Abstract
Many findings indicate that measuring the serum concentration of soluble 70-kD heat shock protein (soluble HSP70) may provide important information in cardiovascular, inflammatory, and pregnancy-related diseases; however, only scarce data are available in cancer. Therefore, using a commercial ELISA kit, we measured soluble HSP70 concentration in the sera of 179 patients with colorectal cancer. We investigated the relationship between soluble HSP70 concentration and mortality, during 33.0 (24.4–44.0) months long follow-up. High (>1.65 pg/ml, median concentration) soluble HSP70 level was a significant (hazard ratio: 1.88 (1.20–2.96, p = 0.005) predictor of mortality during the follow-up period. When we compared the soluble HSP70 levels in patients with non-resected primary tumors as compared to those who were recruited into the study 4–6 weeks after the tumor resection they were found to be significantly (p = 0.020) higher in the former group. Since the patients with non-resected primary tumors had also distant metastasis and died early, we limited the further analysis to 142 patients with no distant metastasis at the beginning of the follow-up. This association remained significant even after multiple Cox-regression analysis had been performed to adjust the data for age and sex (p = 0.028); age, sex, and TNM-T stage (p = 0.041); age, sex, and TNM-N stage (p = 0.021); age, sex, and histological grade (p = 0.023); or age, sex, and tumor localization (p = 0.029). Further analysis showed that the significant association between high HSP70 levels and poor survival is in the strongest in the group of <70-year-old female patients (HR: 5.52 (2.02-15.15), p = 0.001), as well as in those who were in a less advanced stage of the disease at baseline. These novel findings indicate that the serum level of soluble HSP70 might prove a useful, stage-independent prognostic marker in colorectal cancer without distant metastasis.
Keywords: Colorectal cancer, Mortality, Heat shock proteins, HSP70, Predictive marker
Introduction
Heat shock proteins (HSPs) are intracellular, evolutionary conserved proteins with a most important role in maintaining homeostasis of the cells by holding and folding other proteins as well as by protecting the genetic information. Heat shock proteins are usually divided into families according to their molecular weight; currently, 10 kD (HSP10) 27 kD (HSP27), 40 kD (HSP40), 60 kD (HSP60), 70 kD (HSP70), 90 kD (HSP90), and 110 kD (HJSP110) heat shock protein families are known. All these HSPs—HSP27, HSP70, and HSP60—primarily play essential, but diverse roles in tumorigenesis and metastasis formation, by promoting autonomous cell proliferation and inhibiting programmed cell death (Calderwood et al. 2006). Due to the loss of p53 function and the greater expression of the proto-oncogens HER2 and c-Myc, the transcription of heat shock proteins is increased in certain tumor cells (Calderwood et al. 2006).
Although HSPs are intracellular, they can be released from the cells and become detectable in the blood of healthy individuals as soluble HSP (Pockley et al. 1998, 1999). Since the serum levels of soluble HSPs can markedly decrease or increase in different diseases, measurement of HSP concentration may provide clinically important information. Most studies focused on soluble HSP70, the concentration of which can be reliably measured using commercial kits. Measurement of soluble HSP70 was found useful for studying the pathomechanism of cardiovascular disease, and since its level was found to be decreased in patients with coronary artery disease, this HSP is considered cardio-protective (Pockley et al. 2003; Zhu et al. 2003). Accordingly, low HSP70 levels are associated with greater longevity (Terry et al. 2006). On the other hand, infection, inflammation, and necrosis are usually associated with elevated levels of soluble HSP70 (Njemini et al. 2003; Dybdahl et al. 2005; Satoh et al. 2006). Recently, our group reported on the importance of measuring sHSP70 level in pregnancy and pregnancy-related diseases (Molvarec et al. 2006, 2007; Madach et al. 2008).
Abundance of data indicate that the HSP70 content of different cancers is a useful marker of the course of the disease and may predict progression (reviewed in Ciocca and Calderwood (2005)). On the other hand, there are only scarce data on the measurement of soluble HSP70 in cancer. In a prospective study performed in Japan, baseline serum concentration of soluble HSP70 was associated with an increased risk of lung cancer (Suzuki et al. 2006). Plasma level of soluble HSP70 was found to be a potential biomarker for prostate cancer, although its clinical utility is uncertain (Abe et al. 2004). In the present work, we measured serum levels of soluble HSP70 in the blood of patients with colorectal cancer. There are many data indicating that HSP70 expression is high in colorectal tumors and it is related to progression of the disease (Hwang et al. 2003; Dundas et al. 2005; Rau et al. 1999). Moreover, the expression of HSP70 on the membrane of colorectal tumor cells is associated with different routes of metastasis and it is a predictor of patient survival (Pfister et al. 2007). To our best knowledge, however, no data are available on the levels of soluble HSP70 in colorectal cancer and therefore, we decided to investigate this in our study. Clinical follow-up of 179 patients for a median period of 33 months was undertaken along with the recording of clinical events. The relationship between soluble HSP70 levels and patient survival was analyzed. High-baseline concentration of soluble HSP70 in the serum was found to be a useful clinical marker predicting low chance of survival independently of sex and age, as well as most importantly, of clinical predictive markers, such as TNM stages.
