Abstract
The increasing incidence of multidrug-resistant (MDR) pulmonary infections in the cystic fibrosis (CF) population has prompted the investigation of innovative silver based therapeutics. The functionalization of the naturally occurring xanthine theobromine at the N1 nitrogen atom with an ethanol substituent followed by the methylation of the N9 nitrogen atom gives the N-heterocyclic carbene precursor 1-(2-hydroxyethyl)-3,7,9-trimethylxanthinium iodide. The reaction of this xanthinium salt with silver acetate produces the highly hydrophilic silver carbene complex SCC8. The in vitro antimicrobial efficacy of this newly synthesized complex was evaluated with excellent results on a variety of virulent and MDR pathogens isolated from CF patients. A comparative in vivo study between the known caffeine derived silver carbene SCC1 and SCC8 demonstrated the ability of both complexes to improve the survival rates of mice in a pneumonia model utilizing the clinically isolated infectious strain of Pseudomonas aeruginosa PA M57-15.
Introduction
The investigation of transition metal complexes of N-heterocyclic carbenes (NHCs) for applications other than catalysis has been gaining attention in recent years.1,2 Of particular interest is the use of silver carbene complexes (SCCs) as a new class of broad spectrum antimicrobials for the treatment of pulmonary infections.3–14 Much of the research in this area has focused on the development and testing of SCCs on virulent and multidrug-resistant (MDR) pathogens associated with cystic fibrosis (CF), although the in vitro efficacy of SCCs against biosafety level 3 pathogens has also recently been reported.15
CF is a genetic disorder affecting more than 60 000 people worldwide. The disease arises from a mutation in the genetic encoding of the cystic fibrosis transmembrane conductance regulator (CFTR), ultimately affecting the lungs and digestive system of those afflicted.16,17 The CFTR gene is responsible for the production of the protein that regulates the transport of Cl− and Na+ across cell membranes. Mutations of the CFTR gene in CF patients causes problems with the production of this regulatory protein. The result is a disruption of the normal transfer of Cl− and Na+ across cell membranes leading to the dehydration and increased viscosity of cellular secretions.18 In the lungs of CF patients this increase in mucus viscosity interferes with its natural expulsion eventually leading to airway obstruction which creates an excellent environment for bacterial growth.
Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia complex organisms are amongst the most prevalent pathogens found in the lungs of CF patients and are responsible for the majority of morbidity and mortality associated with the disease.19 Current treatments for these infections include the use of large continuous doses of β-lactam antibiotics and aminoglycosides, in particular nebulized tobramycin. Of major concern is the development of antibiotic resistance to standard treatments which is becoming increasingly more prevalent due to the ability of these pathogens to form biofilms.19 We have recently published several articles demonstrating the exceptional in vitro and in vivo antimicrobial efficacy of SCCs against a variety of relevant CF pathogens.4–15 In continuing our pursuit to address the need for more effective antimicrobials to treat virulent CF infections, herein we report the synthesis and characterization of a highly water soluble theobromine derived SCC (SCC8) along with an in vitro antimicrobial efficacy studies and in vivo efficacy study comparing SCC8 to a previously reported caffeine derived SCC (SCC1).
Results and discussion
Synthesis of theobromine derived SCC8
Based upon previous results obtained from the synthesis of an electronically stabilized silver NHC complex of methylated caffeine (SCC1, Fig. 1), it was determined that in general xanthine derivatives could serve as useful building blocks for SCC antimicrobials.5 Like caffeine, theobromine is a naturally occurring molecule which is produced by the cacao tree and is the chief alkaloid found in chocolate. The synthetic advantage of using theobromine as a NHC building block is the availability of the N1 position for substitution (Fig. 2). The N1 nitrogen atom of theobromine is a much better nucleophile when deprotonated than the N9 nitrogen atom, a feature that aids in the isolation of singular substitution products. The addition of an alcohol to the N1 position of theobromine was selected in anticipation that such a functional group would produce an SCC with greater water solubility than SCC1 which is a modest 11 mg/mL.
Fig. 1.
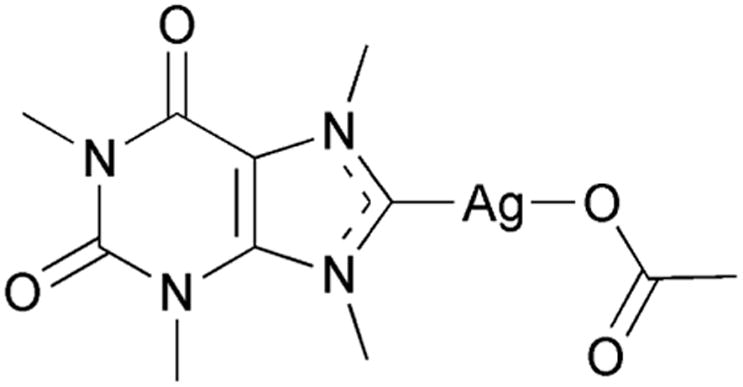
Molecular structure of SCC1.
