Abstract
Phenyl substituted arsenic compounds are widely used as feed additives in the poultry industry and have become a serious environmental concern. We have demonstrated that phenylarsonic acid (PA) is readily degraded by TiO2 photocatalysis. Application of the Langmuir–Hinshelwood kinetic model for the initial stages of the TiO2 photocatalysis of PA yields an apparent rate constant (kr) of 2.8 µmol/L·min and the pseudo-equilibrium constant (K) for PA is 34 L/mmol. The pH of the solution influences the adsorption and photocatalytic degradation of PA due to the surface charge of TiO2 photocatalyst and speciation of PA. Phenol, catechol and hydroquinone are observed as the predominant products during the degradation. The roles of reactive oxygen species, •OH, 1O2, O2−• and hVB+ were probed by adding appropriate scavengers to the reaction medium and the results suggest that •OH plays a major role in the degradation of PA. By-products studies indicate the surface of the catalyst plays a key role in the formation of the primary products and the subsequent oxidation pathways leading to the mineralization to inorganic arsenic. TiO2 photocatalysis results in the rapid destruction of PA and may be attractive for the remediation of a variety of organoarsenic compounds.
Keywords: Titanium dioxide, heterogeneous photocatalysis, phenylarsenic acid, degradation
1. Introduction
Inorganic arsenic species As(III) and As(V) are naturally occurring toxic substances, which threaten the health of millions of people because of the consumption of water contaminated with inorganic arsenic [1]. Due to its high toxicity in humans, the U. S. Environmental Protection Agency (EPA) has lowered the maximum contaminant level of inorganic arsenic in drinking water from 50 to 10 µg/L [2]. While the treatment and removal of As(III) and As(V) from drinking water have received extensive attention [3–5], the wide spread use and environmental threat of organoarsenic species have received much less attention.
Organoarsenic compounds typically exist in the pentavalent oxidation state and have been introduced into the environment through agricultural applications [6,7]. Methylated arsenicals are widely used as herbicides for cotton farming and golf course maintenance with the application of 5,000 tons/year worldwide [8]. Phenylated arsenic compounds including 4-hydroxy-3-nitrophenylarsenic acid (roxarsone), 4-aminophenylarsenic acid (p-arsanilic acid) etc [9] are commonly utilized in the broiler poultry industry as feed additives to control cecal coccidiosis. Initially these compounds were believed to be nontoxic to domestic livestock, and excreted unchanged following ingestion by the animals [10]. Arsenic contaminated poultry litter, is sold and distributed as fertilizer, leading to large areas of arsenic contaminated soils. The PA feed additives were reported to be inert in the environment, however recent reports indicate these nontoxic organoarsenic compounds may be converted into more mobile and toxic inorganic arsenic species [11–14]. Biotransformation also produces toxic inorganic arsenic compounds from organoarsenicals [15]. The biological and environmental transformations of organoarsenicals can lead to the contamination of local water supplies with a complex mixture of arsenic containing substrates.
TiO2 photocatalytic oxidation, an advanced oxidation process (AOP), is a promising technology for environmental purification. TiO2 is the most extensively used semiconductor photocatalyst because of its photocatalytic activity, chemical stability, nontoxicity and low cost. When TiO2 is subjected to UV light (λ > 385 nm), an electron and a hole pair (eCB−/hVB+) is generated. Photocatalytic reactions take place primarily on the surface of TiO2, where the photogenerated electrons and holes are trapped [16]. The electron reacts with adsorbed oxygen, yielding superoxide radical (O2 −•), which can act as an oxidant or reductant. In the aqueous media, the hole typically reacts with adsorbed H2O, hydroxide or surface titanol groups (−TiOH) to produce hydroxyl radical (•OHabs), which is a powerful oxidant [17]. The reactive species (i.e., hVB+, eCB−, •OH, O2 −•, •O2H, H2O2, 1O2, etc.) generated during TiO2 photocatalysis can lead to destruction of problematic pollutants.
A number of different oxidative methods have been employed to convert arsenite to arsenate, including H2O2, UV/H2O2, oxygen and ozone (O3), MnO2, Fenton’s reagent (Fe(II)/H2O2), and UV/Fe(III), however each method has specific limitations and disadvantages [18]. TiO2 photocatalysis leads to the remediation of pollutants with UV irradiation under mild conditions [19, 20]. The TiO2 photocatalytic oxidation (PCO) of As(III) to As(V) occurs in an adsorbed state and via a rapid process [21], but recent reports dispute the roles of •OH, O2−• and hole during TiO2 photocatalysis of As(III) [18, 22–26]. TiO2 is a powerful adsorbent for arsenic species, and thus TiO2 photocatalysis has attracted tremendous interest for treatment of toxic arsenic species [27, 28]. Methylated arsenic species, monomethylarsonic acid (MMA) and dimethylarsonic acid (DMA) are oxidized by •OH and mineralized to As(V) upon TiO2 photocatalysis [29]. The TiO2 photocatalytic oxidation of inorganic arsenic, As(III) involves direct oxidation of the arsenic atom to yield As(IV) which is rapidly converted to arsenate, As(V) [30]. However, TiO2 photocatalysis of methylated arsenic species, appears to involve hydrogen abstraction pathways (leading to oxidation of the carbon atom) as the initial oxidation step to yield a carbon centered radical which can undergo a series of oxidative processes leading to inorganic arsenate. Although recent studies demonstrated that PA is very reactive toward radiolytically generated hydroxyl and sulfate radicals in homogenous solution [31], to the best of our knowledge, TiO2 photocatalysis of phenylated arsenic species has not been reported in the literature. Extensive comparisons have been made between hydroxyl radical reactivities generated via radiolysis (homogeneous conditions) and via TiO2 photocatalysis (heterogeneous conditions). Unlike radiolysis, which leads to clean generation of hydroxyl radicals, TiO2 photocatalysis leads to the formation of several potential oxidants at surface of the photocatalyst. During TiO2 photocatalysis reactions occur at or very near the surface and the observed reaction pathways can be dramatically influenced by adsorption properties of the reactants as well as the reactivity of the reactant in the adsorbed state. With this in mind we chose to conduct for the first time detailed by-products and kinetic studies in the TiO2 photocatalytic degradation of PA to better understand the role the surface in the TiO2 photocatalysis of PA. The mineralization of PA to As(V) is rapid and appears to occur via surface adsorbed •OH. Solution pH influences the degradation and the TiO2 surface influences the partitioning of the reaction pathways.
