Abstract
Nck is a ubiquitously expressed, primarily cytosolic adapter protein consisting of one SH2 domain and three SH3 domains. It links receptor and nonreceptor tyrosine kinases to actin cytoskeleton reorganizing proteins. In T lymphocytes, Nck is a crucial component of signaling pathways for T cell activation and effector function. It recruits actin remodeling proteins to T cell receptor (TCR)-associated activation clusters and thereby initiates changes in cell polarity and morphology. Moreover, Nck is crucial for the TCR-induced mobilization of secretory vesicles to the cytotoxic immunological synapse. To identify the interactome of Nck in human T cells, we performed a systematic screen for interaction partners in untreated or pervanadate-treated cells. We used GST fusion proteins containing full length Nck, the combined SH3 domains or the individual SH3 and SH2 domains to precipitate putative Nck interactors from cellular lysates. Protein bands were excised from gels, processed by tryptic in-gel digestion and analyzed by mass spectrometry. Using this approach, we confirmed previously established interactions (e.g., with Slp76, CD3ɛ, WASP, and WIPF1) and identified several novel putative Nck-binding proteins. We subsequently verified the SH2 domain binding to the actin-binding protein HIP55 and to FYB/ADAP, and the SH3-mediated binding to the nuclear proteins SFPQ/NONO. Using laser scanning microscopy, we provide new evidence for a nuclear localization of Nck in human T cells. Our data highlight the fundamental role of Nck in the TCR-to-cytoskeleton crosstalk and point to yet unknown nuclear functions of Nck also in T lymphocytes.
Keywords: Nck, adapter protein, protein–protein interaction, SH2 domain, SH3 domain, T cell, HIP55, FYB, SFPQ, NONO
Introduction
Nck (noncatalytic region of tyrosine kinase) adapter proteins are almost exclusively composed of one SH2 (Src homology 2) domain and three SH3 domains.1,2 The Nck family comprises two members (Nck1/Nckα and Nck2/Nckβ, also termed Grb4) that are roughly 68% identical with the largest differences located in the linker regions between the interaction modules.1 Nck1 and Nck2 are functionally redundant in many aspects and neither Nck1 nor Nck2 knock-out mice exhibit an apparent phenotype. In contrast, however, Nck1/Nck2 double knock-out mice die in utero.3 Nevertheless, some studies indicate nonoverlapping functions of Nck1 and Nck2 in certain cell types.4
The modular architecture of adapter proteins such as Nck allows for numerous individual and potentially simultaneous protein–protein interactions. Over the past decades, more than 60 interaction partners for Nck have been described in different cellular systems.1 A main function of Nck is to link receptor and receptor-associated tyrosine kinases with proteins that directly or indirectly regulate remodeling and reorganization of the actin cytoskeleton. In this scenario, the SH2 domain mostly mediates binding to phosphorylated receptors or associated phosphoproteins. Larger protein complexes are formed by individual interactions of the different SH3 domains with proline-based recognition motifs. In this way, Nck participates in an array of receptor-initiated signaling processes. In essence, many aspects of tissue development and homeostasis, activation and effector function of immune cells but also malignant transformation and invasiveness of tumor cells depend on regulated changes of cell polarity, morphology and migration that are often regulated through protein complexes formed by Nck.1,2
T cell activation and effector function rely on an initial cell–cell contact followed by dynamic cellular activation ultimately leading to the executionary “kiss of death” in case of cytotoxic effector cells.5 Because individual T cells can eliminate multiple targets consecutively,6 a rapid rearrangement of established contacts is mandatory. According to the multitude of regulated changes of cell polarity, morphology and migration required during T cell activation and effector function, it is also necessary that an engagement of the T cell receptor (TCR) activates multiple actin-regulatory proteins that work in concert to drive actin polymerization at the immunological synapse (IS).5
At the molecular level, TCR ligation results in the activation of the T cell-specific Src-type kinases Fyn and Lck which phosphorylate the crucial ITAMs (immunoreceptor tyrosine-based activation motifs) within the TCR-associated CD3- and zeta-chains to serve as docking-sites for the two SH2 domains of the Syk-type kinase ZAP70 (zeta-chain-associated protein kinase of 70 kDa). Activated ZAP70 phosphorylates an array of downstream substrates like Slp76 (SH2 domain containing leukocyte protein of 76 kDa). Phosphorylated Slp76 binds the crucial guanine nucleotide exchange factor Vav, and Vav in turn activates the Rho family GTPases Cdc42 (cell division cycle 42) and Rac (Ras-related C3 botulinum toxin substrate) that facilitate the activation of WASP (Wiskott-Aldrich syndrome protein) family members. The main function of the multidomain adapter protein WASP is the activation of the Arp2/3 (actin-related protein 2/3) complex that finally leads to the formation of branched actin filament networks.5 In this context, Nck binds phosphorylated Slp76 via its SH2 domain and recruits WASP via SH3-mediated interactions. Thus, Slp76 functions as a scaffold that guides Nck and WASP into proximity of Vav1 and Cdc42-GTP.7 Of note, it was also proposed that Nck directly binds to a proline-rich sequence within the CD3ɛ chain that only becomes accessible upon TCR ligation due to an induced conformational change. This mechanism might display an alternative means to more directly link T cell activation to the cytoskeleton.8
Furthermore, Nck interacts with an extended proline-rich stretch within the cytoplasmic part of the prototypic death factor FasL9,10 and is critically involved in the recruitment of FasL and/or its storage granules to the cytotoxic IS.9 Besides its established function in the regulation of the actin cytoskeleton, Nck has been implicated in pathways regulating cell cycle arrest after DNA damage including a translocation of Nck to the nucleus.11 It has also been suggested that the set of nuclear binding partners significantly differs from cytosolic interactors.12 However, so far, only very few nuclear Nck-interacting proteins have been described.
We performed a systematic search for interaction partners of the four individual interaction modules of Nck in primary and leukemic human T cells. To this end, we precipitated putative binding partners from untreated or pervanadate-treated PHA (phytohemagglutinin) blasts, Jurkat and Hut78 cells with GST fusion proteins containing full length Nck, the combined three SH3 domains or the individual SH3 and SH2 domains. We identified a total of 141 individual bands representing 22 different proteins. We not only verified known interactions but also identified several interesting novel Nck-binding proteins.
Results
Identification of Nck interaction partners
GST fusion proteins containing full length Nck1 (FL), all three SH3 domains (SH3.1-3, aa 1-251), the individual SH3 domains (SH3.1, aa 5-60; SH3.2, aa 109-164; SH3.3, aa 193-251) and the SH2 domain (aa 282-377) were used to precipitate putative Nck-interacting proteins from the leukemic T cell lines Je6.1, JFL and Hut78 or from human T cell blasts. For the precipitation of Nck SH2-interacting proteins, T cell blasts were pretreated with pervanadate to maximize tyrosine phosphorylation. Because available protein stains differ with respect to sensitivity, reactivity and MS (mass spectrometry) compatibility, we used Coomassie staining, conventional silver staining and the fluorescent dyes Flamingo Pink or Sypro Ruby in independent experiments to enhance the recovery of identified Nck-interacting partners. To visualize potential contaminations in a given preparation, 50 μg of each fusion protein were loaded in the absence of cellular lysate and separated by SDS-PAGE (not shown). Impurities were taken into consideration for the subsequent analysis by MALDI-TOF (matrix-assisted laser desorption/ionization time-of-flight) mass spectrometry. In total, we performed eight independent pull-down experiments with T cell blasts, six experiments with the Jurkat variant JFL, one experiment with the Jurkat cell line Je6.1 and two experiments with the T cell lymphoma line Hut78. Representative experiments are shown in Figure 1 for T cell blasts (A) and for JFL cells (B).