Materials and methods
Patients
This study was performed in the outpatient oncology clinic of the Third Department of Internal Medicine, Semmelweis University, between October 2000 and March 2005. One-hundred and seventy nine consecutive patients, diagnosed with colorectal cancer, willing to give informed consent for the study, were enrolled independently of the stage of their tumor. In the majority of cases, the primary tumor was removed surgically according to relevant international guidelines; patients were enrolled 4–6 weeks after surgery. In 16 cases, the primary tumor could not be removed before enrollment, these patients had advanced, metastatic tumor and were referred for primary chemotherapy. All but 31 patients received adjuvant or first-line, 5-FU-based chemotherapy according to current national guidelines. Those who did not receive chemotherapy had very early stage tumor (Dukes A, B1 n = 23) or had refused treatment (n = 8). The patients were prospectively followed for 33.0 (24.4–44.0) months (yielding an acceptable drop-out rate of 8/187 = 4.3%). Reasons for leaving the study included moving to another location or were unknown. Baseline clinical characteristics of the patients are summarized in Table 1.
Table 1.
Demographic and clinical characteristic of patients with colorectal cancer
| Number of patients | 179 |
| Males/females | 101/78 |
| Age at baseline, years (mean ± S.D.) | 65.6 ± 10.1 |
| TNM-T stage, 0 /1/2/3/4/not available | 1/4/35/104/19/16 |
| TNM-N stage, 0/1/2/not available | 71/68/19/21 |
| TNM-M stage, 0/1/not available | 135/33/11 |
| Grade 1/2/3/not available | 17/86/36/40 |
| Tumor resection yes/no | 16/163 |
| Localization of the primary tumor | |
| Colon | 107 |
| Sigmoid | 11 |
| Rectum | 49 |
| Colon + rectum | 3 |
| Sigmoid + rectum | 1 |
| Colorectal + other tumor | 8 |
Staging of the patients was done according to the system described in the paper of Greene (Greene 2007). Briefly, TNM-T stage 0: no evidence of primary tumor, stage 1: tumor invades submucosa, stage 2: tumor invades muscularis propria, stage 3: tumor invades through the muscularis propria into the subserosa, or into non-peritonealized pericolic or perirectal tissues, stage 4: tumor directly invades other organs or structures, and/or perforates visceral peritoneum. TNM-N stage 0: no regional lymph node metastasis, stage 1: metastasis in one to three regional lymph nodes, stage 2: metastasis in four or more regional lymph node. TNM-M stage 0: no distant metastasis, stage 1: distant metastasis
Laboratory methods
Soluble HSP70 levels in the serum were measured using the ELISA kit of R&D Systems (DYC163E, Minneapolis, MN, USA), according to the manufacturer’s instructions as described in detail earlier (Molvarec et al. 2006). The detection range of the assay was 0.05 to 10 ng/ml; intra-/inter-assay variability was <10%/<16%, respectively. Serum concentration of the IgG type antibodies against HSP70 were determined as described in Molvarec et al. (2009).
Statistical analysis
The non-parametric Mann–Whitney test was used for group comparisons. Categorical data were compared using Fisher’s exact test or the χ2 test for trend. Multiple logistic regression and Cox hazard models were used to evaluate potential confounders and to correct the p values of univariate analyses. The power of our study to observe a 0.7 ng/ml difference in mean HSP70 levels (controls versus patients, Fig. 1) with a type I error of alpha = 0.05 was >0.99; whereas it was 0.78 for the difference between survivors and non-survivors (a difference of 0.52 ng/ml).
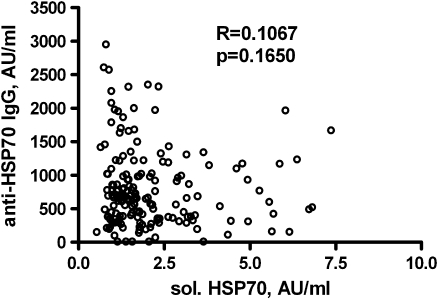
Fig. 1.
Relationship between the levels of soluble HSP70 and the IgG type anti-HSP70 antibodies in the sera of 179 patients with colorectal cancer
All the tests were two-tailed. Statistical analysis was performed using the GraphPad Prism 3.0 (GraphPad Software Inc, San Diego, CA, www.graphpad.com) and SPSS 13.0 (SPSS Inc., Chicago, IL) software.
