Abstract
Previous studies have shown that inhibiting the activity of the proteasome leads to the accumulation of damaged or unfolded proteins within the cell. In this study, we report that proteasome inhibitors, lactacystin and carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (MG132), induced the accumulation of ubiquitinated proteins as well as a dose- and time-dependent increase in the relative levels of heat shock protein (HSP)30 and HSP70 and their respective mRNAs in Xenopus laevis A6 kidney epithelial cells. In A6 cells recovering from MG132 exposure, HSP30 and HSP70 levels were still elevated after 24 h but decreased substantially after 48 h. The activation of heat shock factor 1 (HSF1) may be involved in MG132-induced hsp gene expression in A6 cells since KNK437, a HSF1 inhibitor, repressed the accumulation of HSP30 and HSP70. Exposing A6 cells to simultaneous MG132 and mild heat shock enhanced the accumulation of HSP30 and HSP70 to a much greater extent than with each stressor alone. Immunocytochemical studies determined that HSP30 was localized primarily in the cytoplasm of lactacystin- or MG132-treated cells. In some cells treated with higher concentrations of MG132 or lactacystin, we observed in the cortical cytoplasm (1) relatively large HSP30 staining structures, (2) colocalization of actin and HSP30, and (3) cytoplasmic areas that were devoid of HSP30. Lastly, MG132 treatment of A6 cells conferred a state of thermotolerance such that they were able to survive a subsequent thermal challenge.
Keywords: Heat shock protein, Xenopus, Ubiquitin, Proteasome, Confocal microscopy, KNK437
Introduction
A cellular response to environmental stress or disease states can cause an increase in the production of abnormal proteins that are targeted for degradation (Ross and Pickart 2004; Morimoto 2008). In eukaryotic cells, the proteasome is the site for degradation of most proteins and is necessary for viability. Proteasomes are present in the cytoplasm and nucleus, and some particles are associated with the endoplasmic reticulum and with the cytoskeleton (Rivett et al. 1992; Scherrer and Bey 1994). It is an essential component of the ATP-dependent proteolytic pathway, which assists the degradation of many enzymes, transcriptional regulators, and other critical regulatory proteins. Proteins are marked for degradation by the addition of ubiquitin molecules (Haas and Rose 1982; Neutzner et al. 2008). The ubiquitinated substrate is then hydrolyzed by the 26S proteasome via an ATP-dependent mechanism. The impairment of the ubiquitin–proteasome system has been associated with a number of diseases including Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis, and Parkinson's disease (Masliah et al. 2000; Taylor et al. 2002; Ross and Pickart 2004). Additionally, some studies have shown that impairment of the ubiquitin–proteasome system can cause an upregulation of heat shock protein (HSP) gene expression in yeast, Drosophila, rainbow trout, and mammalian cultured cells (Bush et al. 1997; Lee and Goldberg 1998b; Pritts et al. 2002; Stangl et al. 2002; Le Goff et al. 2004; Dembla-Rajpal et al. 2004; Lundgren et al. 2005; Awasthi and Wagner 2005; Noonan et al. 2007, 2008).
HSPs consist of a number of families including HSP70 and the small HSPs (sHSPs; Feige et al. 1996; Morimoto 1998, 2008). Cytoplasmic stress-inducible HSP70, a member of the HSP70 family, acts as a molecular chaperone to protect unfolded proteins from aggregation and to assist their folding into a native and functional conformation (Morimoto et al. 1994; Feige et al. 1996; Morimoto 1998, 2008). The sHSPs (16–42 kDa), which are quite divergent except for a conserved α-crystallin domain, can form highly polymeric structures that appear to be necessary for function within the cell (Buchner et al. 1998; MacRae 2000; Van Montfort et al. 2002; Heikkila 2004). Proposed sHSP functions in vivo include the acquisition of thermotolerance, resistance against apoptosis, actin capping/decapping activity, cellular differentiation, and modulation of redox parameters (Arrigo and Landry 1994; Arrigo 1998; MacRae 2000; Van Montfort et al. 2002). Finally, the accumulation or mutation of sHSPs has been associated with a variety of disease states including muscle myopathy, cataracts, multiple sclerosis, Alzheimer’s disease, and other neuropathologies (Quinlan and Van Den Ijssel 1999; Bova et al. 1999; Irobi et al. 2004). Stress-inducible hsp gene expression is activated by heat shock factor 1 (HSF1) which interacts with the heat shock element (HSE) found in the 5′ upstream regulatory regions of hsp genes (Feige et al. 1996; Morimoto 1998; Katschinski 2004; Voellmy 2004; Tonkiss and Calderwood 2005). HSF1 preexists in the cell as an inactive monomer and forms a hyperphosphorylated trimer upon heat or chemical stress, which permits its binding to the HSE thus facilitating hsp gene transcription. HSF1 activation occurs in response to the accumulation of unfolded, misfolded, or damaged protein (Voellmy 2004; Tonkiss and Calderwood 2005).
Our laboratory has characterized hsp gene expression in embryos and cultured cells of the aquatic frog, Xenopus laevis (Heikkila et al. 1997; Lang et al. 1999; 2000; Ovakim and Heikkila 2003; Heikkila 2003; 2004; Gellalchew and Heikkila 2005; Manwell and Heikkila 2007; Young et al. 2009). These studies examined a number of different aspects of heat shock and chemical stress-induced expression of hsp30 and hsp70 genes during early frog development as well as in an A6 kidney epithelial cell line. For example, an analysis of the intracellular localization of heat shock-, sodium arsenite-, or cadmium-induced HSP30 in A6 cells revealed that it was localized primarily in the cytoplasm and perinuclear regions (Gellalchew and Heikkila 2005; Manwell and Heikkila 2007; Voyer and Heikkila 2008; Woolfson and Heikkila 2009; Young et al. 2009). HSP30 appears to act as a molecular chaperone in Xenopus cells since it was capable of inhibiting heat-induced aggregation of client protein and maintaining them in a soluble and folding competent state (Fernando and Heikkila 2000; Abdulle et al. 2002; Fernando et al. 2002). While the function of most of the other HSPs, including HSP70, has not been elucidated directly in Xenopus, it is likely, based on findings in other organisms, that their primary roles are that of molecular chaperones.
In the present study, we show, for the first time in an amphibian system, that inhibition of the ubiquitin–proteasome system can induce the accumulation of ubiquitinated protein and an upregulation of hsp gene expression. The exposure of X. laevis A6 kidney epithelial cells to the proteasome inhibitors, lactacystin or carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (MG132), enhanced the levels of both HSP30 and HSP70 as well as their respective mRNAs in a dose- and time-dependent pattern. Furthermore, this response was controlled, at least in part, at the level of HSF activation since pretreatment of cells with the HSF inhibitor KNK437 blocked this response. Also, exposure of A6 cells to simultaneous MG132 and mild heat shock enhanced the accumulation of HSP30 and HSP70 to a much greater extent than observed with each stressor alone. Immunocytochemical analysis revealed that proteasomal inhibition-induced HSP30 accumulation occurred primarily in the cytoplasm in a punctate pattern supplemented with larger HSP30 staining structures. Finally, pretreatment of cells with MG132 conferred a state of thermotolerance since the treated cells were capable of withstanding a subsequent thermal stress.