Fig. 2.
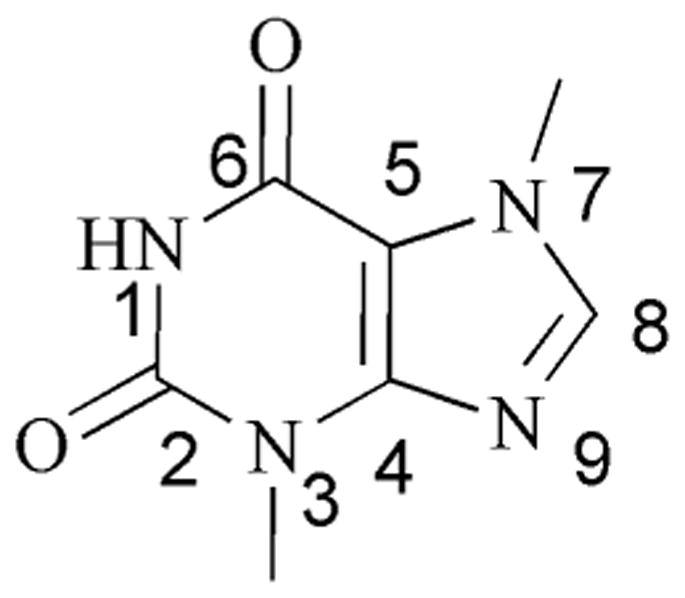
Numbering scheme for theobromine.
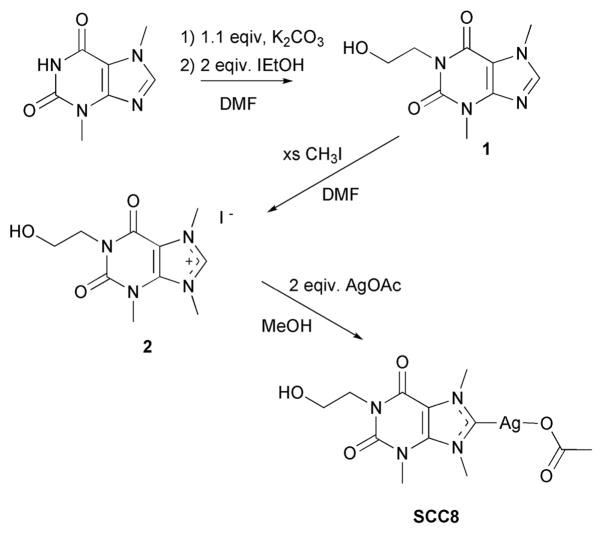
The synthesis of SCC8 was carried out in three steps utilizing theobromine as the main chemical building block (Scheme 1). The N1 nitrogen atom of theobromine was first deprotonated with potassium carbonate followed by condensation with 2-iodo-ethanol to give 1-(2-hydroxyethyl)-3,7-dimethylxanthine 1. Initially it was noticed that the use of stronger bases such as potassium hydroxide produced multiple products in this step. Subsequent methylation afforded the carbene precursor 1-(2-hydroxyethyl)-3,7,9-trimethylxanthinium iodide 2 which was reacted with two equivalents of silver acetate to give SCC8 in 74% yield. The addition of the ethanol functionality off the N1 nitrogen atom served to enhance the solubility of SCC8 ten fold (123 mg/mL) over that of SCC1.
Scheme 1.
Synthesis of silver carbene SCC8.
1H and 13C NMR were used to confirm the formation of the silver complex. The most notable change in the 1H NMR is the loss of the xanthinium hydrogen resonance at 9.33 ppm. The 13C NMR further confirmed the structure of SCC8 with an observed shift of the C8 carbon atom from 139.6 to 186.5 ppm.
Crystals of SCC8 suitable for single crystal X-ray diffraction studies were grown by slow evaporation from a concentrated sample in methanol, Fig. 3. The asymmetric unit contains one full molecule of SCC8. The silver atom is bonded to the carbene carbon atom (C1-Ag1 = 2.072(4) Å) and one of the oxygen atoms of the acetate counter ion (O4-Ag1 = 2.118(3) Å). Bond distances and angles are consistent with those of previously reported for other xanthine derived carbene silver acetate complexes (Ccarbene-Ag = 2.067(3)−2.096(4) Å).5,15 The solid state structure of SCC8 also shows a short intermolecular Ag-Ag interaction of approximately 3.2 Å, which is shorter than the sum of the two van der Waals radii (3.44 Å) of the silver atoms but longer than the 2.89 Å bond distance observed in metallic silver.20,21 NHC silver complexes with argentophilic interactions of similar distances and shorter have been previously reported in the literature.3,11,15,22,23
Fig. 3.
