Abstract
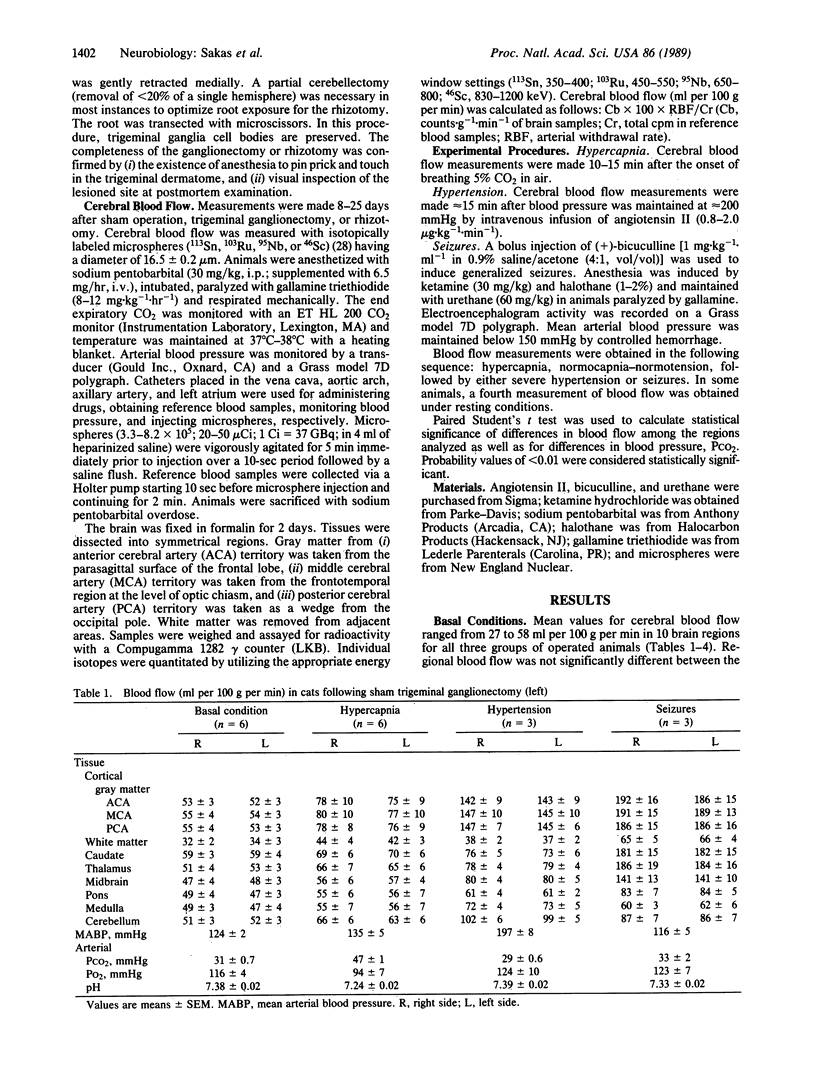
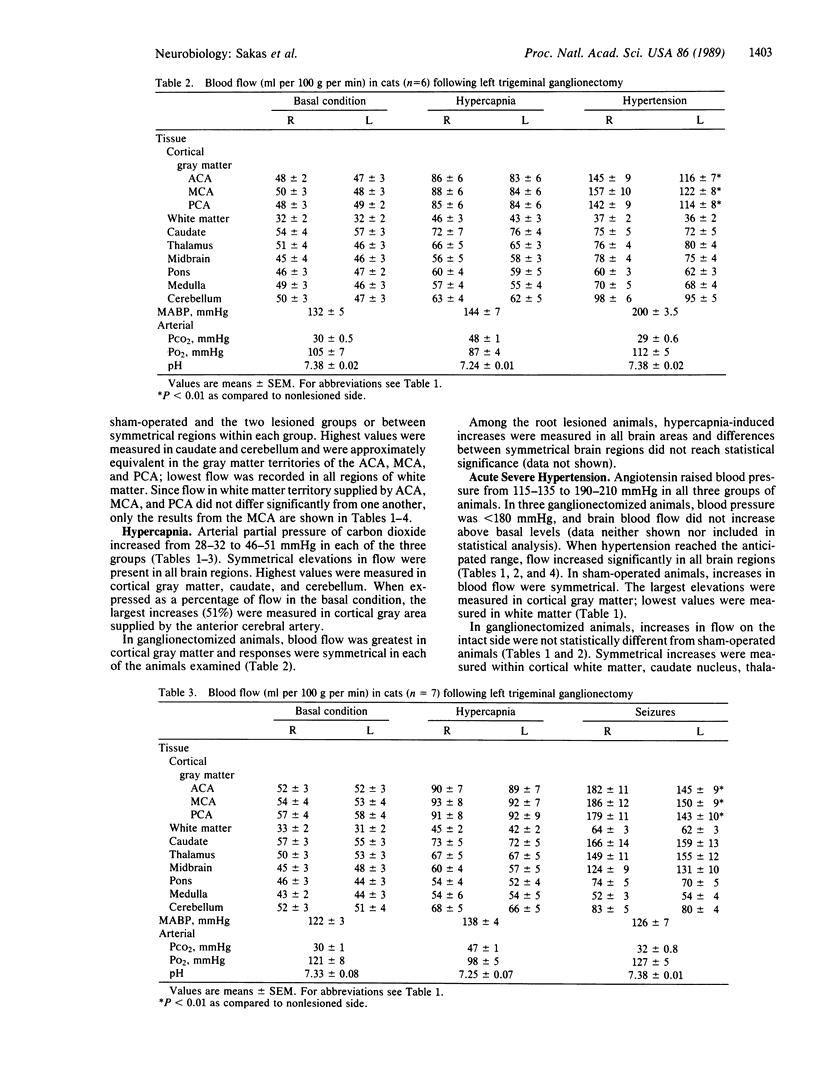
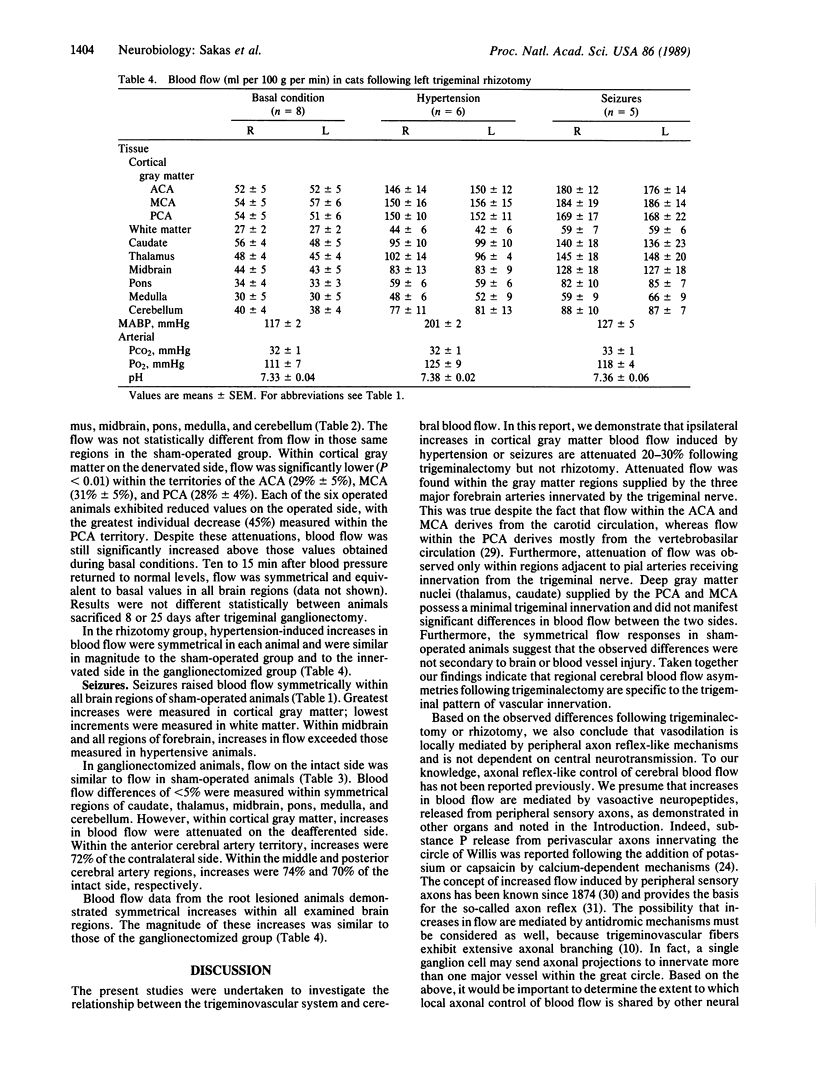
Cerebral blood flow was measured and compared in 10 symmetrical brain regions following unilateral trigeminal ganglionectomy (n = 13), sham operation (n = 6), or trigeminal root section (rhizotomy) (n = 8) in cats. Multiple determinations were obtained in anesthetized and paralyzed animals using radiolabeled microspheres during (i) normocapnia-normotension, (ii) hypercapnia (5% CO2/95% room air), (iii) angiotensin-induced acute severe hypertension (190 greater than mean arterial blood pressure less than 210 mmHg), or (iv) bicuculline-induced seizures. Flow was symmetrical in all brain regions at rest and during increases induced by hypercapnia in the three groups. During severe hypertension or seizures, marked elevations developed bilaterally (approximately 93% and approximately 130%, respectively). In ganglionectomized animals, increases due to hypertension or seizures were attenuated by 28-32% on the denervated side within cortical gray matter regions corresponding to the anterior, middle, and posterior cerebral arteries. Flow was symmetrical within all brain regions in sham-operated animals and in the rhizotomy group, despite comparable increases in regional cerebral blood flow induced by angiotensin. Hence, the trigeminal nerve mediates blood flow adaptations during severe hypertension and seizures. Furthermore, since trigeminal cell bodies and peripheral axons are destroyed or degenerate following ganglionectomy but not following rhizotomy, local "axon reflex-like" mechanisms mediate these increases in cerebral blood flow.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbab M. A., Wiklund L., Svendgaard N. A. Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA-HRP tracing in the rat. Neuroscience. 1986 Nov;19(3):695–708. doi: 10.1016/0306-4522(86)90293-9. [DOI] [PubMed] [Google Scholar]
- Busija D. W., Heistad D. D. Effects of cholinergic nerves on cerebral blood flow in cats. Circ Res. 1981 Jan;48(1):62–69. doi: 10.1161/01.res.48.1.62. [DOI] [PubMed] [Google Scholar]
- Busija D. W., Heistad D. D. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol. 1984;101:161–211. doi: 10.1007/BFb0027696. [DOI] [PubMed] [Google Scholar]
