Abstract
Objective
Previous study supports the presence of reduced volume and elevated response to emotional stimuli in amygdala in adolescents with bipolar disorder (BD). In the present study, structural and functional magnetic resonance imaging (MRI) scans were obtained during the same neuroimaging session in order to examine amygdala structure-function relationships in adolescents with BD. We hypothesized that amygdala volume would be inversely associated with amygdala response to emotional stimuli, such that BD participants with the smallest amygdala volumes would exhibit the highest amygdala response.
Method
51 adolescents [21 with BDI, 30 healthy comparison (HC) adolescents, ages 10-18] underwent structural and functional MRI scans. Amygdala volume (N=49) and signal change (N=44) during emotional face processing were compared between groups and structure-function correlations were examined within the BD group (N=16).
Results
Adolescents with BD displayed decreased amygdala volume (p=.009) and increased amygdala response to emotional faces (p=.043). There was no significant interaction between diagnosis and emotion type. A significant inverse association between amygdala volume and activation during emotional face processing was observed (r=−.54, p=.029).
Conclusions
Decreased volume and increased response to emotional stimuli in amygdala in BD adolescents are consistent with previous reports. This study represents the first report, to our knowledge, of the two findings in the same adolescent BD sample and supports an amygdala structure-function relationship characterized by an inverse association between volume and response to emotional stimuli. This preliminary finding requires replication and suggests a possible pathophysiological link between abnormalities in amygdala structure and response to emotional stimuli in BD.
Keywords: bipolar disorder, adolescents, amygdala, magnetic resonance imaging
Deficits in emotional processing are core features of bipolar disorder (BD) in youth and adulthood, suggesting that amygdala dysfunction may be an early, central and persistent feature of the disorder and may reflect vulnerability to the illness. Children, adolescents and adults with BD who are suffering from an acute mood state or are euthymic have difficulty processing facial emotions, with abnormalities present for both positive and negative stimuli.1-6 Difficulties processing emotional stimuli, and emotional faces in particular, implicate the amygdala, as a key role of the amygdala in processing both positive and negative emotional stimuli has been demonstrated in animal and human research.7-9 This includes evidence in humans that the amygdala contains populations of cells that respond to faces and particularly facial emotion.10 Thus, emotional processing deficits in pediatric BD, and the centrality of the amygdala in emotional processing, implicate an important role for amygdala abnormalities early in the disorder.
Indeed, one of the most consistent neuroimaging findings in pediatric BD is reduced amygdala volume.11-16 A longitudinal, within-subject study demonstrates that these decreases in amygdala volume persist during adolescence and young adulthood in BD.11 Thus, the presence of amygdala structural abnormalities during childhood and adolescence in those with BD provides further evidence that the amygdala may play an important role early in the disorder.
While study of amygdala morphology in children and adolescents with BD has been rather extensive, functional magnetic resonance imaging (fMRI) study of amygdala function in youth with BD is more limited. Although few in number, the fMRI studies of adolescents with BD also demonstrated amygdala abnormalities. Similar to findings in adult BD, fMRI scanning, performed during processing of emotional face stimuli or emotional words, demonstrated amygdala hyper-responsivity in adolescents with BD who were either in an acute mood state or euthymic at time of scanning.17-19 Elevated amygdala activation in response to emotional stimuli across euthymia and acute mood states suggests that excessive amygdala response may be an early and salient trait feature of the disorder.
Given the findings of decreased amygdala volume and increased amygdala response in youth with BD, a critical next step for neuroimaging research in this population is investigation of the association between amygdala structural and functional abnormalities. Siegle et al.20 reported an inverse relationship between sustained amygdala response to emotional stimuli and amygdala volume; such that those adults suffering from major depressive disorder who had the most sustained amygdala activation to emotional stimuli exhibited the smallest amygdala volumes. In order to empirically address a possible link between structural and functional amygdala abnormalities in adolescents with BD, we conducted high resolution structural and functional MRI during the same scanning session in this population and healthy comparison (HC) adolescents. We hypothesized that volumetric reductions and elevated activation during emotional processing would be evident in the amygdala in adolescents with BD relative to HCs. Furthermore, we hypothesized that there would be a significant inverse association between the structural and functional amygdala abnormalities in individuals with BD, such that those adolescents with BD with the smallest amygdala volume would exhibit the highest elevations in amygdala activation. As different responses have been observed in the amygdala in association with specific facial emotions,21, 22 we planned to study the relationships between amygdala structural and functional abnormalities in BD separately for each emotion.