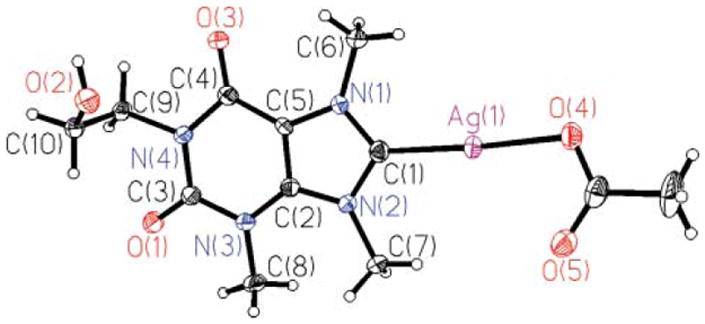
Thermal ellipsoid plot of silver complex SCC8 with thermal ellipsoids shown at 50% probability.
MIC and MBC of SCC8
The minimum inhibitory concentration (MIC) of SCC8 against strains of P. aeruginosa, Alcaligenes xylosoxidans, Stenotrophomonas maltophilia, S. aureus, and Burkholderia species was determined by broth microdilution (Table 1).
Table 1.
MIC and MBC values for SCC8
| Species strain | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|
| Pseudomonas aeruginosa | ||
| PA O1-Va | 1 | 2 |
| PA M57-15b | 1 | 6 |
| PA HP3c | 1 | 1 |
| PA SB05c | 1 | 2 |
| Alcaligenes xylosoxidans | ||
| AX RE05c | 1 | 2 |
| AX OT05c | 1 | 1 |
| Stenotrophomonas maltophilia | ||
| SM BE06c | 1 | 2 |
| SM AH06c | 1 | 8 |
| Staphylococcus aureus | ||
| SA EH06d | 1 | 6 |
| SA LL06d | 1 | 6 |
| Burkholderia multivorans | ||
| BM DP06c | 1 | 2 |
| BM CS06c | 2 | 4 |
| Burkholderia cenocpeacia | ||
| J2315e | 1 | 8 |
| Burkholderia dolosa | ||
| AU4459e | 1 | 6 |
| Burkholderia thailandensis | ||
| E264f | 1 | 1 |
| Burkholderia gladioli | ||
| BG CM06c | 1 | 2 |
| Escherichia coli | ||
| J53g | 2 | 2 |
| J53+pMG101h | >10 | <10 |
Common laboratory strain.
Mucoid strain from a CF patient.
MDR strain from a CF patient.
MRSA strain from a CF patient.
Epidemic clinical strain.
Wild type strain.
Silver sensitive strain.
Strain harboring silver resistance plasmid.
A 10 mg/mL stock solution of SCC8 in sterile water was prepared and diluted 1:1 into M-H containing 105 colony forming units (CFU) of each bacterial strain to yield the appropriate test concentration (1, 2, 4, 6, 8, or 10 μg of SCC8/mL). The E. coli strain J53 is known to be sensitive to killing by silver cations and serves as a positive control. In contrast, the J53 + pGM101 is a J53 derivative that harbors the pGM101 plasmid originally conferring silver-resistance to a burn ward isolate of Salmonella and serves as a negative control.24 The MIC90 of SCC8 was 1 μg/mL for non-silver resistant organisms tested (Table 1). The MIC for J53 lacking the silver resistant plasmid was 2 μg/mL, whereas the MIC of SCC8 for J53 containing pMG101 was greater than 10 μg/mL demonstrating that the antimicrobial activity of SCC8 is primarily due to the silver moiety. Determination of the minimum bactericidal concentration (MBC) of SCC8 against the tested strains was performed. With the exception of the silver resistant E. coli strain, SCC8 appeared to be bactericidal for all of the strains tested, which indicates that SCC8 is capable of killing numerous bacterial strains at clinically achievable concentrations.
