Abstract
Heat shock protein (HSP)70 provides a spectrum of protection against any of a variety of stresses, preventing damage measured at the level of molecules, cells, as well as whole organism. We have previously reported that lipopolysaccharide (LPS)-induced lethality in rats is prevented by a previous exposure to a mild thermal stress and that a thermal stress sufficient to induce HSP70 expression in the liver is accompanied by an inhibition of endotoxin-mediated cytokines and modulation of febrile response. However, the effect of HSP70 upregulation on cytokine expression in animals is unknown. The aim of the present study was to demonstrate the effect of HSP70 overexpression with adenovirus administration on LPS-induced increase in cytokines levels in animals. In the present study, Sprague–Dawley rats were infected with either the control AdTrack or Ad70 virus that directs the expression of human HSP70. After a 5-day incubation, animals were injected with either saline alone or LPS (50 μg/kg). Four hours later, blood samples were drawn and plasma levels of interleukin (IL)-6 or tumor necrosis factor (TNF)-α were measured by enzyme-linked immunosorbent assay. Our data demonstrate for the first time that HSP70 overexpression with adenovirus injection prevented the LPS-induced increase in TNF-α and IL-6 levels in rats. Repression of LPS-induced cytokines expressions by HSP70 upregulation was associated with inhibited IκBα degradation and nuclear factor kappa-B (NF-κB) p65 nuclear translocation in liver, suggesting that HSP70 overexpression may regulate LPS-induced cytokines expression through NF-κB pathway. We conclude that the effects of heat stress-induced increase in HSP70 protein expression on LPS-induced cytokine elaboration in whole animals can be reproduced by the actions of a single gene product.
Keywords: HSP70, LPS, TNF-α, IL-6
Introduction
Exposure of either cells or animals to elevated temperatures for even relatively brief periods of time induces a physiologic adaptation termed tolerance that induces a protection against a subsequent severe stress (Moseley 1994). The ability of cells exposed to a thermal stress to survive the potentially lethal effects of exposure to any number of subsequent insults (heat, radiation, heavy metals, etc.) has been taken as evidence for the global nature of the protective pathways induced by elevated temperatures (Mosser and Martin 1992; Ryan et al. 1992; Hotchkiss et al. 1993; Mailhos et al. 1993; Musch et al. 1996; Chu et al. 1997; Murata et al. 1999).
The expression of members of a superfamily of heat shock proteins (HSPs) are markedly induced after exposure of cells or animals to elevated temperatures (Lu et al. 1993; Kukreja et al. 1994; Wagner et al. 1999). The best characterized of these is HSP70, whose levels increase dramatically within 2 to 4 h after heat (Dokladny et al. 2006b). HSP70 is an ATPase that together with HSP40 is responsible for the maintenance of proper protein structure during stress, as well as the renaturation of damaged proteins during a subsequent recovery period (Craig et al. 1994; Liang and MacRae 1997). An important issue is whether some or all of the tolerant phenotype seen in cells or animals exposed to elevated temperatures are mediated by the actions of HSPs.
The exogenous expression of HSP70 in cells mimics at least some aspect of the tolerant phenotype. Cells overexpressing HSP70 are resistant to multiple apoptotic stimuli, similar to the effects of heat preconditioning (Mosser and Martin 1992; Ryan et al. 1992; Hotchkiss et al. 1993; Mailhos et al. 1993; Musch et al. 1996, 1999; Chu et al. 1997). In addition, repression of HSP70 expression has been shown to increase the sensitivity of both cells and animals to apoptosis. As an example, inhibition of HSP70 expression enhances tumor necrosis factor (TNF)-α-induced cytotoxicity, while overexpression of HSP70 causes cells to become resistant to TNF-α (Gabai et al. 2002; Van Molle et al. 2002). Multicellular physiological functions are also modulated by HSP70 expression. The maintenance of epithelial barrier function in an in vitro model system after thermal shock is enhanced by a previous conditioning heat exposure (Moseley et al. 1994; Dokladny et al. 2006b). We have shown that exogenous expression of HSP70 increases basal levels of transepithelial resistance of MDCK monolayers. In addition, the pattern of the fall and recovery of epithelial integrity is profoundly influenced by elevated expression of HSP70 (Dokladny et al. 2006b).
There is growing evidence that HSPs play a major protective role in whole animals as well. We reported that heat conditioning at levels sufficient to induce HSP70 expression renders animal resistant to a subsequent, otherwise lethal, endotoxin challenge (Ryan et al. 1992). It has previously been shown that HSPs regulate cytokine expression in blood mononuclear cells (Ding et al. 2001; Wischmeyer et al. 2003). In parallel, we found that the lipopolysaccharide (LPS)-induced cytokine response was muted in heat-conditioned animals, and pretreatment with d-galactosamine that blocked the heat-induced accumulation of HSPs in the liver resulted in a significant increase in LPS-induced cytokine plasma concentration (Kluger et al. 1997; Dokladny et al. 2001). Taken together, these data suggest that the endotoxin resistance seen in heat-conditioned animals was mediated to at least some extent by the actions of members of the heat shock family. However, the role of HSP70 in modulation of LPS-induced cytokine expression in animals is not known. We hypothesize that HSP70 overexpression modulates the LPS-induced increase in cytokine level in animals. In order to determine the role of HSP70 on the endotoxin response, we constructed an adenoviral system (Dokladny et al. 2006b) that was used to express exogenous HSP70 in infected animals. Our data demonstrate for the first time that HSP70 overexpression modulated cytokine level in animals in a manner similar to heat conditioning. We also demonstrate that HSP70-mediated modulation of cytokine expression after LPS exposure was associated with inhibited IκBα degradation and nuclear factor kappa-B (NF-κB) p65 nuclear translocation, suggesting that HSP70 may regulate LPS-induced cytokine level through NF-κB pathway.
Materials and methods
Experimental animals Sprague–Dawley rats weighing 200–220 g were obtained from Harlan (Indianapolis, IN, USA) and were housed one per cage in a room maintained at 25°C with a 12:12-h light/dark cycle. Animals were provided ad libitum tap water and laboratory rodent chow (Harlan-Teklad, Madison, WI, USA). All experimental procedures using rats were approved by the University of New Mexico Office of Animal Care and Compliance. All virus and LPS injections were performed at 8:30 a.m., to control for alterations in physiological functions, including body temperature, that occur during the day.