Materials and methods
Cell culture and treatments
A6 cells (CCL-102; American Type Culture Collection) were grown at 22°C in 55% Leibovitz l-15 media containing 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Sigma-Aldrich, Oakville, ON, Canada) in T75 cm2 BD Falcon tissue culture flasks (VWR International, Mississauga, ON, Canada). Flasks of A6 cells were treated with 1 to 20 μM lactacystin (Sigma-Aldrich) or 1 to 50 μM MG132 (Sigma-Aldrich) at 22°C for periods of time ranging from 2 to 24 h. Stock solutions of the proteasome inhibitors were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich). In experiments employing the HSF1 inhibitor, N-formyl-3,4-methylenedioxy-benzylidene-γ-butyrolactam (KNK437; Calbiochem, Gibbstown, NJ, USA) A6 cells were pretreated with 100 μM of the inhibitor prepared in DMSO for 6 h at 22°C before heat shock or proteasome inhibitor treatment. KNK437 at a concentration of 100 μM has been used in a number of previous studies to suppress hsp gene expression by inhibiting HSF–HSE binding activity in eukaryotic systems including mouse, human, and Xenopus cultured cells with no detectable effect on cell viability (Ohnishi et al. 2004; Manwell and Heikkila 2007; Voyer and Heikkila 2008; Takahashi et al. 2008). Some flasks of A6 cells were heat shocked for 2 h by immersion in a water bath set at 33°C. After the different treatments, cells were rinsed with 65% Hanks balanced salt solution (HBSS; Sigma-Aldrich) followed by the addition of 1 mL of 100% HBSS. Cells were removed by means of a rubber scraper, transferred to a 1.5-mL microfuge tube, and pelleted by centrifugation for 1 min at 13,200 rpm. The harvested A6 kidney epithelial cell pellets were frozen and stored at −80°C.
RNA isolation and Northern blot analysis
A6 cell RNA was isolated using the QIAgen RNeasy Mini Kit (Qiagen, Mississauga, ON, Canada) as detailed in the manufacturer’s instructions. Spectrophotometry and electrophoresis with ethidium bromide staining were used to assess RNA concentration and integrity. For Northern blot analysis, total RNA was electrophoresed on 1.2% (w/v) formaldehyde/agarose gels (Lang et al. 1999; Sambrook and Russell 2001) and then transferred onto a positively charged nylon membrane (Roche Diagnostics, Laval, QC, Canada). RNA was UV cross-linked to the membrane with an ultraviolet cross-linker (UltraLum Inc., Claremont, CA, USA). The membrane was then stained with 1× Reversible Blot Stain (Sigma-Aldrich) to ensure equal loading and quality of transfer of the RNA. Digoxigenin-labeled antisense hsp30 and hsp70 riboprobes were prepared as described previously (Lang et al. 1999; 2000). The RNA blot prehybridization, hybridization, and washing procedures were outlined in Lang et al. (1999). Chemiluminescent detection was performed according to the manufacturer’s instructions (Roche Diagnostics, Mississauga, ON, Canada). The images were visualized by means of a DNR chemiluminescent imager (DNR Bio-Imaging Systems Ltd., QC, Canada). Densitometric analyses within the range of linearity were conducted using ImageJ (Version 1.38) software on the data obtained from at least three separate experiments.
Protein isolation and immunoblot analysis
Total protein was isolated from A6 cells as described previously by Young et al. (2009). Protein concentrations were determined by means of a bicinchoninic acid protein assay according to the manufacturer’s instructions (Thermo Scientific, Rockford, IL, USA). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed using a BioRad Mini Protean III gel system (BioRad, Mississauga, ON, Canada) and 12% polyacrylamide gels. Protein samples were transferred to nitrocellulose membranes (BioRad) using a Trans-Blot Semi-dry Transfer Cell (BioRad). Ponceau S (Sigma-Aldrich) staining of the membrane was employed to assess the efficiency of transfer for each sample. Immunodetection was carried out using either the polyclonal rabbit anti-Xenopus HSP30 antibody (Fernando and Heikkila 2000; 1:2,000 dilution), the polyclonal rabbit anti-Xenopus HSP70 antibody (Gauley et al. 2008; 1:150 dilution), the polyclonal rabbit anti-actin (Sigma-Aldrich; 1:200 dilution), or monoclonal mouse anti-ubiquitin (Zymed, San Francisco, CA, USA; 1:150 dilution). Membranes were incubated with alkaline phosphatase-conjugated goat antirabbit secondary antibody (BioRad; 1:3,000 dilution). For detection, the blots were immersed in alkaline phosphatase detection buffer (50 mM Tris, 50 mM NaCl, 25 mM MgCl2, pH 9.5) with 0.33% nitroblue tetrazolium chloride and 0.17% 5-bromo-4-chloro-3-indolyl phosphate, toluidine salt. Densitometric analyses of appropriately stained blots to maintain the signals within the range of linearity were performed as described in the previous section.
Immunocytochemistry and laser scanning confocal microscopy
Immunofluorescence analysis was performed as previously described (Gellalchew and Heikkila 2005; Manwell and Heikkila 2007; Young et al. 2009). A6 cells were grown on glass coverslips for 24 h at 22°C. A6 cells were then incubated with either 5, 10, or 15 μM lactacystin for 15 h or 5, 10, or 30 μM MG132 for 24 h at 22°C. Additionally, cells were treated with 30 μM for 24 h followed by recovery in fresh media for 48 h. In thermotolerance studies, A6 cells were incubated at the challenge temperature of 37°C for 1 h with or without a prior 12-h incubation with 30 μM MG132 at 22°C. In some experiments, A6 cells were incubated for 6 h with 100 μM KNK437 at 22°C before the thermotolerance regimen. Following these treatments, cells were rinsed with phosphate buffered saline (PBS) and fixed in 3.7% paraformaldehyde (BDH, Toronto, ON, Canada) for 10 min and then rinsed three times with PBS. The cells were permeabilized with 0.3% Triton X-100 (Sigma-Aldrich) in PBS for 10 min followed by three washes with PBS for 5 min each. Cells were then incubated with 3.7% bovine serum albumin (BSA; Fisher Scientific, Ottawa, ON, Canada) for 1 h at 22°C or overnight at 4°C. Cells were subsequently incubated with affinity-purified rabbit anti-Xenopus HSP30 antibody (1:500) in 3.7% BSA for 1 h. After three washes for 3 min each in PBS, cells were indirectly labeled with a fluorescent-conjugated secondary antibody, goat antirabbit Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA), at 1:2,000 in 3.7% BSA for 30 min in the dark. The cells were then probed for F-actin with rhodamine-tetramethylrhodamine-5-isothiocyanate phalloidin conjugated TRITC (Molecular Probes) at 1:60 in PBS for 15 min in the dark followed by three PBS washes for 5 min each. The coverslips were mounted on glass slides with VectaShield mounting media containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA, USA) which stains nucleic acids allowing visualization of nuclei. Coverslips were permanently attached to slides using clear nail polish and then stored at 4°C. Slides were examined by laser scanning confocal microscopy (LSCM) using a Zeiss Axiovert 200 microscope and LSM 510 META software (Carl Zeiss Canada Ltd., Mississauga, ON, Canada).