2. Experiments
2.1 Materials
Analytical-reagent grade chemicals were used as received. Millipore filtered water (18 MΩ·cm) was used to prepare all solutions. Phenylarsonic acid (PA), 2-hydroxylphenylarsonic acid (2-OH-PA) and 4-hydroxylphenylarsonic acid (4-OH-PA) were purchased from Avocado were used as authentic samples for chromatographic comparison. Titanium dioxide (Degussa P 25), a mixture of 80% anatase and 20% rutile with an average surface area of 50 m2/g, was used as the photocatalyst.
2.2 Sample preparation
TiO2 concentration employed for our studies was 0.1 g/L and [PA]0 = 38 µmol/L unless otherwise stated. In general 100 mL of solution was prepared and the resulting TiO2 suspension was dispersed using an ultrasonic cleaning bath for 15 minutes. The solution was magnetically stirred in the dark. One hour of stirring in the dark was sufficient to reach adsorption equilibrium prior to irradiation. The suspension was transferred to pyrex cylindrical reactor (12 × 1 in, 160 mL capacity, with a vented Teflon screw top). The solution was saturated with the desired gas by purging (air, oxygen or argon) for 15 min prior to irradiation and continued until the end of the reaction. The majority of experiments were performed at air-saturated conditions. Irradiation of suspension was conducted in a Rayonet photochemical reactor (Southern New England Ultra Violet Company, www.rayonet.org), model RPR-100, equipped with a cooling fan and 15 phosphor coated low-pressure mercury lamps. Pyrex cylindrical reaction vessel (12 × 1 in, 160 mL capacity) with the suspension was clamped in the center of the photochemical reactor. The lamps had a spectral energy distribution with a maximum intensity at λ = 350 nm, and yielding an incident light intensity of 5.2 ± 0.1 × 106 photon/sec/cm3. Ferrioxalate actinometry was used to measure the light intensity [32]. Five mL samples were periodically withdrawn, filtered through a 0.45 µm filter, and analyzed by HPLC-ICP-MS.
2.3 Analysis
Total arsenic concentrations were determined by inductively coupled plasma mass spectroscopy (ICP-MS, HP 4500). The plasma and auxiliary gas flow rate for the ICP-MS were maintained at 15.4 and 1.0 L/min, respectively. Arsenic speciation was determined by continuous high-performance liquid chromatography (HPLC) coupled with ICP-MS detection. An anion-exchange column, Dionex AS 7 (250 mm × 4 mm, 5 µm particle size) was used for the separation of arsenic species. The mobile phase was HNO3 aqueous solution (pH 2.0), and the flow rate was 0.5 mL/min. On the basis of LC-ICP-MS data and correlation of the chromatographic behavior of the products to authentic compounds, the identity of the products appearing in the chromatogram were was assigned as follows; peak 1 = As(III) (180 sec), peak 2 = PA (380 sec), peak 3 = 2-hydroxylphenylarsonic acid (460 sec), peak 4 = 4-hydroxylphenylarsonic acid (520 sec), peak 5 = dihroxylphenylarsonic acid (600 sec), peak 6 = As(V) (730 sec). Although dihydroxyl-substituted isomer is not commercially available for confirmation, it was previously assigned based on the MS data [31].
The analysis for phenolic compounds was conducted using GC (HP 5890 Series II). The aqueous reaction solutions were extracted by methylene chloride (HPLC grade) and subsequently analyzed on GC. The injection port temperature was maintained at 250 °C and the injection volume was 1 µL. The column was DB-5 (30 m × 0.53 mm), and the film thickness was 0.25 µm. The carrier gas was helium with 99.9999% purity at a constant flow rate of 2.7 mL/min, and the temperature of the FID detector was 320 °C. The initial oven temperature was 40 °C for 3 min, ramped at 5 °C/min to 200 °C. Authentic standards were used to confirm the chromatographic behavior of the products.
3. Results and discussions
3.1 PA adsorption on TiO2 photocatalyst (dark)
In order to better understand the adsorption process and its role on the TiO2 photocatalytic degradation process, the adsorption of PA in dark was studied as a function of initial PA concentration without adjusting solution pH. The concentration of PA varied from 5 to 100 µmol/L at a constant TiO2 concentration of 0.1 g/L. Our initial studies established that the adsorption equilibrium was maintained by stirring the dispersion in dark for 60 min.
Langmuir adsorption isotherms, which are often used to describe the heterogeneous adsorption process [33], were employed to assess the PA adsorption capacity of TiO2. Unique adsorption sites, monolayer adsorption, and no interaction between the adsorption sites are the underlying assumptions used in deriving the Langmuir isotherm. Langmuir adsorption isotherm can be described by equation:
| (1) |
Where Qad is the specific adsorbed quantity of a model compound and Ceq is the initial concentration; Qsat is the saturation (maximum) adsorption capacity and Kad is the adsorption constant.