METHODS
Participants
Participants consisted of 21 adolescent outpatients with BD I (47.6% female, mean age 15.10 years +/− 2.05 SD) and 30 HC adolescents (36.7% female, mean age 14.17 years +/− 2.10 SD). These data have not been reported previously, excluding presentation in part at the Sixty-third Annual Scientific Meeting and Convention of the Society of Biological Psychiatry.23 Participants with BD were referred from a University Medical Center, a Veterans Affairs Healthcare System, and the surrounding community, and the HC participants were recruited from the community. Participants were without a history of other neurological disorders, loss of consciousness for greater than five minutes, or significant medical illness, excluding one participant with treated hypothyroidism. The HC adolescents did not meet criteria for DSM-IV Axis I diagnoses24 and had no family history of mood or psychotic disorders in their first-degree relatives as assessed by the Family History Screen for Epidemiologic Studies.25
The revised Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSAD-PL)26 was administered to the participants and a parent/guardian. Final DSM-IV diagnoses were established by the consensus of clinical and structured interviews by a board certified psychiatrist and a psychologist, each expert in childhood mood disorders. At the time of the neuroimaging scan, 11 (52.4%) BD participants met DSM-IV criteria for an elevated mood episode (manic, mixed or hypomanic), 2 (9.5%) for a depressive episode and 8 (38.1%) were euthymic. Psychotropic medications prescribed to the participants with BD at the time of scanning included lithium carbonate (4, 19.0%), anticonvulsants (9, 42.9%), atypical antipsychotics (11, 52.4%), antidepressants (6, 28.6%), stimulants (5, 23.8%), benzodiazepines (1, 4.8%) and levothyroxine (1, 4.8%). Four (19.0%) of the BD participants were unmedicated at the time of scanning.
Following a complete description of the research, written informed consent was obtained from parents/guardians and participants who were 18 years old. Written informed assent was obtained from minors. This research was approved by the Institutional Review Boards of a University Medical Center and the Department of Veterans Affairs.
Structural and Functional Magnetic Resonance Imaging Data Acquisition
High resolution structural MRI and fMRI scanning were performed on a 3-Tesla Siemens Trio MR scanner (Siemens, Erlangen, Germany). A three-dimensional Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1-weighted sequence was used to acquire sagittal images with parameters: TR = 1500 ms, TE = 2.83 ms, FOV = 256 × 256 mm2, matrix = 256 × 256, slice thickness = 1.0 mm without gap, 160 slices, 2 averages. In the same imaging session, fMRI data were acquired with a single-shot echo planar imaging (EPI) sequence in alignment with the anterior commissure-posterior commissure (AC-PC) plane with parameters: TR = 2000 ms, TE = 25 ms, FOV = 240 × 240 mm2, matrix = 64 × 64, flip angle = 80°, slice thickness = 3.0 mm without gap, 32 slices.
Structural Magnetic Resonance Imaging Data Processing
High resolution structural images were processed and analyzed with Statistical Parametric Mapping 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm). Briefly, the SPM5 segmentation function was implemented for bias correction, spatial normalization and segmentation of the original structural images in the same model.27 Bias correction produced images that had more uniform intensities. SPM5 tissue probability maps (voxel size 2 × 2 × 2 mm3) were used to guide the normalization and segmentation. During the spatial normalization, a ‘modulation’ step28 was used to ensure that the overall amount of each tissue class was not altered. Finally, the segmented, normalized and modulated gray matter images were smoothed with a 12-mm, full width at half maximum (FWHM), isotropic Gaussian kernel. The bilateral amygdala region of interest (ROI) was delineated on the tissue possibility map of gray matter using BioImage suite software (http://www.bioimagesuite.org). The mean amygdala volume values obtained from voxel-based morphometry,28 were extracted from the bilateral amygdala ROI.
