Abstract
Thyrotropin-releasing hormone (TRH, pGlu-His-Pro-NH2) and the structurally related [Glu2]TRH (pGlu-Glu-Pro-NH2) are endogenous peptides with a plethora of actions in the central nervous system. Many centrally-mediated effects of TRH are shared with those of [Glu2]TRH, although the involvement of different receptors are presumed. The analeptic action is the best-known TRH-related central nervous system effect. While [Glu2]TRH itself is analeptic, its co-administration with TRH into mice produced a dose-depended attenuation of TRH-evoked reversal of barbiturate-induced sleeping time. This finding is in agreement with our previous observations that [Glu2]TRH significantly attenuates TRH-induced hippocampal extracellular acetylcholine release. Taken together, [Glu2]TRH may be considered as a negative modulator for the cholinergic effect of TRH in the mouse brain.
Keywords: thyrotropin-releasing hormone, endogenous analogue, analeptic effect, acetylcholine
1. Introduction
Thyrotropin-releasing hormone (TRH; pGlu-His-ProNH2) and [Glu2]TRH (pGlu-Glu-ProNH2) are structurally related endogenous peptides (Fig. 1) that are identified both in neuronal and non-neuronal tissues [3, 6]. It has been well-established that TRH is not only a hypothalamic regulatory hormone, but also a neumodulator and/or neurotransmitter with a plethora of suprahypothalamic actions in the mammalian central nervous system (CNS) [15, 19]. Many CNS-effects of TRH is shared with its metabolically stable analogues such as [Glu2]TRH [22] in which the basic histidyl residue (His) of TRH is replaced with the acidic glutamyl residue (Glu). Specifically, [Glu2]TRH manifests analeptic, neuroprotective, antidepressant, anticonvulsant and many other activities in the CNS. However, it is devoid of TSH-releasing activity and, thus, does not elevate thyroid-hormone levels [14, 15]. Additionally, [Glu2]TRH, is not metabolized by thyroliberinase, an enzyme that specifically and rapidly inactivates TRH within minutes in blood [11, 20, 27]; therefore, [Glu2]TRH is stable in rat serum for at least 4 h and is cleared only by the kidneys [12, 13]. This peptide is believed not to be derived from prepro-TRH [3], and does not bind with an appreciable affinity to the two known TRH-receptor isoforms (TRHR1 and TRHR2) [4, 9]. Altogether, it is reasonable to assume that pharmacological effects evoked by this TRH-like peptide, which are also shared with TRH, do not involve the activation of the known TRH receptors but, rather, involve its own receptor or other, yet to be identified TRH receptor(s) in the CNS.
Fig. 1.
Chemical structure of TRH and [Glu2]TRH.
Many CNS-effects of TRH are mediated via neurotransmitters [10]. The robust effect of TRH on the stimulation of acetylcholine (ACh) synthesis and the subsequent increase of extracellular ACh levels in the CNS have been well known [7, 17, 21]. A vast array of CNS-actions of TRH such as analeptic activity has been proposed to occur primarily through cholinergic mechanisms [10, 28, 30], although GABAergic [5], dopaminergic [16] and noradrenergic [16] components of this effect have been implicated in this effect without direct stimulation of the cognate receptors. [Glu2]TRH itself is analeptic [9, 22, 25], although this peptide was found less efficacious than TRH to antagonize pentobarbital-induced anesthesia in mice [22].
Previously, we have shown that local perfusion of [Glu2]TRH via in vivo microdialysis into the rat hippocampus did not change basal levels of extracellular ACh implying that, unlike TRH, this TRH-related peptide does not possess an intrinsic ACh-stimulatory effect [17]. However, a significant attenuation of TRH-induced ACh release was observed, when [Glu2]TRH was co-perfused with TRH in equimolar dose. Accordingly, [Glu2]TRH was found to oppose the cholinergic effect of TRH in the rat CNS. This finding implies that various CNS-effects associated with [Glu2]TRH may not directly involve a cholinergic mechanism. It is noteworthy that the diastereomer [D-Glu2]TRH had no effect on basal extracellular ACh levels and TRH-evoked increase of this neurotransmitter [17]. An inhibitory effect of [Glu2]TRH on TRH-induced growth-hormone release from avian pituitary in birds has also been reported, suggesting that [Glu2]TRH may act as a TRH receptor antagonist or negative modulator within this axis [8].