The dark adsorption of PA is nicely described by Langmuir adsorption isotherm with coefficient R2 = 0.9844, shown in Fig. 1. Qsat is 123 ± 10 µmol/g for PA adsorption on TiO2 photocatalyst. Xu et al reported Qsat for MMA and DMA of 86 and 37 µmol/g respectively [29]. Similar values for PA and MMA are consistent with their analogous structures. Based on the estimated density of surface hydroxyl groups of 4.8 OH/(nm)2 present on Degussa P25 TiO2 [34], the maximum adsorption capacity of PA on the TiO2 surface is 400 µmol/g assuming no steric constraints and a bidentate adsorption mode. The maximum adsorbed concentration observed under our experimental conditions corresponds to 30 ± 3 % monolayer coverage for PA. We consider 30 % surface coverage to be relatively high given the theoretical (improbable) assumption that every surface hydroxyl group can contribute to PA adsorption. In addition, the catalyst undergoes rapid agglomeration that significantly reduces the effective surface area and number of available adsorption sites [35]. The equilibrium, Kad between the adsorption and desorption of PA on the surface of TiO2 is 0.0367 L/µmol.
Fig. 1.
Langmuir isotherm dark adsorption of PA on TiO2 photocatalyst, [TiO2] = 0.1 g/L. Error bars represent one standard deviation based on triplicate experiments.
3.2 TiO2 photocatalytic degradation of PA
TiO2 photocatalysis was carried out as an aqueous suspension of TiO2 (0.1 g/L) and PA at room temperature. The solution was purged with air, oxygen or argon prior to the irradiation. The solution (100 mL) was irradiated in a Rayonet reactor with 15 phosphor-coated low-pressure mercury lamps emitting UV light (350 nm). The results are illustrated in Fig. 2. Degradation of PA in the absence of TiO2 or UV is negligible. The 10–20 % decrease in PA concentration without UV irradiation is due to PA adsorption onto the TiO2 surface. The degradation is also relatively slow under argon-saturated conditions. Under our experimental conditions, TiO2, dissolved oxygen and UV light are required for effective degradation of PA.
Fig. 2.
Time profiles of PA degradation in the TiO2 suspension under various reaction conditions. [PA]0 = 38 µmol/L; [TiO2] = 0.1 g/L; λ = 350 nm. ( w/o TiO2
w/o TiO2  w/o UV,
w/o UV,  Ar-saturated,
Ar-saturated,  air-saturated,
air-saturated,  O2-saturated)
O2-saturated)
While TiO2 photocatalysis involves heterogeneous processes, the initial degradation often follows 1st order kinetics [29, 31]. With this in mind, plots of ln(C0/Ct) vs. time were constructed for TiO2 photocatalysis of PA under Ar, air and O2 saturated conditions. The 1st order PA degradation plots exhibit linear relationships and kAr= 0.04 ± 0.01 min−1, kair = 0.36 ± 0.04 min−1, and kO2 = 0.68 ± 0. 01 min−1.
3.3 Heterogeneous Kinetics
The Langmuir–Hinshelwood (L–H) model is often applied to obtain kinetic parameters for reactions occurring at a solid–liquid interface during photocatalysis [36]. Application of the L–H model enables the observed kinetics to be separated into reactivity and adsorption components. One of the simplest representations of the L–H model is given by:
| (2) |
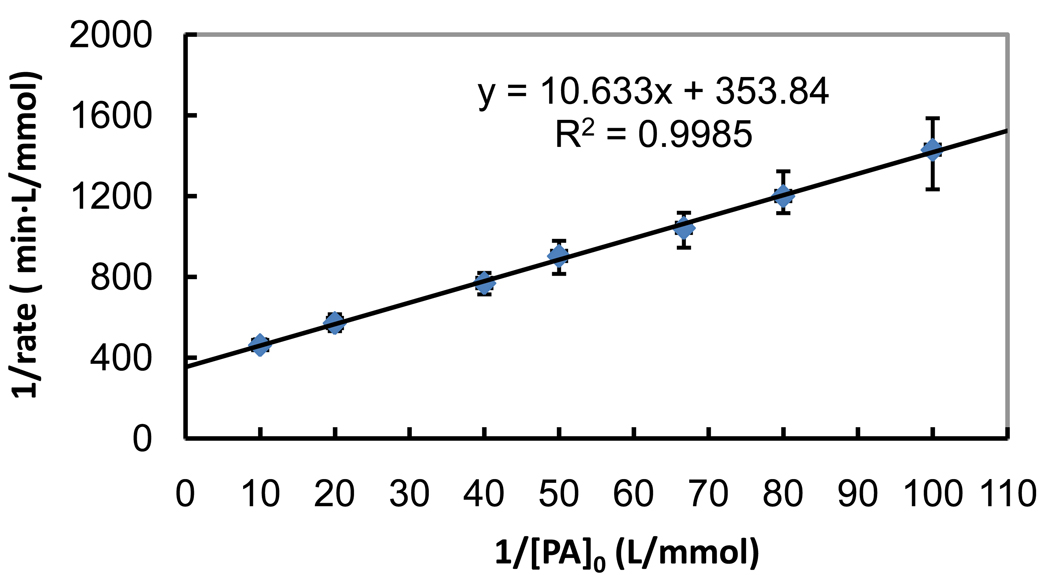
Where r0 is the initial rate (observed) of disappearance of substrate, C0 is the initial substrate concentration, kr is a reactivity coefficient relating to oxidation events at the catalyst’s surface, and K is pseudo-equilibrium constant related to surface adsorption [37]. The apparent kinetic parameters kr and K can be obtained from a plot of 1/r0 vs. 1/C0.
| (3) |
To minimize interference from by-products, the L–H model is generally applied to the first 10–20 % of reaction and low initial concentrations are employed to minimize the potential for interference from multilayer adsorption. The photocatalytic experiments were performed over a range of initial PA concentrations (10 to 100 µmol/L) and a constant TiO2 concentration of 0.1 g/L under uniform photon flux (light intensity: 1.12 ± 0.1 × 106 photon/sec/cm3).
