Abstract
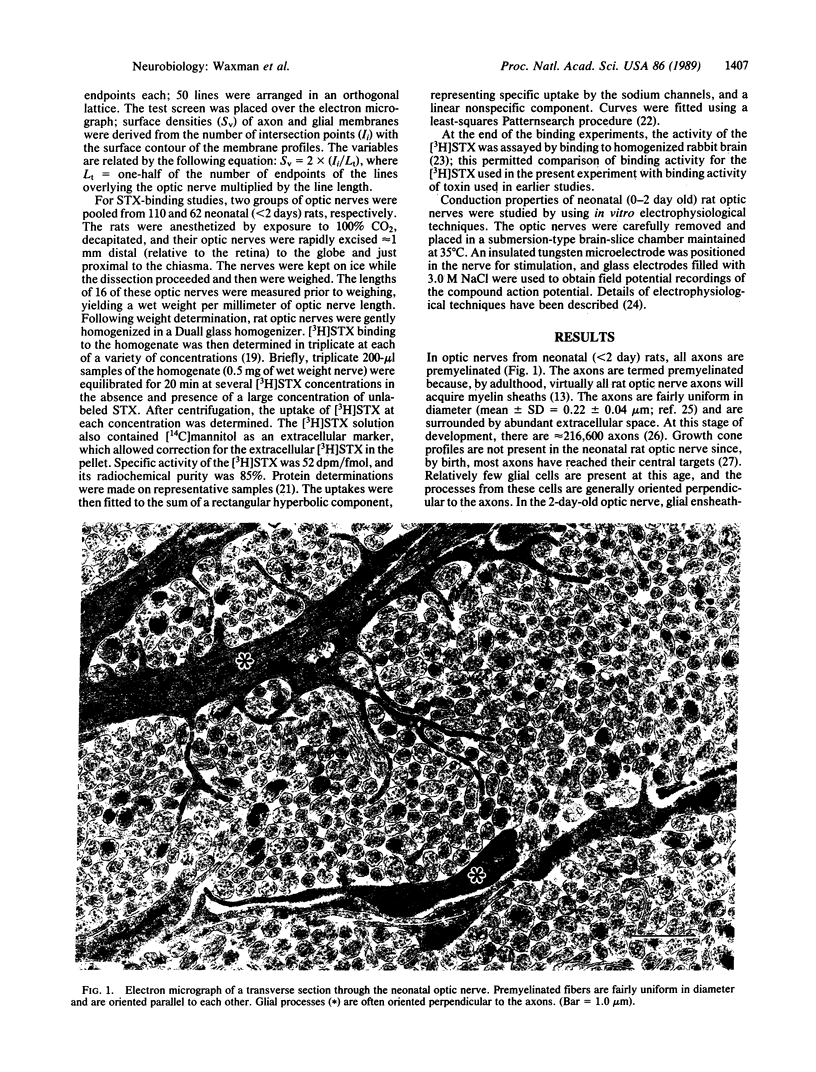
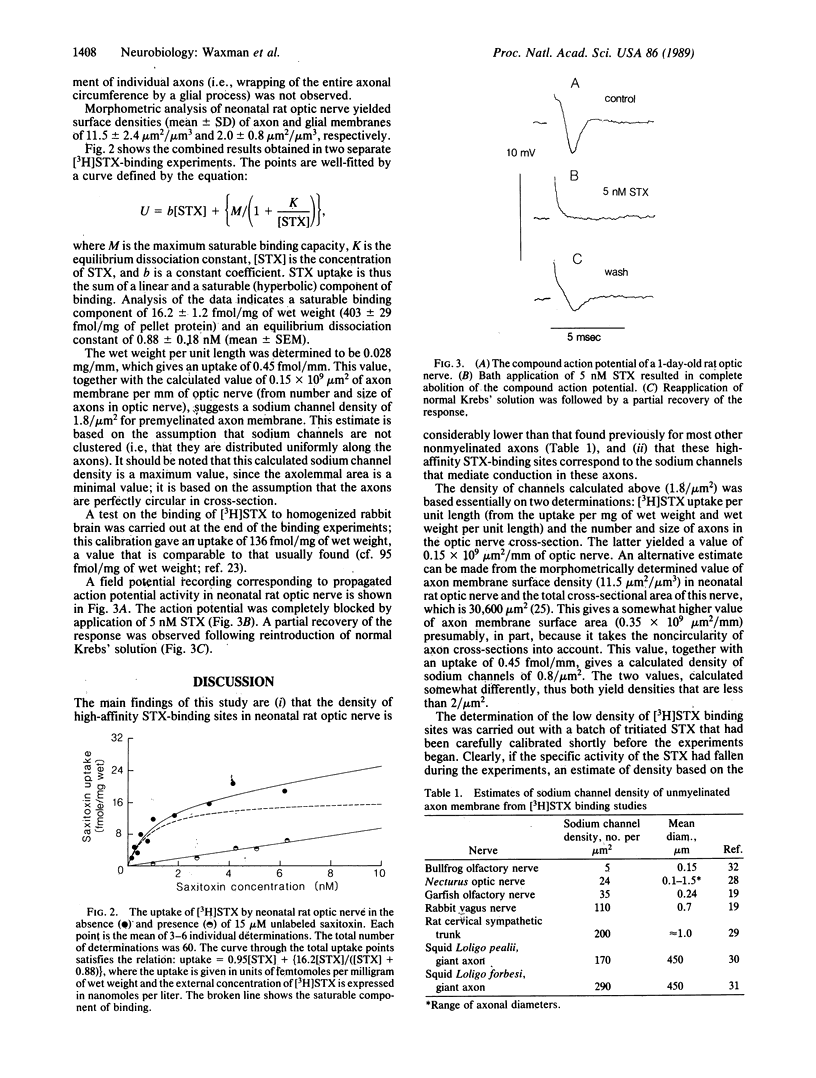
The density of sodium channels in premyelinated axons was estimated from measurements of the binding of [3H]saxitoxin to neonatal rat optic nerve. The maximum saturable binding capacity of the nerve was 16.2 +/- 1.2 fmol/mg of wet weight, with an equilibrium dissociation constant of 0.88 +/- 0.18 nM (mean +/- SEM). These values correspond to a high-affinity saxitoxin-binding site density of approximately 2/microns 2 within premyelinated axon membrane. Action potential propagation in neonatal rat optic nerve is completely blocked by 5 nM saxitoxin, indicating that action potential electrogenesis is mediated by channels that correspond to high-affinity saxitoxin-binding sites. These results demonstrate that action potential conduction is supported by a low density of sodium channels in this system. Since the internodal axon membrane of myelinated fibers may contain a low density of sodium channels, it is possible that restoration of conduction in some demyelinated fibers may not require additional sodium channel incorporation into the demyelinated axon membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H. Conduction velocity and gating current in the squid giant axon. Proc R Soc Lond B Biol Sci. 1975 Apr 29;189(1094):81–86. doi: 10.1098/rspb.1975.0043. [DOI] [PubMed] [Google Scholar]
- BISHOP G. H., SMITH J. M. THE SIZE OF NERVE FIBERS SUPPLYING CEREBRAL CORTEX. Exp Neurol. 1964 Jun;9:483–501. doi: 10.1016/0014-4886(64)90056-1. [DOI] [PubMed] [Google Scholar]
- Black J. A., Foster R. E., Waxman S. G. Rat optic nerve: freeze-fracture studies during development of myelinated axons. Brain Res. 1982 Oct 28;250(1):1–20. doi: 10.1016/0006-8993(82)90948-9. [DOI] [PubMed] [Google Scholar]
- Black J. A., Waxman S. G., Ransom B. R., Feliciano M. D. A quantitative study of developing axons and glia following altered gliogenesis in rat optic nerve. Brain Res. 1986 Aug 13;380(1):122–135. doi: 10.1016/0006-8993(86)91436-8. [DOI] [PubMed] [Google Scholar]
- Black J. A., Waxman S. G., Smith M. E. Macromolecular structure of axonal membrane during acute experimental allergic encephalomyelitis in rat and guinea pig spinal cord. J Neuropathol Exp Neurol. 1987 Mar;46(2):167–184. doi: 10.1097/00005072-198703000-00005. [DOI] [PubMed] [Google Scholar]
- Bostock H., Sears T. A. Continuous conduction in demyelinated mammalian nerve fibers. Nature. 1976 Oct 28;263(5580):786–787. doi: 10.1038/263786a0. [DOI] [PubMed] [Google Scholar]
