5′-CATGAATGAATTGTCTTCATC-3′ (reverse) for NCU09602.3; 5′-AGCTTGAACCTCTTCGACAA-3′ (forward) and 5′-AGATTTTTTATTGTAAACCC-3′ (reverse) for NCU08693.3; and 5′-TTTAATCTGCCCATACTCCCG-3′ (forward) and 5′-TTCTTGACGTGCTCCTCAAA-3′ (reverse) for NCU01499.3. Mycelia of the strains cultivated for 15 h at 30°C or incubated for 1 or 2 h at 45°C were used for RNA preparation. Approximately 15 µg of total RNA, extracted with the TRIzol® reagent (Invitrogen, Carsbad, CA), was electrophoresed on 1.5% agarose gel containing formaldehyde, blotted onto Hybond-N+ membranes, and hybridized with purified DNA probes labeled with [α-32P]dCTP using the Random Primers DNA Labeling System (Invitrogen). Unincorporated nucleotides were removed with Sephadex G-50 Chromatography. Autoradiograms were scanned using a ScanJet 4C Scanner (Hewlett Packard) and analyzed using ImageQuant 5.1 software (Molecular Dynamics). Pixel intensities for each gene were quantified and normalized to a corresponding 28S rRNA blot.
Results and discussion
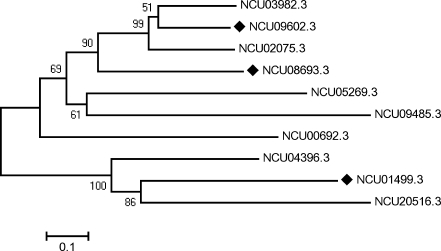
Transcription of 70-kDa class heat shock protein genes is associated with the folding and translocation of proteins across membranes as well as with cell division and developmental stage progression, acquisition of thermotolerance, cell rescue, and a myriad of other physiological processes. Moreover, genes encoding the HSP70 protein family exhibit complex patterns of expression that may respond to growth or stress conditions. In spite of this, heat induction of HSP70 genes in response to extracellular pH changes is poorly understood, and to the best of our knowledge, current understanding is restricted to N. crassa (Gras et al. 2009; Leal et al. 2009). Transcription of the N. crassa hsp70-2 (NCU08693.3) gene was previously demonstrated in germinating conidia of the 74A strain incubated for 5 h at 30°C in either low- or high-Pi medium at pH 5.4. This transcript did not accumulate to significant levels at pH 7.8 (Leal et al. 2009). Based on the identification of HSP70 protein signature motifs, the structurally related genes NCU09602.3 (hsp70-1), NCU08693.3 (hsp70-2), and NCU01499.3 (hsp70-3; Fig. 1) were chosen for transcriptional analysis. The HSP70-1 and HSP70-2 proteins are homologous to the heat shock proteins Ssa2 (cytosolic) and Ssc1 (mitochondrial), respectively, from Saccharomyces cerevisiae (Craig et al. 1989; Daugaard et al. 2007; Deocaris et al. 2006; Ellwood and Craig 1984; Hartl and Hayer-Hartl 2002; Kregel 2002; Mayer and Bukau 2005; Werner-Washburne et al. 1989). HSP70-3 is probably unique to ascomycetes and, thus, differs from the heat shock proteins HSP70-1 and HSP70-2, which are highly similar in eukaryotic organisms (Georg and Gomes 2007). However, the predicted intracellular localization of this heat shock protein is controversial because searches using the TargetP program indicated mitochondrial localization (http://bioinformatics.albany.edu/~ptarget/), whereas those by the BaCelLo and Wolf pSORT programs (http://gpcr.biocomp.unibo.it/bacello/pred.htm; http://wolfpsort.org/) suggested cytoplasmic localization.
Fig. 1.