- Couture R., Cuello A. C. Studies on the trigeminal antidromic vasodilatation and plasma extravasation in the rat. J Physiol. 1984 Jan;346:273–285. doi: 10.1113/jphysiol.1984.sp015021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture R., Gaudreau P., St-Pierre S., Regoli D. The dog common carotid artery: a sensitive bioassay for studying vasodilator effects of substance P and of kinins. Can J Physiol Pharmacol. 1980 Oct;58(10):1234–1244. doi: 10.1139/y80-187. [DOI] [PubMed] [Google Scholar]
- D'Alecy L. G., Rose C. J. Parasympathetic cholinergic control of cerebral blood flow in dogs. Circ Res. 1977 Sep;41(3):324–331. doi: 10.1161/01.res.41.3.324. [DOI] [PubMed] [Google Scholar]
- Davis K. D., Dostrovsky J. O. Activation of trigeminal brain-stem nociceptive neurons by dural artery stimulation. Pain. 1986 Jun;25(3):395–401. doi: 10.1016/0304-3959(86)90244-7. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol. 1981 Sep;318:251–258. doi: 10.1113/jphysiol.1981.sp013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FJALLBRANT N., IGGO A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961 May;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad D. D., Marcus M. L., Gross P. M. Effects of sympathetic nerves on cerebral vessels in dog, cat, and monkey. Am J Physiol. 1978 Nov;235(5):H544–H552. doi: 10.1152/ajpheart.1978.235.5.H544. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Marcus M. L., Sandberg S., Abboud F. M. Effect of sympathetic nerve stimulation on cerebral blood flow and on large cerebral arteries of dogs. Circ Res. 1977 Sep;41(3):342–350. doi: 10.1161/01.res.41.3.342. [DOI] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Dietrich W. D., Navari R. M., Povlishock J. T., Ghatak N. R., Ellis E. F., Patterson J. L., Jr Mechanism of cerebral arteriolar abnormalities after acute hypertension. Am J Physiol. 1981 Apr;240(4):H511–H527. doi: 10.1152/ajpheart.1981.240.4.H511. [DOI] [PubMed] [Google Scholar]
- Lambert G. A., Bogduk N., Goadsby P. J., Duckworth J. W., Lance J. W. Decreased carotid arterial resistance in cats in response to trigeminal stimulation. J Neurosurg. 1984 Aug;61(2):307–315. doi: 10.3171/jns.1984.61.2.0307. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Leniger-Follert E. Mechanisms of regulation of cerebral microflow during bicuculline-induced seizures in anaesthetized cats. J Cereb Blood Flow Metab. 1984 Jun;4(2):150–165. doi: 10.1038/jcbfm.1984.23. [DOI] [PubMed] [Google Scholar]