- Bostock H., Sears T. A. The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J Physiol. 1978 Jul;280:273–301. doi: 10.1113/jphysiol.1978.sp012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coria F., Silos I., Fernandez R., Monton F., Lafarga M. Demyelination-induced plasticity in the axon membrane: an ultrastructural cytochemical study of lead neuropathy in the rat. Neurosci Lett. 1985 Aug 5;58(3):359–364. doi: 10.1016/0304-3940(85)90081-3. [DOI] [PubMed] [Google Scholar]
- Crespo D., O'Leary D. D., Cowan W. M. Changes in the numbers of optic nerve fibers during late prenatal and postnatal development in the albino rat. Brain Res. 1985 Mar;351(1):129–134. doi: 10.1016/0165-3806(85)90238-x. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Ulfhake B. Relations between cell body size, axon diameter and axon conduction velocity of triceps surae alpha montoneurons during the postnatal development in the cat. J Comp Neurol. 1979 Dec 15;188(4):679–686. doi: 10.1002/cne.901880410. [DOI] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester J., Peters A. Nerve fibres in optic nerve of rat. Nature. 1967 Apr 15;214(5085):245–247. doi: 10.1038/214245a0. [DOI] [PubMed] [Google Scholar]
- Foster R. E., Connors B. W., Waxman S. G. Rat optic nerve: electrophysiological, pharmacological and anatomical studies during development. Brain Res. 1982 Mar;255(3):371–386. doi: 10.1016/0165-3806(82)90005-0. [DOI] [PubMed] [Google Scholar]
- Foster R. E., Whalen C. C., Waxman S. G. Reorganization of the axon membrane in demyelinated peripheral nerve fibers: morphological evidence. Science. 1980 Nov 7;210(4470):661–663. doi: 10.1126/science.6159685. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vijverberg H. P., Romey G., Vigne P., Lazdunski M. Different functional states of tetrodotoxin sensitive and tetrodotoxin resistant Na+ channels occur during the in vitro development of rat skeletal muscle. Pflugers Arch. 1984 Oct;402(2):121–128. doi: 10.1007/BF00583323. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., STAMPFLI R. Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibers. J Physiol. 1951 Feb;112(3-4):496–508. doi: 10.1113/jphysiol.1951.sp004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C., Waxman S. G. Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the development of nodes of Ranvier and axoglial relations. J Comp Neurol. 1984 Mar 20;224(1):25–37. doi: 10.1002/cne.902240103. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic channels in nerve membranes. Prog Biophys Mol Biol. 1970;21:1–32. doi: 10.1016/0079-6107(70)90022-2. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. The optimum density of sodium channels in an unmyelinated nerve. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):297–300. doi: 10.1098/rstb.1975.0010. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lund R. D., Bunt A. H. Prenatal development of central optic pathways in albino rats. J Comp Neurol. 1976 Jan 15;165(2):247–264. doi: 10.1002/cne.901650209. [DOI] [PubMed] [Google Scholar]
- Oldfield B. J., Bray G. M. Differentiation of the nodal and internodal axolemma in the optic nerves of neonatal rats. J Neurocytol. 1982 Aug;11(4):627–640. doi: 10.1007/BF01262428. [DOI] [PubMed] [Google Scholar]
- Pellegrino R. G., Ritchie J. M. Sodium channels in the axolemma of normal and degenerating rabbit optic nerve. Proc R Soc Lond B Biol Sci. 1984 Aug 22;222(1227):155–160. doi: 10.1098/rspb.1984.0056. [DOI] [PubMed] [Google Scholar]
- Pellegrino R. G., Spencer P. S., Ritchie J. M. Sodium channels in the axolemma of unmyelinated axons: a new estimate. Brain Res. 1984 Jul 9;305(2):357–360. doi: 10.1016/0006-8993(84)90442-6. [DOI] [PubMed] [Google Scholar]
- Poolos N. P., Mauk M. D., Kocsis J. D. Activity-evoked increases in extracellular potassium modulate presynaptic excitability in the CA1 region of the hippocampus. J Neurophysiol. 1987 Aug;58(2):404–416. doi: 10.1152/jn.1987.58.2.404. [DOI] [PubMed] [Google Scholar]
- Rasminsky M., Sears T. A. Internodal conduction in undissected demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):323–350. doi: 10.1113/jphysiol.1972.sp010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M. Distribution of saxitoxin-binding sites in mammalian neural tissue. Ann N Y Acad Sci. 1986;479:385–401. doi: 10.1111/j.1749-6632.1986.tb15584.x. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Characterization of exchange-labeled saxitoxin and the origin of linear uptake by excitable tissue. Mol Pharmacol. 1977 Nov;13(6):1136–1146. [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B., Strichartz G. R. A new method for labelling saxitoxin and its binding to non-myelinated fibres of the rabbit vagus, lobster walking leg, and garfish olfactory nerves. J Physiol. 1976 Oct;261(2):477–494. doi: 10.1113/jphysiol.1976.sp011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of labelled saxitoxin to the sodium channels in normal and denervated mammalian muscle, and in amphibian muscle. J Physiol. 1977 Jul;269(2):341–354. doi: 10.1113/jphysiol.1977.sp011905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears T. A., Bostock H., Sheratt M. The pathophysiology of demyelination and its implications for the symptomatic treatment of multiple sclerosis. Neurology. 1978 Sep;28(9 Pt 2):21–26. doi: 10.1212/wnl.28.9_part_2.21. [DOI] [PubMed] [Google Scholar]