The L–H kinetic parameters were determined from the slope and intercept of the linear fit of the 1/r0 vs. 1/C0 (Fig. 3). Under our experiment conditions, kr is 2.8 µmol/L·min and K is 34 L/mmol for PA. The L–H kinetic parameters for MMA under similar conditions were kr= 1.8 µmol/L·min and K = 33 L/mmol [29]. MMA and PA have analogous structures except MMA has a single methyl group and PA has a single phenyl group neither of which is expected to have a significant influence on the adsorption. The L–H equilibrium constants of MMA and PA are essentially the same, however the reactivity, kr, of MMA is significantly lower than PA. The absolute hydroxyl radical rate constants in homogenous media for •OH with MMA and PA are ~108 and ~1010 M−1s−1 respectively [31]. While the TiO2 photocatalytic degradation of MMA and PA may both involve •OH, the difference in observed reactivity implies different reaction pathways are involved for MMA and PA. The pathway to MMA likely involves hydroxyl radical abstraction of hydrogen from the methyl group of MMA [38], while in the case of PA hydroxyl radical likely reacts via addition to the aromatic ring (typically 10–100 times faster than the hydrogen abstraction pathway). The final oxidation product of PA is arsenate. Arsenate is strongly adsorbed onto the TiO2 surface [21] and may adversely influence the adsorption and degradation kinetics at high initial PA concentrations.
Fig. 3.
Langmuir-Hinshelwood plot of TiO2 photocatalytic degradation of PA. [PA]0 = 38 µmol/L; [TiO2]=0.1 g/L; air-saturated; λ =350nm. Error bars represent one standard deviation based on triplicate experiments.
3.4 pH effects
TiO2 photocatalysis of PA was conducted under acidic, neutral and basic pH conditions. The solution pH was adjusted from 3 to 11 using 0.1 M HNO3 and 0.1 M NaOH prior to the irradiation. PA exhibits highest adsorption at acidic and neutral pH. PA adsorption decreases dramatically under basic conditions indicative of repulsion of the negative charges on PA and the TiO2 surface under basic conditions. TiO2 has a point of zero charge in solution pH 6.8. Under acidic conditions, the positive charge of the TiO2 surface increases as the pH decreases; above pH 6.8, the negative charge at the surface of TiO2 increases with the increasing pH. The pKa values of PA are 3.8 and 8.5, and PA exists predominantly as a mono-anion between pH 3.8 and 8.5, and as di-anion at pH > 8.5. PA is strongly adsorbed on the surface of TiO2 under acidic conditions because of the electrostatic attraction of the negative change associated with PA and the positive charge associated with the TiO2. Under basic conditions PA and TiO2 process negative charges which will create electrostatic repulsion and reduce the level of surface adsorption. Such pH effects have been observed for a variety of ionizable substrates [29, 39].
The L–H behavior and the observed pH effects indicate the transformations of PA, during TiO2 photocatalysis, involve reactions at or very near the surface and thus are strongly dependent on the interaction between substrate and TiO2 [40]. We also evaluated the degradation kinetics as a function of solution pH and the results are summarized in Table 1. Solution pH can have a dramatic effect on the adsorption properties of TiO2, esp. surface charge and aggregation. While higher adsorption of the negatively charged substrates (like PA) would be expected to increase as the surface of the TiO2 becomes more positively charged (under acidic pH) we observed the maximum adsorption at almost neutral conditions. The rate and extent of TiO2 aggregation can be dramatically influenced by the pH, ionic strength, and composition of the suspension. A recent report indicates the surface areas decrease under acid pH [41]. The reaction rates under acidic are modestly slower than at neutral conditions. When aggregation increases the surface area of TiO2 decreases which could lead to lower observed adsorption and modestly slower degradation under acidic conditions. Although the adsorption was lower at pH 9 compared to acidic and neutral conditions, the increased anionic character of PA at pH 9 may enhance its reactivity towards hydroxyl radical, an electrophilic species. The observed decrease in degradation observed at pH = 11 under our conditions is attributed to a dominating effect of the electrostatic repulsion between the highly negatively charged surface of the TiO2 and negatively charged PA.
Table 1.
1st order kinetics parameters for TiO2 photocatalytic degradation of PA.
| Saturation gasa | pH | k (min−1)b | τ1/2 (min) | R2 |
|---|---|---|---|---|
| argon | 6.5 | 0.04 ± 0.01 | 17.3 | 0.96 |
| air | 6.5 | 0.36 ± 0.04 | 1.9 | 0.97 |
| oxygen | 6.5 | 0.68 ± 0.10 | 1.0 | 0.98 |
| air | 3 | 0.33 ± 0.03 | 2.1 | 0.99 |
| air | 5 | 0.33 ± 0.03 | 2.1 | 0.99 |
| air | 9 | 0.44 ± 0.05 | 1.6 | 0.98 |
| air | 11 | 0.23 ± 0.02 | 3.0 | 0.98 |
Gas is purged in the reaction prior and during irradiation.
[PA]0 = 38 µmol/L; [TiO2] = 0.1 g/L; λ = 350 nm.
3.5 Scavenging experiments for reactive oxygen species
TiO2 PCO involves a complex interdependent series of reaction processes and a number of reactive oxygen species (ROS). In an attempt to assess the reaction processes and the roles of individual ROS, it is instructive to briefly discuss the sequence of events initiated during TiO2 photocatalysis. Upon absorption of a photon an electron from the conduction band is promoted to the valence band creating an electron (eCB−) / hole (hVB +) pair (eq 4).
| (4) |
In the absence of electron or hole trap, recombination of the eCB−/hVB+ pair occurs. Oxygen can act as an eCB− trap leading to superoxide anion radical (eq 5), while extending the lifetime of the hVB+, thus promoting oxidation processes. In aqueous media, the hVB+ is trapped by surface hydroxyl groups to form surface bound •OH groups as illustrated in eq 6 and 7.
| (5) |
| (6) |
| (7) |
While superoxide anion radical, O2−•, and hydroxyl radical, •OH, are formed, •OH is generally believed to play the primary role in promoting oxidation during the TiO2 photocatalysis [36]. Scavengers were employed to probe the roles of O2−• and •OH during the PA degradation process.