Event-Related Emotional Face Paradigm
This task has been used previously by our group and was demonstrated to elicit robust amygdala signal change.29 The task is an adaptation of the block design emotional face paradigm described in Blumberg et al.21 which was used to detect significant differences in amygdala activation between individuals with and without BD. Modifications include implementing an event-related design and addition of a female-male button press. The gender discrimination task results in implicit processing of the emotional face stimuli and has generated robust amygdala activation.29, 30 Participants viewed faces from the Ekman series31 depicting happy, fearful or neutral expressions that were displayed using Psyscope software32 and were instructed to press a button to make a female-male determination. The faces were each on-screen for two seconds and separated by four, eight or twelve second intervals when a cross-hair fixation point was displayed. The face stimuli included five women and five men, with each individual exhibiting all three of the expressions for a total of 30 faces per run. The duration of each run was 4 minutes and 50 seconds and data were averaged over four runs. Ordering of the face stimuli was systematically varied to control for sequential dependencies and was counterbalanced for facial expression, sex, the identity of the face and the length of the interval between stimuli.
Functional Magnetic Resonance Imaging Data Processing
Functional MR image preprocessing was completed using SPM99 software33 as described previously.29 Two images at the beginning of each fMRI run were discarded to account for hemodynamic delay. The remaining images within each run were then realigned to the first volume of that run to correct for movement. Following motion correction, the images were spatially normalized to a standard EPI template from the Montreal Neurological Institute (MNI). FMRI data were resampled to voxels 4 × 4 × 4 mm3 during normalization. Finally, the images were smoothed with a 12-mm, FWHM, isotropic Gaussian kernel.
Event-related response amplitudes were estimated for each participant using the general linear model33 for each of the three event types: fearful, happy, and neutral expressions. This created statistical images of the blood oxygen level-dependent (BOLD) signal change associated with the fearful and happy faces minus the neutral face for each individual subject. The bilateral amygdala region of interest (ROI) was defined by the Wake Forest University (WFU) toolbox.34 The mean percent signal change in the bilateral amygdala ROI was obtained in each participant for the two emotional face types (fear and happy, with neutral subtracted from each) using the MarsBar ROI toolbox.35
Statistical Analysis
Statistical analyses were conducted using SAS software, version 9.1 (SAS Institute, Cary, NC). Amygdala volumes and mean percent signal change values were tested for normality using Kolmogorov-Smirnov test statistics and normal probability plots and were then averaged for hemisphere.
Amygdala volumetric analysis: was conducted using an analysis of covariance (ANCOVA) model that included diagnostic group (HC vs. BD) as a between-subjects factor and age as a covariate. SPM5 was used to display significant group differences in structure from the analysis described above.
Amygdala activation analysis: was conducted using a linear mixed model to assess the effects of diagnostic group and the two emotional stimulus types on the mean percent signal change in the entire amygdala ROI. In this model, diagnostic group (HC vs. BD) represented a between-subjects factor and emotion type (fear and happy, with neutral subtracted from each) was included as a within-subjects factor. The dependent measure employed for this repeated measure analysis was the average percent signal change for the entire amygdala ROI for each emotion type. The interaction between diagnostic group and emotion was modeled and age served as a covariate. The correlation structure of the data was modeled by unstructured variance-covariance matrix for observations on the two emotions. The latter variance-covariance structure was the best fitting according to the Akaike Information Criterion. SPM99 was used to display significant group differences in function from the analysis described above.
In the ANCOVA and linear mixed models described above, least square means and standard errors were computed from each model and plotted to interpret diagnosis effects. Post-hoc exploratory analyses were performed for potential main effects of mood state and presence or absence of psychotropic medication at time of scanning on amygdala volume and mean percent signal change among BD participants.
Potential associations between amygdala volume and activation among BD participants were examined using correlation analysis. Pearson product moment correlations were performed between amygdala volume and amygdala percent signal change in participants with BD for each emotion type (fear and happy, with neutral subtracted from each) and for the average across the two emotion types. All statistical analyses conducted were two-tailed.
RESULTS
The HC and BD groups did not differ statistically in age (p = 0.12) or sex distribution (p = 0.43). With regard to amygdala volume, two participants with BD displayed excessive movement and were excluded from volumetric analyses. There was a significant main effect of diagnosis such that amygdala volumes were significantly smaller in adolescents with BD relative to HC adolescents [F(1,46) = 7.44, p = .009]. Figure 1 shows areas of reduced volume, displaying the localization of clusters of difference within the amygdala ROI for the significant comparison from the ANCOVA model (p < .01). An ANOVA using only diagnosis, without other predictors, also yields a significant main effect of diagnosis, such that amygdala volumes were significantly smaller in adolescents with BD relative to HC adolescents [F(1,47) = 8.34, p = .006).