Figure 1.
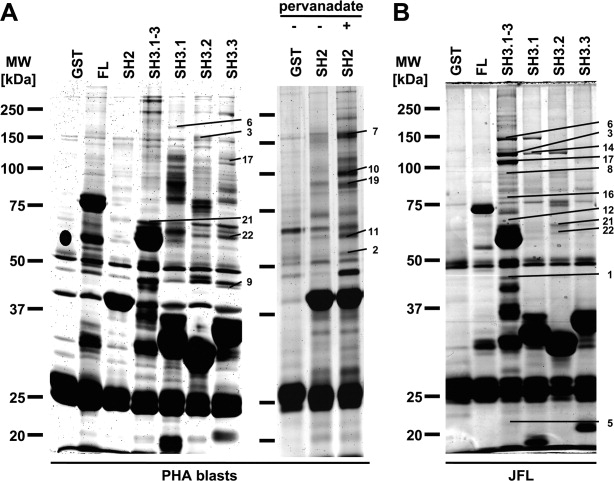
Pull-down of Nck-interacting proteins. Precipitation experiments with GST fusion proteins containing full length Nck1 (FL), the SH2 domain (SH2), all three SH3 domains (SH3.1-3), the individual SH3 domains (SH3.1, SH3.2, SH3.3) and GST as control. The respective fusion proteins were used to precipitate putative Nck-associated proteins from human T cell blasts with or without pervanadate-treatment (A) or from Jurkat T cells JFL (B). After separation by SDS-PAGE and protein visualization, bands that did not appear in controls were excised and subjected to MALDI-TOF analysis. The Flamingo Pink-stained gel (A, left gel) displays a master gel combining the results of eight independent experiments using Coomassie staining, conventional silver staining and Flamingo Pink staining. The Flamingo Pink-stained gel (A, right gel) displays a master gel combining the results of two independent experiments also using Coomassie staining. The silver-stained gel (B) displays a master gel combining the results of six independent experiments using Coomassie staining, conventional silver staining and staining with Flamingo Pink or Sypro Ruby. Numbers correspond to those provided in Tables I and II.
Bands that did not appear in fusion protein controls were excised from the gels. Proteins precipitated by GST alone were also identified to disclose false-positive Nck interaction partners. Moreover, although Nck and GST were identified several times in various precipitates, these identifications were also excluded from the results. In total, based on 17 independent experiments, we identified 141 bands representing 22 individual proteins with a MASCOT score greater than 65 based on individual peptide fragments. A complete list of proteins precipitated with Nck is given in Table I. The frequency of identification in total and with respect to the different cell lines is indicated as well as the most accurate identification as displayed by the number of identified peptides and the MASCOT score (Table I). Table II attributes the found interactions to the respective fusion protein used for precipitation and thus the involved interaction domain (Table II). A complete list of all identified bands including MASCOT score, number of identified peptides, experimental and predicted molecular weights, NCBI and Uniprot accession numbers, precipitating fusion protein as well as the used cell line and protein stain is provided as a Supporting Information Table SI. In Figure 1, identified protein bands are assigned to the respective protein by numbers. Of note, the gels shown display “master gels.” Thus, faint bands might have been more abundant and broad bands better separated in individual experiments from which the respective protein was identified.
Table I.
Proteins Precipitated with Nck Fusion Proteins, Identified by Mass Spectrometry
| Number of Identifications |
||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Protein | Total | T Cell Blasts | JFL | Je6.1 | Hut 78 | Identified Peptides | MASCOT Score |
| 1 | ACTB | 26 | 18 | 8 | – | – | 12 | 522 |
| 2 | ACTR3 | 1 | 1 | – | – | – | 3 | 99 |
| 3 | CBL | 8 | 2 | 6 | – | – | 18 | 357 |
| 4 | CBR1 | 4 | – | – | – | 4 | 8 | 538 |
| 5 | CD3ɛ | 2 | – | 2 | – | – | 4 | 116 |
| 6 | CYFIP2 | 18 | 8 | 7 | 3 | – | 7 | 260 |
| 7 | FYB | 1 | 1 | – | – | – | 7 | 86 |
| 8 | Git1 | 4 | – | 4 | – | – | 7 | 232 |
| 9 | GMPPA | 1 | 1 | – | – | – | 6 | 190 |
| 10 | HCLS1 | 1 | 1 | – | – | – | 8 | 182 |
| 11 | HIP55 | 2 | 2 | – | – | – | 5 | 117 |
| 12 | hnRNPK | 4 | 2 | 2 | – | – | 5 | 289 |
| 13 | MICAL1 | 2 | 2 | – | – | – | 13 | 87 |
| 14 | NCKAP1L | 5 | 2 | 1 | 2 | – | 4 | 199 |
| 15 | NONO | 1 | – | 1 | – | – | 3 | 101 |
| 16 | PABPC1 | 1 | – | 1 | – | – | 3 | 108 |
| 17 | SFPQ | 11 | 4 | 4 | – | 3 | 20 | 384 |
| 18 | SH3PXD2B | 1 | – | – | 1 | – | 19 | 122 |
| 19 | Slp76 | 3 | 3 | – | – | – | 9 | 241 |
| 20 | WASL | 4 | – | 3 | 1 | – | 2 | 91 |
| 21 | WASP | 27 | 16 | 10 | – | 1 | 12 | 529 |
| 22 | WIPF1 | 14 | 10 | 4 | – | – | 10 | 269 |
Table II.
List of Proteins Precipitated with Nck Fusion Proteins Containing Full Length (FL) Nck or Individual SH2/SH3 Binding Modules
| No. | Protein | FL | SH2 | SH3.1-3 | SH3.1 | SH3.2 | SH3.3 |
|---|---|---|---|---|---|---|---|
| 1 | ACTB | 6 | 4 | 4 | 2 | 6 | 4 |
| 2 | ACTR3 | – | 1 | – | – | – | – |
| 3 | CBL | – | – | 4 | – | 4 | – |
| 4 | CBR1 | 2 | – | 2 | – | – | – |
| 5 | CD3ɛ | – | – | 1 | 1 | – | – |
| 6 | CYFIP2 | 3 | – | 6 | 9 | – | – |
| 7 | FYB | – | 1 | – | – | – | – |
| 8 | Git1 | – | – | 2 | – | 2 | – |
| 9 | GMPPA | – | – | – | – | – | 1 |
| 10 | HCLS1 | – | 1 | – | – | – | – |
| 11 | HIP55 | 1 | 1 | – | – | – | – |
| 12 | hnRNPK | 1 | – | 1 | – | 2 | – |
| 13 | MICAL1 | – | – | – | – | 2 | – |
| 14 | NCKAP1L | – | – | 2 | 1 | – | 2 |
| 15 | NONO | – | – | – | – | – | 1 |
| 16 | PABPC1 | – | – | 1 | – | – | – |
| 17 | SFPQ | – | – | 6 | – | – | 5 |
| 18 | SH3PXD2B | – | – | – | – | 1 | – |
| 19 | Slp76 | 1 | 2 | – | – | – | – |
| 20 | WASL | – | – | 2 | – | – | 2 |
| 21 | WASP | 5 | – | 7 | 1 | 8 | 6 |
| 22 | WIPF1 | 2 | – | – | – | 8 | 4 |
The individual SH3 domains show a distinct but partly overlapping set of interaction partners. For example, the actin-regulatory Wiskott-Aldrich-syndrome protein (WASP, allocated number 21) was seen in precipitates of all three SH3 domains, whereas the cytoplasmic FMR1-interacting protein 2 (CYFIP2, (6)) was exclusively present in precipitates of fusion proteins containing the first SH3 domain (SH3.1, SH3.1-3, FL). The heterogeneous nuclear ribonucleoprotein K (hnRNPK, (12)), the G protein-coupled receptor kinase interactor 1 (Git1, (8)) and the E3 ubiquitin ligase CBL (3) precipitated with the second SH3 domain, whereas the splicing factor proline/glutamine-rich SFPQ (17) and non-POU domain containing, octamer-binding NONO (15) were identified from precipitates of the third SH3 domain. The WAS/WASL interacting protein family member 1 (WIPF1, (22)) was precipitated with the second and third SH3 domains, but never with the first one. Cytoplasmic actin was identified in precipitates of all fusion proteins used, highlighting that many Nck SH2- and SH3-interactors are actin-binding proteins. Most proteins including CYFIP2, hnRNPK, SFPQ, and WASP were identified in leukemic as well as primary T cells. However, for example, Git1 and Carbonyl reductase 1 (CBR1, (4)) were only detected in precipitates from leukemic T cells. Of note, in some cases the Nck SH3.1-3 construct precipitated significantly more proteins than the full length protein, which might indicate a negative influence of the SH2 domain on particular SH3-mediated interactions. Moreover, the array of proteins precipitated with the SH3.1-3 construct does not simply reflect the sum of proteins precipitated with the individual SH3 domains. This suggests that in some cases a cooperative interaction with more than one SH3 domain may be necessary for tight complex formation.