In the present study, our aim was to investigate whether [Glu2]TRH has a modulatory effect on TRH–evoked analeptic action in mice upon intravenous (i.v.) administration. Analepsia (reversal of sedation) is undoubtedly the best-known action of TRH in the CNS [10, 19, 28]. TRH and related peptides can antagonize the sedation and hypothermia produced by various drugs. This arousal is commonly measured by the reduction of barbiturate-induced narcosis and the effect is apparently mediated primarily via the activation of cholinergic neurons in the hypothalamus [28] but also via non-cholinergic mechanisms in other regions of the brain [5].
2. Materials and Methods
2.1. Instruments and materials
All chemicals were reagent- or peptide-synthesis grade. Solvents were obtained from Fisher Scientific (Atlanta, GA). 9-Fluorenylmethyloxycarbonyl (Fmoc)-Rink Amide resin, Fmoc-amino acids, TRH and [Glu2]TRH were purchased from Bachem BioSciences (Torrance, CA). Purification of [D-Glu2]TRH was done by reversed-phased high-performance liquid chromatography (RP-HPLC) on a system consisting of a SP 200 binary gradient pump (Thermo Fisher, San Jose, CA), a Rheodyne (Cotati, CA) model 7125 injector valve equipped with a 5-mL sample loop, and a SP 100 UV/VIS detector (Thermo Fisher) operated at 220 nm. The 100 mm × 25 mm i.d. Waters (Milford, MA) RCM DeltaPack C18 column was operated at 3.0 mL/min flow rate. Analytical RP-HPLC was performed on a Surveyor system operated by ChromQuest 4.0 Chromatography Workstation Software (Thermo Fisher) using an Alltech (Deerfield, IL) Econosil C18 column (150 mm × 3.2 mm i.d., 5-μm particles), a flow rate of 0.5 mL/min and UV detection at 220 nm. The mobile phases were mixed from 0.1 % (v/v) trifluoroacetic acid (TFA) in H2O (solvent A) and 0.08% (v/v) of TFA in CH3CN (solvent B). Mass spectra (MS) were acquired on a quadrupole ion trap instrument (LCQ, Thermo, San Jose, CA, USA) operated with the manufacturer’s XCalibur 1.4 software. Atmospheric pressure chemical ionization (APCI) was used with vaporizer temperature of 450 °C, capillary temperature of 150 °C, discharge current of 5 μA, and sheath and auxiliary gas (nitrogen) flow of 60 and 10 arbitrary units, respectively. Elemental analysis was performed by Atlantic Microlab, Inc. (Norcross, GA) confirming ≥ 95% purity of the test compounds.
Male Swiss-Webster mice (30±2 g body weight) obtained from Charles River Laboratories (Wilmington, MA) were used for the experiments. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Florida before initiation of the study. The animals were housed five per cage with free access to water and food with a normal day/night cycle. Each animal was tested only once.
2.2. Solid-phase synthesis of [D-Glu2]TRH
Routine manual solid-phase peptide synthesis on 1 g of pre-loaded (Fmoc)-Pro-Rink-MBHA-Amide resin (0.48 meq/g) utilizing standard Fmoc-chemistry with benzotriazole-1-yloxy-tris-pyrrolidinophosphonium hexafluoro-phosphate/1-hydroxybenzotriazole/N,N′-diisopropyl-ethylamine (PyBOP/HOBt/DIPEA) (1:1:2) activation in N,N-dimethylformamide [15, 19, 21, 24] was used to obtain the diastereomer of [Glu2]TRH, pGlu-D-Glu-Pro-NH2. Fmoc-D-Glu was protected on the side-chain as tert-butyl (tBu) ester. Double coupling (each for 1 h) was applied. The tripeptide was cleaved from the solid support with a mixture of TFA, water and triisopropyl silane (95:2.5:2.5, 10 mL, 1 h). After lyophilization, the crude peptide was purified by preparative RP-HPLC. Purity was confirmed by analytical RP-HPLC, RP-HPLC-MS and combustion analysis. MS (APCI): (M+H)+ m/z 355. Combustion analysis calc. for C15H22N4O6 × 1 H2O: C, 48.38, H, 6.45; N, 15.05; found: C, 48.23; H, 6.28; N, 14.85.