Expression constructs The HSP70 coding region was amplified from pRc/RSV-HSP72 provided by Dr. Anne Knowlton (University of California Davis) using primer sets that were designed to add HindIII and XbaI sites to the 5′ and 3′ ends, respectively, and inserted into the pRc/CMV vector to construct a vector termed RP70. In a similar manner, the coding region was amplified using primers to add NotI and XbaI sites and cloned into pAdTrack (Ad70). Recombination with the adenovirus backbone was performed in BJ5183-AD-1 cells (Stratagene, La Jolla, CA, USA) and the resulting construct was transfected into 293 cells to produce virus directing the expression of exogenous HSP70. Recombination with the parent pAdTrack (AdTrack) plasmid created the construct used as a control in all experiments.
Virus injections Rats were anesthetized with isoflurane (Phoenix Pharmaceuticals, Inc., St. Joseph, MO, UAS) and were injected through the tail vein with either control adenovirus or adenovirus directing the expression of HSP70 (a total dose of 3 × 1011 IFUs per animal in a volume of approximately 0.2 ml). Five days later, animals were injected intraperitoneally with either phosphate buffered saline (PBS) alone or PBS containing LPS (Escherichia coli endotoxin 0111:B4, Sigma, St. Louis, MO, USA) at a dose of 50 μg/kg.
Tissue preparation and Western blot analysis Tissue samples from the liver, kidney, and heart were quickly removed and frozen in liquid nitrogen and then stored at −20°C for subsequent analysis. Samples were homogenized with a TissueRaptor (QIAGEN Instruments AG, Hombrechtikon, Switzerland) for 15 s in lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 500 μM NaF, 2 mM ethylenediaminetetraacetic acid (EDTA), 100 μM vanadate, 100 μM phenylmethylsulfonyl fluoride (PMSF), 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 40 mM paranitrophenyl phosphate, 1 μg/ml aprotinin, and 1% Triton X-100). Tissue lysates were centrifuged to yield a clear lysate. Supernatant was collected, and protein measurement was performed using Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Laemmli gel loading buffer was added to the lysate containing 15–20 μg of protein and boiled for 7 min, after which proteins were separated on a sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins from the gel were transferred to the membrane (Trans-blot transfer medium, nitrocellulose membrane; Bio-Rad Laboratories) overnight. The membrane was incubated for 2 h in blocking solution (5% dry milk in Tris buffered saline (TBS)–Tween 20 buffer) following the incubation with appropriate primary antibodies in blocking solution. HSC70 (#SPA-816), HSP70 (#SPA-810, Clone: C92F3A-5), and heat shock transcription factor 1 (HSF-1) primary antibodies were purchased from Stressgen (Victoria, BC, Canada). NF-κB p65 (Catalog # 51-0500) antibody was purchased from Invitrogen (Carlsbad, CA, USA). IκBα (Catalog#I0505) antibody was purchased from Sigma (St. Louis, MO, USA). After being washed in TBS–Tween buffer, the membrane was incubated in appropriate secondary antibodies. Horseradish peroxidase-conjugated secondary antibodies for Western blot analysis were purchased from Invitrogen (Carlsbad, CA, USA). The membrane was developed using the Santa Cruz Western Blotting Luminol Reagents (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on the Kodak BioMax MS film (Fisher Scientific, Pittsburgh, PA, USA).
Body temperature Core body temperature was measured using a biotelemetry system (Data Science International, St. Paul, MN, USA). Transmitters (TA10TA-F40) were implanted intraperitoneally using aseptic procedures under isoflurane anesthesia and rats were allowed to recover for 7 days. Implantations of the transmitters were performed as previously described (Kluger et al. 1997; Dokladny et al. 2001). All transmitters used in the present study were calibrated and shipped as sterile (ready to use). Transmitters relay signals whose frequencies are proportional to body temperature to receiver boards located under the cage allowing for the measurement at 15 min intervals in undisturbed animals. Temperature measurements were made over an 8-h experimental period after the injection of LPS.
Cytokine measurements Four hours following an injection of LPS, animals underwent isoflurane anesthesia and blood was collected in heparin-coated tubes (BD, Franklin Lakes, NJ, USA). Samples were spun at 2,000 rpm in a clinical centrifuge and stored in small aliquots at −20°C. Supernatants were used for cytokine assays using enzyme-linked immunosorbent assay (ELISA) kits (Quantikine®; Rat TNF-α—catalog # RTA00 and Rat interleukin (IL)-6—catalog # R6000B) purchased from R&D Systems (Minneapolis, MN, USA) and were processed according to the manufacturer’s instructions.
Cytoplasmic and nuclear protein extracts One hour after LPS exposure, animals underwent isoflurane anesthesia and tissue samples from the liver were quickly removed and frozen in liquid nitrogen and then stored at −20°C for subsequent analysis. Nuclear extracts were prepared from liver sections using a method described previously (Li et al. 2006a). Briefly, samples were homogenized with a TissueRaptor (QIAGEN Instruments AG, Hombrechtikon, Switzerland) in 0.8 ml ice-cold buffer A (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.9, 2 mM MgCl2, 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol (DTT), 0.5 mM PMSF). Following 20-min incubation on ice, 50 μl of 10% Nonidet P-40 solution was added; mixture was vortexed for 30 s and centrifuged at 13,000 rpm for 1 min at 4°C (cytoplasmic fraction). The crude nuclear pellet was resuspended in 200 μl ice-cold buffer B (20 mM HEPES, pH 7.9, 25% glycerol (v/v), 0.42 M NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, and 0.5 mM PMSF), incubated on ice for 30 min, and centrifuged at 13,000 rpm for 15 min at 4°C. The supernatant containing nuclear protein extract was collected and stored at −70°C. Protein concentration was performed using a Bio-Rad protein assay kit and Western blot analysis was performed to detect changes in NF-κB p65 translocation into the nuclear fraction and IκBα degradation in cytoplasmic fraction as described above.
Cell assays RAW264 cells (murine macrophages-like cells) and A549 cells (human lung epithelial carcinoma cell line) were obtained from the American Type Tissue Culture Collection (Manassas, VA, USA) and were maintained in Dulbecco’s Modified Eagle Medium (Sigma-Aldrich Inc., St Louis, MO, USA) supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum in a humidified atmosphere containing 5% CO2. Glutamine, penicillin, and streptomycin were purchased from GIBCO-BRL (Grand Island, NY, USA). Fetal calf serum was purchased from Atlanta Biologicals (Lawrenceville, GA, USA). Stock cultures were maintained in 100 mm dishes and were subcultured every 3–4 days. Cultures for all assays were maintained in 60 mm tissue culture plates.For reporter gene assays, cells were transfected with an NF-κB-dependent luciferase construct (Panomics, Inc., Fremont, CA, USA). After 48-h incubation, cells were stimulated with LPS (1 μg/ml) and luciferase levels were measured after further 12-h incubation.For HSF-1 activation determined by Western blot analysis and binding assay, cells were treated with LPS (1 μg/ml) for 1 h or heated to 42°C for 2 h followed by an hour of recovery at 37°C. Cultures were harvested and nuclear extracts were prepared. HSF-1 binding to a heat shock element (HSE) or HSF-1 Western blots were performed essentially as previously described (Dokladny et al. 2006a).For the HSF-1-dependent reporter gene activity, A549 cells (human lung epithelial carcinoma cell line) were transfected with HSF-1-dependent reporter construct. Following a 24-h incubation period, one group of cells was exposed to high temperature (42°C for 2 h) and allowed to recover for 24 h at 37°C. A second group of cells was transfected with HSF-1-dependent reporter gene with a vector expressing the exogenous HSP70. Following a 48-h incubation period, the luciferase activity was determined.