We also identified potential interaction partners of the SH2 domain of Nck. Because SH2-mediated interactions depend on the phosphorylation of tyrosines within the respective binding motive, we inhibited phosphatases with pervanadate to yield a maximal tyrosine phosphorylation. Candidate binding proteins were precipitated from lysates of pervanadate-treated and untreated cells with full length Nck, the isolated SH2 domain or GST alone as control. As shown in Figure 1(A), the SH2 domain precipitated the actin-related protein 3 (ACTR3, also named Arp3 (2)), HPK1 (hematopoietic progenitor kinase 1)-interacting protein of 55 kDa (HIP55) (10), Fyn-binding protein (FYB) (7), the hematopoietic cell-specific Lyn substrate 1 (HCLS1, (10)) and Slp76 (19) after treatment with pervanadate.
Verification of selected interactions
HIP55
The interaction of Nck with the actin-binding adapter protein HIP55 (also termed DBNL (drebrin-like), mAbp1 (mammalian actin-binding protein 1) or SH3P7 (SH3 domain-containing protein 7)) has not been described before. We thus performed additional pull-down experiments from untreated and pervanadate-treated T cell blasts, transferred the proteins on nitrocellulose and carried out Western blots with an anti-HIP55 antibody. As expected, only full length Nck and the SH2 domain but not the SH3.1-3 fusion protein precipitated HIP55. Moreover, HIP55 was precipitated exclusively from the lysates of pervanadate-treated cells [Fig. 2(A)].
Figure 2.
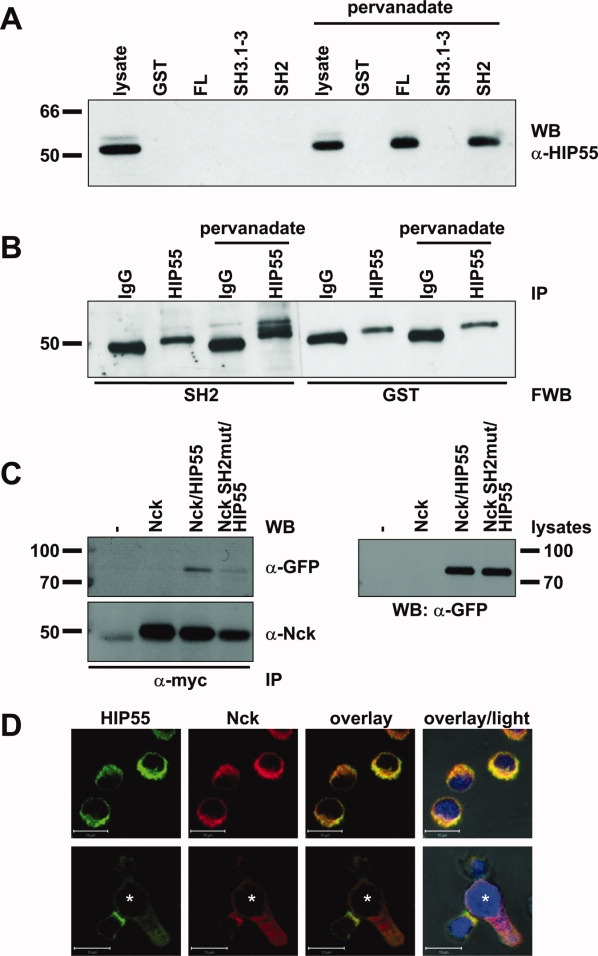
Verification of the Nck/HIP55 interaction. (A) Precipitation with GST fusion proteins containing full length Nck1 (FL), all three SH3 domains (SH3.1-3), the SH2 domain (SH2) and GST as control from lysates of untreated and pervanadate-treated T cell blasts. Proteins were transferred to nitrocellulose followed by an anti-HIP55 immunoblot. (B) After immunoprecipitation (IP) of HIP55 from lysates of untreated and pervanadate-treated T cell blasts (using IgG1 as control), the proteins were transferred to nitrocellulose followed by incubation with the Nck SH2 domain and GST as control, an anti-GST mAb and HRP-conjugated secondary antibodies in a Far Western blot (FWB). (C) Myc-tagged Nck variants were transiently co-expressed with EGFP-tagged HIP55 (murine) in 293T transfectants. 18–24 h after transfection, cells were treated with pervanadate for 2 min. and lysed. Lysates were subjected to immunoprecipitation with anti-myc mAb 9B11 and separated by SDS-PAGE. Proteins were transferred to nitrocellulose followed by Western blot with anti-GFP pAb A11122 to visualize coprecipitated proteins (upper left panel). The blots were stripped and precipitated Nck was detected by Western blot using anti-Nck pAb C-19 (lower left panel). To monitor expression of EGFP-tagged HIP55 25 μg of the cellular lysates used for immunoprecipitation were subjected to SDS-PAGE and transferred to nitrocellulose followed by Western blot with anti-GFP pAb A11122 (right panel). (D) Subcellular distribution of Nck and HIP55 in cloned T cells that were either left untreated or exposed to superantigen-stimulated B-LCL (*) for 30 min. before fixation and staining with anti-HIP55 mAb and AlexaFluor488-conjugated anti-mouse antibody and anti-Nck pAb sc-19 and AlexaFluor555-conjugated anti-rabbit antibody. ProLong gold antifade mounting medium contained DAPI to visualize nuclei. Transmitted light was recorded to visualize cell shapes. Scale bars represent 10 μm.