The results presented earlier demonstrate O2 plays a key role in TiO2 photocatalytic degradation of PA. Oxygen is critical as an electron scavenger to inhibit recombination of the electron/hole pair, thus promoting oxidative processes such as the formation of hydroxyl radical. As a result of this process O2−• is produced, which can also lead to oxidation pathways. Superoxide anion radical has the potential to react directly by oxidative pathways, it can also produce singlet oxygen or decompose to H2O2, which is transformed to hydroxyl radical during TiO2 photocatalysis. The addition of superoxide dismutase (SOD) to solution, which is an effective O2−• scavenger (eq. 8) [38], showed no effect on TiO2 photocatalytic degradation of PA under our experimental conditions.
| (8) |
Tert-butyl alcohol readily reacts with •OH as illustrated by eq. 9 [38, 42]. Inhibition of the TiO2 photocatalytic oxidation of methylated arsenic acids is observed upon addition tert-butyl alcohol [29, 38].
| (9) |
The 1st order rate constant for TiO2 photocatalyzed degradation of PA decreased by 67 % from 0.36 ± 0.04 min−1 to 0.12 ± 0.02 min−1 in the presence of 5 vol% t-BuOH. While hydroxyl radical mediated processes can be completely inhibited in the presence of excess t-BuOH, modest degradation of PA is still observed. Since TiO2 photocatalysis typically involves reactions at or near the surface, the adsorption properties of the substrates, t-BuOH and PA, can have a pronounced influence on the competition for the surface generated hydroxyl radicals. The target compound, PA, is at much lower concentrations than the scavenger, t-BuOH, but due to its stronger adsorption properties (localization near the production of oxidant), degradation still occurs.
The formation of singlet oxygen during TiO2 photocatalysis has been recently reported [43]. Azide has been used as both a singlet oxygen and hydroxyl radical scavenger, represented by eq. 10 and 11 [38]. Addition of azide during photocatalysis decreases the degradation rate of PA to a rate of 0.17 ± 0.03 min−1.
| (10) |
| (11) |
Strong inhibition of the process by t-BuOH and azide provide convincing evidence for the participation of •OH in the oxidation of PA. Modest differences in the level of inhibition between the hydroxyl radical scavengers may be related to adsorption properties and/or slightly differences in the experimental conditions [44]. If singlet oxygen played a significant role in the degradation processes the level of inhibition by azide is expected to be greater than that observed for t-BuOH which only inhibits hydroxyl radical mediated processes. It does not appear that singlet oxygen plays a significant role under our experimental conditions.
Photoexcitation of TiO2 leads to the formation of a hole, which can react with adsorbed H2O or −TiOH, to yield hydroxyl radical on the surface of TiO2. Formic acid has been used as hole scavenger during TiO2 photocatalysis, eq. 12. The hole is consumed by HCO2−, which can eliminate or intercept the generation of hydroxyl radical on TiO2 [45]. Formic acid under neutral and acidic conditions is strongly adsorbed onto the surface of TiO2. Upon addition of formic acid (0.5 g/L) to the solution the degradation rate of PA is almost completely inhibited (k = 0.01 ± 0.01 min−1).
| (12) |
The results for the different scavengers are summarized in Fig. 5. Among the scavengers employed, formic acid leads to almost complete inhibition of the PA degradation, t-BuOH and N3− lead to > 50% decrease in degradation rate. The competitive adsorption of formic acid with PA results in more effective inhibition of hVB+ and/or •OH compared to less strongly adsorbed scavengers, t-BuOH and N3−. The degradation of PA decreases in the presence of •OH and hole scavengers. Hydroxyl radical also plays an important role in the TiO2 photocatalytic degradation of MMA and DMA to arsenate, and arsenite to arsenate [29, 38, 21, 22].
Fig. 5.