Results
Serum soluble HSP70 concentration in patients with colorectal cancer according to stage of the disease and grade of cancer
We studied whether baseline serum concentration of soluble HSP70 is dependent on the stage of the disease or on the grade of cancer (Table 2). No significant differences were found between patients in different stages of the disease. Patients with high histological tumor grade had higher soluble HSP70 levels, than those with low-grade tumors, but the difference was only marginally significant. There was no significant correlation between the serum levels of soluble HSP70 and IgG anti-HSP70 antibodies (Fig. 1).
Table 2.
Soluble HSP70 levels in the sera of 142 patients with colorectal cancer without distant metastasis, according to stages of the disease or grade of cancer
| Stage or grade | Number of patients | Soluble HSP70, ng/ml median (25th–75th percentile) | p values Kruskal–Wallis test |
|---|---|---|---|
| TNM-T | 37 | ||
| 0, 1 or 2 | 92 | 1.68 (1.32-2.17) | |
| 3 | 9 | 1.60 (1.14-2.36) | 0.540 |
| 4 | 4 | 1.57 (0.99-2.74) | |
| Not available | |||
| TNM-N | |||
| 0 | 68 | 1.60 (1.18-2.15) | |
| 1 | 54 | 1.73 (1.24-2.71) | 0.581 |
| 2 | 14 | 1.55 (1.21-2.14) | |
| Not available | 6 | ||
| Grade of cancer | |||
| Low (1 or 2) | 90 | 1.58 (1.1.7) | |
| High (3) | 22 | 1.94 (1.44) | 0.078a |
| Not available | 30 |
aMann–Whitney test
As it was mentioned above, the primary tumor could not removed before enrollment in 16 cases. We compared soluble HSP70 levels in these patients to those with resected tumors. The median soluble HSP70 level (IQ range) of the patients was significantly (p = 0.02) higher in the first (2.34 (1.91–4.51) AU/ml) than in the second group (1.64 (1.18–2.25) AU/ml.
Survival of patients with low and high soluble HSP70 levels
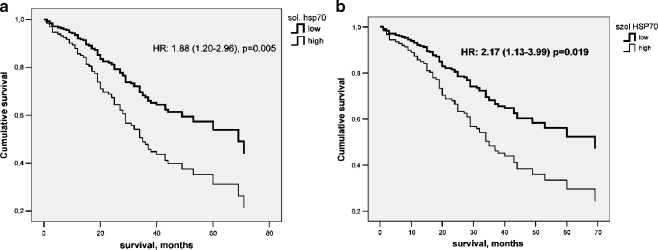
Next, we compared soluble HSP70 levels of the 95 patients who survived and of the 84 patients who did not survive the follow-up period. Median (25th–75th percentile) soluble HSP70 levels were: 1.51 (1.16–2.17) ng/ml and 1.84 (1.22–3.16) ng/ml, respectively (p = 0.014, Mann–Whitney test). Next, the 179 patients were divided into two groups, according to median serum HSP70 level (1.65 ng/ml). The low HSP70 group had serum soluble HSP70 concentration below, whereas the high HSP70 group had levels equal to or higher than the median. Then, we compared the survival of the patients in the two groups during the 33.0 (24.0–44.0) months long observation period (Fig. 2a).
Fig. 2.
Survival of colorectal cancer patients with low (<median 1.65 ng/l) or high (≥1.65 ng/ml) soluble HSP70 levels over a 33.0 (24.0–44.0) months long follow-up period. Hazard ratio (HR) with 95% CI for Cox-regression analysis is indicated. a All 178 patients, b 142 patients without distant metastasis
Patients in the high HSP70 group had a significantly—almost twice—higher chance to die during the follow-up period, as compared to patients in the low HSP70 group (Fig. 2a).
As it was described above, the original group of the 178 patients was heterogeneous: 16 patients had tumors at the onset of the study while tumors were resected in the rest of the patients 4–6 weeks earlier. The reason of non-resection of the tumor was that these 16 patients had very advanced metastatic cancer. Considering this fact, we decided to change the form of evaluation and restricted the analysis to patients who had not distant metastasis at the time of the recruitment to the study. This approach restricted the number of patients from 178 to 142. Since all patients of this group belonged to the TNM-M = 0 stage, this stage system was not considered at further analysis.