Phylogenetic analysis of Neurospora crassa hsp70 genes. Multiple alignments of 10 HSP70 amino acid sequences using the N. crassa genome database was performed using ClustalW (http://www.ebi.ac.uk/clustalw). A phylogenetic tree was constructed from these alignments using the neighbor-joining method with the software package MEGA version 3.1 (http://www.megasoftware.net). Three of them were chosen for transcription analysis (filled diamonds). The number at the nodes represents the percentage of bootstrap values (1,000 replicates). The scale bar represents the phylogenetic distance of 0.1 amino acid substitution per site
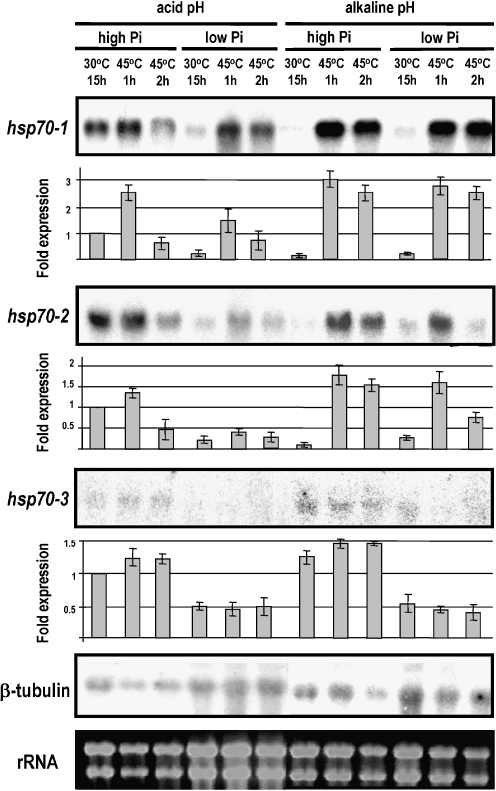
Transcription of the hsp70-1 and hsp70-2 genes in the mycelia of N. crassa grown for 15 h in high-Pi medium at pH 5.4 and 30°C, which are optimal conditions for fungal growth, was strongly reduced at alkaline pH values in either high- or low-Pi culture. However, alkaline pH had a strong effect on the heat shock induction of both these hsp genes, which accumulated to a higher level (at least 20-fold) and for a long duration in either sufficient or low-Pi medium (Fig. 2). Therefore, extracellular phosphate changes had a limited effect on heat shock induction at alkaline pH values. In contrast, transcription of the hsp70-1 and hsp70-2 genes in the mycelia of N. crassa grown for 15 h in high-Pi medium at pH 5.4 and 30°C was strongly reduced in low-Pi cultures in an acidic milieu. Furthermore, although these two hsp genes revealed similar transcription patterns, the hsp70-2 gene showed very poor induction upon heat shock (maximum of 2-fold) in either high- or low-Pi cultures at acidic pH values (Figs. 2 and 3). Thus, we hypothesize that the hsp70-2 gene is associated with conidial germination at 30°C under low-Pi and acidic pH conditions, which are conditions under which the secretion of Pi-repressible acid phosphatases occurs (Freitas et al. 2007; Silva et al. 2008). The hsp70-3 gene follows a different pattern of induction. Transcription of this gene is depressed in mycelia cultured under low-Pi conditions at either acidic or alkaline pH values but is not induced by heat shock (Fig. 2).
Fig. 2.