Results
Effect of proteasome inhibitors on ubiquitinated protein levels and hsp gene expression
The effectiveness of the proteasome inhibitors, lactacystin and MG132, in A6 cells was determined by examining the relative level of cellular proteins conjugated to ubiquitin. An increased level of ubiquitinated protein was used in other studies to confirm proteasome inhibition (Mimnaugh et al. 1997; Melikova et al. 2006). The levels of ubiquitinated proteins in heat shocked A6 cells, as determined by immunoblot analysis using an anti-ubiquitin antibody, were similar to control cells (Fig. 1a). However, the relative accumulation of ubiquitinated protein was higher in cells treated with the proteasome inhibitor, MG132 for 15 or 24 h or with 15 μM lactacystin for 15 h.
Fig. 1.
Effect of proteasome inhibitors on ubiquitinated protein and hsp mRNA levels. a Analysis of ubiquitinated protein levels in cells treated with heat shock or proteasome inhibitors. Cells were either maintained at 22°C (C), heat shocked (HS) at 33°C for 2 h followed by a 2-h recovery period at 22°C, treated with 30 μM MG132 for either 15 or 24 h, or treated with 15 μM lactacystin (Lacta) for 15 h. Protein was transferred to nitrocellulose membranes from SDS-polyacrylamide gels and probed with an anti-ubiquitin antibody as described in “Materials and methods” section. A section of a representative Ponceau S stained membrane that brackets a 42-kDa band (asterisk) is included to demonstrate efficient protein transfer. b, c The effect of proteasome inhibitors on hsp mRNA levels. Cells were either maintained at 22°C or exposed to different concentrations of lactacystin (1 to 20 μM for 15 h; a) or MG132 (1 to 50 μM for 24 h; b) at 22°C. Cells were harvested and total RNA was isolated and quantified. Total RNA (10 μg) was analyzed by Northern hybridization analysis using hsp30 and hsp70 antisense riboprobes as described in “Materials and methods” section. The bottom figure in each panel exhibits a reversible blot stain showing 18S and 28S ribosomal RNA, which confirms equal loading and quality of transfer. In each of the panels, the data are representative of five separate experiments
In the next phase of this study, we examined whether treatment of cells with proteasome inhibitors could induce the expression of hsp30 and hsp70 genes. As shown in Fig. 1b, exposure of cells to 1 μM lactacystin induced a relatively weak accumulation of hsp30 mRNA which increased with increasing concentrations of lactacystin from 5 to 20 μM. A similar finding was observed with hsp70 mRNA although it was more readily detectable at 1 μM lactacystin than hsp30 mRNA. A comparable result was observed with cells treated with MG132 (Fig. 1c). Both hsp30 and hsp70 mRNA were weakly detected with 1 μM MG132 followed by elevated levels of these messages at concentrations ranging from 5 to 50 μM. Additionally, the relative levels of hsp30 mRNA reached maximum levels at 30 and 50 μM MG132, whereas hsp70 mRNA attained peak levels at 20–50 μM.
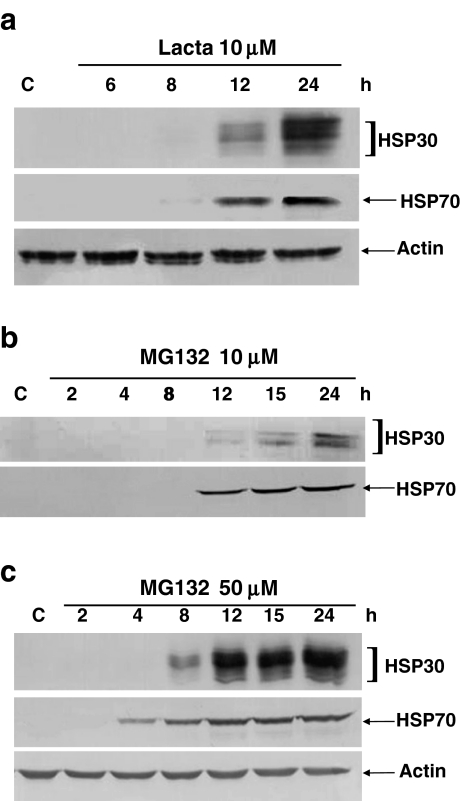
In time course studies, relatively low levels of HSP30 and HSP70 accumulation were first detected in cells treated with 10 μM lactacystin for 8 h. The accumulation of these proteins increased between 8 and 24 h (Fig. 2a). Again, the relative levels of actin remained relatively constant during lactacystin treatment. In cells treated with 10 μM MG132 (Fig. 2b), HSP30 and HSP70 accumulation were first detected after 12 h of treatment. There was a gradual increase in the relative levels of these HSPs, with the highest accumulations observed at 24 h. In cells treated with 50 μM MG132, the accumulation of HSP30 and HSP70 was first detectable at 8 and 4 h, respectively (Fig. 2c). The relative levels of both of these HSPs then increased from 12 to 24 h. Again, the relative levels of actin were not affected by MG132 treatment.
Fig. 2.
Time course of proteasome inhibitor-induced HSP30, HSP70, and actin protein. Cells were maintained at 22°C or treated with lactacystin (10 μM; a), 10 μM MG132 (b), or 50 μM MG132 (c) for different time intervals ranging from 2 to 24 h in duration. Protein was transferred to nitrocellulose membranes from SDS-polyacrylamide gels and probed with anti-HSP30, anti-HSP70, or anti-actin polyclonal antibodies as described in “Materials and methods” section. In each of the panels, the data are representative of four different experiments
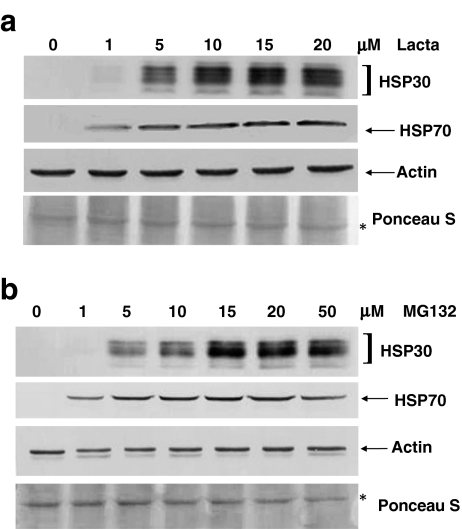
An examination of the effect of proteasome inhibitor concentration on HSP levels was carried out by means of immunoblot analysis. As shown in Fig. 3a, a relatively small amount of HSP30 accumulation was detected in cells treated with 1 μM lactacystin. HSP30 accumulation gradually increased in cells treated with concentrations ranging from 1 to 10 μM lactacystin with the highest relative levels of this protein occurring from 10 to 20 μM. The accumulation pattern of HSP70 was similar, except that it was more readily detected in cells treated with 1 μM lactacystin as observed with Northern blot analysis. Lactacystin treatment had no discernible effect on the accumulation of actin. In MG132-treated cells, there was a very low level of HSP30 accumulation at 1 μM which then increased on a relative basis from 5 to 20 μM MG132 (Fig. 3b). The highest relative amount of HSP30 protein was observed in cells treated with 20 to 50 μM MG132. Cells exposed to 1 μM MG132 had a low level of HSP70 protein accumulation, which increased when the concentration of MG132 was raised to 5 μM. A relatively constant level of accumulation of this protein occurred from 5 to 30 μM MG132 with a slight decrease detected at 50 μM. Also, the relative levels of actin were not noticeably affected by MG132 treatment.