To prove that the association with HIP55 relies on a direct interaction, we precipitated endogenous HIP55 from lysates of untreated and pervanadate-treated T cell blasts with the anti-HIP55 antibody and performed a Far Western blot analysis with the Nck SH2 domain and GST as control [Fig. 2(B)]. We detected a clear band at about 55 kDa (slightly above the heavy chain of the precipitating antibody and below an unspecific band, that is also present in the precipitation with IgG1) only after precipitation of HIP55 from stimulated cells and not in the respective controls. By stripping and reprobing the blot with an anti-HIP55 antibody we clearly assigned this band to HIP55 (not shown). To further verify the interaction of Nck with HIP55, myc-tagged WT Nck and a construct carrying a point mutation within the SH2 domain (R308K) were expressed together with EGFP-tagged HIP55 (murine) in 293T cells. Twenty-four hours after transfection, the cells were treated with pervanadate and cell lysates were subjected to immunoprecipitation with an anti-myc mAb. GFP staining revealed coprecipitation of HIP55 only in the presence of WT Nck but not when the SH2 domain of Nck was mutated, although comparable amounts of HIP55 were expressed [Fig. 2(C)]. We next analyzed the subcellular distribution of Nck and HIP55 in T cell blasts [Fig. 2(D), upper panels]. As expected, both Nck and HIP55 are localized in the cytosol, thus potentially enabling an interaction. As Nck is a key adapter in the TCR-induced actin-reorganization during T cell activation and effector function, we also analyzed the subcellular distribution of Nck and HIP55 in a T cell/target cell contact situation [Fig. 2(D), lower panels]. As reported before, Nck reorients toward the target cell. Interestingly, HIP55 is also enriched in direct proximity of the IS.
FYB
We precipitated the adapter protein FYB, also named SLAP130 (Slp76-associated adaptor protein of 130 kDa) or ADAP (adhesion and degranulation promoting adaptor protein), from pervanadate-treated T cell blasts with the Nck SH2 domain. To verify this interaction, we performed pull-down experiments from untreated and pervanadate-treated T cell blasts and subsequent Western blots with an anti-FYB antibody. As expected, only full length Nck and the SH2 domain but not the SH3.1-3 fusion protein precipitated FYB. Interestingly, the interaction (especially with the SH2 domain) could also be observed in untreated cells indicating that a fraction of FYB might be constitutively phosphorylated in human T cell blasts. However, pervanadate-treatment slightly increased the amount of precipitated FYB [Fig. 3(A)]. Of note, the fusion proteins were not used at equimolar concentrations and therefore the experiments do not allow predictions on quantitative binding preferences.
Figure 3.
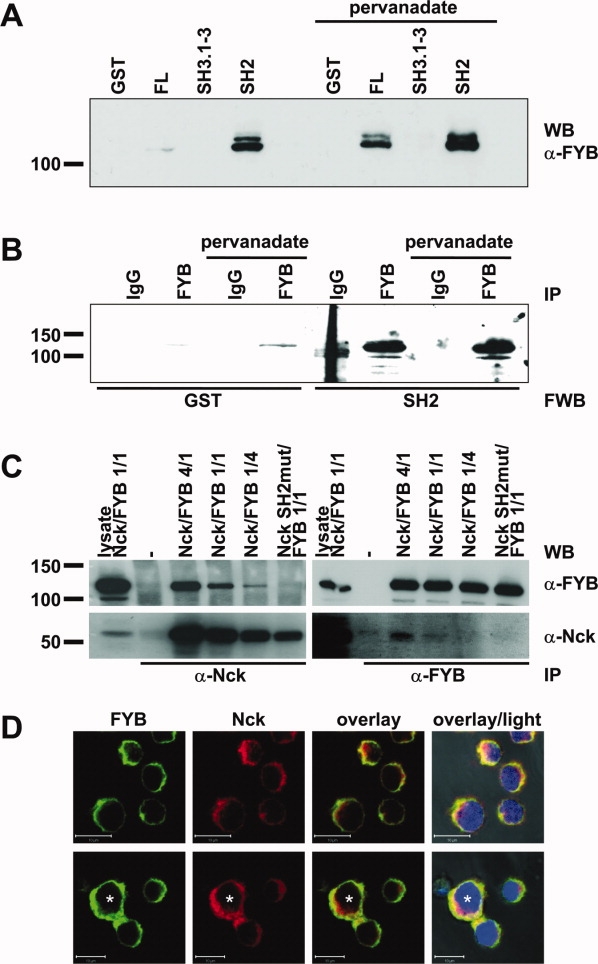
Verification of the Nck/FYB interaction. (A) Precipitation with GST fusion proteins containing full length Nck1 (FL), all three SH3 domains (SH3.1-3), the SH2 domain (SH2), and GST as control from lysates of untreated and pervanadate-treated T cell blasts. Proteins were transferred to nitrocellulose followed by an anti-FYB immunoblot. (B) FYB was immunoprecipitated (IP) from lysates of untreated and pervanadate-treated T cell blasts (using IgG1 as control). Subsequently, the proteins were transferred to nitrocellulose followed by incubation with the Nck SH2 domain and GST as control, an anti-GST mAb and HRP-conjugated secondary antibodies in a Far Western blot. (C) Myc-tagged Nck variants were transiently co-expressed with FYB at different ratios in 293T transfectants. 18–24 h after transfection, cells were treated with pervanadate for 2 min. and lysed. Lysates were split into two aliquots, subjected to parallel immunoprecipitation with anti-Nck mAb 280C10 and anti-FYB mAb 460114 and separated by SDS-PAGE. As a control, 25 μg of cellular lysate were also subjected to SDS-PAGE. Proteins were transferred to nitrocellulose followed by Western blot with with anti-Nck pAb C-19 and anti-FYB mAb 460114 to visualize coprecipitated proteins. To monitor protein expression in transfectants, the blots were stripped and precipitated proteins were detected by Western blot as indicated. (D) Subcellular distribution of Nck and FYB in cloned T cells that were either left untreated or exposed to superantigen-stimulated B-LCL (*) for 30 min. before fixation and staining with anti-FYB mAb and AlexaFluor488-conjugated anti-mouse antibody and anti-Nck pAb sc-19 and AlexaFluor555-conjugated anti-rabbit antibody. ProLong gold antifade mounting medium contained DAPI to visualize nuclei. Transmitted light was recorded to visualize cell shapes. Scale bars represent 10 μm.
Again we performed a Far Western blot with the Nck SH2 domain and GST as a control after immunoprecipitation of endogenous FYB from the lysates of untreated and pervanadate-treated T cells [Fig. 3(B)]. With the SH2 domain, we visualized a band at about 120 kDa that could be clearly assigned to FYB after stripping and reprobing with an anti-FYB antibody. This band was significantly stronger than the very weak band observed in the GST Far Western blot although comparable amounts FYB had been precipitated (not shown). As in the previous pull-down experiments, we only observed a slight increase in the association of Nck with FYB after pervanadate-treatment. To further verify the interaction of Nck with FYB, myc-tagged WT Nck and a SH2 domain-mutated variant were expressed together with FYB at different ratios in 293T cells. Twenty-four hours after transfection, the cells were treated with pervanadate and cell lysates were subjected to immunoprecipitation with anti-Nck or anti-FYB mAbs. The anti-Nck immunoprecipitation followed by FYB staining revealed coprecipitation of FYB in the presence of WT Nck but not when the SH2 domain of Nck was mutated. Conversely, anti-FYB mAb coprecipitated WT but not SH2-mutated myc-tagged Nck [Fig. 3(C)]. Because FYB was described as a mediator of TCR-triggered integrin activation, we next analyzed the distribution of FYB in a T cell/target cell contact situation. Confocal laser-scanning microscopy revealed that FYB mainly localizes to the cytosol and like Nck translocates to the area of intercellular contact upon exposure to target cells [Fig. 3(D)].