When the relationship between the serum level of soluble HSP70 and survival was studied in these 142 patients, a comparable hazard ratio (2.17 (1.13–3.99) vs. 1.88 (1.20–2.96)) of non-survival was found for the patients with high soluble HSP70 levels (Fig. 2b)
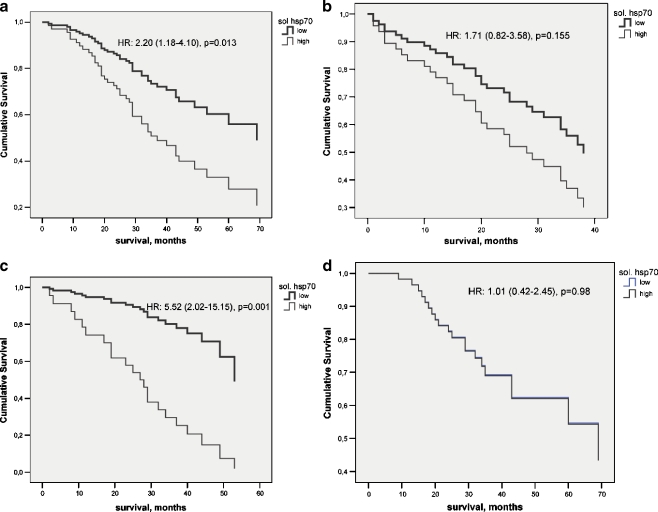
Next, we studied in these 142 patients whether the association between soluble HSP70 level and survival is dependent on the age and sex of the patients. Age groups were defined as less than 70 years or ≥70 years (Fig. 3). Marked differences were observed: this association was restricted to the younger age group. Within the younger age group, female patients had a high (5.52 (2.02–15.15), and highly significant (p = 0.001) hazard ratio for non-survival while in the male patients of this group, non-significant (p = 0.980) hazard ratio was obtained
Fig. 3.
Survival of patients with no distant metastasis with low (<median 1.65 ng/l) or high (≥1.65 ng/ml) soluble HSP70 levels over a 33.0 (24.0–44.0)-month-long follow-up period. a Less than 70-year-old patients; b≥70-year-old patients; c <70-year-old female patients; d <70-year-old male patients. Hazard ratios (HR) with 95% CI for Cox-regression analysis are indicated
Multivariate analysis of the association between soluble HSP70 levels and survival
In addition to HSP level, patient survival during the almost 4-years-long follow-up period was correlated with TNM-T (p = 0.014), and TNM-N (p < 0.001) stages. Tumor grade, by contrast, was not related to survival (p = 0. 148).
Multivariate analysis was performed to ascertain whether the association observed between high soluble HSP70 concentration and excessive mortality (calculated by univariate analysis) remains significant after adjustment for various demographic and clinical confounders (Table 3).
Table 3.
Relationship between the serum level of soluble HSP70 and the mortality of 142 patients with colorectal cancer without distant metastasis during the 33.0 (24.0-44.0) months long follow-up period. Cox-regression analysis adjusted to different variables
| Model | Variable | Odds ratio (OR) | 95% CI | p value |
|---|---|---|---|---|
| Unadjusted | Soluble HSP70 higha/lowb | 2.17 | 1.13–3.99 | 0.019 |
| Adjusted for age and sex | Soluble HSP70 higha/lowb | 2.14 | 1.09–4.22 | 0.028 |
| Adjusted for age, sex and TNM-T stage | Soluble HSP70 higha/lowb | 2.07 | 1.03–4.17 | 0.041 |
| TNM-T stage | 1.60 | 0.82–3.13 | 0.168 | |
| Adjusted for age, sex and TNM-N stage | Soluble HSP70 higha/lowb | 2.50 | 1.15–5.43 | 0.021 |
| TNM-N stage | 2.26 | 1.36–3.75 | 0.002 | |
| Adjusted for age, sex and grade | Soluble HSP70 higha/lowb | 2.12 | 1.17–4.22 | 0.023 |
| grade | 1.01 | 0.91–1.33 | 0.851 | |
| Adjusted for age, sex and localization (colon/sigmoid/rectal) | Soluble HSP70 higha/lowb | 2.18 | 1.08–4.18 | 0.029 |
| localization | 1.33 | 0.95–1.85 | 0.097 | |
| Adjusted for age, sex, and the type of chemotherapy (FU-based or other) | Soluble HSP70 higha/lowb | 2.10 | 1.06–4.15 | 0.033 |
| Type of chemotherapy | 0.75 | 0.38–1.49 | 0.413 |
a>1.65 ng/l
b≤1.65 ng/ml
Different regression models were developed. As evident from Table 3, high soluble HSP70 levels were found to be significantly associated with poor survival, even after adjustment for sex, age, and TNM-T and TNM-N stages; sex, age, and tumor grade; age, sex, and tumor localization, or age, sex, and the type of chemotherapy (FU-based or other). It seems therefore that high serum HSP70 concentration is associated with a low survival rate independently of disease stage, histological tumor grade, or cancer localization.