Fig. 3.
Examination of proteasome inhibitor-induced increases in HSP30 and HSP70 levels. Cells were maintained at 22°C or treated with various concentrations of lactacystin (1 to 20 μM for 15 h; a) or MG132 (1 to 50 μM for 24 h; b). Total protein was isolated and analyzed by immunoblotting as detailed in the legend of Fig. 2. In each of the panels, the data are representative of four separate experiments
In recovery experiments, cells were treated with 30 μM MG132 for 24 h and were then allowed to recover in fresh (l)-15 media for different time periods ranging from 8 to 48 h (Fig. 4). The accumulation of HSP30 significantly (p < 0.01) decreased after 8 h of recovery and remained relatively constant from 8 to 24 h (Fig. 4a). After 48 h of recovery from MG132 exposure, the relative levels of HSP30 decreased substantially. The accumulation of HSP30 after 24 h of recovery was fourfold higher than after 48 h as determined by densitometric analysis (Fig. 4b). The accumulation of HSP70 also decreased significantly (p < 0.05) after 8 h of recovery and remained relatively constant from 8 to 24 h. Between 24 and 48 h of recovery, the relative accumulation of HSP70 also decreased by fourfold. It should be noted that similar experiments were not carried out with lactacystin since it is an irreversible proteasome inhibitor.
Fig. 4.
HSP30 and HSP70 protein levels in cells recovering from MG132 treatment. a Cells were either maintained at 22°C or exposed to 30 μM MG132 for 24 h followed by different recovery intervals in fresh (l)-15 media ranging from 8 to 48 h. Total protein was isolated and analyzed by immunoblotting as described in the legend of Fig. 2. b Image J (Version 1.38) software was used to perform densitometric analysis as outlined in “Materials and methods” section. Significant differences between 0 h of recovery and the longer recovery times are indicated as *p < 0.05 or Δp < 0.01 at an n value of 3
Localization of proteasome inhibitor-induced HSP30 accumulation
The effect of lactacystin and MG132 on the localization of HSP30 in A6 cells was determined by immunocytochemistry and LSCM (Figs. 5 and 6). HSP70 was not investigated using this technique since the affinity-purified, polyclonal anti-HSP70 antibody, which was utilized successfully in immunoblot analysis, was unable to specifically detect HSP70 by immunocytochemistry. A low amount of HSP30 accumulation was detected in 30% of cells incubated with 5 μM lactacystin for 15 h at 22°C (Fig. 5). The relative accumulation of HSP30 increased with 10 and 15 μM concentrations of lactacystin. In cells treated with different concentrations of MG132, a gradual increase in the relative level of HSP30 was noted from 1 to 30 μM (Fig. 6). With both proteasomal inhibitors, HSP30 was localized primarily in the cytoplasm, with a small amount of staining in the nucleus and no detectable accumulation in the nucleolus. Furthermore, relatively large circular HSP30 staining structures were observed in the cytoplasm of 50% of cells treated with 15 μM lactacystin or 70% of cells incubated with 30 μM MG132 (Figs. 5 and 6, white arrows). Additionally, approximately 50% of cells treated with 30 μM MG132 contained large areas within the cytoplasm that were devoid of HSP30 (Fig. 6, yellow arrows). The higher concentrations of either lactacystin or MG132 also displayed an increase in actin stress fiber disorganization and a change in general shape or morphology from cuboidal or short columnar to long columnar. Furthermore, we also noted a colocalization of F-actin and HSP30 in the cortical areas of the cytoplasm of cells treated with 10 and 15 μM lactacystin or 30 μM MG132 (Figs. 5 and 6, yellow colored regions of panels). Finally, in recovery experiments, cells that were incubated for 48 h in fresh media after an exposure to 30 μM MG132 for 24 h displayed an actin cytoskeleton pattern that was similar to control cells (Fig. 6, bottom row).
Fig. 5.
Cellular localization of lactacystin-induced HSP30 by LSCM. Cells were grown on glass coverslips and were maintained at 22°C (C) or treated with 5, 10, or 15 μM lactacystin for 15 h at 22°C. Actin and nuclei were stained directly with phalloidin conjugated to TRITC (red) and DAPI (blue), respectively. HSP30 was indirectly detected with an anti-HSP30 antibody and a secondary antibody conjugated to Alexa-488 (green). The white arrows indicate large circular cytoplasmic foci of HSP30 observed in some cells (bottom set of panels) treated with 15 μM lactacystin for 15 h. The 10-μm white scale bars are indicated at the bottom right section of each panel. These data are representative of five separate experiments
Fig. 6.
Detection of MG132-induced HSP30 localization by LSCM. Cells were grown on glass coverslips and were maintained at 22°C or treated with 5, 10, or 30 μM MG132 at 22°C for 24 h. Additionally, cells were treated with 30 μM MG132 for 24 h followed by a 48 h recovery in fresh media (bottom row). Actin and nuclei were stained directly with phalloidin conjugated to TRITC (red) and DAPI (blue), respectively. HSP30 was indirectly detected with an anti-HSP30 antibody and a secondary antibody conjugated to Alexa-488 (green). The fourth row contains an example of some cells treated with 30 μM MG132 for 24 h which showed distinct patterns of HSP30 staining. An area of HSP30 staining delineated by a red rectangle was enlarged (fourth row, third panel). The white arrows indicate large circular cytoplasmic foci of HSP30 while the yellow arrow points to an example of distinct areas within the cytoplasm where HSP30 was not detected. The 5-, 10-, or 20-μm white scale bars are indicated at the bottom right section of each panel. These data are representative of four different experiments
Involvement of HSF1 activation in MG132-induced HSP30 and HSP70 accumulation
In the present study, a HSF1 inhibitor, KNK437, was employed to determine if proteasome inhibitor-induced accumulation of HSP30 and HSP70 in A6 cells involved HSF1 activation. Previously, we demonstrated that KNK437 pretreatment inhibited both heat shock and chemical stress-induced hsp gene expression in Xenopus A6 cells (Manwell and Heikkila 2007; Voyer and Heikkila 2008). As shown in Fig. 7, when cells were pretreated with KNK437 prior to a heat shock or MG132 treatment, there was an almost complete inhibition of HSP30 and HSP70 accumulation. Subsequent densitometric analysis demonstrated that KNK437 caused inhibition of MG132-induced HSP30 and HSP70 accumulation by 99.1% and 99.8%, respectively (data not shown).
Fig. 7.