Gene expression analysis. Northern blot analysis of hsp70-1 (NCU09602.3), hsp70-2 (NCU08693.3), and hsp70-3 (NCU01499.3) transcripts using total RNA obtained from N. crassa mycelia. The wild-type strain was cultured for 15 h at 30°C in low- or high-Pi liquid medium (0.1 and 10 mM Pi, respectively) at pH 5.4 or 7.8. The effect of heat shock was assayed in mycelia incubated for 1 or 2 h at 45°C. The ethidium bromide-stained rRNA band is shown as a loading control. The β-tubulin gene was hybridized as additional loading control on the northern blot. Bars show fold expression determined from the intensity measured by densitometric analysis. Data are average values ± standard deviation (SD) obtained from two independent experiments
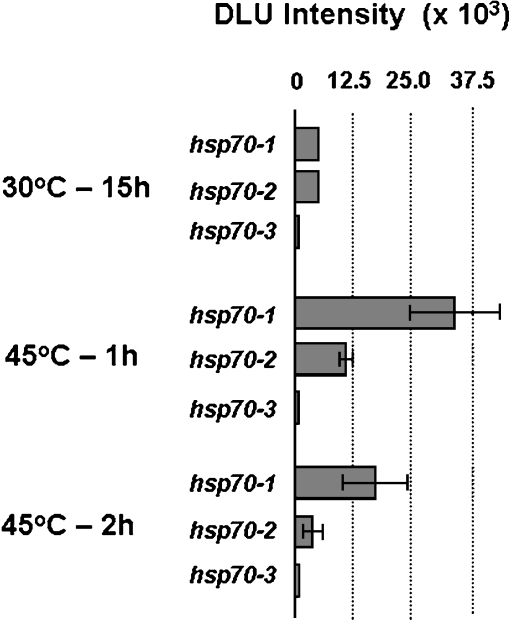
Fig. 3.
Relative gene expression levels of the three hsp70 gene family members in response to low-Pi cultures at pH 5.4 (northern blots are shown in Fig. 2). The images were captured by the Cyclone Storage Phosphor System (Packard) at 600 DPI resolution and analyzed with the OptiQuant program version 3.0 (Packard). The signal intensities of these image areas were expressed in Digital Light Units (DLU) per mm and normalized for the screen background. We assumed identical DLU values for both hsp70-1 and hsp70-2 transcripts expressed in mycelia incubated for 15 h at 30°C. The bars indicate the fold expression determined from the intensities measured by densitometric analysis. Data are average values ± standard deviation (SD) obtained from two independent experiments
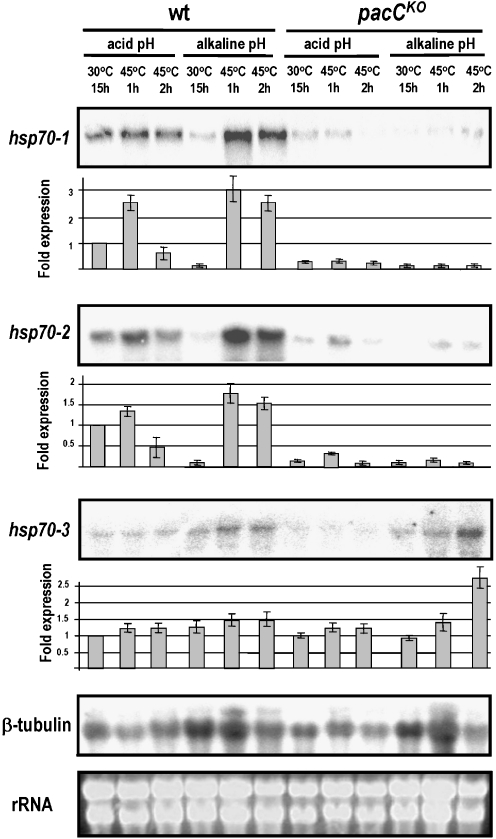
Expression of the hsp70-1 and hsp70-2 genes in mycelia grown under high-Pi conditions and at acidic pH values as well as their induction by heat shock at either acidic or alkaline pH values is strongly reduced in a pacC- background. In other words, transcription of both these hsp genes is positively regulated by PACC at 30°C and 45°C (Fig. 4). In contrast, induction of the hsp70-3 gene by heat shock occurs in the absence of PACC and only at alkaline pH values (Fig. 4). These results suggest that in N. crassa, the transcriptional regulator PACC has novel metabolic functions regardless of the extracellular pH.
Fig. 4.