In vivo efficacy of SCC8 and SCC1
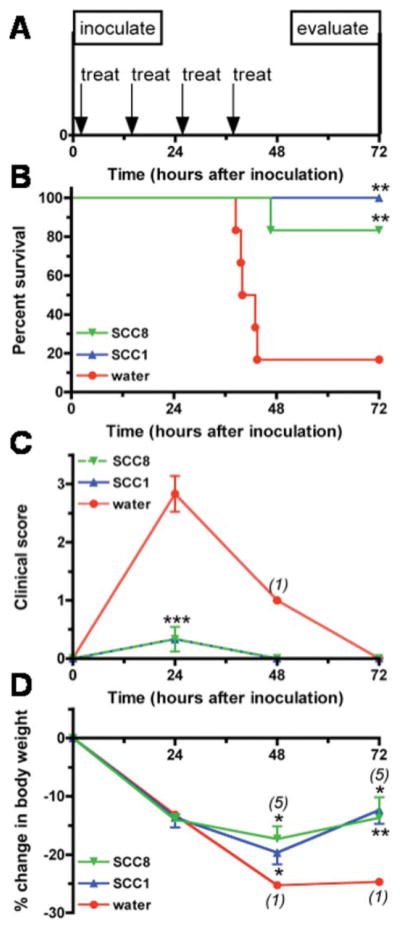
To compare the in vivo efficacy of SCC1 and SCC8 in a mouse pneumonia model, four 50 mg doses of aerosolize SCC1 and SCC8 were delivered 12 hours apart in a nose-only fashion to P. aeruginosa-infected mice in a multi-dosing chamber (Fig. 4A). Treatment with SCC1 resulted in 100% survival for an 83% survival advantage compared with water-treated animals (Fig. 4B: 6/6, 100% SCC1 survival versus 1/6, 17% water; p = 0.0043) and treatment with SCC8 resulted in 83% survival for a 66% survival advantage (Fig. 4B: 5/6, 83% SCC8 survival versus 1/6, 17% water; p = 0.0043). The small difference in survival between SCC1 and SCC8 may be explained by the slightly decreased molar dose of Ag(I) delivered to the SCC8 group based on the differences in molecular weights of the two compounds (MW SCC1: 375.13, SCC8: 405.16. Thus, 14.4 mg Ag(I) delivered as SCC1 and 13.3 mg Ag(I) as SCC8 an 8% difference). The differences in survival between SCC1 and SCC8 were not evident in the clinical appearance of the animals, as both treatments resulted in significant and identical improvements in clinical scores by 24 hours after inoculation compared to the water-treated group (SCC1 versus water and SCC8 versus water both p < 0.001 by ANOVA). The weight loss was identical at 24 hours in all of the treatment groups, but by 48 hours, the slope of the weight loss curve in the SCC1 and SCC8-treated animals had decreased and the mean weight loss in those groups differed significantly from that of the single surviving water-treated mouse (SCC1 versus water, p = 0.0395 and SCC8 versus water, p = 0.0212 by 1 sample t-test). By 72 hours, the SCC1 and 5 surviving SCC8-treated mice were gaining weight (SCC1 versus water, p = 0.0037, SCC8 versus water, p = 0.0356 by 1 sample t-test).
Fig. 4.
Effect of nebulized SCC1 and SCC8 on survival, clinical scores, and weight loss in a mouse model of Pseudomonas pneumonia. A) Treatment protocol. Mice received treatment with nebulized SCC1, SCC8 or water one hour after infection with P. aeruginosa PA M57-15, and again every 12 h for a total of 4 doses. Mice were evaluated on day 3 (72 h). B) Kaplan-Meier survival curves. The survival of mice treated with SCC1, SCC8 and water were 100%, 83% and 17%, respectively (SCC1 vs. water p = 0.0043, SCC8 vs. water p = 0.0096). C) Clinical scores. Clinical scores for each of the three treatment groups are displayed as mean ± SD. Numbers in parentheses indicate the number of surviving animals available for analysis, if less than the initial group size of 6. At the 24 hour time point, SCC1 vs. water and SCC8 vs. water both p < 0.001 by ANOVA. D) Weight loss. Displayed are the mean % weight changes from inoculation weight ± SD for SCC1, SCC8 and water-treated mice. The SCC1 and SCC8 treatment groups were compared at 48 and 72 hours to the single living water-treated animal by 1 sample t-test (48 h: SCC1 vs. water, p = 0.0395, SCC8 vs. water, p = 0.0212; 72 h: SCC1 vs. water, p = 0.0037, SCC8 vs. water, p = 0.0356).
The gross pathology of the lungs mirrored the outcome of the SCC1, SCC8 and water-treated animals. The lungs of the SCC1 and surviving SCC8-treated animals were pink and collapsed upon opening the chest cavity, whereas the lungs of 4 water-treated animals that were opened at the time of death (3 of those that died before 48 hours that were observed at the precise moment of death and the sole survivor) were inflamed and stiff. Spleens were harvested from the 4 water-treated animals and the SCC1-and all SCC8-treated animals to assay for bacteria as a marker of dissemination from the lung. All of the animals that received only nebulized water had bacteria in the spleen (Fig. 5). The only animal that had bacteria in the spleen among the SCC1- and SCC8-treated animals was the single SCC8 animal that died. Thus, the SCC1 and SCC8 animals had a significantly less dissemination of bacteria from the lungs to the spleen (SCC1 versus water, p = 0.0067; SCC8 versus water, p = 0.0357 by Fisher’s exact test of the contingency table). Thus, as we have seen in previous studies, survival appears related to bacteremia, as all of the animals that died had bacteria recovered from their spleens.13,14 These findings indicate that treatment with SCC1 and SCC8 can effectively decrease the likelihood of bacteremia and death in a P. aeruginosa pneumonia model.
Fig. 5.