2.3. Analeptic effect
Eight to eighteen Swiss-Webster mice (30 ± 2 g body weight) were used in each group, according to our previous publications [19, 22–25]. Test compounds were dissolved in saline. The vehicle alone (1.5 mL/kg body weight; control group) or test compounds at various dose in saline were injected through the tail vein of mice. After 10 min, each animal received an intraperitoneal (i.p.) injection of sodium pentobarbital solution at a dose of 40 or 60 mg/kg body weight. Sleeping time was recorded from the onset of loss of the righting reflex until this reflex was regained by the animal. ED50 for [Glu2]TRH was calculated using the Scientist software, version 2.01 (Micromath, St. Louis, MI, USA), by fitting the results of the dose–response experiments to an equation similar to the one introduced by Cheng and Prusoff [2]:
| (1) |
where Δ and Δmax are the average decrease and maximal measured average decrease in sleeping time (min) compared to control, respectively, Di is the dose (μmol/kg body weight), and h is the Hill coefficient [2].
2.4. Statistical analysis
Results were expressed as mean values with standard deviations (SDs, in the factorial experiment [1]) or standard errors (SEMs, in dose–response studies). The comparisons between drug-treated and control animals were made by analysis of variance (ANOVA) followed by post hoc Tukey test for multiple comparisons. Differences were considered significant at P<0.05.
3. Results
To select experimental conditions appropriate for testing the modulation of TRH-evoked arousal by [Glu2]TRH in mice, a preliminary study was performed by using different TRH and pentobarbital doses in a 2×2 factorial experiment [1] (Table 1) based on our previously optimized paradigm for the timing of drug injections [19, 22]. Specifically, we have found that i.p. pentobarbital administration 10 min after i.v. TRH produced the most robust analeptic response. The complex relationship between sleeping time measured by the assay at the given doses of the systemically administered agents, as implicated by earlier studies [18, 28], has been confirmed by statistical analysis summarized in the footnote of Table 1. Both TRH (factor A) and pentobarbital (factor B) doses had significant impact, and interaction (A*B) between the factors was also revealed. Nevertheless, the pentobarbital dose had the largest influence in the paradigm. For the experiments testing the modulation of TRH’s analeptic effect by [Glu2]TRH, we selected 10 μmol/kg body weight TRH (i.v.) and 60 mg/kg body weight i.p. pentobarbital doses based on considering coefficients of variation (CVs) to maximize statistical power of our model.
Table 1.
Sleeping times obtained after different doses of TRH and pentobarbital (2 × 2 factorial experiment). Sleeping time was recorded from the onset of the loss of the righting reflex until this reflex was regained. Pentobarbital was injected 10 min after administration of TRH [20, 23]. Sleeping times (min) are given as averages ± standard deviations (SDs, n=8–10), along with the corresponding coefficients of variation (CVs, expressed as percentages).
| TRH (A) (μmol/kg body weight, i.v.)a | Pentobarbital (B) (mg/kg body weight, i.p.) |
|
|---|---|---|
| 40 | 60 | |
| Sleeping time ± SD,b min (CV, %) | ||
| 10 | 31 ± 2 (8) | 52 ± 4 (7) |
| 20 | 26 ± 3 (10) | 38 ± 5 (13) |
Saline controls: 46 ± 3 min and 90 ± 5 for 40 mg/kg and 60 mg/kg body weight pentobarbital, respectively.