Gene expression analysis. Northern blot analysis of hsp70-1 (NCU09602.3), hsp70-2 (NCU08693.3), and hsp70-3 (NCU01499.3) transcripts using total RNA obtained from N. crassa mycelia. The wild-type (wt) and pacCKO strains were cultured for 15 h at 30°C in high-Pi liquid medium (10 mM Pi) at pH 5.4 or 7.8. The effect of heat shock was assayed in mycelia incubated for 1 or 2 h at 45°C. The ethidium bromide-stained rRNA band is a loading control. The β-tubulin gene acted as additional loading control for the northern blots. Bars show fold expression, determined from the intensity measured by densitometric analysis. Data are average values ± standard deviation (SD) obtained from two independent experiments
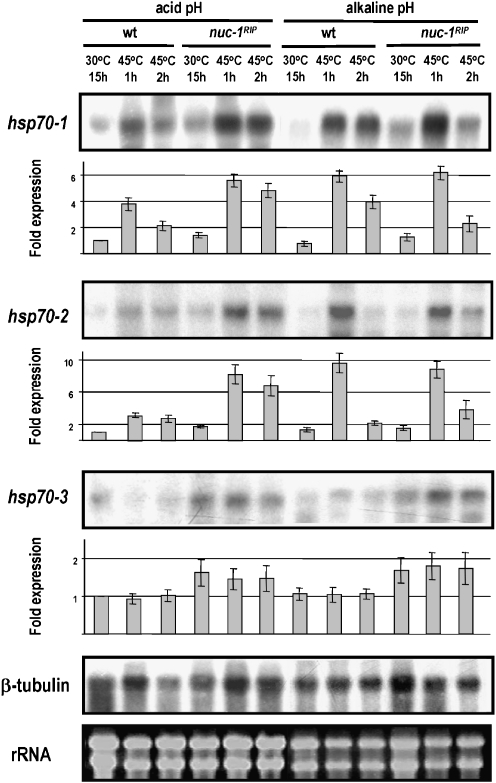
Heat shock induction of the hsp70-1 and hsp70-2 genes was also observed in the nuc-1RIP strain cultured under low Pi conditions at acidic and alkaline pH values. Moreover, although the levels of the transcripts encoding the HSP70-3 protein were slightly elevated in the nuc-1RIP strain, no induction by heat shock was observed (Fig. 5). It is worth noting that culturing the nuc-1RIP strain in a low-Pi medium at acidic pH values is highly stressful to the cells because both the PREG/PGOV complex and the nuc-1 gene are silenced (Gras et al. 2007, 2009; Leal et al. 2007, 2009; Metzenberg 1979). This suggests that the transcription of these hsp70 genes is independent of the nuc-1 gene. Therefore, the nuc-1RIP analysis seems to be more useful as a control for the observed positive regulation by PACC.
Fig. 5.
Gene expression analysis. Northern blot analysis of hsp70-1 (NCU09602.3), hsp70-2 (NCU08693.3), and hsp70-3 (NCU01499.3) transcripts using total RNA obtained from N. crassa mycelia. The wild-type (wt) and nuc-1RIP strains were cultured for 15 h at 30°C in a low-Pi liquid medium (0.1 mM Pi) at pH 5.4 or 7.8. The effect of heat shock was assayed in mycelia incubated for 1 or 2 h at 45°C. The ethidium bromide-stained rRNA band is a loading control. The β-tubulin gene acted as additional loading control for the northern blots. The bars show fold expression determined from the intensity measured by densitometric analysis. Data are average values ± standard deviation (SD) obtained from two independent experiments
In this study, we showed that the hsp70-1 and hsp70-2 genes of N. crassa are preferentially expressed in an acidic milieu but are induced by heat shock regardless of the extracellular pH. Furthermore, transcription of both these hsp genes is positively regulated by the transcription factor PACC, regardless of the pH, whereas heat shock induction of the hsp70-3 gene occurs in a pacC- background and only at alkaline pH values. Together with previously published data (Freitas et al. 2007; Gras et al. 2009; Leal et al. 2009; Nozawa et al. 2003a), these results indicate that the sensing of alkaline pH by the conserved PACC-signaling cascade is not the sole function of this protein. The previously reported exclusive activation of the A. nidulans PacC protein at alkaline pH values was observed in cultures under nonphysiological conditions, i.e., when the culture medium was very complex and when both high Pi-repressible and salt-stress conditions existed in which the synthesis of Pi-repressible enzymes was fully repressed (Freitas et al. 2007; Peñas et al. 2007; Perez-Esteban et al. 1993). Thus, the physiological activation of PACC also occurs in minimal medium supplemented with glucose as the carbon source at pH 5.4 (Silva et al. 2008). Moreover, transcription of these hsp70 genes might be directly or indirectly modulated by the transcriptional regulator PACC. PACC is homologous to Rim101p (Candida albicans and S. cerevisiae) and PacC (A. nidulans), which are transcription factors that regulate pH-conditioned gene expression in these eukaryotic microorganisms (Lamb and Mitchell 2003; Ramon and Fonzi 2003; Tilburn et al. 1995). PacC binds to 5′-GCCARG-3′ sequences upstream of pH-conditioned genes and either activates or represses transcription. The initial guanine residue in this consensus sequence is critical for PacC binding (Tilburn et al. 1995). The Rim101p binding site is 5′-NCCAAG-3′, which is preferentially followed by A or C in the adjacent 3′ position (Ramon and Fonzi 2003). The presence of this pentanucleotide followed by A or C was identified in the sequences upstream of the N. crassa hsp70-1, hsp70-2, hsp70-3, hsf2 (NCU08480.3), and hsf3 (NCU02413.3) genes, whereas this binding consensus was absent in the sequence upstream of the N. crassa hsf1 (NCU08512.3) gene. Thus, the hsf1 gene might not be under the direct control of PACC. Eukaryotic heat shock factors (HSFs) regulate constitutive and stress-inducible transcription of various genes, including the hsp genes and, thereby, play a central role in the regulation of numerous cellular reprogramming events (Hahn et al. 2006; Hashikawa et al. 2007; Sakurai and Takemori 2007; Thompson et al. 2008). HSF proteins recognize continuous and discontinuous repeats of 5′-nGAAn-3′ in target genes; these repeats are present in the sequences upstream of the hsp70-1, hsp70-2, and hsp70-3 genes. Therefore, interactions between the PACC, HSF, and HSP proteins are of great complexity including their competition for the target genes. In conclusion, regulation of these three structurally related hsp70 genes by the PACC protein depends upon specific culture conditions such as the incubation temperature and extracellular pH changes, which are novel aspects of the heat-sensing network of N. crassa.
Acknowledgements
This work was supported by grants from the Brazilian funding agencies FAPESP, CNPq, CAPES, and FAEPA. We thank Mendel Mazucato and Carlos A. Vieira for technical assistance.
References
- Britton ME, Kapoor M. The oligomeric state, complex formation, and chaperoning activity of hsp70 and hsp80 of Neurospora crassa. Biochem Cell Biol. 2002;80:797–809. doi: 10.1139/o02-166. [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Caddick MX, Brownlee AG, Arst HN. Regulation of gene-expression by pH of the growth-medium in Aspergillus nidulans. Mol Gen Genet. 1986;203:346–353. doi: 10.1007/BF00333978. [DOI] [PubMed] [Google Scholar]
- Christis C, Lubsen NH, Braakman I. Protein folding includes oligomerization—examples from the endoplasmic reticulum and cytosol. FEBS J. 2008;275:4700–4727. doi: 10.1111/j.1742-4658.2008.06590.x. [DOI] [PubMed] [Google Scholar]
- Craig EA, Kramer J, Shilling J, Werner-Washburne M, Holmes S, Kosic-Smithers J, Nicolet CM. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989;9:3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones. 2006;11:116–128. doi: 10.1379/CSC-144R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ. Molecular chaperones: assisting assembly in addition to folding. Trends Biochem Sci. 2006;31:395–401. doi: 10.1016/j.tibs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Ellwood MS, Craig EA. Differential regulation of the 70 K heat shock gene and related genes in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:1454–1459. doi: 10.1128/mcb.4.8.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faircloth LM, Churchill PF, Caldwell GA, Caldwell KA. The microtubule-associated protein, NUD-1, exhibits chaperone activity in vitro. Cell Stress Chaperones. 2009;14:95–103. doi: 10.1007/s12192-008-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GC, Shafiq M, Briggs DC, Knowles PP, Collister M, et al. Redox-mediated substrate recognition by Sdp1 defines a new group of tyrosine phosphatases. Nature. 2007;447:487–492. doi: 10.1038/nature05804. [DOI] [PubMed] [Google Scholar]