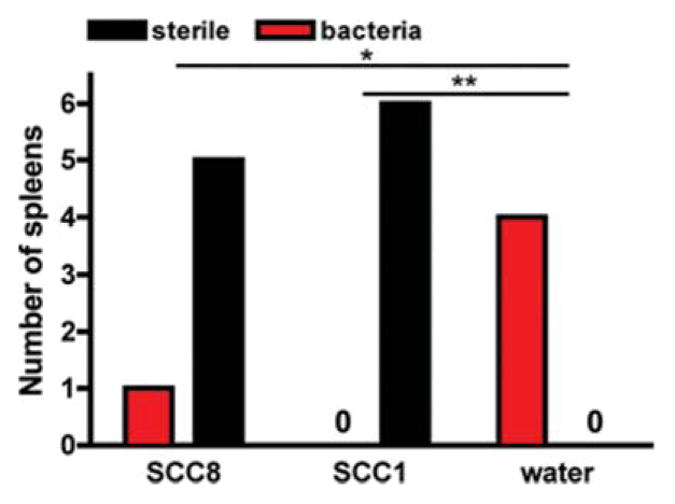
Effects of nebulized SCC1 and SCC8 on the likelihood of bacterial dissemination to the spleen in a mouse model of Pseudomonas pneumonia. Bacteremia in all of the mice plotted as number of spleens in each treatment group (SCC1 vs. water, p = 0.0067; SCC8 vs. water, p = 0.0357).
Experimental
General considerations
2-Iodoethanol, iodomethane, theobromine, and silver acetate were purchased from Alfa Aesar and used as received. All solvents were purchased from Fisher Scientific and used without further purification. All reactions were carried out under aerobic conditions. 1H and 13C NMR data were obtained on a Varian 500 MHz instrument. The spectra were referenced to deuterated solvents. Elemental analyses were preformed by the University of Illinois microanalysis laboratory. Mass spectrometry analyses were preformed by the Ohio State University mass spectrometry and proteomics facility. Crystal structure data sets were collected on a Bruker SMART Apex CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Unit cell determination was achieved by using reflections from three different orientations.
1-Hydroxyethyl-3,7-dimethylxanthine (1)
Theobromine (9.0 g, 50 mmol) and potassium carbonate (7.6 g, 55 mmol) were dissolved in DMF (150 mL). The solution was brought to reflux at which time 2-iodoethanol (3.9 mL, 50 mmol) was added. The solution was stirred for 1 h and an additional equivalent of 2-iodoethanol (3.9 mL, 50 mmol) was added. The reaction was then stirred at reflux for 17 h. Volatiles were removed by rotary evaporator and the crude material dissolved methanol and cooled to give 1 as colorless crystals (7.6 g, 68%). Mp: 201–203 °C. 1H NMR (500 MHz, DMSO-d6) δ 3.39 (s, 3H, CH3), 3.50 (q, 2H, CH2), 3.87 (s, 3H, CH3), 3.94 (t, 2H, CH2), 4.70 (t, 1H, OH), 7.98 (s, 1H, CH). 13C{1H} NMR (125 MHz, DMSO-d6) δ 29.3 (CH3), 33.1 (CH3), 42.4 (CH2), 57.7 (CH2), 106.6 (C), 142.8 (CH), 148.1 (C), 150.9 (C=O), 154.4 (C=O). Found: C, 48.00; H, 5.33; N, 24.25. Calc. for C9H12N4O3: C, 48.19; H, 5.40; N, 25.00%. TOF-MS-EA+: m/z 247 (M+ of C9H12N4O3Na).
1-(2-Hydroxyethyl)-3,7,9-trimethylxanthinium iodide (2)
Compound 1 (2.0 g, 8.9 mmol) was added to iodomethane (30 mL, 92.7 mmol) in a pressure tube and DMF was added dropwise until the solution appeared clear. The tube was sealed and heated at 80 °C for 2 d with stirring. Volatiles were removed by rotary evaporator and the resulting solid was washed with acetone to give 2 as a pale yellow solid (2.4 g, 66%). Mp: 175-176 °C. 1H NMR (500 MHz, DMSO-d6) δ 3.54 (t, 2H, CH2), 3.73 (s, 3H, CH3), 3.98 (t, 2H, CH2), 4.05 (s, 3H, CH3), 4.15 (s, 3H, CH3), 4.47 (bs, 1H, OH), 9.33 (s, 1H, CH). 13C{1H} NMR (125 MHz, DMSO-d6) δ 31.3 (CH3), 35.6 (CH3), 36.9 (CH3), 43.4 (CH2), 57.2 (CH2), 107.7 (C), 139.3 (C), 139.6 (CH) 150.0 (C=O), 153.2 (C=O). Found: C, 32.47; H, 4.00; N, 14.53. Calc. for C10H15N4O3I: C, 32.79; H, 4.13; N, 15.30%. TOF-MS-EA+: m/z 239 (M+ of C10H15N4O3).
X-Ray crystal structure analysis: formula C10H15IN4O3, Mw = 366.16, colorless crystal 0.37 × 0.29 × 0.19 mm, a = 15.0967(18) Å, b = 10.4071(13) Å, c = 8.708(10) Å, α = 90°, β = 104.119(2)°, γ = 90°, V = 1326.8(3) Å3, Dcalc = 1.833 Mg cm−3, μ = 2.421 mm−1, Z = 4, monoclinic, space group P21/c (No. 14), λ = 0.71073 Å, T = 100 K, ω and ϕ scans, 10406 reflections collected, 2684 independent (Rint) 0.0498, 167 refined parameters, R1/wR2 (I ≥ 2σ(I)) = 0.0410/0.1014 and R1/wR2 (all data) = 0.0489/0.1060, maximum (minimum) residual electron density 1.963 (−1.374) e Å−3.