Effect of the HSF1 inhibitor, KNK437 (KNK), on the levels of HSP30 and HSP70 protein in cells treated with MG132. Cells were maintained at 22°C or subjected to 33°C for 2 h (followed by a 2-h recovery at 22°C) or 100 μM KNK437 for 6 h at 22°C. Additionally, cells were pretreated with 100 μM KNK437 for 6 h at 22°C and then exposed to a 33°C heat shock for 2 h (followed by a 2-h recovery at 22°C) or 30 μM MG132 for 12 h. Total protein was isolated and analyzed by immunoblotting as detailed in the legend of Fig. 2. These data are representative of three separate experiments
Effect of concurrent MG132 and elevated temperature on HSP accumulation
The effect of treating cells simultaneously with combined MG132 plus heat shock on the accumulation of HSP30 and HSP70 is illustrated in Fig. 8. Cells exposed to a 30°C heat shock for 8 h or 30 μM MG132 for 8 h at 22°C displayed a relatively low level of HSP30 and HSP70 accumulation (Fig. 8a). However, when these two stressors were applied concurrently for 8 h, the accumulation was higher than the sum of the accumulation of each stressor alone as determined by densitometric analysis (Fig. 8b). In cells treated with 30 μM MG132 for 8 h, the relative accumulation of HSP30 and HSP70 increased as the temperature was elevated from 22°C to 30°C. The highest accumulation was observed at 30 μM MG132 plus 28 or 30°C heat shock for 8 h.
Fig. 8.
Analysis of HSP30 and HSP70 protein levels in cells exposed to elevated temperatures plus MG132. a Cells were maintained at 22°C or treated concurrently with 30 μM MG132 plus different temperatures ranging from 22°C to 30°C for 8 h. Total protein was isolated and analyzed by immunoblotting as outlined in the legend of Fig. 2. b Image J (Version 1.38) software was used to perform densitometric analysis as outlined in the legend of Fig. 4. Significant differences between the maximum signal and other treatments are indicated as *p < 0.05 or Δp < 0.01. These data are representative of three separate experiments
The effect of MG132 on the acquisition of thermotolerance in A6 cells
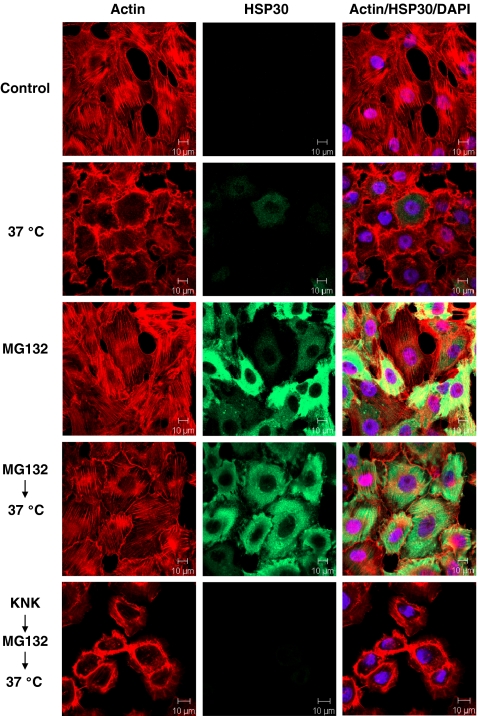
A6 cells were pretreated with MG132 prior to a thermal challenge at 37°C to assess whether this agent could confer a state of thermotolerance. Shifting the incubation temperature of cells from 22°C directly to a 37°C thermal challenge for 1 h resulted in the collapse of the actin cytoskeleton (Fig. 9). Previously, it was established that pretreatment of A6 cells with a 33°C heat shock resulted in HSP accumulation and an acquired state of thermotolerance (Manwell and Heikkila 2007). In this study, cells treated with 30 μM MG132 for 12 h had a relatively high accumulation of HSP30. Therefore, these conditions were employed in the present thermotolerance experiments. Approximately 95% of cells pretreated with 30 μM MG132 for 12 h prior to a thermal challenge displayed intact stress fibers. Thus, MG132 pretreatment was effective at conferring thermotolerance in A6 cells. The accumulation of HSPs may be responsible for this acquired state of thermotolerance by MG132 since KNK437 pretreatment, which inhibited the accumulation of HSP30 and HSP70, also resulted in cytoskeletal collapse (Fig. 9, last row).
Fig. 9.
Cytoprotective effects of pretreating cells with MG132 prior to a 37°C thermal challenge. Cells were grown on glass coverslips and were maintained at 22°C, subjected to a 37°C thermal challenge for 1 h or exposed to 30 μM MG132 for 12 h. Additionally, cells were subjected to 30 μM MG132 for 12 h prior to a 37°C thermal challenge for 1 h. In the last row, cells were pretreated with 100 μM KNK437 for 6 h before exposure to 30 μM MG132 for 12 h and a subsequent thermal challenge at 37°C. The thermal challenges at 37°C were followed by a 2-h recovery period at 22°C. Actin and nuclei were stained directly with phalloidin conjugated to TRITC (red) and DAPI (blue), respectively. HSP30 was indirectly detected with an anti-HSP30 antibody and a secondary antibody conjugated to Alexa-488 (green). These data are representative of four separate experiments
Discussion
The present study has shown, in an amphibian system, that exposure to the proteasomal inhibitors, MG132 or lactacystin, induced an increase in the levels of ubiquitinated protein and an upregulation of hsp30 and hsp70 gene expression. Moreover, this accumulation occurred in a dose- and time-dependent manner. Previously, enhanced hsp gene expression in response to proteasome inhibition was described in yeast, rainbow trout, Drosophila, mouse, rat, dog, and human cultured cells (Bush et al. 1997; Lee and Goldberg 1998b; Pritts et al. 2002; Stangl et al. 2002; Le Goff et al. 2004; Dembla-Rajpal et al. 2004; Lundgren et al. 2005; Awasthi and Wagner 2005; Noonan et al. 2007, 2008). For example, in yeast, inhibition of protein degradation by MG132 caused a coordinate induction of a number of HSPs, including HSP70 and HSP104 (Lee and Goldberg 1998b), while in Drosophila cell lines, genome microarrays uncovered that proteasomal inhibition by MG132 treatment enhanced the expression of HSP27 (Lundgren et al. 2005). Furthermore, in rainbow trout liver cells, MG132 treatment produced an increase in the accumulation of HSP70 (Le Goff et al. 2004). Finally, in mammalian systems, proteasomal inhibition induced HSP70 in Madin–Darby canine kidney cells; HSP27, HSP60, HSP70, and HSP90 in neonatal rat cardiomyocytes; HSP72 in Caco-2 human intestinal epithelial cells; and HSP27 and αB-crystallin in αTN4-1 murine lens epithelial cells (Bush et al. 1997; Stangl et al. 2002; Pritts et al. 2002; Awasthi and Wagner 2005).
The molecular and cellular mechanisms that lead to stress-inducible hsp gene expression during proteasomal inhibition are unclear. Since the proteasome degrades about 80–90% of all proteins, inhibition of this process results in a substantial increase in the concentration of total cellular protein (Lee and Goldberg 1998a). Damaged or aged proteins, normally degraded by the proteasome, are prone to misfolding, which can result in the exposure of their hydrophobic amino acid residues and subsequent aggregation. The accumulation of unfolded cellular protein by proteasomal inhibition may therefore trigger the activation of HSF. In the present study, KNK437 inhibited HSP30 and HSP70 accumulation suggesting that the activation of HSF1 was associated with MG132-induced hsp gene expression. This finding is supported by the similar pattern of hsp mRNA and HSP accumulation in response to different concentrations of lactacystin or MG132 in A6 cells. In other studies, HSF activation in response to MG132 was reported in the chicken erythroblast cell line, HD6, where the activities of HSF1, HSF2, and HSF3 were upregulated (Kawazoe et al. 1998). Moreover, in mouse embryonic fibroblast cells, both MG132 and lactacystin induced hyperphosphorylation, trimerization, and HSE-binding activity of HSF1 (Kim et al. 1999).