- Lewis T. Nocifensor System of Nerves. Br Med J. 1937 Feb 27;1(3973):431–435. doi: 10.1136/bmj.1.3973.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Han D. H., Moskowitz M. A. Pia arachnoid contains substance P originating from trigeminal neurons. Neuroscience. 1983 Aug;9(4):803–808. doi: 10.1016/0306-4522(83)90268-3. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Liszczak T. M., King J. C., Moskowitz M. A. Immunoelectron microscopic study of substance P-containing fibers in feline cerebral arteries. Brain Res. 1986 Mar 26;369(1-2):12–20. doi: 10.1016/0006-8993(86)90508-1. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Mayberg M. R., Moskowitz M. A. Immunohistochemical evidence for a substance P-containing trigeminovascular pathway to pial arteries in cats. Brain Res. 1983 May 23;268(1):162–166. doi: 10.1016/0006-8993(83)90402-x. [DOI] [PubMed] [Google Scholar]
- Marcus M. L., Heistad D. D., Ehrhardt J. C., Abboud F. M. Total and regional cerebral blood flow measurement with 7-10-, 15-, 25-, and 50-mum microspheres. J Appl Physiol. 1976 Apr;40(4):501–507. doi: 10.1152/jappl.1976.40.4.501. [DOI] [PubMed] [Google Scholar]
- Markowitz S., Saito K., Moskowitz M. A. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987 Dec;7(12):4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. A., Basbaum A. I., Kwiat G. C., Goetzl E. J., Levine J. D. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987 Aug;22(2):651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- Mayberg M. R., Zervas N. T., Moskowitz M. A. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984 Feb 10;223(1):46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- Mayberg M., Langer R. S., Zervas N. T., Moskowitz M. A. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981 Jul 10;213(4504):228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Uddman R., Kingman T. A., Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz M. A., Brody M., Liu-Chen L. Y. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience. 