Interaction between high HSP70 levels and the demographic and clinical characteristics of patients in predicting mortality
As high mortality was predicted not only by high soluble HSP70 levels, but by advanced stage of the disease (according to different staging systems) and no difference was found between the HSP70 levels of patients in different stages (Table 2), it seemed worthwhile to test whether soluble HSP70 levels interact with demographic variables and tumor stage in predicting mortality. The interaction tool of the multiple logistic regression was used for this calculation. In addition to age and sex (Fig. 3), highly significant (p < 0.001) interactions were found with the TNM-T and TNM-N stages. These observations indicate that the strength of the association between high soluble HSP70 levels and patient survival can be quite different in different stages of the disease, whereas neither the grade, nor the localization of the tumor influences this association. Therefore, sex- and age-adjusted association between high soluble HSP70 levels and survival of patients in different TNM stages was calculated. High HSP70 levels were found to be associated with low survival rate in patients in lower (0 to 3) TNM-T stages (hazard ratio—HR, 2.60 [1.22–5.59], p = 0.014), but not in those in the highest (4) TNM-T stage (p = 0.470). Similarly, a significant association was observed in patients in the lower (0 or 1), but not in the higher (2) TNM-N stages (HR, 3.58 [1.52–8.47], p = 0.004 vs. p = 0.971). This indicates that the association between higher mortality and high soluble HSP70 levels exists in all patients, except those who were in an advanced stage of the disease already at baseline.
Discussion
This report discusses the predictive value of measuring baseline levels of soluble HSP70 in the sera of patients with colorectal cancer. High concentration of this protein was associated with a poor survival—patients with a serum concentration above the median (1.65 ng/ml) had more than twice higher age-, gender-, and localization-adjusted chance not to survive the almost 3-years-long follow-up period. Since the original group was found to be heterogeneous as for the presence of absence of tumor at baseline, we repeated the analysis by limiting it to the patients (142/179) who had no distant metastasis (TNM-M = 0) at recruitment. Since comparable findings were obtained (Fig. 2), further analysis was restricted to that group. Importantly, this association between high soluble HSP70 level and poor survival was found to be independent of disease stage defined by TNM staging systems, even after adjustment for individual stages. The adjusted chance for the survival of patients with high HSP70 serum concentration was approximately half of those with lower HSP70 levels. Moreover, we found an interaction between high HSP70 serum level and stage of the disease—that is, the association between high HSP70 level and survival could be detected only in the group of patients with less advanced disease at baseline (i.e., with tumors not invading other organs or structures and/or propagating beyond the visceral peritoneum, as well as with four or more tumor-free regional lymph nodes).
Interestingly, we found that the association between serum HSP70 and mortality is dominant in women under the age of 70. It was observed in many large studies including a meta-analysis that the risk of colorectal cancer in current users of postmenopausal hormones is significantly 30% to 40% lower compared with non-users (Newcomb and Storer 1995; Grodstein et al. 1999; Rossouw et al. 2002; Newcomb et al. 2007). It seems that the estrogen is the active agent (Newcomb and Storer 1995). It is tempting to speculate that high level of serum HSP70 may in some way be modulating the protective function of estrogen in pre-menopausal women. In animal experiments high gender-related differences were found in the HSP70 expression, indeed which were mostly due to the estrogen (Voss et al. 2003; Nickerson et al. 2006)
Our findings are in line with the results of the prospective study by Suzuki et al. (2006). These authors measured the serum levels of soluble HSP70 and CRP in a case-control study conducted in Japan and studied the relationship between these serum markers and the clinical course of lung cancer. Odds to develop lung cancer by patients in the highest quartile of soluble HSP70 levels was significantly higher than that found in the rest of the patients; in males, the odds ratio was close to 2.5. No similar study has been published yet for other types of cancer.
Abundant data have been published on the increased expression of HSP70 in several tumors, such as breast cancer (Takahashi et al. 1994; Lazaris et al. 1997; Vargas-Roig et al. 1997), ovarian cancer (Elpek et al. 2003), carcinoma of the uterine cervix (Kaur and Ralhan 1995; Park et al. 1999), lung cancer (Zhong et al. 2003), prostate cancer (Abe et al. 2004; Cornford et al. 2000), and other types of cancer (reviewed in Ciocca and Calderwood (2005)) . Colorectal tumors also exhibit increased HSP70 expression (Hwang et al. 2003; Dundas et al. 2005; Pfister et al. 2007).