A: F(1,29)=60.5, p<0.0001; B: F(1,29)=164.1, p<0.0001; A*B: F(1,29)=13.4, p=0.001 by analysis of variance (ANOVA).
As mentioned in the introduction, [Glu2]TRH was also found to possess intrinsic analeptic activity [9, 22, 25]. However, unlike TRH, this endogenous peptide does not produce an increase in extracellular ACh levels [17], and arousal in mice after its i.v. injection is less profound than that of TRH (Table 2) under the experimental conditions selected from the above 2 × 2 factorial experiment (Table 1). Accordingly, TRH produced almost 50% of decrease in the sleeping time compared to the vehicle (saline) treated control group upon using 60 mg/kg body weight i.p. pentobarbital 10 min after injection of 10 μmol/kg body weight of test compounds (Table 2 and Fig. 2, bars with hatch pattern of vertical lines). At the same time, exposing mice to equimolar dose of [Glu2]TRH decreased the sleeping time by approximately 20% compared to the vehicle treated group (Table 2). Dose-response studies conducted at various doses of [Glu2]TRH (5, 10, 15, 25, 50, and 100 μmol/kg body weight i.v., respectively) showed a statistically significant (p <0.05) difference in sleeping times at each doses compared to the saline-treated control group, and afforded an ED50 (dose producing 50% of the maximum response) of 23 ± 6 μmol/kg body weight under the given experimental conditions (Fig. 2, bars with hatch pattern of horizontal lines). Non-linear fitting to equation (1) [2] presumed to describe dose–response relationship yielded h ≅ 1. Interestingly, when [Glu2]TRH or TRH was administered intracisternally into rats at a dose of 10 μg after the animals received pentobarbital first [9], the two peptides were practically equipotent in reducing the pentobarbital-induced sleeping time. Nevertheless, the goal of our study was not to compare the analeptic potency of these two structurally similar peptides, but to demonstrate their potential interplay in the CNS through a pharmacological paradigm.
Table 2.
Barbiturate induced sleeping time in mice after i.v. administration of test compounds (equimolar doses of 10 μmol/kg body weight) when pentobarbital (60 mg/kg, i.p.) was injected 10 min after injection of test compound. Data was given as average ± SEM.
| Compound | Sleeping time (min) |
|---|---|
| Vehicle | 79±2 |
| TRH | 40±2* |
| [Glu2]TRH | 65±2*,† |
| [D-Glu2]TRH | 76±2 |
| [Glu2]TRH + TRH | 59±3*,† |
| [D-Glu2]TRH + TRH | 43±2* |
Statistically significant difference (P < 0.05, n=12–18) from saline control,
Statistically significant difference from TRH (alone).
Fig. 2.
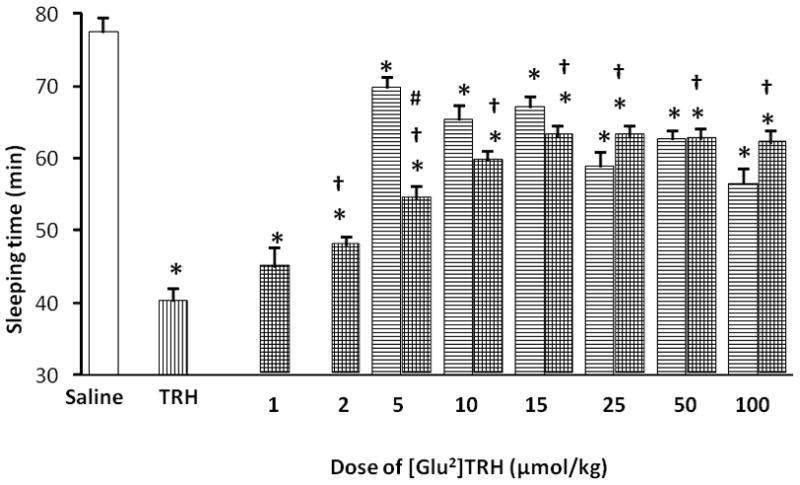
Sleeping time measured after i.v. administration of TRH (bars with hatch pattern of vertical lines) or [Glu2]TRH (bars with hatch pattern of horizontal lines) at equimolar dose (10 μmol/kg body weight) followed by injection of pentobarbital (60 mg/kg body weight, i.p.) 10 min later, as well as reversal of TRH-induced analeptic effect (10 μmol/kg body weight) by various doses of [Glu2]TRH upon co-administration with TRH (bars shaded with cross-hatch pattern). Open bar shows sleeping time measured for the control (saline) group. The sleeping time was recorded from the onset of the loss of the righting reflex until the reflex was regained. Data are shown as average ± SEM for n=12–18 at each doses. Statistical evaluation was done by analysis of variance (ANOVA) followed by post hoc Tukey test. Differences were considered significant with P<0.05; * indicates statistically significant differences from the control group (saline); † indicates statistically significant differences from the TRH group; # indicates statistically significant differences from the [Glu2]TRH group.