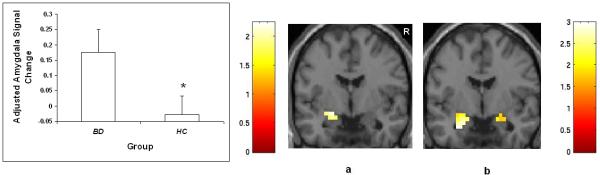
Figure 1.
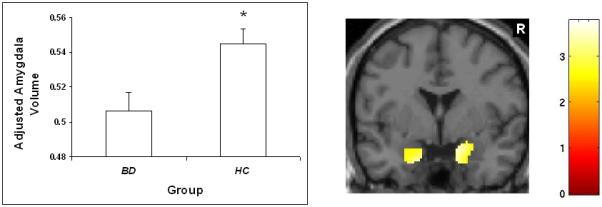
Amygdala volume in adolescents with and without BD
The graph displays least square (ls) mean age-adjusted amygdala volume ± standard error in adolescents with BD and HC adolescents. The coronal image (y = 0mm, MNI coordinates) shows the bilateral amygdala regions where volume was significantly smaller in adolescents with BD relative to HC adolescents at threshold p < 0.01. The color bar represents the range of T values.
R = right.
With regard to amygdala activation, there were three participants with BD and four HC participants who displayed excessive movement and were excluded from functional analyses. There was a significant main effect of diagnosis [F(1,41) = 4.36, p = 0.043], derived from greater amygdala activation amongst adolescents with BD when processing emotional face stimuli, relative to HC adolescents. Figure 2 shows BOLD contrast maps from SPM99 displaying the localization of clusters of difference within the amygdala ROI for the significant comparisons from the linear mixed model (p < .05). There was also a significant main effect of emotion [F(1,42) = 8.83, p = 0.005], derived from an overall greater activation in all participants, irrespective of group, to fearful faces compared to happy faces. There was no significant interaction between diagnosis and emotion type. An analysis including only diagnosis and emotion without other predictors does not generate a significant association between diagnosis and amygdala activation [F(1,42) = 2.81, p =.10). The response to positive emotional stimuli in the individuals with BD was inversely associated with age(r = −.51, p = .03, N = 18). Post-hoc analyses revealed that, amongst the BD participants, no significant effects on amygdala volume or activation during processing of emotional faces were detected in relation to clinical factors including presence or absence of psychotropic medication or mood state (euthymic, elevated or depressed).
Figure 2.
Amygdala percent signal change in adolescents with and without BD
The graph displays least square (ls) mean age-adjusted percent signal change ± standard error in the amygdala during processing of emotional face stimuli in adolescents with BD and HC adolescents. The coronal images (y = −4mm, MNI coordinates) show the amygdala regions where activation was increased in the BD group compared to the HC group when processing (a) happy faces and (b) fearful faces at threshold p < 0.05. The color bars represent the range of T values.
R = right.
Correlational analysis conducted among the BD participants revealed a significant inverse association between amygdala volume and amygdala activation during the processing of happy faces (r = −.59, p = .015, N = 16) (Figure 3). There was no significant association between amygdala volume and activation during the processing of fearful faces (r = −.37, p = .158, N = 16). A significant inverse association was observed between amygdala volume and the average amygdala activation derived from response to both emotion types (r = −.54, p = .029, N = 16).
Figure 3.
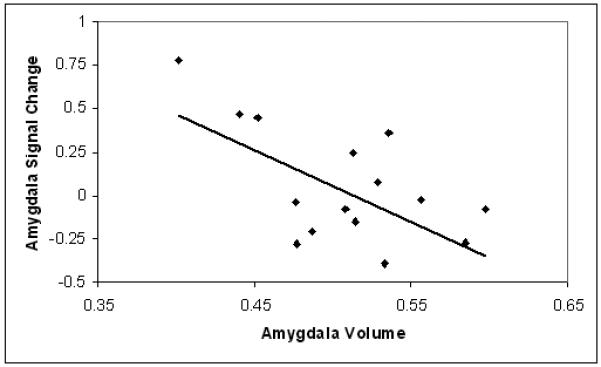
Association between amygdala volume and activation during happy face processing among adolescents with BD
The scatter plot displays the mean percent signal change in the amygdala during processing of happy faces as a function of mean amygdala volume for each participant with BD (r = −.59, p = 0.015).