Isolation of mononuclear cells Isolation of mononuclear cells was performed according to the manufacturer’s instruction manual (HISTOPAQUE®-1077, Sigma-Aldrich Inc., St Louis, MO, USA) with minor modifications. Three (males), nonsmoking, healthy subjects (mean ± standard error (SE), age 31 ± 4 years, body weight 77.7 ± 7.2 kg) participated in this study. The host institution’s human subject research committee approved the study. Prior to participation, subjects were informed regarding the possible risks and discomforts involved, and their written consent was obtained. Venous whole blood was collected into sterile syringes (Becton Dickinson & Company, Franklin Lakes, NJ, USA) and immediately transferred into vacutainers (BD Biosciences, Franklin Lakes, NJ, USA) containing sodium heparin. Equal volumes (∼15 ml) of HISTOPAQUE®-1077 and whole blood were added to the 50-ml conical tube followed by centrifugation at 2,100 rpm for 35 min at room temperature. After centrifugation, the plasma layer was aspirated with a Pasteur pipet and discarded. The opaque interface containing mononuclear cells was collected, transferred into a clean conical centrifuge tube, and resuspended with 10 ml of Rapid Prototyping and Manufacturing Institute (RPMI) Medium 1640 (Invitrogen Corp., Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Invitrogen Corp., Grand Island, NY, USA). The mixture was centrifuged at 2,000 rpm for 10 min. After centrifugation, the supernatant was discarded and mononuclear cell pellet was resuspended with RPMI Medium. Three million cells were placed in tissue culture plates (Phoenix Research Products, Hayward, CA, USA) and incubated for 24 h at 37°C. After incubation, mononuclear cells were infected either with control adenovirus or adenovirus directing the expression of HSP70 (a total dose of 10 × 1010 IFUs per plate) for 48 h. After incubation with the virus, cells were exposed to either PBS or LPS (1 μg/ml) for 1 h.Cultures were harvested and nuclear extracts were prepared according to the manufacturer’s instruction manual (Active Motif, Carlsbad, CA, USA). Western blot analysis was performed to detect changes in NF-κB p65 translocation into the nuclear fraction.
Data analysis For the body temperature measurements, the experiment was conducted under a repeated measures design. Twelve rats had their temperature monitored every 15 min for 24 h before the injection and every 15 min for 8 h after the injection. The animals were grouped into four groups: adenovirus (HSP70) treatment (yes or no) and LPS treatment (yes or no). In the first analysis, mixed model was used. In the model, temperature from pre-injection to postinjection at the same time of the day for each of the experimental group was compared to the change in temperature for the AdTrack/Sal group. The covariance parameter estimate 0.656 shows that on average, one temperature is correlated with the next one temperature in time. The high correlation supports the use of this model. The type 3 test of the fixed effects confirms if experimental group is significant overall. The P value 0.0319 indicates that there are significant effects of the experimental group. The least squares means test determines whether the change from pre-injection temperature to postinjection temperature is statistically significant. For two groups AdTrack/LPS and Ad70/LPS, the least squares means test indicated that the change in temperature is statistically significant with P values 0.043 and 0.0056, respectively. The next analysis (differences of least squares means) compared the changes of all groups to the control group (AdTrack/Sal). The Ad70/LPS and AdTrack/LPS groups are significantly different from the control (P < 0.05). Results for cytokines are expressed as means ± SE. Statistical significance of differences between mean values was assessed with Student's t tests for unpaired data. All reported significance levels represent two-tailed P values. A P value of <0.05 was used to indicate statistical significance.
Results
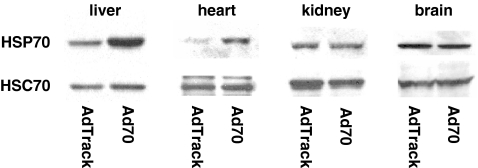
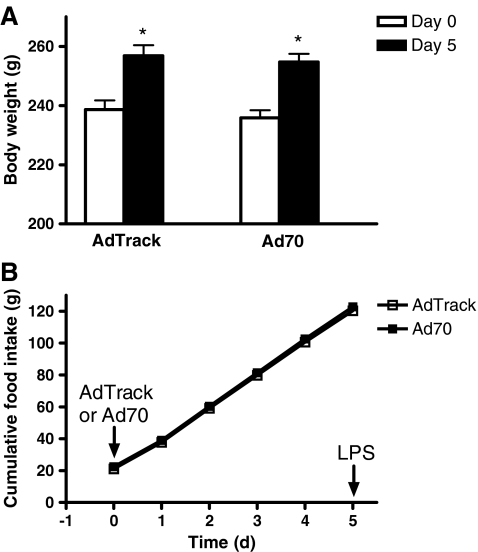
Expression of HSP70 in virus-infected animals Animals were injected with either control adenovirus or adenovirus directing the expression of HSP70. Five days later, they were sacrificed and livers, hearts, and kidneys were dissected and an aliquot was taken for Western blots analysis. HSP70 protein expression after virus infection is shown in Fig. 1. There was a marked induction of HSP70 in the livers and hearts of animals infected with Ad70 compared to animals that received the control adenovirus, while no increase in HSP70 protein expression was observed in kidneys and brains in Ad70-infected animals. Expression of HSC70, the constitutively expressed relative of HSP70, was equivalent in animals injected with either Ad70 or the control AdTrack virus. The relative increase in HSP70 measured after infection with Ad70 is equivalent to what we observed earlier in heat-conditioned animals (Dokladny et al. 2001). It was previously shown that viral and bacterial infections or inflammation trigger physiological and behavioral responses of the host organisms. These responses may include decrease in food intake and body weight loss, lethargy, and increase in body temperature (Conn et al. 1995; Kozak et al. 1995; Elander et al. 2007). In order to ensure that adenovirus infection caused no sickness behavior, animals were injected with either control or HSP70 overexpressing virus and body weight and food intake was monitored 5 days after adenovirus injection. Body weight was measured before (day 0) and 5 days after either control or Ad70 virus injection (day 5) and data are presented in Fig. 2. Between day 0 and day 5, both control virus- and Ad70 virus-injected animals gained weight—18.2 ± 1.4 g (P < 0.01) in control and 18.8 ± 2.3 g (P < 0.01) in HSP70-injected animals. At day 5, there was no difference in body weight of animals injected either with control or Ad70 virus (P = 0.653). Food intake was measured daily after virus administration (Fig. 2b). Food intake was identical in all animals independent of the virus that was injected.