Time profiles of PA degradation in the TiO2 suspension with various scavengers. [PA]0 = 38 µmol/L; [TiO2]=0.1 g/L; λ =350 nm; air-saturated except argon-saturated. ( argon-saturated;
argon-saturated;  air-saturated;
air-saturated;  with 4000 U/mL SOD;
with 4000 U/mL SOD;  with 5 vol% t-BuOH;
with 5 vol% t-BuOH;  with 0.2 g/L N3−;
with 0.2 g/L N3−;  with 0.5 g/L formic acid)
with 0.5 g/L formic acid)
3.6 Product studies
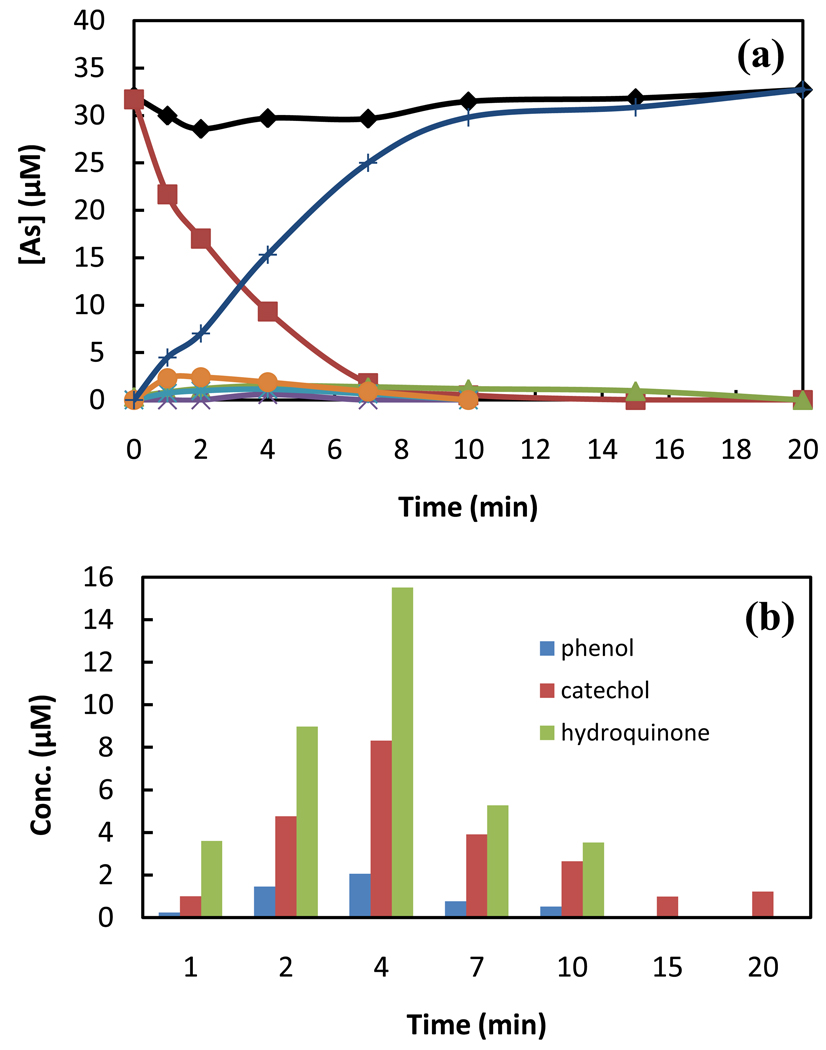
Product studies were carried out to elucidate the reaction pathways involved in the TiO2 photocatalytic degradation of PA. Under homogeneous conditions, •OH reacts with PA via hydroxylation of the aromatic ring leading to phenolic products and inorganic arsenic species [31]. The final arsenic species produced during TiO2 photocatalytic degradation of PA is arsenate. The mass balance for total arsenic throughout the degradation process is 80 – 90 % because of adsorption on TiO2. HPLC-ICP-MS analyses and comparison of chromatographic behavior with authentic samples was used to assign the identity of a number of the reaction products. Details of the analytical methods used for product identification are included in the experimental section. Monohydroxylated and dihydroxylated phenylarsonic acids are intermediate products present at relatively low concentrations, (<10 %) during TiO2 photocatalysis as illustrated in Fig. 6 (a). Computational and product studies suggest •OH preferentially adds to the ortho- and para- positions of PA, yielding 2- and 4-hydroxyl adducts as the major initial products during radiolysis (homogeneous conditions) [31]. In contrast, phenol, catechol and hydroquinone are major degradation products from TiO2 PCO of PA (heterogeneous conditions) confirmed by GC analyses of a methylene chloride extract of the reaction solution. The distribution of phenolic products is illustrated in Fig. 6 (b). The dihydroxylated products, catechol (ortho isomer) and hydroquinone (para isomer), are major products, but we were not able to detect resorcinol, the meta isomer. The observed phenolic products could also be the result of direct oxidation via hVB+, but ipso addition of H2O to the corresponding radical cation is not expected to yield the predominant observed products. The presence of phenols and inorganic arsenite and arsenate in the solution requires the cleavage of the arsenic–carbon bond in the PA molecule. The concentration of As(III) is low in solution and arsenate is the final product for the PA degradation.
Fig. 6.
(a) Relative concentrations of arsenic species in the solution during the TiO2 photocatalytic degradation. (♦ total As;  PA;
PA;  As(III);
As(III);  2-OH-PA;
2-OH-PA;  4-OH-PA;
4-OH-PA;  DiOH-PA, dihydroxylated phenlyarsonic acid;
DiOH-PA, dihydroxylated phenlyarsonic acid;  As(V) ). (b) Relative concentrations of organic species in the solution [PA]0 = 38 µmol/L; [TiO2] = 0.1 g/L; air-saturated; λ = 350 nm.
As(V) ). (b) Relative concentrations of organic species in the solution [PA]0 = 38 µmol/L; [TiO2] = 0.1 g/L; air-saturated; λ = 350 nm.
The major observed products during TiO2 PCO of PA are phenol, catechol and hydroquinone accounting for ~70% of the organic reaction products at a treatment time of 4 mins, while only low levels of phenol were detected under radiolytic treatment [31]. As(III) is not observed during TiO2 PCO of methylated species [29] thus cleavage of arsenic-carbon bond does not appear to involve the same type of cleavage pathway as observed for PA.
The strong adsorption of PA and hydroxylated PA to the TiO2 surface where hydroxyl radicals are localized will enhance the probability for dihydroxylation. Preferential formation of the ortho- and para- dihydroxylated products is indicative of •OH mediated oxidation. The dihydroxylated products could be formed from reactions of phenol with hydroxyl radical or the result of dihydroxylation of PA followed by cleavage of the carbon-arsenic bond. The formation of catechol and hydroquinone initially and through-out the degradation processes suggest that arsenic-bond bond cleavage follows dihydroxylation. While benzenetriols can be generated during the photocatalytic oxidation of substrates with benzene rings we did not observed such products by GC analyses. Successive •OH attack of the aromatic ring leads to carbonyl compounds, which can be mineralized to carbon dioxide and water [46].