1-(2-Hydroxyethyl)-3,7,9-trimethylxanthin-8-ylidene silver acetate (SCC8)
Xanthinium salt 2 (1.8 g, 4.9 mmol) was dissolved in methanol (200 mL) and silver acetate (1.6 g, 9.8 mmol) was added with stirring. The reaction was stirred 1.5 h during which time a yellow precipitate of AgI was formed. The reaction mixture was filtered and the volatiles removed by rotary evaporation. The resulting solid was washed with acetone and dried to afford SCC8 as a white solid (1.5 g, 74%). Mp: 212-214 °C. 1H NMR (500 MHz, DMSO-d6) δ 1.74 (s, 3H, CH3 of OAc), 3.54 (t, 2H, CH2), 3.75 (s, 3H, CH3), 3.98 (t, 2H, CH2), 4.07 (s, 3H, CH3), 4.20 (s, 3H, CH3). 13C{1H} NMR (125 MHz, DMSO-d6) δ 23.4 (CH3 of OAc), 31.5 (CH3), 37.8 (CH3), 39.1 (CH3), 43.2 (CH2), 57.4 (CH2), 108.9 (C), 140.5 (C), 150.4 (C=O), 153.2 (C=O), 173.6 (C=O of OAc), 186.5 (C-Ag). Found: C, 35.48; H, 4.14; N, 13.40. Calc. for C12H17N4O5Ag: C, 35.64; H, 4.24; N, 13.86%. TOF-MS-EA+: m/z 345/347 (M+ of C10H14N4O3Ag).
X-Ray crystal structure analysis: formula C12H17AgN4O5, Mw = 405.17, colorless crystal 0.43 × 0.08 × 0.07 mm, a = 23.896(3) Å, b = 8.2797(11) Å, c = 14.8598(19) Å, α = 90°, β = 94.104(2)°, γ =90°, V = 2932.5(6) Å3, Dcalc = 1.835 Mg cm−3, μ = 1.405 mm−1, Z = 8, monoclinic, space group C2/c (No. 15), λ = 0.71073 Å, T = 100 K, ω and ϕ scans, 11365 reflections collected, 2985 independent (Rint) 0.0476, 204 refined parameters, R1/wR2 (I ≥ 2σ(I)) = 0.0395/0.0931 and R1/wR2 (all data) = 0.0535/0.1001, maximum (minimum) residual electron density 1.160 (−0.658) e Å−3.
Bacteria
The laboratory strain PAO1-V was provided by Dr Maynard Olson (University of Washington, Seattle). The mucoid clinical isolate of P. aeruginosa PA M57-15 was provided by Dr Thomas Ferkol (Washington University, St. Louis, MO). The PA HP3, PA SB05, AX RE05, AX OT05, SM BE06, SM AH06, SA EH06, SA LL06, BM DP06, BM CS06, and BG CM06 strains were cultured from the sputum of cystic fibrosis patients at St. Louis Children’s Hospital. Burkholderia cepacia complex organisms J2315, and AU4459 were provided by Dr John Lipuma (University of Michigan, Ann Arbor, MI). Burkholderia thailandensis strain E264 was provided by Dr Josie Gray (University of Washington, Seattle). The silver sensitive and silver resistant E. coli strains J53 and J53+pMG101, were provided by Dr Simon Silver (University of Chicago, Chicago, IL).24 All bacterial strains were maintained as glycerol stocks at −80 °C.
Drugs
Stocks of SCC1 (Ksp of 11 mg/mL) and SCC8 (Ksp of 125 mg/mL) were reconstituted in sterile water (Milli-Q Synthesis System, Millipore Corp., Billerica, MA) to a concentration of 10 mg/mL and aliquots of 1 mL were stored at −80 °C until use.
In vitro antimicrobial activity
Minimal inhibitory concentrations (MICs) were determined by broth microdilution method as previously described by a standard Clinical and Laboratory Standards Institute (CLSI) protocol.13 Briefly, bacteria were streaked from frozen glycerol stocks onto TSA or blood agar plates and incubated overnight at 37 °C. Colonies from the fresh plates were suspended in the CLSI standard M-H broth to an optical density at 650 nm (OD650) of 0.2 and grown at 37 °C in a shaking incubator at 200 rpm to an OD650 of 0.4, which corresponds to ~5 × 108 colony forming units (CFU)/mL. The bacteria were diluted in the broth to a concentration of 105 in 100 μL, which was added to triplicate wells of a 96-well plate, containing 100 μL of SCC8 diluted in sterile water to various concentrations from 10 mg/mL stock. The final concentrations tested were 1, 2, 4, 6, 8, and 10 μg/mL. The plates were incubated overnight at 37 °C. The MIC was the lowest of these concentrations, at which each of the triplicate wells in each 96-well plate was clear. Each triplicate measurement was performed at least in duplicate for a minimum of 6 separate measurements. The MBC of SCC8 was determined by plating the wells with growth inhibition (clear) on TSA or blood agar plates and noting the lowest concentration that resulted in no growth after an overnight incubation at 37 °C.