Fig. 1.
Expression of HSP70 in virus-infected animals. Rats were infected with 3 × 1011 IFUs of either the control AdTrack virus or Ad70 that directs the expression of human HSP70. After a 5-day incubation, the animals were sacrificed and samples of liver, heart, kidney, and brain were prepared for Western blot analysis to measure the relative expression of HSP70 and HSC70
Fig. 2.
The effect of AdTrack or Ad70 virus injections on body weight or cumulative food intake. Rats were infected with 3 × 1011 IFUs of either the control AdTrack virus or Ad70 that directs the expression of human HSP70 and body weight (a) and cumulative food intake (b) were measured. Day 0 before virus injection, Day 5 5 days after virus injection and before LPS injection. Data represent means ± SE (n = 6). *P < 0.05 vs. day 0 in each group
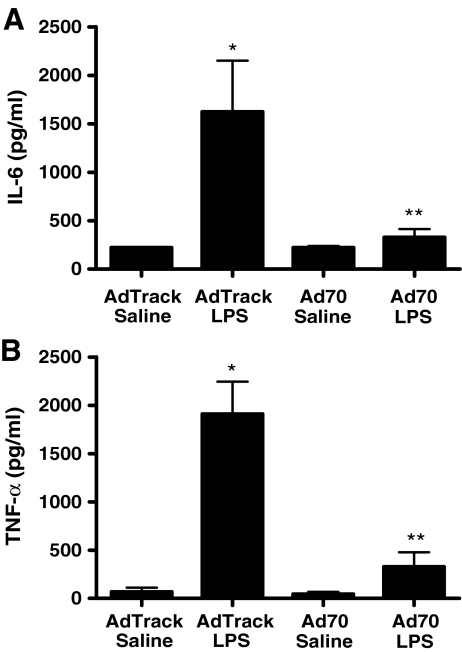
Modulation of cytokine levels after exogenous HSP70 expression The elaboration of IL-6 in LPS-treated animals is shown in Fig. 3a. In control rats, injection of LPS caused approximately a fivefold increase in plasma IL-6 concentrations. While virally mediated HSP70 expression did not alter basal levels of IL-6, the LPS-mediated increase in levels of the cytokine was abolished in animals with elevations in HSP70 expression. Similar results were seen when we measured plasma levels of TNF-α, as shown in Fig. 3b. Injection of LPS in animals infected with the control adenovirus caused over a tenfold increase in plasma levels of TNF-α. Expression of exogenous HSP70 essentially eliminated the ability of the endotoxin to induce cytokine expression.
Fig. 3.
LPS-dependent cytokine induction in virus-infected animals. Rats were infected with 3 × 1011 IFUs of either the control AdTrack virus or Ad70 that directs the expression of human HSP70. After a 5-day incubation, animals were injected with either saline alone or saline containing LPS at a dose of 50 μg/kg. Four hours later, blood samples were drawn and plasma levels of IL-6 (a) or TNF-α (b) were measured by ELISA. a Data represent means ± SE (n = 6). *P < 0.05 vs. AdTrack/saline. **P < 0.05 vs. AdTrack/LPS. b Data represent means ± SE (n = 6). *P < 0.001 vs. AdTrack/saline. **P < 0.01 vs. AdTrack/LPS
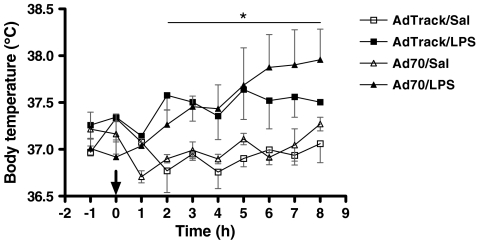
Effects of HSP70 on LPS-induced fever Modulation of the febrile response to LPS by HSP70 expression is shown in Fig. 4. The body temperature of animals that received the control virus and then were treated with LPS was elevated within 2 h of endotoxin administration and remained above control levels for the duration of the experiment. The temperatures of the animals expressing elevated levels of HSP70 that were subsequently injected with LPS were approximately the same as that in LPS-treated control animals for the first 5 h after administration of the endotoxin. Between hours 5 and 8, exogenous expression of HSP70 somewhat augmented the febrile response after LPS treatment, in a manner similar to that seen in heat-conditioned animals exposed to LPS (Dokladny et al. 2001), although the observed trend did not reach the level of significance (P = 0.08).
Fig. 4.