Replacement of the Glu with its D-diastereomer (D-Glu) in [Glu2]TRH resulted in a complete loss of analeptic response under the experimental conditions used (Table 2). When [D-Glu2]TRH was co-injected with equimolar dose of TRH (10 μmol/kg body weight, each agent), there was no significant change in sleeping times compared to that of TRH alone (43 ± 2 min and 40 ± 2 min, respectively) either. In all, [D-Glu2]TRH is neither an analeptic nor an inhibitor/negative modulator, in this regard. These observations are in agreement with those concluded for the ACh release studies [17], implying that Xaa must be an L-amino acid to induce these CNS effects with a peptide having pGlu-Xaa-Pro-NH2 sequence.
A different outcome was observed, however, upon co-administration of [Glu2]TRH and TRH (Fig. 2, bars shaded with cross-hatch pattern). Specifically, [Glu2]TRH dose-dependently attenuated TRH-induced analeptic action. The effect of [Glu2]TRH was tested at various doses (1, 2, 5, 10, 15, 25, 50, and 100 μmol/kg body weight, respectively), while keeping the doses of TRH and pentobarbital constant at 10 μmol/kg (i.v.) and 60 mg/kg body weight (i.p.), respectively. As shown in Fig. 2 (bars shaded with cross-hatch pattern), there was no synergism between the two peptides having intrinsic analeptic activity; rather, a negative modulatory effect of [Glu2]TRH could be implicated. Although i.v. co-administration of 1 μmol/kg body weight of [Glu2]TRH and 10 μmol/kg body weight of TRH did not produce statistically significant difference in sleeping time compared to that of TRH alone (45 ± 3 min and 40 ± 2 min, respectively, see Fig. 2), increasing the [Glu2]TRH doses to ≥2 μmol/kg body weight did afford a profound reversal of the TRH-evoked analeptic effect. Actually, the measured sleeping times were statistically not different at ≥10 μmol/kg body weight of [Glu2]TRH from those of obtained by [Glu2]TRH alone at the same dose. In essence, the reversal of pentobarbital-induced narcosis elicited by TRH was inhibited by equimolar or higher doses of [Glu2]TRH under the experimental conditions we used (Fig. 2, bars shaded with cross-hatch pattern).
4. Discussion
In the present study, we investigated the influence of [Glu2]TRH on TRH-evoked analeptic action. The impact of TRH and related peptides on certain CNS effects has been associated with the augmentation of various neurotransmitter systems, mostly involving cholinergic neurons [10, 15, 19, 30]. However, the lack of specific TRH antagonists has hampered the elucidation of the mechanisms underlying of the diverse CNS-actions of TRH. Thus far, only benzodiazepines have showed weak antagonism [26]; however, they also produce CNS depression limiting thereby their usefulness in this regard [15]. Previously, we have shown that local perfusion of [Glu2]TRH via in vivo microdialysis into the rat hippocampus did not change the basal level of extracellular ACh; co-perfusion of [Glu2]TRH with TRH, on the other hand, yielded a significant attenuation of TRH-induced ACh release [17]. At the same time, the diastereomer pGlu-D-Glu-Pro-NH2 affected neither the basal extracellular nor TRH-induced increase of ACh levels, which indicates receptor-mediated mechanism of action for [Glu2]TRH. On the other hand, CNS effects of the latter endogenous peptide alone does not arise through a cholinergic mechanism and, therefore, may implicate indirect mechanisms via GABAergic, dopaminergic and noradrenergic receptors [5, 16]. Nevertheless, [Glu2]TRH may be involved in the control of the cholinergic effects of TRH as a negative modulator of the latter.