Mice
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) at 5 weeks of age were used for these studies that were approved by the Washington University School of Medicine Animal Studies Committee. Animals were housed in a barrier facility under pathogen-free conditions until they were inoculated with bacteria.
Delivery
SCC1, SCC8, and water were delivered via an Aeroneb Lab apparatus (Aerogen Inc., Galway, Ireland) connected to a multi-dosing animal chamber. The Aerogen nebulizer is based on micropump technology that produces 4 to 6 μm particles in a low velocity aerosol. The multi-dosing chamber is a square plexiglass box with inner dimensions of 8 × 8 × 4.5 inches height. The nebulizer is mounted in the center of the lid.
Pulmonary infection model
Mice (16–23 g) were anesthetized using a cocktail composed of a 1:1 ratio of ketamine HCL (100 mg/mL, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine sterile solution (20 mg/mL, Bedford, OH). Each mouse received an intramuscular injection of approximately 1 μL/gm body weight of the cocktail mix. A 100 μL aliquot of PA M57-15 in LB (1.9 × 106 CFU) was delivered to each mouse intranasally. Beginning 1 hour after inoculation, the treatment groups of 6 mice each received 5 mL of either SCC1 (50 mg total dose), SCC8 (50 mg total dose), or water via 15-minute nebulization exposure periods every 12 hours for a total of four doses. To ensure nose-only delivery of the drugs, mice were individually placed in CH-247 tubes (CH Technologies, Westwood, NJ) prior to placement into a multi-dosing chamber. Mice were weighed and scored based on their clinical appearance daily. Three days after infection (72 hour after the initial inoculation), mice were euthanized with carbon dioxide and their lungs and spleens were visualized for signs of inflammation. Spleens were harvested, homogenized in 1 mL of LB (PowerGen 125 homogenizer, Fischer Scientific) and 10 μL aliquots of the homogenate were plated in duplicate on TSA plates and grown overnight at 37 °C. Presence or absence of bacterial colonies was noted to determine bacteremia.
Clinical score
Mice were examined for features of activity, fur and posture. The activity scores were as follows: 0 for normal activity, 1 for slow, but spontaneous walking, 2 for movement only with stimulus and 3 for lack of movement with stimulus. The fur was noted to be either normal (score of 0) or ruffled (score of 1). The mice were noted to have normal posture (score of 0) or to be hunched (score of 1). Thus, clinical scores ranged from 0 for a well animal to 5 for a moribund animal.
Statistical analysis
All analyses were performed using Prism 4. The in vivo survival curves in the infection model were compared using a log-rank test. Changes in animal weights at 24 hours after inoculation were compared by ANOVA. The average clinical scores for the three groups at 48 and 72 hours after inoculation were compared by 1 sample t-test using the single living water-treated mouse as the test mean. Data are given as mean ± standard deviation. The risk of bacteremia between the three treatment groups was compared by Fisher’s exact test of a contingency table (number of animals with bacteria in the spleen versus number of animals cleared).
Conclusion
The studies presented demonstrate the potential to take advantage of naturally occurring chemical moieties for the synthesis of new silver carbene complexes. In particular, theobromine serves as an excellent chemical building block for such products, in part due to its ease of functionalization at the N1 nitrogen atom upon deprotonation. The addition of alcohols such as a hydroxyethyl group at this position appears to greatly enhance the water solubility of the resulting silver carbene complex. In fact SCC8 demonstrated a ten fold enhancement in water solubility over an analogous caffeine derivative. Like previously reported SCCs, SCC8 showed single digit μg/mL concentration MICs in vitro on all relevant CF pathogens tested including MDR strains. In vivo efficacy studies conducted have demonstrated that nebulized silver carbene complexes such as SCC1 and SCC8 have great potential in aiding in the future treatment of virulent and MDR pulmonary infections associated with CF.
Acknowledgments
This work was supported by the Washington University School of Medicine, The University of Akron, The University of Akron Center for Silver Therapeutics Research, The Goodyear Tire & Rubber Company, the National Institute of Allergies and Infectious Diseases (1 R01 A106785601) and The National Institute of General Medical Sciences (1 R01 GM86895-01). We wish to thank the Ohio Board of Regents and the National Science Foundation for funds used to purchase the 500 MHz NMR instrument (CHE-0341701 and DMR-0414599) and the Bruker-Nonius Apex CCD X-ray diffractometer (CHE-0116041) used in this research.