- Freitag DG, Ouimet PM, Girvitz TL, Kapoor M. Heat shock protein 80 of Neurospora crassa, a cytosolic molecular chaperone of the eukaryotic stress 90 family, interacts directly with heat shock protein 70. Biochemistry. 1997;36:10221–10229. doi: 10.1021/bi963030g. [DOI] [PubMed] [Google Scholar]
- Freitas JS, Silva EM, Rossi A. Identification of nutrient-dependent changes in extracellular pH and acid phosphatase secretion in Aspergillus nidulans. Genet Mol Res. 2007;6:721–729. [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- Georg RC, Gomes SL. Comparative expression analysis of members of the Hsp70 family in the chytridiomycete Blastocladiella emersonii. Gene. 2007;386:24–34. doi: 10.1016/j.gene.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Gras DE, Silveira HCS, Martinez-Rossi NM, Rossi A. Identification of genes displaying differential expression in the nuc-2 mutant strain of the mold Neurospora crassa grown under phosphate starvation. FEMS Microbiol Lett. 2007;269:196–200. doi: 10.1111/j.1574-6968.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- Gras DE, Silveira HCS, Peres NTA, Sanches PR, Martinez-Rossi NM, Rossi A. Transcriptional changes in the nuc-2A mutant strain of Neurospora crassa cultivated under conditions of phosphate shortage. Microbiol Res. 2009 doi: 10.1016/j.micres.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hahn JS, Neef DW, Thiele DJ. A stress regulatory network for co-ordinated activation of proteasome expression mediated by yeast heat shock transcription factor. Mol Microbiol. 2006;60:240–251. doi: 10.1111/j.1365-2958.2006.05097.x. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hashikawa N, Yamamoto N, Sakurai H. Different mechanisms are involved in the transcriptional activation by yeast heat shock transcription factor through two different types of heat shock elements. J Biol Chem. 2007;282:10333–10340. doi: 10.1074/jbc.M609708200. [DOI] [PubMed] [Google Scholar]
- Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61(4):310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kang S. Functional domains of the transcriptional activator NUC-1 in Neurospora crassa. Gene. 1993;130:259–264. doi: 10.1016/0378-1119(93)90428-6. [DOI] [PubMed] [Google Scholar]
- Kang S, Metzenberg RL. Molecular analysis of nuc-1+, a gene controlling phosphorus acquisition in Neurospora crassa. Mol Cell Biol. 1990;10:5839–5848. doi: 10.1128/mcb.10.11.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Curle CA, Runham C. The Hsp70 gene family of Neurospora crassa—cloning, sequence-analysis, expression, and genetic-mapping of the major stress-inducible member. J Bacteriol. 1995;177:212–221. doi: 10.1128/jb.177.1.212-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Mitchell AP. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol Cell Biol. 2003;23:677–686. doi: 10.1128/MCB.23.2.677-686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal J, Squina FM, Martinez-Rossi NM, Rossi A. The transcription of the gene for iso-orotate decarboxylase (IDCase), an enzyme of the thymidine salvage pathway, is downregulated in the pregc mutant strain of Neurospora crassa grown under phosphate starvation. Can J Microbiol. 2007;53:1011–1015. doi: 10.1139/W07-064. [DOI] [PubMed] [Google Scholar]
- Leal J, Squina FM, Freitas JS, Silva EM, Ono CJ, Martinez-Rossi NM, Rossi A. A splice variant of the Neurospora crassa hex-1 transcript, which encodes the major protein of the Woronin body, is modulated by extracellular phosphate and pH changes. FEBS Lett. 2009;583:180–184. doi: 10.1016/j.febslet.2008.11.050. [DOI] [PubMed] [Google Scholar]