Unusual membrane expression of HSP70 occurs only on the surface of tumor cells, but not in normal tissues (Multhoff et al. 1995; Gehrmann et al. 2003). In principle, increased membrane expression of HSP70 can be beneficial for the patients, because it can promote lysis of these tumor cells by NK cells (Multhoff et al. 2000). On the other hand, it can be associated with a greater metastatic potential and hence, unfavorable prognosis (Gehrmann et al. 2003; Farkas et al. 2003). In colorectal cancer, Hwang et al. (2003) compared the heat shock protein content of weakly vs. highly metastatic colorectal cell lines and found that HSP70 expression was elevated in the highly metastatic cell line. Over-expression of mortalin, a mitochondrial HSP70, was shown in colorectal adenocarcinomas and found to correlate with poor survival (Dundas et al. 2005). According to recent studies by Pfister et al. (2007), the relationship between HSP70 expression by the tumor cells and patient survival depends on the localization of the tumor. Specifically, HSP70 membrane expression correlated significantly with an improved overall survival in patients with colon cancers, whereas a marginally significant negative association was found in those with lower rectal cancer.
Theoretically, the elevation of soluble HSP70 levels in colorectal cancer patients can be explained by different mechanisms. In principle, it can be associated with the humoral immune response against the protein. In accordance with the earlier work of Pockley et al. performed in patients with hypertension (Pockley et al. 2002), we did not find significant correlation between the serum concentrations of the soluble HSP70 and IgG type anti-HSP70 antibodies in patients with colorectal cancer either. It is also possible that high soluble HSP70 levels results from increased expression of the protein by tumor cells. Under certain circumstances, HSP70 proteins escape from the cytoplasm, appear on the cell membrane and then, detach from the tumor cells (Pockley et al. 1998; Wright et al. 2000) through non-classical (endoplasmic reticulum–Golgi-independent) protein mechanisms, via intact lipid rafts (Broquet et al. 2003). To our best knowledge, however, no comparison of tumor-associated and soluble HSP70 was ever reported. In the present study, we found the serum levels of the soluble HSP70 significantly higher in those patients with non-resected primary tumors (primary chemotherapy) than in those with resected tumors before enrollment, indicating that in these patients, probably a part of the protein is derived from the tumors cells. On the other hand since the majority of the patients was recruited 4–6 weeks after the removal of the tumors, soluble HSP70 protein probable was originated from other sources. Furthermore, it cannot be excluded that a fraction of the tumor-derived protein was still detectable in the circulation after 4–6 weeks.
Accordingly, there is no direct evidence to support the neoplastic origin of high soluble HSP70 concentration in the blood of patients with colorectal cancer. An alternative explanation could be that the increase in HSP70 levels results from injury and necrosis of tumor cells, such as it is seen in other diseases where tissue damage occurs, e.g., in the wound discharge and peripheral blood of patients with soft tissue trauma (Pittet et al. 2002; Flohe et al. 2007), in peripheral and renal vascular disease (Wright et al. 2000)] or after myocardial infarction (Dybdahl et al. 2005; Satoh et al. 2006) and coronary bypass grafting (Dybdahl et al. 2002).
The association between high serum HSP70 levels and poor survival of patients with colorectal cancer is a novel observation for which no definite explanation can be offered at the time being. It is well known that extracellular HSP70 has a major role in anti-tumor immunity, as well as it is an adjuvant to tumor-associated antigens and as such, it may induce specific tumor cell killing by cytotoxic T cells (Calderwood et al. 2005). Thus, it is tempting to speculate that high soluble HSP70 level in the serum may inhibit cellular anti-tumor immunity. Further studies are needed to investigate this assumption directly.
The identification of early, molecular markers, which can predict cancer-related mortality, is an urgent and important goal in the development of cancer diagnostics and therapy. Ideally, identifying patients with high-risk carcinomas and a low chance of survival at initial diagnosis could afford early and individualized therapy that would improve clinical outcome. Our findings indicate that the prognostic value (adjusted odds ratios of about 2; p < 0.01; see Table 3) for the overall survival of the soluble HSP70 measurement is comparable to that of the known tumor markers, such as CEA and CA 19-9 for disease-free survival in early stage colorectal cancer. (Wang et al. 2002, 2007;Ogata et al. 2009) For example, in a recent study by Takagawa et al. (2008) performed in 638 patients with colorectal cancer, high (>10 ng/ml) CEA values were found an independent risk factor for disease-free survival with an adjusted odds ratio of 3.21 (p < 0.001)
Due to the limited size of the study population, our present work should be considered a preliminary investigation only. If, however, our findings will be confirmed by subsequent studies conducted on larger cohorts of patients, measurement of the serum level of soluble HSP70 might prove a useful, non-invasive, and inexpensive predictive marker in patients with colorectal cancer who have no distant metastasis at the time of recruitment
Acknowledgments
This work was supported by the OTKA T049266 grant (GF) and NF72689 grant (ZP) of the Hungarian Research Fund.