As an extension of these studies, we addressed the effect of [Glu2]TRH on the TRH-evoked analeptic effect in this report. We confirmed that [Glu2]TRH itself was analeptic [9, 22, 25]; however, its analeptic action was not only less efficacious than that of TRH under the experimental conditions employed (Table 2 and Fig. 2), but it was apparently mediated by a non-cholinergic mechanism based on earlier ACh release studies [16] and under the experimental conditions we selected according to preliminary experiments (Table 1). When the TRH dose was kept constant at 10 μmol/kg body weight, equimolar or higher dose of [Glu2]TRH completely inhibited the TRH-evoked analeptic effect upon i.v. co-administration (Fig. 2, bars shaded with cross-hatch pattern). Taken together, our results presented here and measured by a pharmacological paradigm have confirmed previous observations obtained via neurochemical measurements utilizing in vivo intracranial microdialysis and continuous peptide influx into the brain that bypassed issues related to different blood-brain barrier penetrations, pharmacokinetics and metabolic stabilities [17]. We have essentially validated that [Glu2]TRH, indeed, opposes the cholinergic effect of TRH in the mouse brain—even upon systemic administration of these peptides. This property of [Glu2]TRH raises the hope that it may be used as a lead for the development of useful pharmacological agents, including selective TRH modulators and/or antagonists, to understand cholinergic mechanisms by which TRH exerts its vast array of CNS effects.
Acknowledgments
The authors are indebted to the inspiring scientific environment at the Department of Medicinal Chemistry, College of Pharmacy, University of Florida, where majority of the research reported here was conducted. This research was supported by a grant (MH59360 to L.P.) from the National Institute of Health. L.P. is currently the Robert A. Welch Professor at the University of North Texas Health Science Center at Fort Worth (endowment BK-0031).
Footnotes
Conflict of interest: The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vien Nguyen, Email: vnguyen@hsc.unt.edu.
Alevtina D. Zharikova, Email: alevtina@ufl.edu.
Katalin Prokai-Tatrai, Email: kprokai@hsc.unt.edu.
Laszlo Prokai, Email: lprokai@hsc.unt.edu.
References
- 1.Box GE, Hunter WG, Hunter JS. Statistics for Experimenters: Design, Innovation, and Discovery. 2. Wiley; 2005. pp. 173–174. [Google Scholar]
- 2.Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 3.Cockle SM, Aitken MA, Beg F, Smyth DG. A novel peptide, pyroglutamylglutamylproline amide, in the rabbit prostate complex, structurally related to thyrotrophin-releasing hormone. J Biol Chem. 1989;264:7788–7791. [PubMed] [Google Scholar]
- 4.Colsona AO, Gershengorn MC. Thyrotropin-releasing hormone analogs. Mini Reviews Med Chem. 2006;6:221–226. doi: 10.2174/138955706775476019. [DOI] [PubMed] [Google Scholar]
- 5.Cott J, Engel J. Antagonism of the analeptic activity of thyrotropin-releasing hormone (TRH) by agents which enhance GABA transmission. Psychopharmacol. 1976;55:145–149. doi: 10.1007/BF00439101. [DOI] [PubMed] [Google Scholar]
- 6.Del Rio-Garcia J, Smyth DG. Distribution of pyroglutamylpeptide amides related to thyrotropin-releasing hormone in the central nervous system and periphery of the rat. J Endocrinol. 1990;127:445–450. doi: 10.1677/joe.0.1270445. [DOI] [PubMed] [Google Scholar]