LPS-induced febrile response in virus-infected animals. Seven days before virus infection (3 × 1011 IFUs of either the control AdTrack virus or Ad70 that directs the expression of human HSP70) rats were implanted with transmitters for core body temperature measurements. Five days after virus infection, at time 0 h (arrow), either saline alone or saline containing LPS (final dose of 50 μg/kg) was injected, and core body temperature was monitored over the subsequent 8-h period. Animals were injected with LPS at 8:30 a.m. 12:12-h light/dark cycle with lights on at 7:00 a.m. Data represent means ± SE (total n = 12). *P < 0.05 AdTrack/LPS or Ad70/LPS vs. AdTrack/Sal
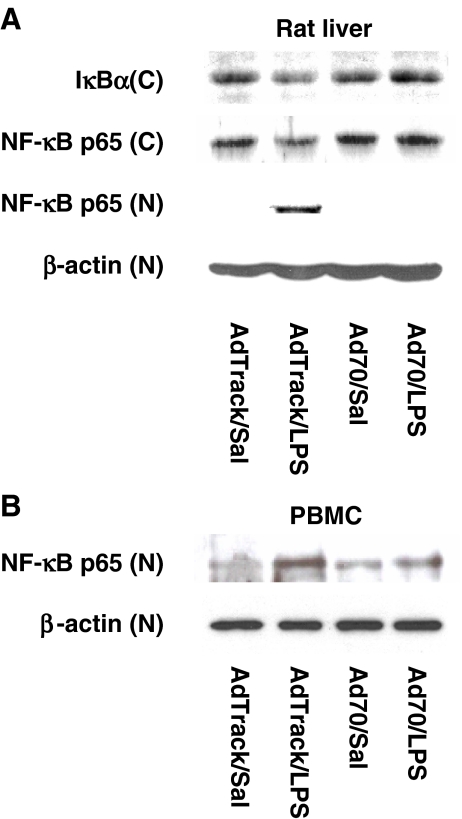
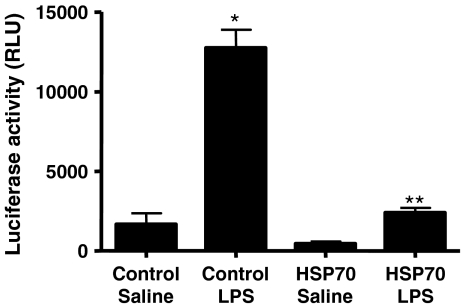
The effect of HSP70 overexpression on LPS-induced NF-κB p65 nuclear translocation The LPS-dependent expression of cytokines in vivo is known to be regulated by an NF-κB-dependent pathway. In multiple systems, HSP70 has been shown to interfere with NF-κB-dependent transcription (Shi et al. 2006). In the following studies, we examined the role of increased expression of HSP70 on LPS-induced activation of NF-κB p65 in both in vivo and ex vivo models in rats and in mononuclear cells from healthy volunteers, respectively (Fig. 5). Animals expressing elevated levels of HSP70 were subsequently injected with LPS or saline for 1 h. Liver samples were collected and NF-κB p65 translocation into the nuclear fraction was determined by Western blot analysis. In control virus-injected animals, exposure to LPS resulted in both NF-κB p65 protein translocation into the nucleus and IκBα degradation in the liver (Fig. 5a). In animals, elevated levels of HSP70 in the liver prevented the LPS-induced increase in IκBα degradation and cytoplasmic-to-nuclear translocation of NF-κB p65, suggesting that HSP70 is involved in regulation of LPS-induced NF-κB protein activation in vivo. Next studies were designed to show the involvement of HSP70 protein in modulating the NF-κB protein translocation to the nucleus in human mononuclear cells. Mononuclear cells obtained from healthy volunteers were collected and incubated at 37°C for 24 h. Then, the cells were exposed to control or HSP70 expressing adenovirus for 48 h. After the exposure, mononuclear cells were treated with LPS (1 μg/ml) for 1 h. Cells were harvested, nuclear fraction was prepared, and NF-κB protein translocation into the nucleus was determined by Western blot analysis. In cells exposed to control adenovirus, NF-κB p65 translocates to the nucleus upon LPS treatment (Fig. 5b). In contrast, adenovirus-directed overexpression of HSP70 prevented the LPS-induced NF-κB p65 nuclear translocation, indicating that HSP70 may be involved in modulation of LPS-induced NF-κB p65 translocation into the nucleus in human mononuclear cells.The experiment shown in Fig. 6 was designed to test whether LPS-induced NF-κB-dependent transcription is modulated by increased expression of HSP70. RAW264 cells, a mouse macrophage cell line, were transfected with an NF-κB-dependent reporter gene either alone or together with a vector directing the expression of exogenous HSP70. Forty-eight hours later, cells were stimulated with LPS for 12 h and luciferase activity was measured in cell extracts. In the absence of HSP70 expression, stimulation with LPS caused a robust enhancement of luciferase activity, reflecting the expected increase in NF-κB-dependent transcription. Co-expression of HSP70 markedly repressed the levels of luciferase expressed in both control and LPS-treated cells. Thus, the ability of HSP70 even in the absence of a thermal stress to repress cytokine production in vivo was paralleled by its ability to repress LPS-stimulated NF-κB transcription in a cell culture model system.
Fig. 5.
The effect of an increased expression of HSP70 on LPS-induced NF-κB activation in in vivo and ex vivo models. a Animals expressing elevated levels of HSP70 were injected with LPS (50 μg/kg) or saline for 1 h. Liver samples were collected and NF-κB p65 activation determined by cytoplasmic-to-nuclear translocation of NF-κB p65 and degradation of IκBα was determined by Western blot analysis. Exposure to HSP70 prevented the LPS-induced NF-κB p65 nuclear translocation and IκBα degradation. C cytoplasmic fraction, N nuclear fraction. b Mononuclear cells (PBMC) from healthy volunteers were collected and allowed to incubate at 37°C for 24 h. Then, cells were exposed to control or HSP70 expressing adenovirus at a total dose of 10 × 1010 IFUs per plate for 48 h. After the exposure, mononuclear cells were treated with LPS (1 μg/ml) for 1 h. Cells were harvested, nuclear fraction was prepared, and NF-κB protein levels were determined by Western blot analysis. Exposure to HSP70 prevented the LPS-induced increase in NF-κB p65 nuclear translocation in mononuclear cells. N nuclear fraction
Fig. 6.