- Li F, Luan W, Zhang C, Zhang J, Wang B, Xie Y, Li S, Xiang J. Cloning of cytoplasmic heat shock protein 90 (FcHSP90) from Fenneropenaeus chinensis and its expression response to heat shock and hypoxia. Cell Stress Chaperones. 2009;14:161–172. doi: 10.1007/s12192-008-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey K. The Fungal Genetics Stock Center: from molds to molecules. Adv Appl Microbiol. 2003;52:245–262. doi: 10.1016/S0065-2164(03)01010-4. [DOI] [PubMed] [Google Scholar]
- Metzenberg RL. Implications of some genetic control mechanisms in Neurospora. Microbiol Rev. 1979;43:361–383. doi: 10.1128/mr.43.3.361-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Barrientos M, Hermosa R, Nicolas C, Cardoza RE, Gutierrez S, Monte E. Overexpression of a Trichoderma HSP70 gene increases fungal resistance to heat and other abiotic stresses. Fungal Genet Biol. 2008;45:1506–1513. doi: 10.1016/j.fgb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Nahas E, Terenzi HF, Rossi A. Effect of carbon source and pH on the production and secretion of acid-phosphatase (EC3.1.3.2) and alkaline-phosphatase (EC3.1.3.1) in Neurospora crassa. J Gen Microbiol. 1982;128:2017–2021. [Google Scholar]
- Nicchitta CV. Cell biology: how to combat stress. Nature. 2009;457:668–669. doi: 10.1038/457668a. [DOI] [PubMed] [Google Scholar]
- Nozawa SR, Ferreira-Nozawa MS, Martinez-Rossi NM, Rossi A. The pH-induced glycosylation of secreted phosphatases is mediated in Aspergillus nidulans by the regulatory gene pacC-dependent pathway. Fungal Genet Biol. 2003a;39:286–295. doi: 10.1016/S1087-1845(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Nozawa SR, May GS, Martinez-Rossi NM, Ferreira-Nozawa MS, Coutinho-Netto J, Maccheroni W, Jr, Rossi A. Mutation in a calpain-like protease affects the posttranslational mannosylation of phosphatases in Aspergillus nidulans. Fungal Genet Biol. 2003b;38:220–227. doi: 10.1016/S1087-1845(02)00521-2. [DOI] [PubMed] [Google Scholar]
- Ogawa N, DeRisi J, Brown PO. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell. 2000;11:4309–4321. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Y, Addison R, Aramayo R, Metzenberg RL. Translocation of Neurospora crassa transcription factor NUC-1 into the nucleus is induced by phosphorus limitation. Fungal Genet Biol. 1996a;20:185–191. doi: 10.1006/fgbi.1996.0034. [DOI] [PubMed] [Google Scholar]
- Peleg Y, Aramayo R, Kang S, Hall JG, Metzenberg RL. NUC-2, a component of the phosphate-regulated signal transduction pathway in Neurospora crassa, is an ankyrin repeat protein. Mol Gen Genet. 1996b;252:709–716. doi: 10.1007/BF02173977. [DOI] [PubMed] [Google Scholar]
- Peñas MM, Hervas-Aguilar A, Munera-Huertas T, Reoyo E, Penalva MA, Arst HN, Jr, Tilburn J. Further characterization of the signaling proteolysis step in the Aspergillus nidulans pH signal transduction pathway. Eukaryot Cell. 2007;6:960–970. doi: 10.1128/EC.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Esteban B, Orejas M, Gomez-Pardo E, Penalva MA. Molecular characterization of a fungal secondary metabolism promoter: transcription of the Aspergillus nidulans isopenicillin N synthetase gene is modulated by upstream negative elements. Mol Microbiol. 1993;9:881–895. doi: 10.1111/j.1365-2958.1993.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Plesofsky NS, Levery SB, Castle SA, Brambl R. Stress-induced cell death is mediated by ceramide synthesis in Neurospora crassa. Eukaryot Cell. 2008;7:2147–2159. doi: 10.1128/EC.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon AM, Fonzi WA. Diverged binding specificity of Rim101p, the Candida albicans ortholog of PacC. Eukaryot Cell. 2003;2:718–728. doi: 10.1128/EC.2.4.718-728.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing L, Monnerjahn C, Meyer U. Differential stress gene expression during the development of Neurospora crassa and other fungi. FEMS Microbiol Lett. 1998;168:159–166. doi: 10.1111/j.1574-6968.1998.tb13268.x. [DOI] [PubMed] [Google Scholar]