The present study also investigated the pattern of hsp gene expression in A6 cells recovering from MG132 treatment. Cells treated with 30 μM MG132 for 24 h had a relatively high accumulation of HSP30 and HSP70, which remained elevated for up to 24 h after the removal of MG132. At 48 h posttreatment, the relative levels of these HSPs decreased substantially. A prolonged accumulation of HSPs in cells following proteasomal inhibition was also documented in rat neonatal cardiomyocytes and Chinese hamster ovary cells (Stangl et al. 2002; Kovacs et al. 2006). The prolonged accumulation of HSPs in recovery experiments may be due to MG132's long half-life in the cell and as such would continue to inhibit proteasome function after MG132 removal (Lee and Goldberg 1998a). Furthermore, it may take the cell additional time to degrade all of the ubiquitinated cellular proteins that accumulated during proteasomal inhibition. The combination of these two possibilities may explain the prolonged accumulation of HSP30 and HSP70 in A6 cells recovering from MG132 exposure.
Immunocytochemistry and LSCM were employed to determine the localization of HSP30 in A6 cells exposed to MG132 or lactacystin. In both instances, HSP30 accumulation occurred primarily in the cytoplasm in a punctate pattern with a lesser amount in the nucleus. The punctate pattern of HSP30 accumulation in A6 cells may be due to the stress-induced formation of HSP30 multimeric structures as previously reported with heat shock treatment of Xenopus cultured cells (Ohan et al. 1998; Gellalchew and Heikkila 2005; Manwell and Heikkila 2007). It was also noted that in A6 cells treated with low concentrations of proteasome inhibitors, only a portion of the cell population showed detectable HSP30. However, this percentage of HSP30 staining cells increased with higher proteasome inhibitor concentrations. In contrast, heat shock treated cells consistently showed HSP30 staining in all A6 cells. At this time, the reason for the unresponsiveness of certain cells within the A6 cell population to proteasome inhibitor-induced HSP30 accumulation is not known. However, we have found a similar situation in A6 cells treated with low concentrations of cadmium chloride or herbimycin A (Voyer and Heikkila 2008; Woolfson and Heikkila 2009). Additionally, in a comparison of two mammalian cultured cell lines treated with 10 μM MG132, only 15% of HeLa cells showed the presence of HSF1 granules in contrast to almost 100% of K562 cells (Holmberg et al 2000).
The current study also determined that treatment of A6 cells with relatively high concentrations of MG132 or lactacystin caused a disruption in the organization of the actin cytoskeleton. Cytoskeletal disruption in response to proteasomal inhibition has been reported in both mammals and plants (Sheng et al. 2006; Csizmadia et al. 2008). In A6 cells, the actin cytoskeleton was also reported to be sensitive to other stresses including heat shock, sodium arsenite, and cadmium chloride exposure (Gellalchew and Heikkila 2005; Manwell and Heikkila 2007; Voyer and Heikkila 2008; Woolfson and Heikkila 2009). While the exact mechanism for proteasome inhibitor-induced cytoskeletal disruption is not known, it has been suggested that the accumulation of unfolded proteins may activate a signal transduction cascade that activates numerous cellular events including cytoskeletal dysregulation (Csizmadia et al. 2008).
Some A6 cells treated with higher concentrations of MG132 or lactacystin displayed relatively large HSP30 staining foci. While the identity of these structures is unknown, it is possible that they may be inclusion bodies containing unfolded protein bound to HSP30, especially given its role as a molecular chaperone (Fernando and Heikkila 2000; Heikkila 2003; 2004). In support of this possibility, Garcia-Mata et al. (1999) reported that unfolded proteins that begin to aggregate first form large cytoplasmic inclusion bodies before producing an aggresome (Garcia-Mata et al. 1999). Also, in experiments with human glioma cells, it was found that HSP27 and αB-crystallin colocalized with inclusion bodies and the aggresome in response to proteasome inhibition (Ito et al. 2002). Another unique HSP30 accumulation pattern observed in some A6 cells treated with high concentrations of MG132 was the presence of distinct regions within the cytoplasm where HSP30 did not accumulate. The identity and the mechanism involved in the formation of this pattern are not known. One possibility is that unfolded proteins localize to the large cytoplasmic HSP30-containing foci. Therefore, the surrounding areas, which contain very little unfolded protein, would have a very low level of HSP30 since it is not required. In support of this possibility, a study by Johnston et al. (1998) showed that the green fluorescent protein-tagged cystic fibrosis transmembrane conductance regulator accumulated within inclusion bodies and the aggresome in response to proteasomal inhibition and was substantially depleted elsewhere in the cytoplasm. Additionally, it is likely that the cortical cytoplasmic areas devoid of HSP30 are associated with localized areas of actin cytoskeletal disruption. This possibility is in line with the potential role of sHSPs in stabilization of the actin cytoskeleton (MacRae 2000; Van Montfort et al. 2002). In support of this theory, we found that relatively high concentrations of lactacystin (10 or 15 μM) or MG132 (30 μM) produced a colocalization of HSP30 and actin in the cortical cytoplasm, an area associated with actin assembly/disassembly. Also, our results support a recent model proposed by Doshi et al. (2009) relating the stability of the actin cytoskeleton to HSP27 in the regulation of movement in heat shocked human cancer cells. This latter study is supported by the studies of Jia et al. (2009) examining the effect of thiolutin, an organosulfur compound, on cytoskeletal structure and cell adhesion of zebrafish endothelial cells.
Finally, we found that MG132-treated A6 cells acquired a state of thermotolerance since they were able to survive a subsequent thermal challenge at 37°C. Thermotolerance following proteasomal inhibition was reported in yeast, rat cardiomyocytes, canine kidney cells, and also human fibroblast cells (Bush et al. 1997; Lee and Goldberg 1998b; Luss et al. 2002; Bonelli et al. 2004). In the present study, hsp gene expression was required for this state of thermotolerance since it was repressed by KNK437. Together, these findings suggest that MG132-induced HSP accumulation can protect cells from injury or other stresses.
In conclusion, the present study has shown, for the first time in amphibian cells, that inhibition of proteasomal activity can induce the upregulation of HSPs, the accumulation of ubiquitinated protein, and a disruption of the actin cytoskeleton. Furthermore, a combination of mild heat shock and proteasomal inhibitors can act synergistically to enhance HSP accumulation. Given the numerous advantages of the Xenopus embryonic system (Heikkila 2004), future work will focus on the impact of proteasomal inhibition on hsp gene expression during early development. Further analysis of the relationship between proteasome dysfunction and hsp gene expression is important given the fact that proteasome inhibitors are being tested clinically to treat a range of human pathologies (Eldridge and O'Brien 2009).
Acknowledgments
This research was supported by a Natural Sciences and Engineering Research Council (NSERC) grant to JJH. JJH is the recipient of a Canada Research Chair in Stress Protein Gene Research.