Repression of NF-κB-dependent reporter gene activity by HSP70. RAW264 cells were transfected with an NF-κB-dependent reporter construct either alone or together with an HSP70 expression vector. After a 48-h incubation period, cells were stimulated with LPS (1 μg/ml) for 12 h, and luciferase activity was measured in cell extracts. Data represent means ± SE (n = 4). *P < 0.001 vs. control/saline. **P < 0.001 vs. control/LPS
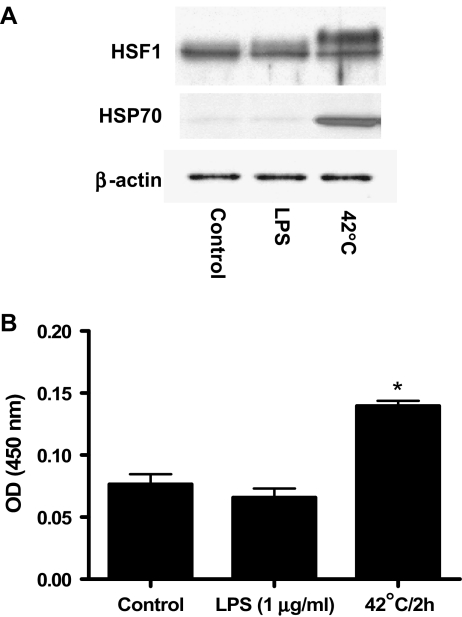
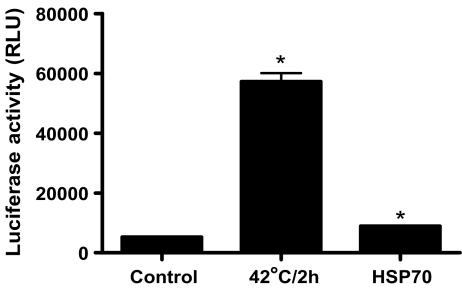
The effect of LPS exposure or heat stress on HSF1 activation and HSP70 protein expression The experiment in Fig. 7 was designed to rule out any complicating regulation of HSF-1 activity by LPS in the effects of the endotoxin on cytokine elaboration. RAW264 cells were maintained under control conditions, treated for 1 h with LPS, or were heated for 2 h at 42°C and then incubated at 37°C for 1 h. HSF-1 activation was determined either by Western blot analysis (Fig. 7a) or DNA binding assay (Fig. 7b). Only heat exposure resulted in significant HSP70 protein expression in RAW264 cells (Fig. 7a). Heat stress caused an electrophoretic shift in HSF-1 reflecting an increase in the activating phosphorylation (Fig. 7a) and increased the amount of HSF-1 that bound to the HSP70 heat shock element by approximately twofold (Fig. 7b). Neither an increase in DNA binding activity nor a shift in HSF-1 mobility was seen in extracts prepared from cells treated with LPS, indicating that the endotoxin did not increase HSF-1-dependent transcription. HSF-1 is the main regulator of heat shock response in eukaryotic cells. Under diverse environmental and physiological stress, HSF1 trimerizes and translocates to the nucleus (Sarge et al. 1993), where it rapidly activates a transcription of a large number of genes involved in regulation of protein homeostasis including HSP70. In order to rule out the possibility that HSP70 activates HSF1, the effect of HSP70 and heat exposure (positive control) on HSF-1-dependent transcription was examined. A549 cells (human lung epithelial carcinoma cell line) were transfected with an HSF-1-dependent reporter construct. Following a 24-h incubation, one group of cells was exposed to heat stress (42°C for 2 h) and allowed to recover for 24 h at 37°C. HSF-1-dependent luciferase activity was then measured. A second group of cells was transfected with HSF-1-dependent reporter gene in the presence of a vector expressing the exogenous HSP70. Following a 48-h incubation period, the luciferase activity was determined. Treatment with heat resulted in a tenfold increase in luciferase activity (Fig. 8), indicating the expected increase in HSF-1-dependent transcription. Cotransfection with HSP70 caused a very small although significant increase in HSF-1-dependent transcription. These data suggest that HSP70 regulates the LPS-induced increase in cytokine expression without a corresponding increase in HSF-1-dependent transcriptional activity.
Fig. 7.
Effects of LPS on HSF-1 activation. RAW264 cells were either exposed to LPS (1 μg/ml for 1 h) or to 42°C for 2 h followed by 1-h recovery period at 37°C. Heat exposure, but not LPS treatment, resulted in a significant increase in HSP70 protein expression and caused an electrophoretic shift in HSF-1 reflecting an increase in the activating phosphorylation determined by Western blot analysis (a). B-actin served as an internal control. Heat stress exposure increased the amount of HSF-1 that bound to the HSP70 heat shock element (b). b Data represent means ± SE (n = 4). *P < 0.001 vs. control
Fig. 8.
The effect of heat exposure or HSP70 on HSF-1-dependent reporter gene activity. A549 cells (human lung epithelial carcinoma cell line) were transfected with an HSF-1-dependent reporter construct. Following a 24-h incubation, one group of cells was exposed to high temperature (42°C for 2 h) and allowed to recover for 24 h at 37°C. HSF-1-dependent luciferase activity was then measured. A second group of cells was transfected with HSF-1-dependent reporter gene in the presence of a vector expressing the exogenous HSP70. Following a 48-h incubation period, the luciferase activity was determined. Data represent means ± SE (n = 3). *P < 0.001 vs. control
Discussion
Heat shock response manifested by an increased expression of HSPs represents a highly conserved response of protection and recovery from stress-induced molecular damage associated with unfolded, misfolded, or aggregated proteins. Expression of HSP70 after a moderate stress protects cells and organisms from the potentially lethal effects of a more severe subsequent stress (Mosser and Martin 1992; Mailhos et al. 1993; Musch et al. 1996). Our previous research indicated that a conditioning heat stress sufficient to cause the accumulation of inducible HSP70 protected animals against an otherwise lethal challenge with endotoxin (Ryan et al. 1992). The prevention of LPS-induced mortality was accompanied by a significant suppression in the circulating concentration of TNF-α and enhanced febrile response induced by endotoxin in heat stress conditioned animals (Kluger et al. 1997). In the present study, we demonstrate for the first time that expression of HSP70 alone significantly attenuates endotoxin-mediated serum inflammatory cytokine accumulation and that this attenuation is linked to an inhibition of NF-κB p65 nuclear translocation in liver. These studies demonstrate that in the intact organism, HSP70 expression in the absence of other conditioning stimuli can have a profound effect on circulating cytokine levels and that this effect is linked to effects of NF-κB and independent of HSF-1 activation.
Our previous data have shown an increase in LPS-induced fever in heat-conditioned animals, suggesting that HSP70 may play an important role in modulating febrile response to LPS (Kluger et al. 1997; Dokladny et al. 2001). In the present study, LPS-induced febrile response in HSP70 administrated animals tended to be higher compared to the control virus injected animals, but the difference did not reach statistical significance. Inhibition of liver HSP70 protein synthesis during the conditioning heat stress using d-galactosamine relieved the repression of cytokine elaboration in response to LPS and resulted in a profound fall in core temperature, indicting the important role of hepatic function in modulating a pathophysiological response to LPS (Dokladny et al. 2001). Although the role of specific cell types was not studied, in our previous model, we speculated that Kupffer cells responding to heat stress-induced HSP70 release attenuated cytokine expression in the response to LPS exposure (Kluger et al. 1997). The results from the present study suggest that HSP70 mediated inhibition of cytokine expression after LPS exposure may occur through inhibition of NF-κB pathway. Hepatic macrophages (Kupffer cells) and alveolar macrophages were shown to be the primary cells involved in effective detoxification of LPS (Mori et al. 1973; Freudenberg et al. 1982). In plasma, LPS is recognized by LPS-binding proteins produced in the liver (Schumann et al. 1990). LPS-binding proteins bring LPS to a receptor CD14 (Wright et al. 1990), thus allowing LPS to be loaded on the LPS receptor complex composed of dimerized Toll-like receptor-4 (TLR4) and MD-2 (Hailman et al. 1994; Tobias et al. 1995). In recent studies, it was shown that LPS-induced fever is triggered via Toll-like receptor 4 on hematopoietic cells (Steiner et al. 2006a), suggesting that hepatic and pulmonary macrophages play a critical role in triggering febrile response. Cyclooxygenase-2 has been documented to play a critical role in triggering fever via enhanced production of prostaglandins (Cao et al. 1997; Li et al. 2001, 2003, 2006b; Ivanov and Romanovsky 2004) that are synthesized by hepatic and pulmonary macrophages (Steiner et al. 2006b). These data indicated that macrophages in the liver and lung recognizing LPS via TLR4 and producing PGE2 through COX-2 pathway played a crucial role in initiating of the fever response (Romanovsky et al. 2006).