Footnotes
CCDC reference numbers 737693 and 737694 for 1-(2-hydroxyethyl)-3,7,9-trimethylxanthinium iodide and SCC8. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b907726j
Notes and references
- 1.Hindi KM, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ. Chem Rev. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn FE, Jahnke MC. Angew Chem Int Ed. 2008;47:3122–3172. doi: 10.1002/anie.200703883. [DOI] [PubMed] [Google Scholar]
- 3.Melaiye A, Sun Z, Zhaohui, Hindi K, Milsted A, Ely D, Reneker DH, Tessier CA, Youngs WJ. J Am Chem Soc. 2005;127:2285–2291. doi: 10.1021/ja040226s. [DOI] [PubMed] [Google Scholar]
- 4.Youngs WJ, Tessier CA, Garrison JC, Quezada CA, Melaiye A, Durmus S, Panzner MJ, Kascatan-Nebioglu A. In: Medicinal Inorganic Chemistry. Sessler JL, Doctrow SR, McMurry TJ, Lippard SJ, editors. American Chemical Society, Oxford University Press; 2005. pp. 414–427. ACS Symposium Series 903. [Google Scholar]
- 5.Kascatan-Nebioglu A, Melaiye A, Hindi K, Durmus S, Panzner MJ, Hogue LA, Mallett RJ, Hovis CE, Coughenour M, Crosby SD, Milsted A, Ely DL, Tessier CA, Cannon CL, Youngs WJ. J Med Chem. 2006;49:6811–6818. doi: 10.1021/jm060711t. [DOI] [PubMed] [Google Scholar]
- 6.Panzner MJ, Tessier CA, Youngs WJ. In: The Chemistry of Pincer Compounds. 1. Morales-Morales D, Jensen CM, editors. Elsevier; Amsterdam: 2007. pp. 139–150. [Google Scholar]
- 7.Kascatan-Nebioglu A, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ. Coord Chem Rev. 2007;251:884–895. [Google Scholar]
- 8.Hindi KM, Siciliano TJ, Durmus S, Panzner MJ, Medvetz DA, Reddy VD, Hogue LA, Hovis CE, Hilliard JK, Mallet RJ, Tessier CA, Cannon CL, Youngs WJ. J Med Chem. 2008;51:1577–1583. doi: 10.1021/jm0708679. [DOI] [PubMed] [Google Scholar]
- 9.US Pat., 2007021401, 2007.
- 10.WO Pat., 2009015112, 2009.
- 11.Garrison JC, Tessier CA, Youngs WJ. J Organomet Chem. 2005;690:6008–6020. [Google Scholar]
- 12.Kascatan-Nebioglu A, Panzner MJ, Garrison JC, Tessier CA, Youngs WJ. Organometallics. 2004;23:1928. [Google Scholar]
- 13.Hindi KM, Ditto AJ, Panzner MJ, Medvetz DA, Hovis CE, Han DS, Hilliard JK, Taylor JB, Yun YH, Cannon CL, Youngs WJ. Biomaterials. 2009;30:3771–3779. doi: 10.1016/j.biomaterials.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon CL, Hogue LA, Vajravelu R, Capps GH, Ibricevic A, Hindi KM, Mallett RJ, Hovis CE, Walter MJ, Kascatan-Nebioglu A, Brody SL, Youngs WJ. Antimicrob Agents Chemother. 2009;53:3285–3293. doi: 10.1128/AAC.00314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panzner MJ, Deeraksa A, Smith A, Wright BD, Hindi KM, Kascatan-Nebioglu A, Torres AG, Judy BM, Hovis CE, Hilliard JK, Mallett RJ, Cope E, Estes DM, Cannon CL, Leid JG, Youngs WJ. Eur J Inorg Chem. 2009;13:1739–1745. doi: 10.1002/ejic.200801159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh MJ, Ramsey BW, Accurso F, Cutting CR. In: The Metabolic and Molecular Basis of Inherited Disease. 8. Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. Vol. 3. McGraw Hill; New York: 2001. pp. 5121–5189. [Google Scholar]
- 17.Beers MH, Berkow R, editors. The Merck Manual of Diagnosis and Therapy. 17. Merck and Co; Whitehouse Station, N.J: 1999. Cystic Fibrosis. Chapter 19, Section 267. [Google Scholar]
- 18.Lyczak JB, Cannon CL, Pier GB. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaulac C, Clement-Major S, Hawari J. J Lagace, Antimicrob Agents Chemother. 1996;40:665–669. doi: 10.1128/aac.40.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu HL, Zhang XM, Yu Q, Wang DQ. Z Kristallogr. 2003;218:307–308. [Google Scholar]
- 21.Baenziger NC, Struss AW. Inorg Chem. 1976;15:1807–1809. [Google Scholar]
- 22.Catalano VJ, Malwitz MA. Inorg Chem. 2003;42:5483–5385. doi: 10.1021/ic034483u. [DOI] [PubMed] [Google Scholar]
- 23.Hahn FE, Radloff C, Pape T, Hepp A. Chem Eur J. 2008;14:10900–10904. doi: 10.1002/chem.200801877. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Matsui K, Lo JF, Silver S. Nat Med. 1999;5:183–188. doi: 10.1038/5545. [DOI] [PubMed] [Google Scholar]