SFPQ/NONO
The nuclear protein SFPQ was precipitated with Nck SH3 domains (SH3.1-3 and SH3.3) from T cell blasts, JFL and Hut78 cells. The anti-SFPQ antibody reacted with three distinct bands migrating at 100 kDa, 65 kDa, and 50 kDa. Nck FL precipitated a major reactive band at 50 kDa [Fig. 4(A)]. The 65 and 100 kDa forms were only observed after longer exposure times (not shown). Again, we used a combination of precipitation and Far Western blot analysis to prove a direct interaction with Nck and clearly show that the Nck SH3.3 domain directly binds to SFPQ as does the SH3.2 domain [Fig. 4(B)]. In our initial screening experiments, we also precipitated the nuclear protein NONO with the Nck SH3.3 domain from JFL cells. However, Far Western blot analyses revealed that this interaction might rather be indirect (not shown), supporting the notion that NONO is part of a heterodimeric complex with SFPQ. Because both SFPQ and NONO are described as primarily nuclear proteins, we compared the subcellular distribution of Nck and SFPQ/NONO in human T cell blasts [Fig. 4(C)]. As observed before, Nck shows a mainly cytosolic distribution. In individual cells (1–5% of the analyzed cells, depending on the duration of stimulation), however, Nck was clearly enriched within the nucleus where it colocalized with SFPQ and NONO. This distribution pattern was verified by the acquisition of stack images and was also observed when the cells were fixed/permeabilized with methanol/acetone (not shown).
Figure 4.
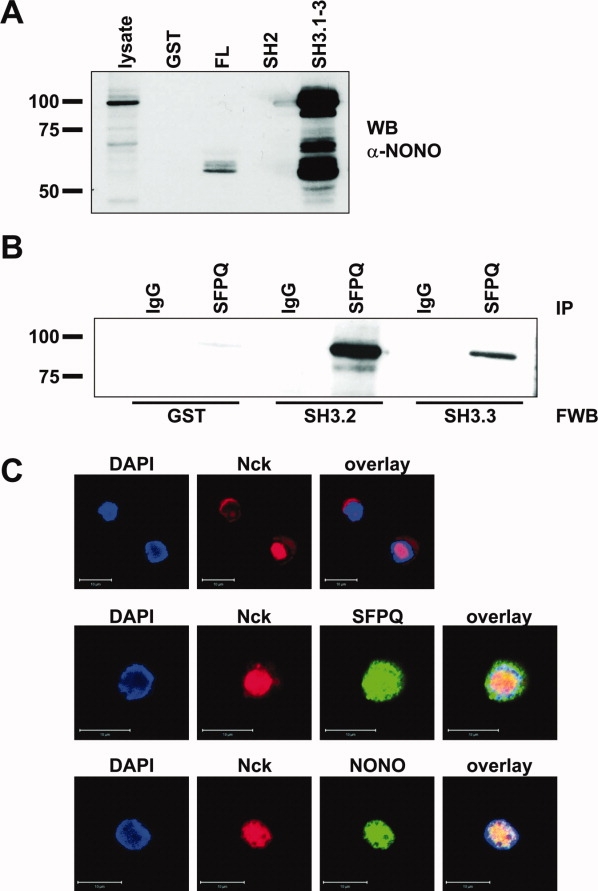
Verification of the Nck/SFPQ interaction. (A) After precipitation with GST fusion proteins containing full length Nck1 (FL), the SH2 domain (SH2), all three SH3 domains (SH3.1-3) and GST as a control from lysates of Jurkat T cells the proteins were transferred to nitrocellulose followed by an anti-SFPQ immunoblot. (B) SFPQ was immunoprecipitated (IP) from lysates of Jurkat T cells (using IgG1 as control) and the proteins were transferred to nitrocellulose followed by incubation with the Nck SH3.2 domain, the Nck SH3.3 domain, and GST as control, an anti-GST mAb and HRP-conjugated secondary antibodies in a Far Western blot (FWB). (C) Subcellular distribution of Nck, SFPQ (middle panel) and NONO (lower panel) in cloned T cells. Cells were fixed and stained with anti-SFPQ mAb or anti-NONO mAb and AlexaFluor488-conjugated anti-mouse antibody and anti-Nck pAb sc-19 and AlexaFluor555-conjugated anti-rabbit antibody. ProLong gold antifade mounting medium contained DAPI to visualize nuclei. Scale bars represent 10 μm.
Discussion
Identification of Nck interaction partners
In this work, we precipitated and identified 22 individual proteins from T cells with Nck1 fusion proteins. Although the two isoproteins Nck1 and Nck2 seem to be functionally redundant in many aspects and especially the interaction domains are highly homologous, some nonoverlapping functions have been described indicating that also the set of interacting proteins might differ to a certain degree. Thus, the described interaction partners are not necessarily attributed to both isoproteins. We confirmed several previously known Nck-interacting proteins, especially those participating in TCR-to-actin-cytoskeleton crosstalk including WASP, WIPF1, Slp76, and CD3ɛ. We also verified the previously described interaction with Git1,13 an ADP ribosylation factor GTPase activating protein (Arf GAP) that is functionally associated with cell attachment, spreading and motility14 and might also be involved in T cell activation and effector function.15 The interaction with the E3 ubiquitin ligase CBL has also been reported in previous studies.16
In contrast, interactions of Nck with the adapter proteins HIP55 and FYB have not been described before. HIP55 is a multidomain adapter protein that contains two independent actin-binding domains, a putative proline-rich motif, a SH3 domain and at least two tyrosine phosphorylation sites. In lymphocytes, antigen engagement of the BCR or TCR induces tyrosine phosphorylation of HIP55, probably by Src or Syk kinases (e.g., Lyn, Syk, or ZAP-70).17,18 RNA interference-induced knockdown of HIP55 inhibited TCR-induced activation of HPK1 (hematopoietic progenitor kinase 1) and the MAPK (mitogen-activated protein kinase) JNK.17 Moreover, T cells from knock-out mice showed similar signaling defects and, although they developed normally, exhibited reduced proliferation and IL-2 secretion. These defects were accompanied by reduced antigen-specific immune responses.19 However, also a negative regulation of TCR signaling has been ascribed to HIP55.20 HIP55 has also been implicated in the internalization of the TCR.20 Thus, Nck might link HIP55 to other molecules participating in the TCR-to-cytoskeleton crosstalk by direct interaction. Because we observed that both Nck and HIP55 are located in the proximity of T cell/target cell interfaces, such an interaction might be involved in T cell activation or effector function.
FYB is an adaptor protein participating in TCR-triggered inside-out activation of T cell integrins, and thus, also plays a role in the formation of the IS. It is expressed in many hematopoietic cells with the exception of B cells21,22 and contains one SH3 domain and a C-terminal SH3-like domain. Upon TCR ligation, FYB is tyrosine-phosphorylated by Fyn23 and subsequently recruited to Slp76. Via its own proline-rich motif, FYB associates with the adapter proteins SKAP1/SKAP55 (Src kinase-associated phosphoprotein 1/of 55 kDa) or SKAP2/SKAP55R (Src kinase-associated phosphoprotein 2/of 55 kDa-related protein).24,25 The formed protein complex allows for membrane-recruitment of the small Ras-like GTPase Rap1 which then triggers integrin activation required for lymphocyte extravasation as well as formation of ISs.26 Accordingly, FYB reorients towards the target cell and like Nck is enriched in the proximity of the IS. Again, the association with Nck might function as an alternative means to link FYB and TCR-triggered integrin activation to the actin-reorganizing machinery. Nevertheless, the exact composition and function of the proposed HIP55/Nck and FYB/Nck complexes during T cell activation and effector function have to be elucidated.