Our previous studies have shown that LPS-induced increased plasma level of TNF-α and IL-6 was inhibited by heat conditioning (Kluger et al. 1997; Dokladny et al. 2001). In the present study, we show for the first time that the LPS-induced increase in pro-inflammatory cytokines was inhibited by expression of HSP70 in vivo, suggesting that the heat stress-induced inhibition of TNF-α was mediated solely by the actions of HSP70 in the absence of HSF-1 activation. Similarly, glutamine-induced increase in heat shock protein prevented LPS-induced increase in inflammatory cytokine release and protected against vascular hyporeactivity (Jing et al. 2007). Consistent with our in vivo data, LPS-stimulated increase in TNF-α and IL-6 was prevented by HSP70 overexpression in cultured macrophages (Snyder et al. 1992; Hagiwara et al. 2007). Additionally, sodium arsenite-induced increase in heat shock proteins (HSP70) in mouse Kupffer cells significantly inhibited LPS-induced cytokine TNF-α and IL-6 production and mRNA expression by reduced NF-κB activation and IκBα degradation (Sun et al. 2005). In previous studies, it has been shown that in human peripheral blood monocyte-derived macrophages, overexpression of HSP70 significantly inhibited LPS-induced increases in TNF-α, IL-1, IL-10, and IL-12, although the synthesis of IL-6 was not inhibited by overexpression of HSP70 (Ding et al. 2001). Similarly, in a macrophage cell line, the LPS-mediated increase in protein and mRNA expression of the pro-inflammatory cytokines TNF-α and IL-1 was inhibited by HSP70 overexpression induced either by a heat shock or by forced expression of the HSP70 gene.
NF-κB is an essential transcription factor that regulates transcription of many genes involved in immune function, inflammation, control of cell growth, and apoptosis (Ghosh et al. 1998; Aggarwal 2004; Hayden and Ghosh 2004; Karin and Greten 2005). In the present study, we show for the first time that HSP70 inhibits LPS-induced NF-κB p65 nuclear translocation and IκBα degradation in vivo, although the cellular and molecular mechanisms by which HSP70 modulates NF-κB activation in animals require future research. Consistent with our in vivo data, heat shock response associated with upregulation of HSP70 protein expression or HSP70 overexpression by gene transfection resulted in an inactivation of NF-κB pathways by inhibiting of NF-κB p65 nuclear translocation and preventing degradation of IκBα in mouse macrophages (Shi et al. 2006). IκB kinase causes the rapid phosphorylation of IκB leading to its subsequent ubiquitination (Muzio et al. 1998; Senftleben and Karin 2002). Pretreatment with heat stress inhibited IκB kinase catalytic activity and subsequent degradation of IκB in irradiated HeLa cells (Curry et al. 1999) and in normal bronchial epithelial cells and type II alveolar epithelial cells (Yoo et al. 2000) preventing IκBα phosphorylation (Shanley et al. 2000). Physical association of HSP70 with a crucial signaling transducer for activating NF-κB pathway tumor necrosis factor receptor-associated factor 6 (TRAF6) was shown as a novel model in preventing TRAF6 ubiquitination and subsequent NF-κB activation in murine cells. Although the effect of HSP70 on LPS-induced NF-κB activation was studied in several in vitro and ex vivo models, its physiological effect in in vivo system still remains unknown. Further studies are needed to delineate the molecular interactions between HSP70 and NF-κB in modulating febrile response and broad spectrum of cytokines in animals. This knowledge may help us understand the regulatory role of HSP70 in inflammatory and infectious diseases.
In conclusion, our data demonstrate for the first time that an overexpression of HSP70 in animals is sufficient to inhibit LPS-induced increases in cytokine expression and modulate the febrile response. Our studies also suggest that HSP70 may modulate the cytokine expression in animals through inhibition of NF-κB pathway.
Acknowledgment
This work was supported by National Institutes of Health Grant AR40771 the NIEHS Center Grant P30-ES012072. The authors thank Dr. N. Kanagy for the use of the telemetry equipment.
References
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Cao C, Matsumura K, et al. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol. 1997;272(6 Pt 2):R1712–R1725. doi: 10.1152/ajpregu.1997.272.6.R1712. [DOI] [PubMed] [Google Scholar]
- Chu EK, Ribeiro SP, et al. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25(10):1727–1732. doi: 10.1097/00003246-199710000-00025. [DOI] [PubMed] [Google Scholar]
- Conn CA, McClellan JL, et al. Cytokines and the acute phase response to influenza virus in mice. Am J Physiol. 1995;268(1 Pt 2):R78–R84. doi: 10.1152/ajpregu.1995.268.1.R78. [DOI] [PubMed] [Google Scholar]
- Craig EA, Weissman JS, et al. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78(3):365–372. doi: 10.1016/0092-8674(94)90416-2. [DOI] [PubMed] [Google Scholar]
- Curry HA, Clemens RA, et al. Heat shock inhibits radiation-induced activation of NF-kappaB via inhibition of I-kappaB kinase. J Biol Chem. 1999;274(33):23061–23067. doi: 10.1074/jbc.274.33.23061. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Fernandez-Prada CM, et al. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16(6):210–219. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Kozak A, et al. Effect of heat stress on LPS-induced febrile response in D-galactosamine-sensitized rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R338–R344. doi: 10.1152/ajpregu.2001.280.2.R338. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Moseley PL, et al. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G204–G212. doi: 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Wharton W, et al. Induction of physiological thermotolerance in MDCK monolayers: contribution of heat shock protein 70. Cell Stress Chaperones. 2006;11(3):268–275. doi: 10.1379/CSC-194R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elander L, Engstrom L, et al. IL-1beta and LPS induce anorexia by distinct mechanisms differentially dependent on microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R258–R267. doi: 10.1152/ajpregu.00511.2006. [DOI] [PubMed] [Google Scholar]
- Freudenberg MA, Freudenberg N, et al. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982;63(1):56–65. [PMC free article] [PubMed] [Google Scholar]
- Gabai VL, Mabuchi K, et al. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22(10):3415–3424. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, et al. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, et al. Changes in cell culture temperature alter release of inflammatory mediators in murine macrophagic RAW264.7 cells. Inflamm Res. 2007;56(7):297–303. doi: 10.1007/s00011-007-6161-z. [DOI] [PubMed] [Google Scholar]