DISCUSSION
We observed both significantly reduced amygdala volume and significantly increased amygdala response to emotional stimuli in a sample of adolescents with BD relative to HCs. The structural and functional neuroimaging data for each participant were acquired in the same session. Moreover, an association was revealed between the structural and functional abnormalities, such that adolescents with BD who had the smallest amygdala volume exhibited the highest amygdala activation when processing emotional stimuli. These findings are consistent with reports in the literature of structural and functional amygdala abnormalities in youth with BD. To the best of our knowledge, this work represents the first report where the two findings are reported in the same sample of adolescents with BD and the first demonstration of an association between these abnormalities in BD. Intriguingly, our findings also provide some early evidence in BD to suggest a pathophysiological link between amygdala structural abnormalities and abnormal responding to emotional stimuli. This is supported by the association between amygdala structure and function.
In the adolescents with BD, age was associated with response to positive emotional stimuli, such that the youngest participants with BD exhibited the highest amygdala activation when processing positive emotional stimuli. Thus, age was used as a covariate in analyses. When age is not included as a covariate in the analyses, while the association between diagnosis and amygdala volume remains statistically significant, the association between diagnosis and amygdala activation is no longer significant.
The pathophysiological mechanisms that may contribute to these amygdala abnormalities cannot be determined based on this work. Decreased amygdala volume reported herein could be a consequence of a number of different cellular processes, including decreased density of neuronal processes, altered ratio of small and large cell types, or loss or atrophy of neurons or glia. Postmortem study of the amygdala in BD has generated report of reduced number and density of neurons, as well as volume, in the lateral nucleus (LN) and reduced neuron density in the accessory basal nucleus (ABN).36 Furthermore, Bezchlibnyk et al.37 found decreased neuron somal size in the LN and the parvocellular portion of the ABN in individuals with BD. Finally, Bowley et al.38 reported decreased glial cell density in the amygdala of participants with BD.
The association between structural and functional abnormalities in the amygdala suggests the presence of a single pathophysiological mechanism that may contribute to these two abnormalities. A plausible pathway towards these outcomes could be disruption of an inhibitory neurotransmitter system in the amygdala, such as gamma-aminobutyric acid (GABA), with release of inhibition resulting in elevated function. GABAergic cell loss in limbic structures in individuals with BD has been reported in postmortem studies.39-41 Loss of inhibitory GABAergic interneurons in amygdala is one potential mechanism that could contribute to both decreased volume and increased excitatory activity in the amygdala in youth with BD. Glial loss has also been postulated to be a cellular mechanism that could disrupt the balance between glutamatergic and GABAergic systems resulting in excessive limbic activity in BD.42, 43 Other possibilities include cellular changes associated with other neurotransmitter systems that modulate amygdala activity, such as serotonin and dopamine,44, 45 or conversely activity changes in amygdala that lead to cellular changes. For example, Siegle et al.20 suggested that excessive amygdala activation may increase excitotoxic effects of glutamate, given the glutamatergic projections within the amygdala, resulting in smaller amygdala volume. Future studies that elucidate the mechanisms underlying the development of the associated structure-function changes in BD may yield new insights into its pathophysiology and potentially suggest novel treatment approaches.
Amygdala abnormalities are not specific to BD and have been reported in other childhood-onset psychiatric conditions, including volumetric abnormalities in childhood-onset schizophrenia46 and unipolar depression.47 Amygdala activation abnormalities have also been reported in a pediatric depressed sample.48 However, in unipolar depression elevated amygdala activation is observed in response to negative stimuli, while in youth with BD we, as well as others, observed elevated amygdala activation in response to both negative and positive stimuli.17 Altered responding to positively-valenced stimuli may be especially important in contributing to the unique predisposition to manic states in pediatric BD.49 This phenotype may be salient to distinguishing youth with BD from those with other psychiatric conditions. While the possible particular relevance of abnormal responding to positive stimuli in pediatric BD is of interest, the absence of an interaction between diagnosis and emotion type and the fact that the inverse association between amygdala volume and activation was only significant for response to positive stimuli and not for response to negative stimuli, highlight the preliminary nature of our findings and the need for replication.