Nuclear localization of Nck
Although Nck contains no nuclear localization signal, a partial nuclear localization of Nck has been observed in certain cell types.11,12 Whereas the nuclear localization of Nck was rather constitutive in murine fibroblasts (NIH3T3) and in a human epithelial carcinoma cell line (A431),12 the translocation of Nck into the nucleus in human cervix carcinoma cells (HeLa) was caused by DNA damage (or by septin knock down).11 Interestingly, the pattern of nuclear binding partners significantly differs from cytosolic interactions.12 However, only few nuclear Nck-interacting proteins have been described so far. In an earlier study, the nucleocytoplasmic RNA-binding protein Sam68 (Src-associated in mitosis 68 kDa protein) was shown to associate with Nck.12 We also picked Sam68 in this study, although it was excluded from the presented results due to the relatively low MASCOT score of 58. Moreover, we frequently identified hnRNPK in our initial pull-down analyses, that has been shown to interact with Nck.27 hnRNPK is predominantly located in the nucleus, but may shuttle between nucleus and cytosol. It is linked to diverse processes including translation, transcription, RNA processing, RNA shuttling and stabilization as well as chromatin remodeling and cell survival.28
As additional nuclear proteins that potentially associate with Nck, we identified SFPQ (also known as polypyrimidine tract binding protein associated splicing factor, PSF) and NONO (also termed nuclear RNA-binding protein of 54 kDa, p54nrb). These proteins were identified in precipitates with Nck SH3.3 domain fusion proteins and contain potential SH3 domain binding sites (as revealed by the ELM tool (Eukaryotic Linear Motif resource for functional sites in proteins: http://elm.eu.org/)). We reproducibly identified SFPQ as a band migrating at 100 kDa, whereas the predicted molecular weight is about 70 kDa. Nevertheless, in other studies the full length form of SFPQ also migrated at the higher molecular weight of 100 kDa.29 The Western blot analyses of Nck precipitates with an anti-SFPQ antibody revealed precipitated bands at 100, 65, and 50 kDa. Whereas the 100 kDa resembles full length SFPQ, the 65 and 50 kDa most likely correspond to cleavage products that have already been described in previous studies.30 SFPQ/NONO are highly homologous DNA/RNA binding proteins, that form a multifunctional heterodimer implicated in nuclear processes such as transcription, nuclear RNA processing, nuclear retention of promiscuously edited RNA, DNA relaxation, and tumorigenesis.31 Of note, the SFPQ/NONO complex has been shown to bridge nuclear N-WASP (by interaction of NONO with N-WASP) with the RNA-polymerase 2. The knock-down of both N-WASP or NONO significantly reduced in vitro transcription supporting the notion that actin dynamics might be associated/required with/for efficient transcription.32 In this context, nuclear Nck might recruit additional effectors/regulators of the actin machinery to the site of gene transcription by its interaction with the SFPQ/NONO complex. Accordingly, we observed a nuclear localization of Nck in individual human T cells (1–5% of the cells depending on the duration of cultivation) and a colocalization with SFPQ and NONO in the nucleus. Nevertheless, the physiological trigger for the nuclear translocation of Nck as well as the precise function of nuclear Nck in T cells remain to be identified.
Taken together, our results clearly highlight the important role of Nck in the T cell compartment. Nck participates in different interdependent pathways of T cell activation and effector function during most stages of T cell selection and maturation. The interactions with HIP55 and FYB underscore the well-established role of Nck as a key adaptor in the TCR-to-actin-cytoskeleton crosstalk, whereas the interaction with the nuclear proteins SFPQ and NONO might point to a new interesting link between the actin cytoskeleton and transcription.
Materials and Methods
Cells
The human T cell lines Jurkat (Je6.1) and Hut78 and the FasL-transfected Jurkat variant JFL were propagated in RPMI 1640 medium (Gibco/Invitrogen, Carlsbad, USA) with 5% (v/v) heat-inactivated FCS (Invitrogen, Karlsruhe, Germany) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin, Biochrom AG, Berlin, Germany) at 37°C in a humidified atmosphere with 5% CO2. Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coat preparations of healthy donors by Ficoll density gradient centrifugation and PHA blasts were generated and cultivated as described previously.33 The human CD4+ T cell clone 12603 was established in our laboratory and maintained as described before.34 293T cells (human embryonic kidney, SV40 transformed) were used for transient protein expression and cultured in DMEM medium (Invitrogen) supplemented with 1% (v/v) l-glutamine (Biochrom) and antibiotics.
Plasmids and transfection
Fragments encoding full length Nck (FL), the combined three SH3 domains (SH3.1-3: aa 1-251), the individual SH3 domains (SH3.1: aa 5-60, SH3.2: aa 109-164, SH3.3: aa 193-251) and the SH2 domain (aa 280-362) in pGEX vectors as well as full length Nck and Nck carrying a point mutation within the SH2 domain (R308K) in pcDNA3.1/myc-His vector were provided by Louise Larose (Montreal, Canada) and are described elsewhere.10,35,36 Full length FYB in pEFBos vector was provided by Stefanie Kliche (Magdeburg, Germany) and is described elsewhere.25 Murine EGFP-tagged HIP55 in pEGFP C1 vector was kindly provided by Jürgen Wienands (Göttingen, Germany).18 Protein expression in Escherichia coli (DH5α) was induced by adding IPTG before fusion proteins were purified from culture supernatants by affinity binding to glutathione sepharose 4B (GE Healthcare, Munich, Germany). 293T cells were transfected in bulk (4 × 106) by standard calcium phosphate precipitation. 18–24 h after transfection, cells were treated with pervanadate before lysis and immunoprecipitation.
Confocal microscopy
Cells were fixed with paraformaldehyde and permeabilized with 1% Triton X-100 as described earlier.9 The following antibodies were used: mouse IgG1 isotype-control MOPC-21 (Abcam, Cambridge, UK), anti-HIP55 mAb clone 7 (BD Biosciences, Heidelberg, Germany), anti-Slap-130 (FYB) mAb clone 460114 (R&D Systems, Minneapolis, MN), anti-p54nrb (NONO) mAb clone 3 (BD Biosciences) and anti-SFPQ mAb clone [B92] (Abcam, Cambridge, UK) with AlexaFluor488-conjugated goat anti-mouse IgG (Invitrogen), anti-Nck pAb C-19 (Santa Cruz Biotechnology, Santa Cruz, CA) with AlexaFluor555-conjugated donkey anti-rabbit IgG (Invitrogen). Stained samples were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen) and analyzed on a laser scanning microscope (LSM 510 Meta, Carl Zeiss Jena, Jena, Germany). Images were acquired via scanning through the x–y-plane with 63× objective lenses and detection settings adjusted so that control samples (stained with control IgG and second step antibodies only) were uniformly negative. Z-stacks were frequently recorded with a stack size of 0.5 μm.
Pervanadate treatment
To increase overall phosphorylation in a given cell population, we blocked phosphatases with pervanadate (diperoxovanadate, resulting from an oxidation of sodium vanadate with peroxide). For pervanadate treatment, 0.1 mM Na3VO4 (Sigma, Deisenhofen, Germany) and 1 mM H2O2 were diluted in culture medium supplemented with 2% FCS and antibiotics. Cells were centrifuged, resuspended with the freshly prepared pervanadate solution and incubated for 2 min. at room temperature.