Footnotes
Judit Kocsis and Balázs Madaras equally contributed to the work; therefore, they share the first authorship.
References
- Abe M, Manola JB, Oh WK, Parslow DL, George DJ, Austin CL, Kantoff PW. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer. 2004;3(1):49. doi: 10.3816/cgc.2004.n.013. [DOI] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278(24):21601. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol. 2005;35(9):2518. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31(3):164. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60(24):7099. [PubMed] [Google Scholar]
- Dundas SR, Lawrie LC, Rooney PH, Murray GI. Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol. 2005;205(1):74. doi: 10.1002/path.1672. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105(6):685. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91(3):299. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elpek GO, Karaveli S, Simsek T, Keles N, Aksoy NH. Expression of heat-shock proteins hsp27, hsp70 and hsp90 in malignant epithelial tumour of the ovaries. Apmis. 2003;111(4):523. doi: 10.1034/j.1600-0463.2003.1110411.x. [DOI] [PubMed] [Google Scholar]
- Farkas B, Hantschel M, Magyarlaki M, Becker B, Scherer K, Landthaler M, Pfister K, Gehrmann M, Gross C, Mackensen A, Multhoff G. Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res. 2003;13(2):147. doi: 10.1097/00008390-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Flohe SB, Bangen JM, Flohe S, Agrawal H, Bergmann K, Schade FU. Origin of immunomodulation after soft tissue trauma: potential involvement of extracellular heat-shock proteins. Shock. 2007;27(5):494. doi: 10.1097/shk.0b013e31802dec51. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Schmetzer H, Eissner G, Haferlach T, Hiddemann W, Multhoff G. Membrane-bound heat shock protein 70 (Hsp70) in acute myeloid leukemia: a tumor specific recognition structure for the cytolytic activity of autologous NK cells. Haematologica. 2003;88(4):474. [PubMed] [Google Scholar]
- Greene FL. Current TNM staging of colorectal cancer. Lancet Oncol. 2007;8(7):572. doi: 10.1016/S1470-2045(07)70185-7. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999;106(5):574. doi: 10.1016/S0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Hwang TS, Han HS, Choi HK, Lee YJ, Kim YJ, Han MY, Park YM. Differential, stage-dependent expression of Hsp70, Hsp110 and Bcl-2 in colorectal cancer. J Gastroenterol Hepatol. 2003;18(6):690. doi: 10.1046/j.1440-1746.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- Kaur J, Ralhan R. Differential expression of 70-kDa heat shock-protein in human oral tumorigenesis. Int J Cancer. 1995;63(6):774. doi: 10.1002/ijc.2910630604. [DOI] [PubMed] [Google Scholar]
- Lazaris A, Chatzigianni EB, Panoussopoulos D, Tzimas GN, Davaris PS, Golematis B. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res Treat. 1997;43(1):43. doi: 10.1023/A:1005706110275. [DOI] [PubMed] [Google Scholar]
- Madach K, Molvarec A, Rigo J, Jr, Nagy B, Penzes I, Karadi I, Prohaszka Z. Elevated serum 70 kDa heat shock protein level reflects tissue damage and disease severity in the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):133. doi: 10.1016/j.ejogrb.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. J Hum Hypertens. 2006;20(10):780. doi: 10.1038/sj.jhh.1002060. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Rigo J, Jr, Nagy B, Walentin S, Szalay J, Fust G, Karadi I, Prohaszka Z. Serum heat shock protein 70 levels are decreased in normal human pregnancy. J Reprod Immunol. 2007;74(1-2):163. doi: 10.1016/j.jri.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Derzsy Z, Kocsis J, Boze T, Nagy B, Balogh K, Mako V, Cervenak L, Mezes M, Karadi I, Prohaszka Z, Rigo J Jr (2009) Circulating anti-heat-shock-protein antibodies in normal pregnancy and preeclampsia. Cell Stress Chaperones in press [DOI] [PMC free article] [PubMed]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61(2):272. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Pfister K, Botzler C, Jordan A, Scholz R, Schmetzer H, Burgstahler R, Hiddemann W. Adoptive transfer of human natural killer cells in mice with severe combined immunodeficiency inhibits growth of Hsp70-expressing tumors. Int J Cancer. 2000;88(5):791. doi: 10.1002/1097-0215(20001201)88:5<791::AID-IJC17>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Newcomb PA, Storer BE. Postmenopausal hormone use and risk of large-bowel cancer. J Natl Cancer Inst. 1995;87(14):1067. doi: 10.1093/jnci/87.14.1067. [DOI] [PubMed] [Google Scholar]