- 7.Giovannini MG, Casamenti F, Nistri A, Paoli F, Pepeu G. Effect of thyrotropin releasing hormone (TRH) on acetylcholine release from different brain areas investigated by microdialysis. Br J Pharmacol. 1991;102:363–368. doi: 10.1111/j.1476-5381.1991.tb12179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey S, Trudeau VL, Ashworth RJ, Cockle SM. pGlutamylglutamylprolineamide modulation of growth hormone secretion in domestic fowl: antagonism of thyrotrophin-releasing hormone action? J Endocrinol. 1993;138:137–147. doi: 10.1677/joe.0.1380137. [DOI] [PubMed] [Google Scholar]
- 9.Hinkle PM, Pekary AE, Senanayaki S, Sattin A. Role of TRH receptors as possible mediators of analeptic actions of TRH-like peptides. Brain Res. 2002;935:59–64. doi: 10.1016/s0006-8993(02)02454-x. [DOI] [PubMed] [Google Scholar]
- 10.Horita A. An update on the CNS actions of TRH and its analogs. Life Sci. 1998;62:1443–1448. doi: 10.1016/s0024-3205(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 11.Kelly JA, Slator GR, Tipton KF, Williams CH, Bauer K. Kinetic investigation of the specificity of porcine brain thyrotropin-releasing hormone-degrading ectoenzyme for thyrotropin-releasing hormone-like peptides. J Biol Chem. 2000;275:16746–16751. doi: 10.1074/jbc.M910386199. [DOI] [PubMed] [Google Scholar]
- 12.Klootwijk W, de Boer RDH, Sleddens-Linkels E, Cockle SM, de Herder WW, Bauer K, Visser TJ, de Greef WJ. Urinary excretion of the TRH-like peptide pyroglutamyl-glutamyl-prolineamide in rats. J Endocrinol. 1997;153:411–421. doi: 10.1677/joe.0.1530411. [DOI] [PubMed] [Google Scholar]
- 13.Klootwijk W, de Herder WW, Kwekkeboom DJ, Lamberts SW, Krenning EP, Visser TJ, de Greef WJ. High serum levels of the thyrotropin-releasing hormone-like peptide pyroglutamyl-glutamyl-prolineamide in patients with carcinoid tumors. J Clin Endocrinol Metab. 1997;82:3068–3073. doi: 10.1210/jcem.81.8.8768836. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd RL, Pekary AE, Sattin E, Amundson T. Antidepressant effects of thyrotropin-releasing hormone analogues using a rodent model of depression. Pharmacol Biochem Behav. 2001;70:15–22. doi: 10.1016/s0091-3057(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 15.Monga V, Meena CL, Kaur N, Jain R. Chemistry and biology of thyrotropin-releasing hormone (TRH) and its analogs. Curr Med Chem. 2008;15:2718–2733. doi: 10.2174/092986708786242912. [DOI] [PubMed] [Google Scholar]
- 16.Mushiroi T, Shibahara R, Tamura M, Shimizu T, Itoh Y, Ukai Y, Yoshikuni Y, Kimura K. Montirelin hydrate (NS-3), a TRH analog, improved the disturbance of consciousness caused by head concussion and pentobarbital in mice. Folia Pharmacol Jpn. 1996;107:237–245. doi: 10.1254/fpj.107.237. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen V, Zharikova AD, Prokai L. Evidence for interplay between thyrotropin-releasing hormone (TRH) and its structural analogue pGlu-Glu-Pro-NH2 ([Glu2]TRH) in the brain: An in vivo microdialysis study. Neurosci Lett. 2007;415:64–67. doi: 10.1016/j.neulet.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 18.Prange J, Breese GR, Cott JM, Martin BR, Cooper BR, Wilson CI, Plotnikoff NP. Thyrotropin releasing hormone: antagonism of pentobarbital in rodents. Life Sci. 1974;14:447–455. doi: 10.1016/0024-3205(74)90359-2. [DOI] [PubMed] [Google Scholar]