- Small R. K., Pfenninger K. H. Components of the plasma membrane of growing axons. I. Size and distribution of intramembrane particles. J Cell Biol. 1984 Apr;98(4):1422–1433. doi: 10.1083/jcb.98.4.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R., Rogart R. B., Ritchie J. M. Binding of radioactively labeled saxitoxin to the squid giant axon. J Membr Biol. 1979 Aug;48(4):357–364. doi: 10.1007/BF01869446. [DOI] [PubMed] [Google Scholar]
- Strichartz G. R., Small R. K., Pfenninger K. H. Components of the plasma membrane of growing axons. III. Saxitoxin binding to sodium channels. J Cell Biol. 1984 Apr;98(4):1444–1452. doi: 10.1083/jcb.98.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Strichartz G. R., Orkand R. K. Sodium channels in axons and glial cells of the optic nerve of Necturus maculosa. J Gen Physiol. 1979 Nov;74(5):629–642. doi: 10.1085/jgp.74.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Bennett M. V. Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat New Biol. 1972 Aug 16;238(85):217–219. doi: 10.1038/newbio238217a0. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Black J. A., Foster R. E. Ontogenesis of the axolemma and axoglial relationships in myelinated fibers: electrophysiological and freeze-fracture correlates of membrane plasticity. Int Rev Neurobiol. 1983;24:433–484. doi: 10.1016/s0074-7742(08)60226-3. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Brill M. H. Conduction through demyelinated plaques in multiple sclerosis: computer simulations of facilitation by short internodes. J Neurol Neurosurg Psychiatry. 1978 May;41(5):408–416. doi: 10.1136/jnnp.41.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch Neurol. 1977 Oct;34(10):585–589. doi: 10.1001/archneur.1977.00500220019003. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Quick D. C. Cytochemical differentiation of the axon membrane in A- and C-fibres. J Neurol Neurosurg Psychiatry. 1977 Apr;40(4):379–385. doi: 10.1136/jnnp.40.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Wood S. L. Impulse conduction in inhomogeneous axons: effects of variation in voltage-sensitive ionic conductances on invasion of demyelinated axon segments and preterminal fibers. Brain Res. 1984 Feb 27;294(1):111–122. doi: 10.1016/0006-8993(84)91314-3. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]