- Rezaie S, Ban J, Mildner M, Poitschek C, Brna C, Tschachler E. Characterization of a cDNA clone, encoding a 70 kDa heat shock protein from the dermatophyte pathogen Trichophyton rubrum. Gene. 2000;241:27–33. doi: 10.1016/S0378-1119(99)00475-8. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–98. doi: 10.1379/1466-1268(1996)001<0097:DOTHSR>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SA, Rossi A. Effect of phosphate levels on the synthesis of acid phosphatases (EC 3.1.3.2) in Neurospora crassa. Genet Res. 1985;45:239–249. doi: 10.1017/S0016672300022230. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Takemori Y. Interaction between heat shock transcription factors (HSFs) and divergent binding sequences: binding specificities of yeast HSFs and human HSF1. J Biol Chem. 2007;282:13334–13341. doi: 10.1074/jbc.M611801200. [DOI] [PubMed] [Google Scholar]
- Selker EU, Garrett PW. DNA-Sequence Duplications Trigger Gene Inactivation in Neurospora crassa. Proc Natl Acad Sci U S A. 1988;85:6870–6874. doi: 10.1073/pnas.85.18.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EM, Freitas JS, Gras DE, Squina FM, Leal J, Silveira HCS, Martinez-Rossi NM, Rossi A. Identification of genes differentially expressed in a strain of the mold Aspergillus nidulans carrying a loss-of-function mutation in the palA gene. Can J Microbiol. 2008;54:803–811. doi: 10.1139/W08-072. [DOI] [PubMed] [Google Scholar]
- Steel GJ, Fullerton DM, Tyson JR, Stirling CJ. Coordinated activation of Hsp70 chaperones. Science. 2004;303:98–101. doi: 10.1126/science.1092287. [DOI] [PubMed] [Google Scholar]
- Thompson S, Croft NJ, Sotiriou A, Piggins HD, Crosthwaite SK. Neurospora crassa heat shock factor 1 is an essential gene; a second heat shock factor-like gene, hsf2, is required for asexual spore formation. Eukaryot Cell. 2008;7:1573–1581. doi: 10.1128/EC.00427-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburn J, Sarkar S, Widdick DA, Espeso EA, Orejas M, Mungroo J, Peñalva MA, Arst HN., Jr The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 1995;14:779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuriki N, Tawfik DS. Chaperonin overexpression promotes genetic variation and enzyme evolution. Nature. 2009;459:668–673. doi: 10.1038/nature08009. [DOI] [PubMed] [Google Scholar]
- Tremmel D, Duarte M, Videira A, Tropschug M. FKBP22 is part of chaperone/folding catalyst complexes in the endoplasmic reticulum of Neurospora crassa. FEBS Lett. 2007;581:2036–2040. doi: 10.1016/j.febslet.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Turkel S, Turgut T, Kayakent N. Effect of osmotic stress on the derepression of invertase synthesis in nonconventional yeasts. Lett Appl Microbiol. 2006;42:78–82. doi: 10.1111/j.1472-765X.2005.01806.x. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Neupert W, Cyr DM. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994;266:1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- Werner-Washburne M, Becker J, Kosic-Smithers J, Craig EA. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989;171:2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier IJ, Khachatourians GG, Ovsenek N. Constitutive and heat-inducible heat shock element binding activities of heat shock factor in a group of filamentous fungi. Cell Stress Chaperones. 1999;4:211–222. doi: 10.1379/1466-1268(1999)004<0211:CAHIHS>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]