Analysis of T cell/target cell interfaces
B-LCL target cells were incubated with 1 ng/mL Staphylococcus enterotoxin A (Toxin Technologies, FL) for 30 min. at 37°C. After three washes with culture medium, target cells and T cells were mixed at a ratio of 1:2 and cocultured for 30 min. in 1 mL of culture medium at 37°C before transfer to poly-l-lysine (Sigma)-pretreated coverslips. After 10 min. on ice, the cells were washed with PBS and processed for fixation as described above.
(Co)-Immunoprecipitation
For precipitation with fusion proteins, cells were centrifuged, washed once with PBS and lysed in cold Nonidet P-40 (NP40) buffer as previously described.10 After centrifugation, cell lysates were incubated for at least 90 min. rotating at 4°C with the respective GST fusion protein (20–40 μg) and glutathione sepharose beads (GE Healthcare). After vigorous washes, the precipitates were resuspended in sample buffer (30% (v/v) glycerol, 6% (w/v) SDS, 200 mM Tris pH 6.8, 0.005% (w/v) bromophenol blue, 50 mM DTT) and incubated for 30 min. at 65°C. After addition of iodoacetamide to a final concentration of 150 mM and incubation at room temperature for 30 min. in the dark, the precipitates were separated by SDS-PAGE. For immunoprecipitation with mouse IgG1 isotype-control MOPC-21, anti-HIP55 mAb clone 7, anti-Slap-130 (FYB) mAb clone 460114, anti-Nck mAb 280C10 (produced in our laboratory according to Ref. 37), anti-SFPQ mAb clone [B92], and anti-myc mAb 9B11 (Cell Signaling Technology, Beverly, MA), Nonidet P-40 lysates were incubated with 1–2 μg of the respective antibody and protein G sepharose beads for at least 90 min. at 4°C and subjected to SDS-PAGE after three washes and boiling in sample buffer. For co-immunoprecipitation experiments, lysates of transfected cells were equally split prior to immunoprecipitation with the respective antibodies.
(Far) Western blot
Proteins were transferred to 0.45 μm nitrocellulose membranes. Protein load and efficacy of transfer (and the presence of fusion proteins used for pull-down analyses) were checked by reversible staining with Ponceau S (not shown). Membranes were routinely blocked with bovine serum albumin in TBST (5%, w/v). The following fusion proteins/antibodies were used for (Far) Western blot: GST, SH2-GST, SH3.2-GST and SH3.3-GST (1–2 μg/mL each), anti-GST mAb B11F8 (produced in our laboratory (23)), anti-HIP55 mAb clone 7, anti-Slap-130 (FYB) mAb clone 460114, anti-SFPQ mAb clone [B92], anti-Nck pAb C-19, anti-GFP pAb A11122 (Invitrogen, Karlsruhe, Germany), and horseradish peroxidase (HRP)-conjugated goat anti-mouse/rabbit secondary antibody (GE Healthcare). For reprobing, membranes were stripped in stripping solution (100 mM 2-mercaptoethanol, 2% SDS (w/v), 60 mM Tris) for 30 min. at 56°C and restained following the standard protocol. ECL (enhanced chemiluminescence) reagents were used for chemiluminescent detection using Hyper Film (GE Healthcare).
Protein staining
Protein bands were visualized using Coomassie staining, conventional silver staining or the fluorescent dyes Flamingo Pink (Bio-Rad Laboratories, Hercules, CA) or Sypro Ruby (Invitrogen). Gels were incubated in a colloidal Coomassie G-250 solution containing 10% (w/v) ammonium sulfate, 10% (v/v) phosphoric acid, 0.12% (w/v) Coomassie G-250 (Sigma) and 20% (v/v) methanol for at least 18 h and destained using deionized water or water with 10% (v/v) ethanol and 2% (v/v) phosphorous acid. For conventional silver staining, the gels were fixed overnight with methanol (50%, v/v)/acetic acid (5%, v/v), and washed for 10 min. each with 50% (v/v) ethanol and deionized water. Gels were treated with sodium thiosulfate (0.02%, w/v) for 1 min. and washed twice with deionized water. After 20 min. of incubation in silver nitrate solution (0.1%, w/v) at 4°C and two additional washes in deionized water, the gels were developed with sodium carbonate (2%, w/v)/formalin (0.04%, v/v) until the desired staining intensity was obtained. The reaction was terminated by addition of 5% (v/v) acetic acid. For staining with Flamingo Pink or Sypro Ruby, gels were fixed in methanol (40%, v/v)/acetic acid (10%) solution for at least 3 h and stained with a Flamingo Pink or Sypro Ruby solution for 24 h. Flamingo Pink stained gels were washed with 0.1% (v/v) Tween20 in water and with pure water. Sypro Ruby stained gels were washed twice with methanol (10%, v/v)/acetic acid (7%, v/v) for 30 min. and twice with water.
Documentation, gel analysis, and spot picking
Coomassie or silver stained protein bands were manually excised from gels using a scalpel or a gel picker device (1.5 and 3 mm). Flamingo Pink and Sypro Ruby fluorescence was scanned on a Typhoon Trio (GE Healthcare) at 532 nm. Individual spots within protein bands were selected and picked under water by an automated Ettan spotpicker (GE Healthcare) with a 2 mm picking head according to the manufacturer's instructions. The picked gels were rescanned to verify the correct location of the cut spots.
In-gel tryptic digestion and mass spectrometry
In-gel tryptic digestion and mass spectrometry were performed as described previously with slight modifications.33 Gel pieces were washed twice with HPLC grade water (Roth, Karlsruhe, Germany) and dehydrated in a first step with 25 mM ammonium bicarbonate (ABC) in 50% acetonitrile (ACN) and shrunk in pure ACN as a second step. The dry gel pieces were then rehydrated with 100 ng sequencing-grade trypsin (Serva, Heidelberg, Germany) in 10 μL of 5 mM ABC. After rehydration, 10 μL of 5 mM ABC were added. For tryptic in-gel digestion, samples were incubated overnight at 37°C. To extract the peptides after the incubation period, 20 μL of 0.3% trifluoroacetic acid (TFA) in ACN were added and the samples were sonicated in a water bath (Sonorex Super RK100; Bandelin, Berlin, Germany) for 15 min. The liquid phases were collected, lyophilized, redissolved in 0.5–1 μL MALDI matrix solution (3.2 mg/mL α-cyanohydroxycinnamic acid (Sigma) dissolved in 65% ACN/0.1% TFA) and spotted onto a stainless steel 192-well MALDI plate and air-dried. The samples were analyzed by peptide mass fingerprinting in reflectron mode using the 4700 Proteomics Analyzer mass spectrometer (Applied Biosystems, Framingham, MA). For MS analyses, typically 750 shots were accumulated for each spot. The peptide mass spectra were processed by internal calibration with autolytic fragments of porcine trypsin using the GPS Explorer software version 3.6 (Applied Biosystems). Database searches with MASCOT were performed using the following parameters: the modification on cysteine residues by amidomethylation was set as obligate, methionine oxidation was considered as a potential modification; the maximum number of missed tryptic cleavages was one; the monoisotopic masses were considered and the mass tolerance was set to ±50 ppm and searched against human proteins in the NCBI database (191694 entries, 2007/02/28, Homo sapiens) using MASCOT V2.0 (Matrix Sciences, London, UK).
Acknowledgments
We thank Louise Larose (Montreal, Canada), Stefanie Kliche (Magdeburg, Germany), and Jürgen Wienands (Göttingen, Germany) for providing essential reagents.