References
- Abdulle R, Mohindra A, Fernando P, Heikkila JJ. Xenopus small heat shock proteins, Hsp30C and Hsp30D, maintain heat- and chemically-denatured luciferase in a folding-competent state. Cell Stress Chaperones. 2002;7:6–16. doi: 10.1379/1466-1268(2002)007<0006:XSHSPH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. J Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Arrigo AP, Landry J. Expression and function of the low-molecular weight heat shock proteins. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1994. pp. 335–373. [Google Scholar]
- Awasthi N, Wagner BJ. Upregulation of heat shock protein expression by proteasome inhibition: an antiapoptotic mechanism in the lens. Invest Opthalmol Vis Sci. 2005;46:2082–2091. doi: 10.1167/iovs.05-0002. [DOI] [PubMed] [Google Scholar]
- Bonelli MA, Alfieri RR, Desenzani S, Petronini PG, Borghetti AF. Proteasome inhibition increases HuR level, restores heat-inducible HSP72 expression and thermotolerance in WI-38 senescent human fibroblasts. Exp Gerontol. 2004;39:423–432. doi: 10.1016/j.exger.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Bova MP, Yaron O, Huang QL, Ding LL, Haley DA, Stewart PI, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci USA. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J, Ehrnsperger M, Gaestel M, Walke S. Purification and characterization of small heat shock proteins. Methods Enzymol. 1998;290:339–349. doi: 10.1016/S0076-6879(98)90030-1. [DOI] [PubMed] [Google Scholar]
- Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- Csizmadia V, Raczynski A, Csizmadia E, Fedyk ER, Rottman J, Alden CL. Effect of an experimental proteasome inhibitor on the cytoskeleton, cytosolic protein turnover, and induction in the neuronal cells in vitro. Neurotoxicology. 2008;29:232–243. doi: 10.1016/j.neuro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Dembla-Rajpal N, Seipelt R, Wang Q, Rymond BC. Protesome inhibition alters the transcription of multiple yeast genes. Biochim Biophys Acta. 2004;1680:34–45. doi: 10.1016/j.bbaexp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Doshi BM, Hightower LE, Lee J. The role of Hsp27 and actin in the regulation of movement in human cancer cells responding to heat shock. Cell Stress Chaperones. 2009;14:445–457. doi: 10.1007/s12192-008-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge AG, O'Brien T (2009) Therapeutic strategies within the ubiquitin proteasome system. Cell Death Differ. doi:10.1038/cdd.2009.82 [DOI] [PubMed]
- Feige U, Morimoto RI, Yahara I, Polla BS. Stress-inducible cellular responses. Basel: Birkhauser; 1996. [Google Scholar]
- Fernando P, Heikkila JJ. Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity. Cell Stress Chaperones. 2000;5:148–159. doi: 10.1379/1466-1268(2000)005<0148:FCOXSH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P, Abdulle R, Mohindra A, Guillemette JG, Heikkila JJ. Mutation or deletion of the C-terminal tail affects the function and structure of Xenopus laevis small heat shock protein, hsp30. Comp Biochem Physiol B Biochem Mol Biol. 2002;133:95–103. doi: 10.1016/S1096-4959(02)00110-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Bebok Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol. 1999;146:1239–1254. doi: 10.1083/jcb.146.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauley J, Young JTF, Heikkila JJ. Intracellular localization of HSP110 in Xenopus laevis A6 kidney epithelial cells. Comp Biochem Physiol A Mol Integr Physiol. 2008;151:133–138. doi: 10.1016/j.cbpa.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Gellalchew M, Heikkila JJ. Intracellular localization of Xenopus small heat shock protein, hsp30, in A6 kidney epithelial cells. Cell Biol Int. 2005;29:221–227. doi: 10.1016/j.cellbi.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Haas A, Rose I. The mechanism of ubiquitin activating enzyme: a kinetic and equilibrium analysis. J Biol Chem. 1982;257:10329–10337. [PubMed] [Google Scholar]
- Heikkila JJ. Expression and function of small heat shock protein genes during Xenopus development. Semin Cell Dev Biol. 2003;14:259–266. doi: 10.1016/j.semcdb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ. Regulation and function of small heat shock protein genes during amphibian development. J Cell Biochem. 2004;93:672–680. doi: 10.1002/jcb.20237. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ, Ohan N, Tam Y, Ali A. Heat shock protein gene expression during early Xenopus laevis development. Cell Mol Life Sci. 1997;53:114–121. doi: 10.1007/PL00000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg CI, Illman SA, Kallio M, Mikhailov A, Sistonen L. Formation of nuclear HSF1 granules varies depending on stress stimuli. Cell Stress Chaperones. 2000;5:219–228. doi: 10.1379/1466-1268(2000)005<0219:FONHGV>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irobi J, Impe K, Seeman P, Jordanova A, Dierick I, Verpoorten N, Michalik A, Vriendt E, Jacobs A, Gerwen V, Vennekens K, Mazanec R, Tournev I, Hilton-Jones D, Talbot K, Kremensky I, Bosch L, Robberecht W, Vandekerckhove J, Broeckhoven C, Gettemans J, Jonghe P, Timmerman V. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Garcia-Mata R, Sztul E, Kato K. Inhibition of proteasomes induces accumulation, phosphorylation, and recruitment of HSP27 and alphaB-crystallin to aggresomes. J Biochem. 2002;131:593–603. doi: 10.1093/oxfordjournals.jbchem.a003139. [DOI] [PubMed] [Google Scholar]
- Jia Y, Wu S-L, Isenberg, JS, Dai S, Sipes JM, Field L, Zeng B, Bandle RW, Ridnour LA, Wink DA, Ramchandran R, Karger BL, Roberts DD (2009) Thiolutin inhibits endothelial cell adhesion by perturbing Hsp27 interactions with components of the actin and intermediate filament cytoskeleton. Cell Stress Chaperones. doi:10.1007/s12192-009-0130-0 [DOI] [PMC free article] [PubMed]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschinski DM. On heat and cells and proteins. News Physiol Sci. 2004;19:11–15. doi: 10.1152/nips.01403.2002. [DOI] [PubMed] [Google Scholar]
- Kawazoe Y, Nakai A, Tanabe M, Nagata K. Proteasome inhibition leads to the activation of all members of the heat shock factor family. Eur J Biochem. 1998;255:356–362. doi: 10.1046/j.1432-1327.1998.2550356.x. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim SH, Li GC. Proteasome inhibitors MG132 and lactacystin hyperphosphorylate HSF1 and induce hsp70 and hsp27 expression. Biochem Biophys Res Commun. 1999;254:264–268. doi: 10.1006/bbrc.1998.9840. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Lentini KM, Ingano LM, Kovacs DM. Presenilin 1 forms aggreesomal deposits in response to heat shock. J Mol Neurosci. 2006;29:9–19. doi: 10.1385/JMN:29:1:9. [DOI] [PubMed] [Google Scholar]