- Hailman E, Lichenstein HS, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179(1):269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R, Nunnally I, et al. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265(6 Pt 2):R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- Jing L, Wu Q, et al. Glutamine induces heat-shock protein and protects against Escherichia coli lipopolysaccharide-induced vascular hyporeactivity in rats. Crit Care. 2007;11(2):R34. doi: 10.1186/cc5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Rudolph K, et al. Effect of heat stress on LPS-induced fever and tumor necrosis factor. Am J Physiol. 1997;273(3 Pt 2):R858–R863. doi: 10.1152/ajpregu.1997.273.3.R858. [DOI] [PubMed] [Google Scholar]
- Kozak W, Zheng H, et al. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am J Physiol. 1995;269(5 Pt 2):R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Kontos MC, et al. Oxidant stress increases heat shock protein 70 mRNA in isolated perfused rat heart. Am J Physiol. 1994;267(6 Pt 2):H2213–H2219. doi: 10.1152/ajpheart.1994.267.6.H2213. [DOI] [PubMed] [Google Scholar]
- Li S, Ballou LR, et al. Cyclooxygenase-2 mediates the febrile response of mice to interleukin-1beta. Brain Res. 2001;910(1–2):163–173. doi: 10.1016/S0006-8993(01)02707-X. [DOI] [PubMed] [Google Scholar]
- Li S, Goorha S, et al. Intracerebroventricular interleukin-6, macrophage inflammatory protein-1 beta and IL-18: pyrogenic and PGE(2)-mediated? Brain Res. 2003;992(1):76–84. doi: 10.1016/j.brainres.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Li G, Qi XP, et al. Verapamil modulates LPS-induced cytokine production via inhibition of NF-kappa B activation in the liver. Inflamm Res. 2006;55(3):108–113. doi: 10.1007/s00011-005-0060-y. [DOI] [PubMed] [Google Scholar]
- Li Z, Perlik V, et al. Kupffer cell-generated PGE2 triggers the febrile response of guinea pigs to intravenously injected LPS. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1262–R1270. doi: 10.1152/ajpregu.00724.2005. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110(Pt 13):1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Lu D, Maulik N, et al. Molecular adaptation of vascular endothelial cells to oxidative stress. Am J Physiol. 1993;264(3 Pt 1):C715–C722. doi: 10.1152/ajpcell.1993.264.3.C715. [DOI] [PubMed] [Google Scholar]
- Mailhos C, Howard MK, et al. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience. 1993;55(3):621–627. doi: 10.1016/0306-4522(93)90428-I. [DOI] [PubMed] [Google Scholar]
- Mori K, Matsumoto K, et al. On the in vivo clearance and detoxification of endotoxin by lung and liver. Ann Surg. 1973;177(2):159–163. doi: 10.1097/00000658-197302000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley PL. Mechanisms of heat adaptation: thermotolerance and acclimatization. J Lab Clin Med. 1994;123(1):48–52. [PubMed] [Google Scholar]
- Moseley PL, Gapen C, et al. Thermal stress induces epithelial permeability. Am J Physiol. 1994;267(2 Pt 1):C425–C434. doi: 10.1152/ajpcell.1994.267.2.C425. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Martin LH. Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol. 1992;151(3):561–570. doi: 10.1002/jcp.1041510316. [DOI] [PubMed] [Google Scholar]
- Murata M, Gong P, et al. Differential metal response and regulation of human heavy metal-inducible genes. J Cell Physiol. 1999;180(1):105–113. doi: 10.1002/(SICI)1097-4652(199907)180:1<105::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Musch MW, Ciancio MJ, et al. Induction of heat shock protein 70 protects intestinal epithelial IEC-18 cells from oxidant and thermal injury. Am J Physiol. 1996;270(2 Pt 1):C429–C436. doi: 10.1152/ajpcell.1996.270.2.C429. [DOI] [PubMed] [Google Scholar]
- Musch MW, Sugi K, et al. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology. 1999;117(1):115–122. doi: 10.1016/S0016-5085(99)70557-3. [DOI] [PubMed] [Google Scholar]
- Muzio M, Natoli G, et al. The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187(12):2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Steiner AA, et al. Cells that trigger fever. Cell Cycle. 2006;5(19):2195–2197. doi: 10.4161/cc.5.19.3321. [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Flanagan SW, et al. Acute heat stress protects rats against endotoxin shock. J Appl Physiol. 1992;73(4):1517–1522. doi: 10.1152/jappl.1992.73.4.1517. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, et al. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13(3):1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann RR, Leong SR, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Karin M. The IKK/NF-kappaB pathway. Crit Care Med. 2002;30(1 Supp):S18–S26. doi: 10.1097/00003246-200201001-00003. [DOI] [PubMed] [Google Scholar]
- Shanley TP, Ryan MA, et al. Heat shock inhibits phosphorylation of I-kappaBalpha. Shock. 2000;14(4):447–450. doi: 10.1097/00024382-200014040-00005. [DOI] [PubMed] [Google Scholar]
- Shi Y, Tu Z, et al. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock. 2006;26(3):277–284. doi: 10.1097/01.shk.0000223134.17877.ad. [DOI] [PubMed] [Google Scholar]
- Snyder YM, Guthrie L, et al. Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. J Leukoc Biol. 1992;51(2):181–187. doi: 10.1002/jlb.51.2.181. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Chakravarty S, et al. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 2006;107(10):4000–4002. doi: 10.1182/blood-2005-11-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Ivanov AI, et al. Cellular and molecular bases of the initiation of fever. PLoS Biol. 2006;4(9):e284. doi: 10.1371/journal.pbio.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Chen D, et al. Heat shock response inhibits NF-kappaB activation and cytokine production in murine Kupffer cells. J Surg Res. 2005;129(1):114–121. doi: 10.1016/j.jss.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Tobias PS, Soldau K, et al. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270(18):10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- Molle W, Wielockx B, et al. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16(5):685–695. doi: 10.1016/S1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- Wagner M, Hermanns I, et al. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol. 1999;277(5 Pt 1):L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- Wischmeyer PE, Riehm J, et al. Glutamine attenuates tumor necrosis factor-alpha release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition. 2003;19(1):1–6. doi: 10.1016/S0899-9007(02)00839-0. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yoo CG, Lee S, et al. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164(10):5416–5423. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]