1983 Aug;9(4):809–814. doi: 10.1016/0306-4522(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Kuo C., Leeman S. E., Jessen M. E., Derian C. K. Desensitization to substance P-induced vasodilation in vitro is not shared by endogenous tachykinin neurokinin A. J Neurosci. 1987 Aug;7(8):2344–2351. [PMC free article] [PubMed] [Google Scholar]
- Moskowitz M. A., Wei E. P., Saito K., Kontos H. A. Trigeminalectomy modifies pial arteriolar responses to hypertension or norepinephrine. Am J Physiol. 1988 Jul;255(1 Pt 2):H1–H6. doi: 10.1152/ajpheart.1988.255.1.H1. [DOI] [PubMed] [Google Scholar]
- Mueller S. M., Heistad D. D., Marcus M. L. Effect of sympathetic nerves on cerebral vessels during seizures. Am J Physiol. 1979 Aug;237(2):H178–H184. doi: 10.1152/ajpheart.1979.237.2.H178. [DOI] [PubMed] [Google Scholar]
- Norregaard T. V., Moskowitz M. A. Substance P and the sensory innervation of intracranial and extracranial feline cephalic arteries. Implications for vascular pain mechanisms in man. Brain. 1985 Jun;108(Pt 2):517–533. doi: 10.1093/brain/108.2.517. [DOI] [PubMed] [Google Scholar]
- O'Connor T. P., van der Kooy D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci. 1988 Jul;8(7):2468–2476. doi: 10.1523/JNEUROSCI.08-07-02468.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T. P., van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci. 1986 Aug;6(8):2200–2207. doi: 10.1523/JNEUROSCI.06-08-02200.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskell G. L., Simons T. Trigeminal nerve pathways to the cerebral arteries in monkeys. J Anat. 1987 Dec;155:23–37. [PMC free article] [PubMed] [Google Scholar]
- Saito K., Greenberg S., Moskowitz M. A. Trigeminal origin of beta-preprotachykinin products in feline pial blood vessels. Neurosci Lett. 1987 Apr 23;76(1):69–73. doi: 10.1016/0304-3940(87)90194-7. [DOI] [PubMed] [Google Scholar]
- Strassman A., Mason P., Moskowitz M., Maciewicz R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986 Aug 6;379(2):242–250. doi: 10.1016/0006-8993(86)90777-8. [DOI] [PubMed] [Google Scholar]
- Wei E. P., Kontos H. A., Christman C. W., DeWitt D. S., Povlishock J. T. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985 Nov;57(5):781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]