3.7 Mechanism of TiO2 photocatalytic degradation of PA
The degradation of most organic compounds on TiO2 is typically initiated by an oxidative path (hole transfer or •OH radical reaction) [47]. The results of our scavenger experiments presented earlier clearly indicate hydroxyl radical plays a major role in the TiO2 PCO of PA. Comparison of the results for arsenite, methylated arsenic species and PA demonstrate distinct differences among the reaction mechanisms for TiO2 PCO of arsenite, methylated arsenic and phenyl arsenic species. The mechanism for TiO2 PCO of inorganic arsenite As(III) occurs by oxidation of the arsenic atom [21, 22], while methylated arsenic species appear to react by hydrogen abstraction [31]. As(III) is observed during hydroxyl mediated oxidation of PA, but there is no report of the formation of As(III) during hydroxyl radical mediated oxidation of the corresponding methylated arsenic species [31, 38].
Addition of •OH to the aromatic ring of PA can occur at four different positions, ortho, para, meta, and ipso. Addition at ortho, meta or para position would yield the corresponding monohydroxylated PA derivatives. While ortho and para isomers are observed products, we were not able to confirm the formation of the meta isomer. We attribute the selective formation of the ortho and para hydroxyl PAs to the electronic directing effect of the arsenate group in the parent compound. Under homogeneous conditions (radiolysis), hydroxyl radical leads to hydroxylation preferably at the ortho and para positions leading to hydroxyl-PA products as the predominant pathway [31], however TiO2 PCO (heterogeneous conditions) appears to promote the formation of catechol and hydroquinone requiring addition of two hydroxyl groups with cleavage of the arsenic-carbon bond. The proposed surface mediated hydroxyl radical reactions are illustrated in Fig. 7. Following initial addition of hydroxyl radical to the aromatic ring of PA, addition of a second hydroxyl group could yield a dihydroxylated PA adduct. Hydroxyl radical could also add to the ipso position (carbon at position of As attachment) to yield a radical intermediate which upon collapse via arsenic-carbon bond homolysis can lead to the observed products, catechol and hydroquinone. The latter reaction appears to be the predominant reaction pathway during TiO2 PCO based on the observed product distribution. The adsorption of the arsenic group onto the TiO2 in the monohydroxylated PA adducts may weaken the arsenic-carbon bond making it more susceptible to elimination compared to radiolysis conditions where catechol and hydroquione were not the predominant products. As(IV), a by-product of the hemolytic arsenic-carbon bond cleavage, readily disproportionates to yield 1:1 ratio of As(III) and As(V) [30], leading to the As(III) observed during TiO2 photocatalytic degradation of PA. As(III) is oxidized to arsenate by molecular oxygen, •OH or hVB+, and converted to As(V) [21, 48].
Fig. 7.
Proposed surface mediated mechanism of TiO2 photocatalytic degradation of PA.
4. Conclusion
In summary, TiO2 photocatalytic degradation of phenylarsonic acid leads to mineralization to As(V). While inorganic arsenic species are generally considered to be more toxic than organoarsenic species, there are well-established methods for removal of arsenate from water. The degradation kinetics for PA is nicely modeled by simple kinetic processes and indicates the reactions occur at or near the surface of TiO2. The adsorption and degradation of PA during TiO2 photocatalysis parallel the electrostatic interactions between the TiO2 surface and PA as a function of solution pH. Experiments conducted in the presence of hydroxyl radical, superoxide anion radical, singlet oxygen and hole scavengers indicate that hydroxyl radical plays a key role during TiO2 photocatalysis of PA. The reaction pathway observed for PA is different than those reported for inorganic arsenic and methylated arsenic species. Different product distributions are observed for radiolysis and TiO2 PCO of PA. A surface mediated reaction mechanism is proposed to explain the formation of the observed reaction products. The TiO2 surface appears to play an important role in the partitioning of the reaction pathways leading to the formation of As(V). TiO2 photocatalysis can be effectively employed to treat and remove the organoarsenic species from contaminated water.
Fig. 4.
pH effects on PA adsorption on TiO2 photocatalyst, [PA]0 = 38 µmol/L, [TiO2] = 0.1 g/L. Error bars represent one standard deviation based on triplicate experiments.
Acknowledgments
K.E.O. gratefully acknowledges support from the NIH/NIEHS (Grant no. S11ES1118). We thank the reviewers for valuable insight and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jain CK, Ali I. Water Res. 2000;34:4304–4312. [Google Scholar]
- 2.Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Science. 2002;296:2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- 3.Adriano DC. Trace elements in the terrestrial environment. Springer: New York; 2001. [Google Scholar]
- 4.Thirunavukkarasu OS, Viraraghavan T, Subramanian KS. Water SA. 2003;29:161–170. [Google Scholar]
- 5.Hlavay J, Polyak KJ. Colloid Interface Sci. 2005;284:71–77. doi: 10.1016/j.jcis.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Science. 2002;296:2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- 7.Welch AH, Lico MS, Hughes JL. Ground Water. 1988;26:333–347. [Google Scholar]
- 8. http://www.atsdr.cdc.gov/toxprofiles/tp2-c6.pdf.