References
- 1.Lettau M, Pieper J, Janssen O. Nck adapter proteins: functional versatility in T cells. Cell Commun Signal. 2009;7:1. doi: 10.1186/1478-811X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14:723–731. doi: 10.1016/s0898-6568(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 3.Bladt F, Aippersbach E, Gelkop S, Strasser GA, Nash P, Tafuri A, Gertler FB, Pawson T. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol. 2003;23:4586–4597. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan S, Chen M, Woodley D, Li W. Nckbeta adapter controls neuritogenesis by maintaining the cellular paxillin level. Mol Cell Biol. 2007;27:6001–6011. doi: 10.1128/MCB.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 6.Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–1079. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 7.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 8.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 9.Lettau M, Qian J, Linkermann A, Latreille M, Larose L, Kabelitz D, Janssen O. The adaptor protein Nck interacts with Fas ligand: Guiding the death factor to the cytotoxic immunological synapse. Proc Natl Acad Sci USA. 2006;103:5911–5916. doi: 10.1073/pnas.0508562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzel J, Sanzenbacher R, Ghadimi M, Lewitzky M, Zhou Q, Kaplan DR, Kabelitz D, Feller SM, Janssen O. Multiple interactions of the cytosolic polyproline region of the CD95 ligand: hints for the reverse signal transduction capacity of a death factor. FEBS Lett. 2001;509:255–262. doi: 10.1016/s0014-5793(01)03174-x. [DOI] [PubMed] [Google Scholar]
- 11.Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell. 2007;130:837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawe DC, Hahn C, Wong AJ. The Nck SH2/SH3 adaptor protein is present in the nucleus and associates with the nuclear protein SAM68. Oncogene. 1997;14:223–231. doi: 10.1038/sj.onc.1200821. [DOI] [PubMed] [Google Scholar]
- 13.Frese S, Schubert WD, Findeis AC, Marquardt T, Roske YS, Stradal TE, Heinz DW. The phosphotyrosine peptide binding specificity of Nck1 and Nck2 Src homology 2 domains. J Biol Chem. 2006;281:18236–18245. doi: 10.1074/jbc.M512917200. [DOI] [PubMed] [Google Scholar]
- 14.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 15.Phee H, Abraham RT, Weiss A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat Immunol. 2005;6:608–617. doi: 10.1038/ni1199. [DOI] [PubMed] [Google Scholar]
- 16.Rivero-Lezcano OM, Sameshima JH, Marcilla A, Robbins KC. Physical association between Src homology 3 elements and the protein product of the c-cbl proto-oncogene. J Biol Chem. 1994;269:17363–17366. [PubMed] [Google Scholar]
- 17.Han J, Kori R, Shui JW, Chen YR, Yao Z, Tan TH. The SH3 domain-containing adaptor HIP-55 mediates c-Jun N-terminal kinase activation in T cell receptor signaling. J Biol Chem. 2003;278:52195–52202. doi: 10.1074/jbc.M305026200. [DOI] [PubMed] [Google Scholar]
- 18.Larbolette O, Wollscheid B, Schweikert J, Nielsen PJ, Wienands J. SH3P7 is a cytoskeleton adapter protein and is coupled to signal transduction from lymphocyte antigen receptors. Mol Cell Biol. 1999;19:1539–1546. doi: 10.1128/mcb.19.2.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han J, Shui JW, Zhang X, Zheng B, Han S, Tan TH. HIP-55 is important for T-cell proliferation, cytokine production, and immune responses. Mol Cell Biol. 2005;25:6869–6878. doi: 10.1128/MCB.25.16.6869-6878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bras S, Foucault I, Foussat A, Brignone C, Acuto O, Deckert M. Recruitment of the actin-binding protein HIP-55 to the immunological synapse regulates T cell receptor signaling and endocytosis. J Biol Chem. 2004;279:15550–15560. doi: 10.1074/jbc.M312659200. [DOI] [PubMed] [Google Scholar]
- 21.Togni M, Lindquist J, Gerber A, Kolsch U, Hamm-Baarke A, Kliche S, Schraven B. The role of adaptor proteins in lymphocyte activation. Mol Immunol. 2004;41:615–630. doi: 10.1016/j.molimm.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Peterson EJ. The TCR ADAPts to integrin-mediated cell adhesion. Immunol Rev. 2003;192:113–121. doi: 10.1034/j.1600-065x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 23.da Silva AJ, Janssen O, Rudd CE. T cell receptor zeta/CD3-p59fyn(T)-associated p120/130 binds to the SH2 domain of p59fyn(T) J Exp Med. 1993;178:2107–2113. doi: 10.1084/jem.178.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Kang H, Raab M, da Silva AJ, Kraeft SK, Rudd CE. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc Natl Acad Sci USA. 1998;95:8779–8784. doi: 10.1073/pnas.95.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marie-Cardine A, Hendricks-Taylor LR, Boerth NJ, Zhao H, Schraven B, Koretzky GA. Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J Biol Chem. 1998;273:25789–25795. doi: 10.1074/jbc.273.40.25789. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Rudd CE. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008;18:486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao ZS, Manser E, Lim L. Interaction between PAK and nck: a template for Nck targets and role of PAK autophosphorylation. Mol Cell Biol. 2000;20:3906–3917. doi: 10.1128/mcb.20.11.3906-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. Bioessays. 2004;26:629–638. doi: 10.1002/bies.20048. [DOI] [PubMed] [Google Scholar]
- 29.Tapia-Paez I, Tammimies K, Massinen S, Roy AL, Kere J. The complex of TFII-I, PARP1, and SFPQ proteins regulates the DYX1C1 gene implicated in neuronal migration and dyslexia. FASEB J. 2008;22:3001–3009. doi: 10.1096/fj.07-104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shav-Tal Y, Lee B, Bar-Haim S, Vandekerckhove J, Zipori D. Enhanced proteolysis of pre-mRNA splicing factors in myeloid cells. Exp Hematol. 2000;28:1029–1038. doi: 10.1016/s0301-472x(00)00510-5. [DOI] [PubMed] [Google Scholar]
- 31.Shav-Tal Y, Zipori D. PSF and p54(nrb)/NonO—multi-functional nuclear proteins. FEBS Lett. 2002;531:109–114. doi: 10.1016/s0014-5793(02)03447-6. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Yoo Y, Okuhama NN, Tucker PW, Liu G, Guan JL. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat Cell Biol. 2006;8:756–763. doi: 10.1038/ncb1433. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H, Gelhaus C, Lucius R, Nebendahl M, Leippe M, Janssen O. Enrichment and analysis of secretory lysosomes from lymphocyte populations. BMC Immunol. 2009;10:41. doi: 10.1186/1471-2172-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lettau M, Qian J, Kabelitz D, Janssen O. Activation-dependent FasL expression in T lymphocytes and natural killer cells. Signal Transduct. 2004;4:206–211. [Google Scholar]
- 35.Kebache S, Zuo D, Chevet E, Larose L. Modulation of protein translation by Nck-1. Proc Natl Acad Sci USA. 2002;99:5406–5411. doi: 10.1073/pnas.082483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lussier G, Larose L. A casein kinase I activity is constitutively associated with Nck. J Biol Chem. 1997;272:2688–2694. doi: 10.1074/jbc.272.5.2688. [DOI] [PubMed] [Google Scholar]
- 37.Lettau M, Beyer A, Janssen O. Novel monoclonal antibodies for the investigation of PCH family proteins. Signal Transduct. 2007;7:320–328. [Google Scholar]