- Lang L, Miskovic D, Fernando P, Heikkila JJ. Spatial pattern of constitutive and heat shock-induced expression of the small heat shock protein gene family, hsp30, in Xenopus laevis tailbud embryos. Dev Genet. 1999;25:365–374. doi: 10.1002/(SICI)1520-6408(1999)25:4<365::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lang L, Miskovic D, Lo M, Heikkila JJ. Stress-induced, tissue-specific enrichment of hsp70 mRNA accumulation in Xenopus laevis embryos. Cell Stress Chaperones. 2000;5:36–44. doi: 10.1379/1466-1268(2000)005<0036:SITSEO>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff P, Drean Y, Person C, Jossic-Corcos C, Ainouche A, Michel D. Intracellular trafficking of heat shock factor 2. Exp Cell Res. 2004;294:480–493. doi: 10.1016/j.yexcr.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/S0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:30–38. doi: 10.1128/mcb.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren J, Masson P, Mirzaei Z, Young P. Identification and characterization of a Drosophila proteasome regulatory network. Mol Cell Biol. 2005;25:4662–4675. doi: 10.1128/MCB.25.11.4662-4675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luss H, Schmitz W, Neumann J. A proteasome inhibitor confers cardioprotection. Cardiovasc Res. 2002;54:140–151. doi: 10.1016/S0008-6363(02)00232-8. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwell LA, Heikkila JJ. Examination of KNK437- and quercetin-mediated inhibition of heat shock-induced heat shock protein gene expression in Xenopus laevis cultured cells. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:521–530. doi: 10.1016/j.cbpa.2007.06.422. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Melikova MS, Kondratov KA, Kornilova ES. Two different stages of epidermal growth factor (EGF) receptor endocytosis are sensitive to free ubiquitin depletion produced by proteasome inhibitor MG132. Cell Biol Int. 2006;30:31–43. doi: 10.1016/j.cellbi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Mimnaugh EG, Chen HY, Davie JR, Celis JE, Neckers L. Rapid deubiquitination of nucleosomal histones in human tumour cells caused by proteasome inhibitors and stress response inducers: effects on replication, transcription, translation, and the cellular stress response. Biochemistry. 1997;36:14418–14426. doi: 10.1021/bi970998j. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Jurivich DA, Kroeger PE, Mathur SK, Murphy SP, Nakai A, Sarge K, Abravaya K, Sistonen LT. Regulation of heat shock gene transcription by a family of heat shock factors. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1994. pp. 417–455. [Google Scholar]
- Neutzner A, Benard G, Youle RJ, Karbowski M. Role of the ubiquitin conjugation system in the maintenance of mitochondrial homeostasis. Ann N Y Acad Sci. 2008;1147:242–253. doi: 10.1196/annals.1427.012. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Giardina C, Hightower LE. Hsp70B' regulation and function. Cell Stress Chaperones. 2007;12:393–402. doi: 10.1379/CSC-278e.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Fournier G, Hightower LE. Surface expression of Hsp70B′ in response to proteasome inhibition in human colon cells. Cell Stress Chaperones. 2008;13:105–110. doi: 10.1007/s12192-007-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohan NW, Tam Y, Heikkila JJ. Heat-shock-induced assembly of Hsp30 into high molecular weight aggregates in Xenopus laevis cultured cells. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:381–389. doi: 10.1016/S0305-0491(97)00364-7. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Takahashi A, Yokota S, Ohnishi T. Effects of a heat shock protein inhibitor KNK437 on heat sensitivity and heat tolerance in human squamous cell carcinoma cell lines differing in p53 status. Int J Radiat Biol. 2004;80:607–614. doi: 10.1080/09553000412331283470. [DOI] [PubMed] [Google Scholar]
- Ovakim D, Heikkila JJ. Effect of histone deacetylase inhibitors on the expression of heat shock protein genes, hsp70 and hsp30, during Xenopus laevis development. Genesis. 2003;36:88–96. doi: 10.1002/gene.10202. [DOI] [PubMed] [Google Scholar]
- Pritts TA, Hungness ES, Hershko DD, Robb BW, Sun X, Luo GJ, Fischer JE, Wong HR, Hasselgren PO. Proteasome inhibitors induce heat shock response and increase IL-6 expression in human intestinal epithelial cells. Am J Physiol Regul Integr Comp Physiol. 2002;282:1016–1026. doi: 10.1152/ajpregu.00492.2001. [DOI] [PubMed] [Google Scholar]
- Quinlan R, Ijssel P. Fatal attraction: when chaperone turns harlot. Nature Med. 1999;5:25–26. doi: 10.1038/4704. [DOI] [PubMed] [Google Scholar]
- Rivett AJ, Palmer A, Knecht E. Electron microscopic localization of the multicatalytic proteinase complex in rat liver and in cultured cells. J Histochem Cytochem. 1992;40:1165–1172. doi: 10.1177/40.8.1619280. [DOI] [PubMed] [Google Scholar]
- Ross CA, Pickart CM. The ubiquitin–proteasome pathway in Parkinson's disease and other neurodegenerative diseases. Trends Cell Biol. 2004;14:703–711. doi: 10.1016/j.tcb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- Scherrer K, Bey F. The prosomes (multicatalytic proteinases; proteasomes) and their relationship to the untranslated messenger ribonucleoproteins, the cytoskeleton, and cell differentiation. Prog Nucleic Acid Res Mol Biol. 1994;49:1–63. doi: 10.1016/S0079-6603(08)60047-1. [DOI] [PubMed] [Google Scholar]
- Sheng X, Hu Z, Lu H, Wang X, Baluska F, Samaj J, Lin J. Roles of the ubiquitin/proteasome pathway in pollen tube growth with emphasis on MG132-induced alterations in ultrastructure, cytoskeleton, and cell wall components. Plant Physiol. 2006;141:1578–1590. doi: 10.1104/pp.106.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl K, Gunther C, Frank T, Lorenz M, Meiners S, Ropke T, Stelter L, Moobed M, Baumann G, Kloetzel PM, Stangl V. Inhibition of the ubiquitin–proteasome pathway induces differential heat-shock protein response in cardiomyocytes and renders early cardiac protection. Biochem Biophys Res Commun. 2002;291:542–549. doi: 10.1006/bbrc.2002.6476. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Yamakawa N, Mori E, Ohnishi K, Yokota S, Sugo N, Aratani Y, Koyama H, Ohnishi T. Development of thermotolerance requires interaction between polymerase-β and heat shock proteins. Cancer Sci. 2008;99:973–978. doi: 10.1111/j.1349-7006.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Calderwood SK. Regulation of heat shock gene transcription in neuronal cells. Int J Hyperthermia. 2005;21:433–444. doi: 10.1080/02656730500165514. [DOI] [PubMed] [Google Scholar]
- Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein α-crystallin family of molecular chaperones. Adv Protein Chem. 2002;59:105–156. doi: 10.1016/S0065-3233(01)59004-X. [DOI] [PubMed] [Google Scholar]
- Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer J, Heikkila JJ. Comparison of the effect of heat shock factor inhibitor, KNK437, on heat shock- and chemical stress-induced hsp30 gene expression in Xenopus laevis A6 cells. Comp Biochem Physiol A Mol Integr Physiol. 2008;151:253–261. doi: 10.1016/j.cbpa.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Woolfson JP, Heikkila JJ. Examination of cadmium-induced expression of the small heat shock protein gene, hsp30, in Xenopus laevis A6 kidney epithelial cells. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:91–99. doi: 10.1016/j.cbpa.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Young JTF, Gauley J, Heikkila JJ. Simultaneous exposure of Xenopus A6 kidney epithelial cells to concurrent mild sodium arsenite and heat stress results in enhanced hsp30 and hsp70 gene expression and acquisition of thermotolerance. Comp Biochem Physiol A Mol Integr Physiol. 2009;153:417–424. doi: 10.1016/j.cbpa.2009.03.024. [DOI] [PubMed] [Google Scholar]