- 9.Jackson BP, Bertsch PM. Environ. Sci. Technol. 2001;35:4868–4873. doi: 10.1021/es0107172. [DOI] [PubMed] [Google Scholar]
- 10.Garbarino JR, Bednar AJ, Rutherford DW, Beyer RS, Wershaw RL. Environ. Sci. Technol. 2003;37:1509–1514. doi: 10.1021/es026219q. [DOI] [PubMed] [Google Scholar]
- 11.Onken BM, Hossner LR. Soil Sci. Soc. Am. J. 1996;60:1385–1392. [Google Scholar]
- 12.Marin AR, Pezeshki SR, Masschelen PH, Choi HS. J. Plant Nutr. 1993;16:865–880. [Google Scholar]
- 13.Moore PA, Daniel TC, Gilmour JT, Shreve BR, Edwards DR, Wood BH. J. Environ. Qual. 1998;27:92–99. [Google Scholar]
- 14.Bednar AJ, Garbarino JR, Ferrer I, Rutherford DW, Wershaw RL, Ranville JF, Wildeman TR. Sci. Total Environ. 2003;302:237–245. doi: 10.1016/s0048-9697(02)00322-4. [DOI] [PubMed] [Google Scholar]
- 15.Castlehouse H, Smith C, Raab A, Deacon C, Meharg AA, Feldmann J. Environ. Sci. Technol. 2003;37:951–957. doi: 10.1021/es026110i. [DOI] [PubMed] [Google Scholar]
- 16.Nartyanov IN, Savinov EN. J. Photochem. Photobiol. A: Chem. 2000;134:219–226. [Google Scholar]
- 17.Gaya UI, Abdullah AH. J. Photochem. Photobiol. C: Photochem. Rev. 2008;9:1–12. [Google Scholar]
- 18.Dutta PK, Pehkonen SO, Sharma VK, Ray AK. Environ. Sci. Technol. 2005;39:1827–1834. doi: 10.1021/es0489238. [DOI] [PubMed] [Google Scholar]
- 19.Fox MA, Dulay MT. Chem. Rev. 2003;93:341–357. [Google Scholar]
- 20.Linsebigler AL, Lu G, Yates JT. Chem. Rev. 1995;95:735–758. [Google Scholar]
- 21.Xu T, Kamat PV, O’Shea KE. J. Phys. Chem. A. 2005;109:9070–9075. doi: 10.1021/jp054021x. [DOI] [PubMed] [Google Scholar]
- 22.Yoon S, Oh S, Yang JE, Lee JH, Lee M, Yu S, Par D. Environ. Sci. Technol. 2009;43:864–869. doi: 10.1021/es801480u. [DOI] [PubMed] [Google Scholar]
- 23.Ryu J, Choi W. Environ. Sci. Technol. 2004;38:2928–2933. doi: 10.1021/es034725p. [DOI] [PubMed] [Google Scholar]
- 24.Leng WH, Cheng XF, Zhang JQ, Cao CN. Environ. Sci. Technol. 2007;41:6311–6312. doi: 10.1021/es070349n. [DOI] [PubMed] [Google Scholar]
- 25.Ryu J, Choi W. Environ. Sci. Technol. 2007;41:6313–6314. [Google Scholar]
- 26.Yoon S, Lee JH. Environ. Sci. Technol. 2005;39:9695–9701. doi: 10.1021/es051148r. [DOI] [PubMed] [Google Scholar]
- 27.Bissen M, Vieillard-Baron MM, Schindelin AJ, Frimmel FH. Chemosphere. 2001;44:751–757. doi: 10.1016/s0045-6535(00)00489-6. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Chow W. Environ. Sci. Technol. 2002;36:3872–3878. doi: 10.1021/es0158197. [DOI] [PubMed] [Google Scholar]
- 29.Xu T, Cai Y, O’Shea KE. Environ. Sci. Technol. 2007;41:5471–5477. doi: 10.1021/es0628349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klaning UK, Bieski BHJ, Sehested K. Inorg. Chem. 1998;28:2717–2724. [Google Scholar]
- 31.Xu T, Kamat PV, Joshi S, Mebel AM, Cai Y, O’Shea KE. J. Phys. Chem. A. 2007;11:7819–7824. doi: 10.1021/jp072135y. [DOI] [PubMed] [Google Scholar]
- 32.Calvert J, Pitts JN. Photochemistry. New York: Wiley; 1966. [Google Scholar]
- 33.Dutta PK, Ray AK, Sharma VK, Millero FJ. J. Colloid Interface Sci. 2004;278:270–275. doi: 10.1016/j.jcis.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Mueller R, Kammler HK, Wegner K, Pratsinis SE. Langmuir. 2003;19:160–165. [Google Scholar]
- 35.O’Shea KE, Pernas E, Saiers J. Langmuir. 1999;15:2071–2076. [Google Scholar]
- 36.Turchi CS, Ollis DF. J. Catal. 1999;122:178–192. [Google Scholar]
- 37.Tada H. Langmuir. 1996;12:966–971. [Google Scholar]
- 38.Xu Z, Jing C, Li F, Meng X. Environ. Sci. Technol. 2008;42:2349–2354. doi: 10.1021/es0719677. [DOI] [PubMed] [Google Scholar]
- 39.Jiang C, Meng X, Liu S, Baidas S, Patraju R, Christodoulatos C, Korfiatis GP. J. Colloid Interface Sci. 2005;290:14–21. doi: 10.1016/j.jcis.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Parra S, Oliverob J, Pulgarin C. Appl. Catal. B: Environ. 2002;36:75–85. [Google Scholar]
- 41.Mandzy N, Grulke E, Druffel T. Powder Technol. 2005;160:121–126. [Google Scholar]
- 42.Subramanian B, Yang Q, Yang Q, Khodadoust AP, Dionysiou DD. J. Photochem. Photobiol. A: Chem. 2007;192:114–121. [Google Scholar]
- 43.Daimon T, Nosaka Y. J. Phys. Chem. C. 2007;111:4420–4424. [Google Scholar]
- 44.Cha HJ, Park OK, Kim YH, Cha HG, Kang YS. Int. J. Nanosci. 2006;5:795–801. [Google Scholar]
- 45.Nguyen VNH, Beydoun D, Amal R. J. Photochem. Photobiol. A: Chem. 2005;171:113–120. [Google Scholar]
- 46.Sobczyñski A, Duczmal L, Zmudziñski W. J. Molecular Catal. A Chem. 2004;213:225–230. [Google Scholar]
- 47.Hoffmann MR, Martin ST, Choi W, Bahnemann DW. Chem. Rev. 1995;95:69–96. [Google Scholar]
- 48.Huang PM, Oscarson DW, Liaw WK, Hammer UT. Hydrobiologia. 1982;91–92:315–322. [Google Scholar]