Preliminary evidence suggests that mood-stabilizing treatments for BD may exert their effects by normalizing structural and functional abnormalities in the amygdala.12, 21 We did not detect significant effects of presence of medication. However, our ability to detect effects could have been limited by inadequate power. Future studies to systematically assess potential influences of treatment on amygdala structure and function are warranted. Replication in larger, more homogenous samples, e.g., a sample of euthymic participants with BD, will be important to address these limitations.
In summary, this study provides evidence to support a structure-function relationship in the amygdala in adolescents with BD characterized by a significant inverse association between volume and response to emotional stimuli. These findings provide preliminary evidence to suggest a possible pathophysiological link between abnormalities in amygdala structure and amygdala response to emotional stimuli in BD.
References
- 1.McClure EB, Pope K, Hoberman AJ, Pine DS, Leibenluft E. Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry. 2003 Jun;160(6):1172–1174. doi: 10.1176/appi.ajp.160.6.1172. [DOI] [PubMed] [Google Scholar]
- 2.Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007 Aug;46(8):1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- 3.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008 Spring;20(2):529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998 Aug 17;32(3):171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 5.Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002 Feb;159(2):302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- 6.Murphy FC, Sahakian BJ, Rubinsztein JS, et al. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 7.Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2007 Nov 12; doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 8.LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992 Apr;2(2):191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 9.Schneider F, Grodd W, Weiss U, et al. Functional MRI reveals left amygdala activation during emotion. Psychiatry Res. 1997 Dec 30;76(2-3):75–82. doi: 10.1016/s0925-4927(97)00063-2. [DOI] [PubMed] [Google Scholar]
- 10.Fried I, Cameron KA, Yashar S, Fong R, Morrow JW. Inhibitory and excitatory responses of single neurons in the human medial temporal lobe during recognition of faces and objects. Cereb Cortex. 2002 Jun;12(6):575–584. doi: 10.1093/cercor/12.6.575. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg HP, Fredericks C, Wang F, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005 Dec;7(6):570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005 Jun;44(6):565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 13.Chen BK, Sassi R, Axelson D, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004 Sep 15;56(6):399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 14.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004 Feb;6(1):43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 15.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005 Jul;62(7):734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 16.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004 May 30;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007 Jul 15;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008 Apr 15;162(3):244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006 Jun 6;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acad Sci. 2003 Apr;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005 Dec;183(3):308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004 Mar 15;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Kalmar JH, Wang F, Chepenik LG, Shah MP, Papademetris X, Blumberg HP. Progressive changes in an amygdala-ventral prefrontal neural system during adolescence in bipolar disorder. Biol Psychiatry. 2008;63(S1):50. [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, D.C.: 2000. Text Revision ed. [Google Scholar]
- 25.Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H. Family psychiatric screening instruments for epidemiologic studies: pilot testing and validation. Psychiatry Res. 1995 Jul 28;57(2):169–180. doi: 10.1016/0165-1781(95)02632-7. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 Jul;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005 Jul 1;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000 Jun;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 29.Shah MP, Wang F, Kalmar JH, et al. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. doi: 10.1038/npp.2008.204. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996 Oct 31;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 31.Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists; Palo Alto, CA: 1979. [Google Scholar]
- 32.Cohen J, MacWhinney B, Flatt M, Provost J. An interactive graphical system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Methods, Research, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- 33.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping. 1995;2:189–210. [Google Scholar]
- 34.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003 Jul;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 35.Matthew B, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2) abstract 497. [Google Scholar]
- 36.Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007 Oct 15;62(8):884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Bezchlibnyk YB, Sun X, Wang JF, MacQueen GM, McEwen BS, Young LT. Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neurosci. 2007 May;32(3):203–210. [PMC free article] [PubMed] [Google Scholar]
- 38.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002 Sep 1;52(5):404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 39.Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001 Sep 15;50(6):395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- 40.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998 Jul 15;44(2):88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 41.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001 Jul;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 42.Krystal JH, Sanacora G, Blumberg H, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 43.Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci. 1998 Nov 15;18(22):9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002 Jan 1;22(1):324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frazier JA, Hodge SM, Breeze JL, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008 Jan;34(1):37–46. doi: 10.1093/schbul/sbm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005 Jan 1;57(1):21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001 Nov;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 49.Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry. 2003 Jun 1;53(11):1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]