- 19.Prokai L. Central nervous system effects of thyrotropin-releasing hormone and its analogues: opportunities and perspectives for drug discovery and development. In: Jucker E, editor. Progress in Drug Research. Vol. 59. Birkhäuser; Basel, Switzerland: 2002. pp. 133–169. [DOI] [PubMed] [Google Scholar]
- 20.Prokai L, Prokai-Tatrai K, Zharikova AD, Nguyen V, Perjési P, Stevens SM. Centrally acting and metabolically stable thyrotropin-releasing hormone analogues by replacement of histidine with substituted pyridinium. J Med Chem. 2004;47:6025–6033. doi: 10.1021/jm020531t. [DOI] [PubMed] [Google Scholar]
- 21.Prokai L, Zharikova AD. Neuropharmacodynamic evaluation of the centrally active thyrotropin-releasing hormone analogue [Leu2]TRH and its chemical brain-targeting system. Brain Res. 2002;952:268–274. doi: 10.1016/s0006-8993(02)03251-1. [DOI] [PubMed] [Google Scholar]
- 22.Prokai-Tatrai K, Nguyen V, Zharikova AD, Braddy AC, Stevens SM, Jr, Prokai L. Prodrugs to enhance central nervous system effects of the TRH-like peptide pGlu-Glu-Pro-NH2. Bioorg Med Chem Lett. 2003;13:1011–1014. doi: 10.1016/s0960-894x(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 23.Prokai-Tatrai K, Perjesi P, Zharikova AD, Li X, Prokai L. Design, synthesis and biological evaluation of novel, centrally-acting thyrotropin-releasing hormone analogues. Bioorg Med Chem Lett. 2002;12:2171–2174. doi: 10.1016/s0960-894x(02)00368-2. [DOI] [PubMed] [Google Scholar]
- 24.Prokai-Tatrai K, Prokai L. Prodrugs of thyrotropin-releasing hormone and related peptides as central nervous system agents. Molecules. 2008;14:633–654. doi: 10.3390/molecules14020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prokai-Tatrai K, Teixido M, Nguyen V, Zharikova AD, Prokai L. A pyridinium-substituted analogue of the TRH-like tripeptide pGlu-Glu-Pro-NH2 and its prodrugs as central nervous system agents. Med Chem. 2005;2:141–152. doi: 10.2174/1573406053175256. [DOI] [PubMed] [Google Scholar]
- 26.Roussel JP, Astier H, Tapia-Arancibia L. Benzodiazepines inhibit thyrotropin (TSH)-releasing hormone-induced TSH and growth hormone release from perifused rat pituitaries. Endocrinology. 1986;119:2519–2526. doi: 10.1210/endo-119-6-2519. [DOI] [PubMed] [Google Scholar]
- 27.Scalabrino GA, Hogan N, O’Boyle KM, Slator GR, Gregg DJ, Fitchett CM, Draper SM, Bennett GW, Hinkle PM, Bauer K, Williams CH, Tipton KF, Kelly JA. Discovery of a dual action first-in-class peptide that mimics and enhances CNS-mediated actions of thyrotropin-releasing hormone. Neuropharmacology. 2007;52:1472–1481. doi: 10.1016/j.neuropharm.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt DE. Effects of thyrotropine releasing hormone (TRH) on pentobarbital-induced decrease in cholinergic neuronal activity. Commun Psychopharmacol. 1977;1:469–473. [PubMed] [Google Scholar]
- 29.Teixido M, Prokai-Tatrai K, Wang X, Nguyen V, Prokai L. Exploratory neuropharmacological evaluation of a bridged thyrotropin-releasing hormone analogue. Brain Res Bull. 2007;73:103–107. doi: 10.1016/j.brainresbull.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarbrough GG. TRH potentiates excitatory actions of acetylcholine on cerebral cortical neurons. Nature. 1976;263:523–552. doi: 10.1038/263523a0. [DOI] [PubMed] [Google Scholar]