- Newcomb PA, Zheng Y, Chia VM, Morimoto LM, Doria-Rose VP, Templeton A, Thibodeau SN, Potter JD. Estrogen plus progestin use, microsatellite instability, and the risk of colorectal cancer in women. Cancer Res. 2007;67(15):7534. doi: 10.1158/0008-5472.CAN-06-4275. [DOI] [PubMed] [Google Scholar]
- Nickerson M, Kennedy SL, Johnson JD, Fleshner M. Sexual dimorphism of the intracellular heat shock protein 72 response. J Appl Physiol. 2006;101(2):566. doi: 10.1152/japplphysiol.00259.2006. [DOI] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Mets T. Elevated serum heat-shock protein 70 levels in patients with acute infection: use of an optimized enzyme-linked immunosorbent assay. Scand J Immunol. 2003;58(6):664. doi: 10.1111/j.1365-3083.2003.01341.x. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Murakami H, Sasatomi T, Ishibashi N, Mori S, Ushijima M, Akagi Y, Shirouzu K. Elevated preoperative serum carcinoembrionic antigen level may be an effective indicator for needing adjuvant chemotherapy after potentially curative resection of stage II colon cancer. J Surg Oncol. 2009;99(1):65. doi: 10.1002/jso.21161. [DOI] [PubMed] [Google Scholar]
- Park CS, Joo IS, Song SY, Kim DS, Bae DS, Lee JH. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol Oncol. 1999;74(1):53. doi: 10.1006/gyno.1999.5429. [DOI] [PubMed] [Google Scholar]
- Pfister K, Radons J, Busch R, Tidball JG, Pfeifer M, Freitag L, Feldmann HJ, Milani V, Issels R, Multhoff G. Patient survival by Hsp70 membrane phenotype: association with different routes of metastasis. Cancer. 2007;110(4):926. doi: 10.1002/cncr.22864. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52(4):611. doi: 10.1097/00005373-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27(6):367. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4(1):29. doi: 10.1379/1466-1268(1999)004<0029:IOHHSP>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20(9):1815. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42(3):235. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Rau B, Gaestel M, Wust P, Stahl J, Mansmann U, Schlag PM, Benndorf R. Preoperative treatment of rectal cancer with radiation, chemotherapy and hyperthermia: analysis of treatment efficacy and heat-shock response. Radiat Res. 1999;151(4):479. doi: 10.2307/3579836. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail. 2006;8(8)):810. doi: 10.1016/j.ejheart.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Seki N, Ando M, Nishino Y, Kondo T, Watanabe Y, Ozasa K, Inoue T, Tamakoshi A. Serum heat shock protein 70 levels and lung cancer risk: a case-control study nested in a large cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1733. doi: 10.1158/1055-9965.EPI-06-0005. [DOI] [PubMed] [Google Scholar]
- Takagawa R, Fujii S, Ohta M, Nagano Y, Kunisaki C, Yamagishi S, Osada S, Ichikawa Y, Shimada H. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann Surg Oncol. 2008;15(12):3433. doi: 10.1245/s10434-008-0168-8. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Mikami T, Watanabe Y, Okazaki M, Okazaki Y, Okazaki A, Sato T, Asaishi K, Hirata K, Narimatsu E, et al. Correlation of heat shock protein 70 expression with estrogen receptor levels in invasive human breast cancer. Am J Clin Pathol. 1994;101(4):519. doi: 10.1093/ajcp/101.4.519. [DOI] [PubMed] [Google Scholar]
- Terry DF, Wyszynski DF, Nolan VG, Atzmon G, Schoenhofen EA, Pennington JY, Andersen SL, Wilcox MA, Farrer LA, Barzilai N, Baldwin CT, Asea A. Serum heat shock protein 70 level as a biomarker of exceptional longevity. Mech Ageing Dev. 2006;127(11):862. doi: 10.1016/j.mad.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Roig LM, Fanelli MA, Lopez LA, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997;21(5):441. [PubMed] [Google Scholar]
- Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol. 2003;285(2):H687. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS, Chen PM. CA19-9 as the most significant prognostic indicator of metastatic colorectal cancer. Hepatogastroenterology. 2002;49(43):160. [PubMed] [Google Scholar]
- Wang JY, Lu CY, Chu KS, Ma CJ, Wu DC, Tsai HL, Yu FJ, Hsieh JS. Prognostic significance of pre- and postoperative serum carcinoembryonic antigen levels in patients with colorectal cancer. Eur Surg Res. 2007;39(4):245. doi: 10.1159/000101952. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15(1):8. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- Zhong L, Peng X, Hidalgo GE, Doherty DE, Stromberg AJ, Hirschowitz EA. Antibodies to HSP70 and HSP90 in serum in non-small cell lung cancer patients. Cancer Detect Prev. 2003;27(4):285. doi: 10.1016/S0361-090X(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23(6):1055. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]